Abstract
Purpose
To investigate the effect of an anti-TNF-α agent (etanercept) on recovery processes in a partial spinal cord injury (SCI) model using clinical and electrophysiological tests.
Methods
Twenty-four New Zealand rabbits were divided into three groups: group 1 [SCI + 2 ml saline intramuscular (i.m.), n = 8], group 2 (SCI + 2.5 mg/kg etanercept, i.m., 2–4 h after SCI, n = 8) and group 3 (SCI + 2.5 mg/kg etanercept, i.m., 12–24 h after SCI, n = 8). Rabbits were evaluated before SCI, immediately after SCI, 1 week after, and 2 weeks after SCI, clinically by Tarlov scale and electrophysiologically by SEP.
Results
Tarlov scores of groups 2 and 3 were significantly better than group 1, 2 weeks after SCI. SEP recovery was significantly better in groups 2 and 3 than group 1, 2 weeks after SCI.
Conclusions
These results show that blocking TNF-α mediated inflammation pathway by an anti-TNF-α agent enhances clinical and electrophysiological recovery processes in partial SCI model.
Keywords: Spinal cord injury, Inflammation, TNF-α, Etanercept, Somatosensory evoked potentials
Introduction
Traumatic spinal cord injury (SCI) is an important cause of neurological disability and there is no available treatment yet. Mechanisms of SCI are described as primary and secondary [1]. In primary injury, there is physical and mechanical trauma to the spinal cord. Potential recovery after this stage depends on residual physical neural connections even if there is total functional loss. In secondary injury in which the presence of ischemia and microvascular damage, glutamatergic excitotoxicity, oxidative stress and inflammation have important roles, most of the cell death occurs and persistent functional loss takes place [1].
Inflammation in SCI starts with the migration of neutrophils and macrophages to the injury site. Production of cytokines or interleukins triggers immune system cells to respond to the injury by initiating and coordinating cellular and humoral responses [1]. Gene expression studies in acute SCI revealed that spreading inflammatory signals starts in early phase after SCI [2].
TNF-α is a cytokine with pleiotropic functions in immunity, inflammation, control of cell proliferation, differentiation and apoptosis, and can be present both as a transmembrane protein or a soluble cytokine [3]. TNF-α is synthesized as a precursor protein, which is produced especially by the activated macrophages and also by T and B lymphocytes, natural killer cells, dendritic cells and monocytes. Within the central nervous system, TNF-α is produced by microglia, astrocytes and certain neuron populations [3, 4]. TNF-α interact with two structurally related, but functionally distinct TNF receptors. TNFR1 is constitutively expressed in practically all cell types except erythrocytes, whereas TNFR2 is generally inducible and mainly expressed by endothelial cells, immune cells and certain neuron populations. TNFR1 can trigger two distinct signaling pathways, of which one of them leads to transcription of pro-inflammatory and antiapoptotic genes and the other leads to apoptosis. TNF-α binding to TNFR1 will activate pro-inflammatory or apoptotic signaling pathways [3, 5]. Signaling through TNFR2 activates proinflammatory and survival signaling pathways [3, 6].
TNF-α and other cytokines are released in response to stress and after injury [7]. SCI-induced TNF-α contributes to cell death via processes that involve the modulation of normal synaptic transmission, inducing cFOS cooperatively with glutamate [1, 7, 8]. During the acute phase of SCI, expression of proinflammatory cytokines which also include TNF-α increase 6–12 h after injury and peak at 4 days causing apoptosis in neurons and oligodendrocytes [1, 2, 9].
Anti-TNF-α agents are clinically in use for the treatment of inflammatory diseases as rheumatoid arthritis, juvenile polyarticular rheumatoid arthritis, inflammatory bowel disease, psoriatic arthritis, ankylosing spondylitis and other systemic autoimmune disorders [3]. Etanercept, which is one of the anti-TNF-α agents, is a genetically engineered fusion protein composed of a dimmer of the extracellular portions of human TNFR2 fused to the Fc portion of human IgG1 [3]. It reduces the effect of TNF functioning as decoy receptor that binds to TNF [10].
