Abstract
Introduction
Three- or four-level anterior cervical discectomy and fusion with autograft and plate fixation have demonstrated relatively good fusion rates and outcomes, but donor site morbidity and the limitations of autograft harvest remain problematic. The purpose of this study is to assess the radiographic and clinical outcomes of three- or four-level anterior cervical discectomy and fusion with a PEEK cage and plate construct.
Methods
This retrospective review included 43 consecutive patients who underwent three- or four-level anterior cervical discectomy and fusion with a PEEK cage and plate construct (three level: 39 cases, four level: 4 cases). The fusion rate, time to fusion, Cobb angle and disc height were assessed radiographically. Clinical outcomes were evaluated with the VAS, NDI, and SF36 scores. Complications were also recorded.
Results
Solid fusion was achieved in all the patients, and mean time to fusion was 13.7 ± 5.1 weeks. The postoperative Cobb angle, lordotic angle, and disc height (5.6°, 10.5° and 3.15 mm, respectively) increased significantly compared to preoperative values (p = 0.038, p = 0.032, and p = 0.0004, respectively), and these improvements were maintained through final follow-up. The postoperative NDI (17.2), VAS (2.8), and SF36 (13.1) scores increased significantly compared to the preoperative scores (p = 0.026, p = 0.0007 and p = 0.041, respectively). Complications included three cases of respiratory difficulty, four cases of dysphagia and one case of hoarseness. There were no cases of donor site morbidity.
Conclusions
Three- or four-level anterior cervical discectomy and fusion with a PEEK cage, and plate construct provide good clinical and radiographic outcomes including high fusion rates, low complication rates, low donor site morbidity, and good maintenance of the lordotic angle and disc height in the treatment of multilevel cervical spondylosis.
Keywords: ACDF, PEEK cage, Three-level fusion, Four-level fusion
Introduction
Anterior cervical discectomy and fusion (ACDF) was originally introduced in 1955 by Robison and Smith, and numerous long-term follow-up studies have shown that it is an effective treatment for cervical spondylotic disease [1]. This technique allows direct decompression of neural structures, restoration of disc height, and stabilization of the affected motion segments [2, 3]. The success of the procedure relies on the development of a solid arthrodesis, although pseudarthrosis is not always associated with inferior outcome [4, 5]. Following anterior discectomy, the placement of a tricortical autograft harvested from the anterior iliac crest has led to excellent fusion rates [1]. However, multilevel anterior cervical discectomy and fusion still remains a difficult problem. The graft complication rate in autogenous bone graft in multilevel fusion is higher than at the single level, and it is clear that success rates decline as the number of levels increases. Therefore, in multilevel ACDF procedures, augmentation with plate fixation may decrease the micromovement of cervical spine, enhance the fusion rate and correct spinal curve to physiological lordosis.
Some surgeons have recommended corpectomy and strut grafting in multilevel cervical disease because high rates of pseudarthrosis have been observed after three- or four-level ACDF [4]. However, instrumented multilevel ACDF allows restoration of cervical lordosis and offers a greater biomechanical stability than long strut graft [5–8]. Three- or four-level anterior cervical discectomy with autograft and plate fixation has produced relatively good fusion rates and outcomes [9–11], but donor site morbidity and the limitations of autograft harvest remain problematic [6]. Equivalent fusion rates have been reported after allografting and autografting are combined using anterior plates and segmental screws [7, 8].
Cervical spine interbody fusion cages are frequently used in the surgical treatment of patients with degenerative cervical spine disease. The cage can provide stability, high fusion rates and low subsidence [12–15]. There are many studies [5, 6, 12] on the favourable results of one- or two-level anterior cervical fusion using a PEEK (polyetheretherketone) cage in degenerative cervical spine disease, including our previous report [5, 12]. However, there are few reports on three- or four-level anterior cervical fusion using a PEEK cage. In addition, the use of an anterior cervical plate and cage has increased fusion rates whilst avoiding donor site morbidity in multilevel ACDF, but these results have not been consistently reproduced [16, 17].
The purpose of this study is to report the radiographic (Table 1) and clinical outcomes of patients that underwent three- or four-level ACDF with a PEEK (polyetheretherketone) cage and plate construct.
Table 1.