Using immunohistochemical and other molecular techniques, Genovese et al. [11] state clearly that etanercept attenuates the degree of TNF-α and IL-1β in the injured spinal cord, the infiltration of the injured spinal cord with neutrophils, cell apoptosis, iNOS and COX-2 expression, nitrotyrosine formation and spinal cord damage.
Animal models for SCI researches are classified into two groups as contusion and transection models [12]. As compression and contusion types of SCI are the most commonly seen injuries in humans, contusion models may be more appropriate for assessment of acute management strategies [12]. This type of SCI can be produced by using a surgical spring-loaded clip, balloon and computer-controlled reproducible impact contusion devices. Balloon compression method has the advantages of reproducibility, adjusting the time and the degree of compression. The majority of compression/contusion type of SCI models result in complete and irreversible neurological transection. In this study, a partial SCI model described and developed in our department is used [13, 14]. Through this model, detailed investigations on anatomical, functional, cellular [13, 14] and biochemical recovery processes and stigmata are possible. Partial SCI models have previously been used to investigate cellular trophism and plasticity, and neurobiological mechanisms of locomotor training programs [15, 16].
The neuroelectrophysiological effect of anti-TNF-α agents in a partial/reversible SCI model has not been studied yet. In this study, we aimed to test and observe the effect of etanercept, one of the available anti-TNF-α agents, on partial SCI by clinical and electrophysiological follow-up in rabbit.
Materials and methods
The investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996) and approval was received from the Institutional Animal Ethics Committee at Cumhuriyet University.
Animals
Twenty-four New Zealand male and female rabbits, weighing 2.5–3.0 kg, were divided into three groups: group 1: control (SCI + 2 ml saline i.m. paraspinally to SCI site, n = 8), group 2: early etanercept (SCI + 2.5 mg/kg etanercept i.m. paraspinally to SCI site, 2–4 h after SCI, n = 8) and group 3: late etanercept (SCI + 2.5 mg/kg etanercept i.m. paraspinally to SCI site, 12–24 h after SCI, n = 8). The animals were allowed access to water and food ad libitum, during the pre-surgery and post-surgery period. The animals were kept at the Animal Care Facility of Cumhuriyet University School of Medicine.
Surgical procedure
All rabbits in the control and etanercept groups were anesthetized via intramuscular injection of xylazine hydrochloride (Rompun, Bayer, Istanbul, Turkey) 5 mg/kg and ketamine hydrochloride (Ketalar, Pfizer, Istanbul, Turkey) 50 mg/kg; breathing was continued spontaneously with room air. Rabbits were positioned prone on the operating table. Under a sterile technique, a midline dorsal incision was done. The laminae and transverse processes of T6–L2 were exposed by gentle blunt dissection of paravertebral muscles. A self-retaining retractor was placed in the operation area and laminectomy was performed at T10. A balloon angioplasty catheter (Brio PTCA balloon catheter-ICV8368, 1.5 × 30 mm, Italy) was placed at the epidural space below the T9 lamina. The balloon was gradually inflated until 4 atm pressure was achieved; this pressure was maintained for 30 min and the balloon was deflated. Following removal of the balloon catheter, paravertebral fascia and skin were sutured.
In this method described, developed and used in our department [13, 14], 30 min compression at 4 atm pressure results in partial and relatively reversible neurological function loss. At the end of the procedures, all rabbits were killed under deep anesthesia.
SEP recording
To assess the electrophysiological status, SEP latencies were recorded before SCI, immediately after SCI, 1 week after SCI, and 2 weeks after SCI. A Dantec-Keypoint 4-channel electromyography device was used for the recordings. SEPs were recorded with thin subcutaneous needle electrodes (0.2 mm diameter) from the parietal region 2 mm lateral to the midline and 2 mm caudal from the coronal suture. A similar reference electrode was inserted to midline over nasion. The posterior tibial nerve contralateral to the skull electrode was stimulated by needle electrodes. The stimulus rate was 5/s with duration of 0.2 ms. Stimulus intensity was fixed slightly above the motor threshold. Bandpass filter was set at 20 Hz–1 KHz. Two trials were performed to demonstrate the consistency of the waveforms.