Radiographic changes
| Preoperative | Final F/U | Δ | P value | |
|---|---|---|---|---|
| Lordotic angle of fused segment (°) | 5.4 ± 10.1 | 11 ± 4.5 | 6 ± 11 | <0.001 |
| Cobb angle (°) (C2–C7) | 12.2 ± 11.2 | 14.1 ± 8.5 | 9.7 ± 11.7 | 0.12 |
| Disc height (mm) | 71.8 ± 7.6 | 75.36 ± 6.7 | 3.5 ± 2.9 | <0.001 |
Methods
Patients
We performed a retrospective review of all the patients undergoing a primary three- or four-level ACDF performed by the senior author (K.J.S.) between April 2002 and March 2009. Inclusion criteria were, (1) diagnosis of cervical spondylosis, (2) disease resistant to conservative treatment consisting of immobilization, anti-inflammatory medications, epidural steroids, and physical therapy for 6 months, (3) follow-up >24 months. Patients were excluded, if they had a history of rheumatoid arthritis or previous cervical spine surgery. Fifty-two patients were identified and 9 were lost to follow-up, leaving 43 patients in the study group. This study was approved by the institutional review board at our institution.
Of the 43 patients, 24 (55.8 %) were male and 19 (44.2 %) were female, with an average age of 61.3 ± 10.1 years (ranges 48–78 years). The average follow-up time was 28.3 months (ranges 24–88 months). All patients underwent three-level (Fig. 1 ) or four-level (Fig. 2) anterior cervical discectomy and fusion with a PEEK cage and plate construct (three-level: 39 cases, four-level: 4 cases), and cages were filled with iliac crest cancellous bone chips. The indication for surgery was radiculopathy in 22 patients (51.1 %) and myelopathy or myeloradiculopathy in 21 patients (48.8 %).
Fig. 1.
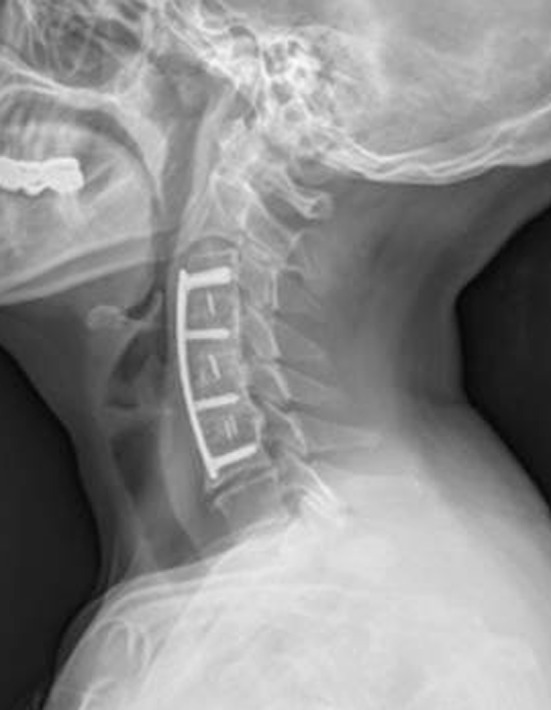
A 66-year-old woman underwent three-level (C3–C4, C4–C5, C5–C6) discectomy and fusion with a PEEK cage filled with autogenous iliac cancellous graft due to cervical spondylotic myelopathy. Two-year postoperative follow-up lateral X-ray showing complete bony fusion and maintenance of the cervical lordotic curve
Fig. 2.