Neurological evaluation
Rabbits were evaluated immediately after SCI, 1 week after SCI, and 2 weeks after SCI by an independent observer according to the Tarlov scoring system as described in Table 1.
Table 1.
Criteria in Tarlov scoring [23]
| Score | Neurological outcome |
|---|---|
| 0 | Spastic paraplegia and no movement of the lower limbs |
| 1 | Spastic paraplegia and slight movement of the lower limbs |
| 2 | Good movement of the lower limbs, but inability to stand |
| 3 | Able to stand, but unable to walk normally |
| 4 | Complete recovery and normal gait/hopping |
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences for Windows (SPSS version 15.0, Chicago, IL, USA). All values were expressed as mean ± SD. Comparison of variables between the groups was performed with Kruskal Wallis test and Mann–Whitney U test. Friedman and Wilcoxon tests were used for comparison of in-group data analysis. A value of P < 0.05 was considered statistically significant.
Results
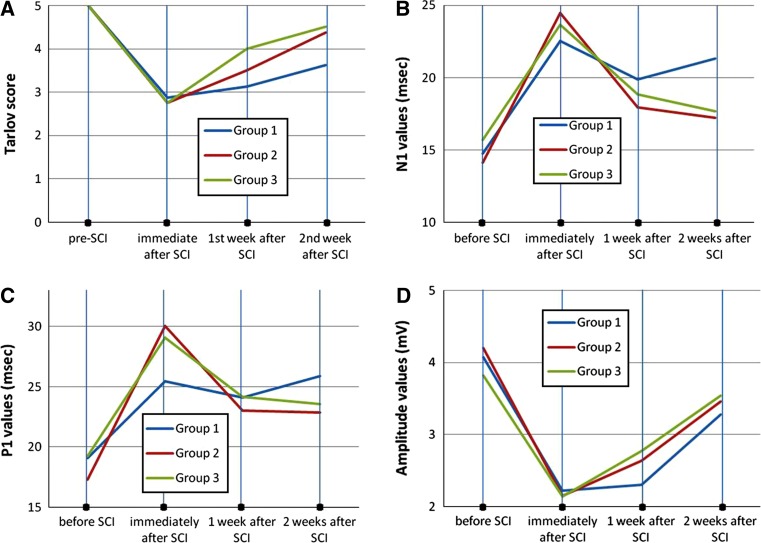
Clinical evaluation results of groups using Tarlov scoring system are shown in Fig. 1a; Table 2. One week after SCI, Tarlov scores of group 3, and 2 weeks after SCI, Tarlov scores of group 2 and 3 were significantly better than group 1 (p = 0.01, p = 0.02 and p = 0.01 respectively).
Fig. 1.
Temporal changes in Tarlov scores (a), time course of N1 latencies (b), time course of P1 latencies (c) and time course of amplitude values (d) following spinal cord injury. The mean for each variable is plotted before SCI and for 2 weeks following SCI. ms millisecond, mV millivolt
Table 2.
The results of Tarlov scoring in the groups (mean ± SD)
| Pre-SCI | Immediately after SCI | 1st week after SCI | 2nd week after SCI | |
|---|---|---|---|---|
| Group 1 | 5 | 2.87 ± 0.59 | 3.12 ± 0.59 | 3.65 ± 0.48 |
| Group 2 | 5 | 2.75 ± 0.66 | 3.50 ± 0.50 | 4.37 ± 0.48* |
| Group 3 | 5 | 2.75 ± 0.43 | 4.00 ± 0.50** | 4.50 ± 0.50** |
SCI spinal cord injury
* p = 0.02, ** p = 0.01 versus group 1
Before SCI, SEP N and P1 latency values between and in every single group did not display statistically significant difference (Fig. 1b, c; Tables 3, 4). N1 and P1 latency values immediately after, and 1 week after SCI compared between groups did not show statistically significant differences. Evaluation of N1 and P1 latency values 2 weeks after SCI showed statistically significant prolongation in group 1 as compared to groups 2 and 3 (p = 0.01 and p = 0.002 for N1 latencies, and p = 0.02 and p = 0.03 for P1 latencies, respectively). There was no significant difference between groups 2 and 3 (Fig. 1b, c; Tables 3, 4).