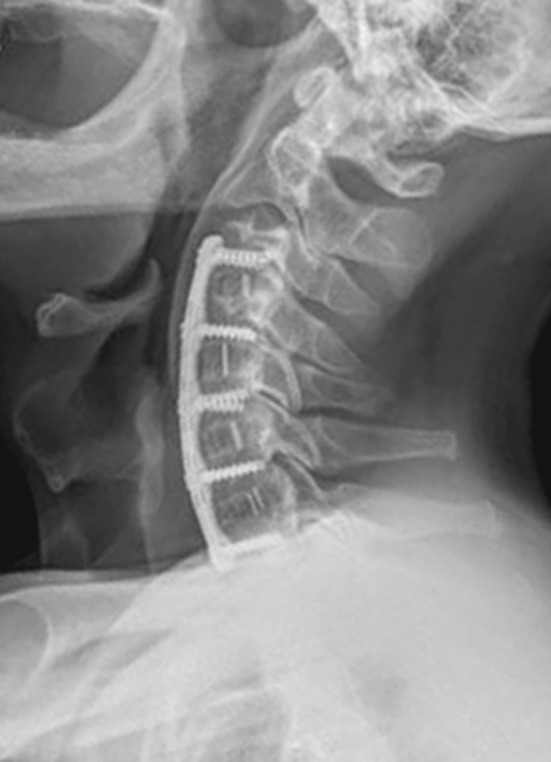
A 59-year-old man underwent four-level (C3–C4, C4–C5, C5–C6, C6–C7) discectomy and fusion with a PEEK cage filled with autogenous iliac cancellous graft due to cervical spondylotic myelopathy. Two-year postoperative follow-up lateral X-ray showing complete bony fusion and maintenance of the cervical lordotic curve
Surgical technique
The modified Robinson technique for ACDF was used and discectomy was performed to the level of the uncovertebral joints and the posterior longitudinal ligament. The latter was excised when ossified or when disc material was present posterior to the ligament. The endplates were decorticated, facilitating removal of posterior osteophytes and providing a highly vascular fusion bed. The nerve roots were decompressed with meticulous foraminotomies.
We used PEEK cages (Stryker, Kalamazoo, MI, USA) filled with cancellous iliac crest autograft. The cancellous iliac crest autograft was harvested through a 1 cm skin incision using a cylindrical coring troca (diameter, 7 mm; AO Synthes, Bettlach, Switzerland). A lateral X-ray of the cervical spine was taken to determine the size of the cage, plate lordosis, and the insertion angle of the screw. A Maxima Anterior Cervical Plate System (U&I Corporation, Seoul, Korea) was used for anterior stabilization. Patients were instructed to wear a Philadelphia brace for 4 weeks and a soft collar brace for an additional 2 weeks.
Outcomes assessment
Radiological follow-up for bone fusion was performed immediately after surgery and then 6 weeks, 3, 6, 9, 12, 18 and 24 months after surgery. The presence or absence of bone fusion was determined using anteroposterior (AP), lateral, and flexion/extension lateral plain radiographs. Fusion was defined as, (1) less than 2° of movement on the lateral flexion/extension views, (2) the presence of bridging trabecular bone between the endplates on the AP/lateral views, (3) no signs of implant failure for the anterior plate system, and (4) less than 50 % radiolucency in the perimeter surrounding the cage. CT scans were used as a secondary measure when the presence of bridging trabecular bone was not observed or was ambiguous on the radiographs. The fusion status of all the patients was separately evaluated based primarily on the presence or absence of contiguous bridging bone on the radiographs by an independent radiologist who was blinded to the treatment group [18].
Cervical lordosis was measured using the Cobb angle, which is formed by the perpendicular line of the upper margin of the upper vertebral body and the lower margin of the lower vertebral body. The change in lordosis or kyphosis was measured prior to surgery and at final follow-up. The change in disc height was measured as the distance between the centre of the superior end plate of the upper vertebral body and the inferior end plate of the lower vertebral body. The difference before surgery and at final follow-up was determined.
Clinical outcomes were assessed by comparing the scores on a 10-point Visual Analogue Scale (VAS), the Korean version of the Neck Disability Index (NDI) and the Short Form Health Survey (SF-36) before surgery and at final follow-up [19–21]. Complications were assessed by reviewing the patients’ medical records (Table 2). Dysphagia was evaluated using the dysphagia score proposed by Bazaz et al. [22]. We considered the occasional sensation of dysphagia with solid foods as moderate and frequent episodes as severe.
Table 2.
Frequency of complications
| Complication | Frequency |
|---|---|
| Respiratory discomfort | 3 (6.9 %) |
| Swallowing difficulty | 4 (9.3 %) |
| Hoarseness | 1 (2.3 %) |
| Donor site problems | |
| Superficial infection | 1 (2.3 %) |
| Pain | 1 (2.3 %) |
Mann–Whitney tests were used to compare outcomes before surgery and at final follow-up. All statistical analyses were performed using SPSS (version 17, SPSS, Chicago, IL, USA), and statistical significance was defined as p < 0.05.