Table 3.
N1 latency results in the groups (mean ± SD)
| N1 (ms) | Pre-SCI | Immediately after SCI | 1st week after SCI | 2nd week after SCI |
|---|---|---|---|---|
| Group 1 | 14.78 ± 1.70 | 22.55 ± 4.36 | 19.87 ± 2.41 | 21.32 ± 0.99 |
| Group 2 | 14.13 ± 1.60 | 24.46 ± 4.77 | 17.96 ± 2.47 | 17.22 ± 2.41* |
| Group 3 | 15.72 ± 1.97 | 23.65 ± 3.23 | 18.87 ± 1.76 | 17.68 ± 0.97** |
SCI spinal cord injury, ms millisecond
* p = 0.01, ** p = 0.002 versus group 1
Table 4.
P1 latency results in the groups (mean ± SD)
| P1 (ms) | Pre-SCI | Immediately after SCI | 1st week after SCI | 2nd week after SCI |
|---|---|---|---|---|
| Group 1 | 19.07 ± 2.31 | 25.45 ± 4.29 | 24.12 ± 2.96 | 25.88 ± 1.30 |
| Group 2 | 17.33 ± 2.04 | 30.02 ± 5.99 | 23.02 ± 2.20 | 22.88 ± 3.44* |
| Group 3 | 19.27 ± 2.02 | 29.06 ± 4.59 | 24.15 ± 1.59 | 23.55 ± 1.73** |
SCI spinal cord injury, ms millisecond
* p = 0.02, ** p = 0.03 versus group 1
When each group was evaluated in itself, before SCI, N1 and P1 latencies showed statistically significant differences when compared with immediately after SCI N1 and P1 values in all groups (for N1, p = 0.002 and for P1, p = 0.003 for group 1; for N1, p = 0.001 and for P1, p = 0.001 for group 2; and for N1, p = 0.001 and for P1, p = 0.001 for group 3). But there was no significant difference between the three post-SCI N1 and P1 latencies in group 1. Groups 2 and 3 showed similar changes. Immediately after SCI, N1 and P1 latencies were significantly reduced when compared with 1 week results in groups 2 and 3 (for N1, p = 0.004 and for P1, p = 0.008 for group 2; and for N1, p = 0.001 and for P1, p = 0.02 for group 3). One week after SCI and 2 weeks after SCI, N1 and P1 latencies showed no statistical significance between groups 2 and 3 (Fig. 1b, c; Tables 3, 4).
The amplitude values did not show significant difference between groups (all values p > 0.05) (Fig. 1d; Table 5).
Table 5.
N1-P1 amplitude latency results in the groups (mean ± SD)
| Amplitude (mV) | Pre-SCI | Immediately after SCI | 1st week after SCI | 2nd week after SCI |
|---|---|---|---|---|
| Group 1 | 4.07 ± 1.42 | 2.22 ± 1.32 | 2.30 ± 1.83 | 3.28 ± 1.10 |
| Group 3 | 4.20 ± 1.81 | 2.15 ± 1.05 | 2.64 ± 1.29 | 3.46 ± 1.27 |
| Group 2 | 3.82 ± 2.07 | 2.14 ± 1.29 | 2.77 ± 1.58 | 3.54 ± 1.86 |
SCI spinal cord injury, mV millivolt
These results show that anti-TNF-α treatment 2–4, 12–24 h after SCI enhances clinical and electrophysiological recovery.
Discussion
Available anti-TNF-α agents are etanercept, infliximab, adalimumab, golimumab and certolizumab [3]. Only two of them, etanercept [11, 17, 18] and infliximab [19, 20], have been tried and results reported in SCI. In our study, we used etanercept to block TNF-α mediated inflammation pathway after SCI. Two studies used mice and extradural compression with aneurysm clip for SCI [11, 17], one study used rat and hemisection of spinal cord for SCI [18], one study used rat and extradural compression with aneurysm clip for SCI [20] and one study used rabbit and aortic occlusion method for SCI [19]. In our study, we used epidural balloon compression method for SCI in rabbits which led to partial and relatively reversible neurological deficit. These studies reported their results using clinical and behavioral tests, and biochemical, histological and immunohistochemical methods.
Tarlov’s scoring is a simple, appropriate and most widely used behavioral test for the evaluation of neurological deficit in laboratory animals. Results obtained from our study demonstrated that etanercept treatment enhanced recovery of traumatic SCI-related neurodeficit.
SEPs, first described in 1947, provide a reliable, reproducible and objective in vivo assessment of the functional integrity of the ascending sensory pathways that project into the dorsal spinal cord. Their utility in SCI studies has been previously demonstrated in animal studies [21]. An increase of more than 10 % in latency with respect to baseline is considered indicative of significant likelihood of spinal cord injury [22]. In our study, we chose SEP as the electrophysiological follow-up method, because our SCI method causes dorsal spinal cord compression which anatomically affects mostly the posterior columns. None of the previous studies that used anti-TNF-α agents in SCI observed electrophysiological effects of anti-TNF-α treatment in SCI. Anti-TNF-α treatment groups (groups 2 and 3) showed significant electrophysiological recovery compared to group 1.
Molecular genetics studies on SCI can provide insights into the pathogenesis of SCI and also timing of anti-inflammatory treatment. Carmel et al. [2] studied the expressions of large numbers of genes and their temporal and spatial relationships and described global patterns of gene expression following acute SCI. One of their striking results was high expression levels of the genes that have roles in the inflammatory pathways after acute SCI. TNF receptor expression levels were found to be higher than normal in the 6th hour after SCI and the expression gradually increased 48 h after SCI. This gives a clue about the timing of treatment with anti-TNF-α agents after SCI. This also provides an explanation why we could not observe different electrophysiological recovery responses between etanercept treated groups (group 2 and group 3). Because TNF-α and its receptors had already started to be expressed by injured neuronal tissues 2 h after SCI, blocking TNF-α effect with etanercept 2–24 h after SCI did not cause statistically significant electrophysiological recovery.
Pathophysiological processes which are involved in SCI have different mechanisms and every single mechanism has different multiple pathways. TNF-α mediated inflammation is one of the inflammatory pathways involved in the formation of inflammatory response to the trauma during SCI. Although our results show only minimal but statistically significant difference between group 1 and groups treated with etanercept (groups 2 and 3), sensitivity of SEP makes them meaningful and these results provide a clue to the importance of prevention of inflammatory response after SCI and the efficacy of the treatment on clinical and electrophysiological improvement.
Conclusion
With this study, we aimed to show the clinical and electrophysiological efficacy of etanercept treatment in a partial (reversible) SCI model using SEP and Tarlov’s score. Our results show that blocking the inflammation process in SCI by anti-TNF-α agent enhances clinical and electrophysiological recovery. These results are preliminary and will be a foundation for further studies using our SCI model and provide evidence of decreased inflammation, which is not sufficiently found in literature. These results also must be confirmed with different spinal cord trauma models, different drug doses, compression pressures and drug timing schedules with further investigations. Our animal study seems to show promising results, but in the real life these results are not replicated.
Acknowledgments
This work is supported by the Scientific Research Project Fund of the Cumhuriyet University under project number T-475.
Conflict of interest
None.