Results
The mean VAS neck and arm pain score decreased from 6.74 ± 1.09 preoperatively to 3.93 ± 1.78 at final follow-up (p < 0.001). The mean NDI score improved from 29.52 ± 8.54 preoperatively to 12.34 ± 6.23 at final follow-up (p = 0.026). The mean SF-36 score improved from 64.7 ± 6.23 preoperatively to 85.8 ± 8.74 at final follow-up (p = 0.041). The mean VAS donor site pain score decreased from 3.24 ± 1.27 immediately after surgery to 0.57 ± 0.64 at final follow-up (p < 0.001).
All the patients achieved a solid fusion, with a mean time to fusion of 13.7 ± 5.1 weeks. The preoperative mean cervical lordosis (C2–C7) was 12.2 ± 11.2, and the mean segmental lordosis was 5.4 ± 10.1. At final follow-up, the mean cervical lordosis was 14.1 ± 8.5 (p = 0.12), and the segmental lordosis was 11 ± 4.5 (p < 0.001). The mean disc height increased significantly (3.15 ± 2.96 mm) compared to preoperative levels (p < 0.001).
Dysphagia occurred in four patients (9.3 %), three of whom had dysphasia 3 months postoperatively, and one of them had dysphagia at 6 months postoperatively. At final follow-up, dysphagia had resolved in all four patients. Three patients presented with respiratory difficulties (3.9 %) during the first 3 days after surgery. None needed endotracheal intubation to sustain their airway, and oxygen was delivered through nasal prongs or a plastic oxygen mask. The incidence of hoarseness was 2.3 % (one case). For this patient, postoperative evaluation was performed by fiberoptic endoscopic evaluation of phonation on the seventh day after surgery, and the hoarseness resolved completely by the 6-week follow-up. One patient had graft-site pain that resolved completely by the 6-month follow-up visit. Another case of graft-site pain was associated with a superficial infection that was treated successfully with a two-week course of antibiotics.
Discussion
Anterior cervical decompression fusion (ACDF) with autogenous bone has been widely used as a surgical treatment for degenerative disc disease of the cervical spine; however, donor site morbidity continues to be a common complication. Numerous methods, such as cage augmentation [23], bone substitutes, allograft, artificial replacement [24], and bone morphogenetic protein have been developed in an effort to avoid this complication. Cage characteristics depend on the composition of the cage. Currently, cages made of polyetheretherketone (PEEK) are primarily used for anterior cervical fusions because it has biomechanical similarities to bone. PEEK is a semicrystalline polyaromatic, linear polymer that provides a good combination of strength, stiffness, toughness, and environmental resistance with biocompatible, non-absorbable, and corrosion-resistant abilities [17, 25, 26]. In addition, the PEEK cage is radiolucent and does not produce radiographic artifacts, leading to easy evaluation of fusion status on radiographs and CT scans. Cage-assisted ACDF has proven to be a safe and effective procedure for the treatment of degenerative cervical disc disease because of its theoretical ability to prevent graft collapse, with the potential advantage of indirect foraminal decompression by restoration and preservation of intervertebral height and lordosis [5, 6, 12, 17, 25, 26].
Many spinal surgeons induce bone fusion by filling the cage with autogenous iliac cancellous bone, achieving good or excellent clinical outcomes and fusion rates [5, 6, 23, 27]. However, others have reported poor clinical outcomes and fusion rates secondary to a high rate of cage subsidence, local kyphosis, and pseudarthrosis [14, 28]. This phenomenon may occur due to insufficient fixation power of the cage alone. Indeed, some surgeons [5, 7, 8, 14, 15] have reported a significantly higher incidence of cage subsidence and subsequent local kyphosis in patients who received a cage alone compared to patients who received plate augmentation. Furthermore, fusion time was significantly delayed in these studies, and the fusion rate was significantly lower amongst patients who had the cage alone compared to those who had plate augmentation.