Footnotes
F. Bayrakli, H. Balaban and U. Ozum contributed equally to the manuscript.
References
- 1.Leal-Filho MB. Spinal cord injury: from inflammation to glial scar. Surg Neurol Int. 2011;2:112. doi: 10.4103/2152-7806.83732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmel JB, Galante A, Soteropoulos P, Tolias P, Recce M, Young W, Hart RP. Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiol Genomics. 2001;7:201–213. doi: 10.1152/physiolgenomics.00074.2001. [DOI] [PubMed] [Google Scholar]
- 3.Caminero A, Comabella M, Montalban X. Tumor necrosis factor alpha (TNF-alpha), anti-TNF-alpha and demyelination revisited: an ongoing story. J Neuroimmunol. 2011;234:1–6. doi: 10.1016/j.jneuroim.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 4.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22:3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson AR, Christensen RN, Gensel JC, Miller BA, Sun F, Beattie EC, Bresnahan JC, Beattie MS. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J Neurosci. 2008;28:11391–11400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–599. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- 9.Sobani ZA, Quadri SA, Enam SA. Stem cells for spinal cord regeneration: current status. Surg Neurol Int. 2010;1:93. doi: 10.4103/2152-7806.74240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalevsky J, Secher T, Ezhevsky SA, Janot L, Steed PM, O’Brien C, Eivazi A, Kung J, Nguyen DH, Doberstein SK, Erard F, Ryffel B, Szymkowski DE. Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection. J Immunol. 2007;179:1872–1883. doi: 10.4049/jimmunol.179.3.1872. [DOI] [PubMed] [Google Scholar]
- 11.Genovese T, Mazzon E, Crisafulli C, Paola R, Muia C, Bramanti P, Cuzzocrea S. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Ther. 2006;316:1006–1016. doi: 10.1124/jpet.105.097188. [DOI] [PubMed] [Google Scholar]
- 12.Talac R, Friedman JA, Moore MJ, Lu L, Jabbari E, Windebank AJ, Currier BL, Yaszemski MJ. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25:1505–1510. doi: 10.1016/S0142-9612(03)00497-6. [DOI] [PubMed] [Google Scholar]
- 13.Tazegül T (2010) Tavşanda deneysel kısmi omurilik hasarı modelinin tanımlanması. Dissertation, Cumhuriyet Üniversitesi
- 14.Yıldız O (2008) Deneysel omurilik basısında riluzole ve magnezyum sülfat tedavisinin etkinliğinin MRG ve patolojik inceleme ile araştırılması. Dissertation, Cumhuriyet Üniversitesi
- 15.Chadi G, Andrade MS, Leme RJ, Gomide VC. Experimental models of partial lesion of rat spinal cord to investigate neurodegeneration, glial activation, and behavior impairments. Int J Neurosci. 2001;111:137–165. doi: 10.3109/00207450108994227. [DOI] [PubMed] [Google Scholar]
- 16.Multon S, Franzen R, Poirrier AL, Scholtes F, Schoenen J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. J Neurotrauma. 2003;20:699–706. doi: 10.1089/089771503767869935. [DOI] [PubMed] [Google Scholar]
- 17.Genovese T, Mazzon E, Crisafulli C, Esposito E, Paola R, Muia C, Bella P, Meli R, Bramanti P, Cuzzocrea S. Combination of dexamethasone and etanercept reduces secondary damage in experimental spinal cord trauma. Neuroscience. 2007;150:168–181. doi: 10.1016/j.neuroscience.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 18.Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, McMahon SB. Effects of etanercept and minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13:673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Guven C, Borcek AO, Cemil B, Kurt G, Yildirim Z, Ucankus NL, Kilic N, Ceviker N. Neuroprotective effects of infliximab in experimental spinal cord ischemic injury. J Clin Neurosci. 2010;17:1563–1567. doi: 10.1016/j.jocn.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Kurt G, Ergun E, Cemil B, Borcek AO, Borcek P, Gulbahar O, Ceviker N. Neuroprotective effects of infliximab in experimental spinal cord injury. Surg Neurol. 2009;71:332–336. doi: 10.1016/j.surneu.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal G, Kerr C, Thakor NV, All AH (2010) Characterization of graded multicenter animal spinal cord injury study contusion spinal cord injury using somatosensory-evoked potentials. Spine (Phila, PA, 1976) 35:1122–1127. doi: 10.1097/BRS.0b013e3181be5fa7 [DOI] [PMC free article] [PubMed]
- 22.Agrawal G, Sherman D, Maybhate A, Gorelik M, Kerr DA, Thakor NV, All AH. Slope analysis of somatosensory evoked potentials in spinal cord injury for detecting contusion injury and focal demyelination. J Clin Neurosci. 2010;17:1159–1164. doi: 10.1016/j.jocn.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Papakostas JC, Matsagas MI, Toumpoulis IK, Malamou-Mitsi VD, Pappa LS, Gkrepi C, Anagnostopoulos CE, Kappas AM. Evolution of spinal cord injury in a porcine model of prolonged aortic occlusion. J Surg Res. 2006;133:159–166. doi: 10.1016/j.jss.2005.10.007. [DOI] [PubMed] [Google Scholar]