It has been suggested that plate augmentation has the potential to lower the rate of pseudoarthrosis in multilevel ACDF, but results have varied widely to date. Geisler et al. [29] reported a 100 % fusion rate in 35 plated multilevel (three- and four-level) ACDFs. Wang et al. [30] found that 18 % (7 of 40) of the patients had pseudarthrosis after three-level plated ACDF, and they observed no difference in fusion rates between plated and nonplated subgroups. Bolesta et al. [3] reported a nonunion rate of 53 % (8/15 patients) for plated ACDF, the highest in the literature. This wide range of reported values can be explained by multiple factors, including differences in surgical technique, the number of levels grafted, the criteria used for pseudarthrosis, the length of brace use, and the length of follow-up. In the current study, solid fusion was achieved in all the patients, with a mean time to fusion of 13.7 ± 5.1 weeks. This is longer than the 6–10 weeks required with ACDF and autogenous bone graft in single-level fusions but shorter than the 3–6 months required for an ACDF with a cage alone [7]. The fusion rate (100 %) we observed at final follow-up was higher than that reported in studies of PEEK cages alone [7]. In addition, the postoperative Cobb angle, lordotic angle, and disc height (5.6°, 10.5°, and 3.15 mm, respectively) increased significantly compared to their preoperative values (p = 0.038, p = 0.032, and p = 0.0004, respectively), increases that were maintained through final follow-up. These results suggest that plate augmentation significantly enhances the stability of the fusion site, produces more desirable fusion rates, and helps maintain cervical lordosis.
Although plate augmentation in ACDF is not universally employed, several reports have illustrated its efficacy in promoting fusion. Song et al. [5] found that ACDF with a cage and plate construct led to a high fusion rate in the treatment of single-level degenerative cervical disease and also led to acceptable intervertebral disc space and lordosis. Emery et al. [4] and Wang et al. [30] studied the development of pseudoarthrosis after multilevel fusion without plate augmentation, and both reported high pseudoarthrosis rates of 44 and 37 %, respectively. Papadopoulos et al. [31] implemented plate augmentation in multilevel fusions and found that only two of their 46 cases (4 %) developed pseudoarthrosis. This drastic difference in pseudoarthrosis rate following multilevel fusions emphasises the importance of using plate augmentation, although the interbody fusion material used in the current study was a cage, which differs from the autogenous iliac bone used in the previous reports.
Although ACDF with a cage alone has the advantages of shorter operation time, minimal blood loss, and relative simplicity of the procedure when compared to ACDF with a cage and plate construct, patients are required to wear a rigid brace after surgery for a longer period, often leading to a significant level of discomfort. Additionally, radiological outcomes of ACDF with a cage and plate construct are comparable to ACDF with autologous tricortical iliac bone, suggesting that the metal plate augmentation procedure could be a method that not only addresses the drawbacks of using a cage alone, but also reduces the donor site morbidity associated with ACDF with autologous tricortical iliac bone.
Many studies have shown that one- or two-level fusions with PEEK cage have outcomes similar to those with autograft [5, 6, 12]. However, there are only a few studies on three- or four-level fusion with a PEEK cage. Notably, there is no study using a PEEK cage and plate augmentation in three- or four-level fusion. Demirkan et al. [17] have reported a favourable fusion rate (90.5 %, 38/42 levels) in cases that used a PEEK cage only. In that study, there were three patients with non-fusion levels, all of whom underwent three- or four-level fusion surgery rather than one- or two-level fusion surgery. This phenomenon is consistent with previous reports [10–12] that additional plate augmentations enhance the fusion rate in cervical fusion with autograft alone. Therefore, plate augmentation is better than the cage alone procedure in cases of multilevel cervical fusion because plate fixation may decrease the micromovement of the surgical area.
Previous studies have reported that donor-site morbidity occurs in 9.4 to 49 % of subjects [32, 33]. Pitzen et al. [34] suggested that filling the cage with local autogenous bone may help to eliminate complications at the iliac crest. Although this technique seems reasonable, we did not employ it due to the inferior quality of local bone compared to iliac crest cancellous bone. The heat that develops during drilling of the endplates and the presence of bone fragments from osteophytes can result in decreased viability of the cells (i.e., osteocytes, osteoblasts and mesenchymal precursor cells), which adversely affects both the rate of fusion and time to fusion. Despite these limitations, Pitzen et al. [34] achieved a fusion rate of 91.3 %; therefore, future studies should evaluate the use of local bone versus iliac crest bone graft in these cervical procedures. In the present study, donor-site pain following the harvest of autogenous cancellous bone using a 7-mm bone punch biopsy was minimal and resolved in all patients within the first week after surgery.
The strengths of our study include the usage of only a PEEK cage in all cases, as opposed to diverse cages, including carbon and titanium mesh, as seen in previous reports. Some limitations of our study include its retrospective nature and failure to include a patient group that underwent ACDF with an autogenous bone and plate construct. Future studies are needed to prospectively compare the results of ACDF with a cage and plate construct and ACDF with an autogenous bone and plate construct.
Conclusion
Three- or four-level anterior cervical discectomy and fusion with a PEEK cage and plate construct provide good clinical and radiographic outcome including a high fusion rate, low complication rate, low donor site morbidity, and good maintenance of lordotic angle and disc height in the treatment of multilevel cervical spondylosis.
Acknowledgment
This project was supported by Dong-Ah Pharmaceutical Company in Korea.
Conflict of interest
None.
References
- 1.Lowery GL, McDonough RF. The significance of hardware failure in anterior cervical plate fixation. Patients with 2- to 7-year follow-up. Spine (Phila Pa 1976) 1998;23:181–186. doi: 10.1097/00007632-199801150-00006. [DOI] [PubMed] [Google Scholar]
- 2.Malloy KM, Hilibrand AS. Autograft versus allograft in degenerative cervical disease. Clin Orthop Relat Res. 2002;394:27–38. doi: 10.1097/00003086-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bolesta MJ, Rechtine GR, Chrin AM. Three- and four-level anterior cervical discectomy and fusion with plate fixation: a prospective study. Spine (Phila Pa 1976) 2000;25:2040–2044. doi: 10.1097/00007632-200008150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Emery SE, Fisher JR, Bohlman HHN. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine (Phila Pa 1976) 1997;22:2622–2624. doi: 10.1097/00007632-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Song KJ, Lee KB. A preliminary study of the use of cage and plating for single-segment fusion in degenerative cervical spine disease. J Clin Neurosci. 2006;13(2):181–187. doi: 10.1016/j.jocn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Cho DY, Liau WR, Lee WY, Liu JT, Chiu CL, Sheu PC. Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery. 2003;52(3):1343–1349. [PubMed] [Google Scholar]
- 7.Kulkarni AG, Hee HT, Wong HK. Solis cage (PEEK) for anterior cervical fusion: preliminary radiological results with emphasis on fusion and subsidence. Spine J. 2007;7(2):205–209. doi: 10.1016/j.spinee.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami M, Tamaki T, Iwasaki H, Yoshida M, Ando M, Yamada H. A comparative study of surgical approaches for cervical compressive myelopathy. Clin Orthop Relat Res. 2000;381:129–136. doi: 10.1097/00003086-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Guo Q, Bi X, Ni B, et al. Outcomes of three anterior decompression and fusion techniques in the treatment of three-level cervical spondylosis. Eur Spine J. 2011;20:1539–1544. doi: 10.1007/s00586-011-1735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Q, Zhou X, Wang X, Cao P, Tsai N, Yuan W. A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J. 2012;21:474–481. doi: 10.1007/s00586-011-1961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uribe JS, Sangala JR, Duckworth EA, Vale FL. Comparison between anterior cervical discectomy fusion and cervical corpectomy fusion using titanium cages for reconstruction: analysis of outcome and long-term follow-up. Eur Spine J. 2009;18:654–662. doi: 10.1007/s00586-009-0897-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song KJ, Taghavi CE, Hsu MS, Lee KB, Kim GH, Song JH. Plate augmentation in anterior cervical discectomy and fusion with cage for degenerative cervical spinal disorders. Eur Spine J. 2010;19:1677–1683. doi: 10.1007/s00586-010-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gercek E, Arlet V, Delisle J, Marchesi D. Subsidence of stand-alone cervical cages in anterior interbody fusion: warning. Eur Spine J. 2003;12(5):513–516. doi: 10.1007/s00586-003-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barsa P, Suchomel P. Factors affecting sagittal malalignment due to cage subsidence in standalone cage assisted anterior cervical fusion. Eur Spine J. 2007;16(9):1395–1400. doi: 10.1007/s00586-006-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmieder K, Wolzik-Grossmann M, Pechlivanis I, Engelhardt M, Scholz M, Harders A. Subsidence of the wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J Neurosurg Spine. 2006;4(6):447–453. doi: 10.3171/spi.2006.4.6.447. [DOI] [PubMed] [Google Scholar]
- 16.Emery SE, Bohlman HH, Bolesta MJ, Jones PK. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to seventeen-year follow-up. J Bone Joint Surg Am. 1998;80(7):941–951. doi: 10.2106/00004623-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Demircan MN, Kutlay AM, Colak A, Kaya S, Tekin T, Kibici K, Ungoren K. Multilevel cervical fusion without plates, screws or autogenous iliac crest bone graft. J Clin Neurosci. 2007;14(8):723–728. doi: 10.1016/j.jocn.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Brantigan JW. Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation. Spine (Phila Pa 1976) 1994;19(11):1271–1279. doi: 10.1097/00007632-199405310-00014. [DOI] [PubMed] [Google Scholar]
- 19.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–415. [PubMed] [Google Scholar]
- 20.Song KJ, Choi BW, Choi BR, Seo GB. Cross-Cultural Adaptation and Validation of the Korean Version of the Neck Disability Index. Spine (Phila Pa 1976) 2010;35(20):E1045–E1049. doi: 10.1097/BRS.0b013e3181df78e9. [DOI] [PubMed] [Google Scholar]
- 21.Hains F, Waalen J, Mior S. Psychometric properties of the neck disability index. J Manipulative Physiol Ther. 1998;21(2):75–80. [PubMed] [Google Scholar]
- 22.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery. Spine. 2002;27:2453–2458. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Topuz K, Colak A, Kaya S, et al. Two-level contiguous cervical disc disease treated with peek cages packed with demineralized bone matrix: results of 3-year follow-up. Eur Spine J. 2009;18:238–243. doi: 10.1007/s00586-008-0869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SW, Limson MA, Kim SB, et al. Comparison of radiographic changes after ACDF versus Bryan disc arthroplasty in single and bi-level cases. Eur Spine J. 2009;18:218–231. doi: 10.1007/s00586-008-0854-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooke NS, Rorke AW, King AT, et al. Preliminary experience of carbon fiber cage prostheses for treatment of cervical spine disorders. Br J Neurosurg. 1997;11:221–227. doi: 10.1080/02688699746285. [DOI] [PubMed] [Google Scholar]
- 26.Munoz FL, de las Heras BG, Lopez VC, et al. Comparison of three techniques of anterior fusion in single-level cervical disc herniation. Eur Spine J. 1998;7:512–516. doi: 10.1007/s005860050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das K, Couldwell WT, Sava G. Use of cylindrical titanium mesh and locking plates in anterior cervical fusion. Technical note. J Neurosurg Spine. 2001;94:174–178. doi: 10.3171/spi.2001.94.1.0174. [DOI] [PubMed] [Google Scholar]
- 28.Kast E, Derakhshani S, Bothmann M, et al. Subsidence after anterior cervical interbody fusion. A randomized prospective clinical trial. Neurosurg Rev. 2009;32:207–214. doi: 10.1007/s10143-008-0168-y. [DOI] [PubMed] [Google Scholar]
- 29.Geisler FH, Caspar W, Pitzen T, Johnson TA. Reoperation in patients after anterior cervical plate stabilization in degenerative disease. Spine. 1998;23(8):911–920. doi: 10.1097/00007632-199804150-00013. [DOI] [PubMed] [Google Scholar]
- 30.Wang JC, McDonough PW, Kanim LEA, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine. 2001;26:643–647. doi: 10.1097/00007632-200103150-00015. [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos EC, Huang RC, Girardi FP, Synnott K, Cammisa FP. Three-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Spine. 2006;8:897–902. doi: 10.1097/01.brs.0000209348.17377.be. [DOI] [PubMed] [Google Scholar]
- 32.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Keller EE, Triplett WW. Iliac bone graft: review of 60 consecutive cases. J Oral Maxillofac Surg. 1987;45:11–14. doi: 10.1016/0278-2391(87)90079-6. [DOI] [PubMed] [Google Scholar]
- 34.Pitzen T, Kiefer R, Munchen D, Barbier D, Reith W, Steudel WI. Filling a cervical spine cage with local autograft: change of bone density and assessment of bony fusion. Zentralbl Neurochir. 2006;67:8–13. doi: 10.1055/s-2006-921404. [DOI] [PubMed] [Google Scholar]


