Abstract
Purpose
How the lumbar neural foramina are affected by segmental deformities in patients in whom degenerative lumbar scoliosis (DLS) is unknown. Here, we used multidetector-row computed tomography (MDCT) to measure the morphology of the foramina in three dimensions, which allowed us to elucidate the relationships between foraminal morphology and segmental deformities in DLS.
Methods
In 77 DLS patients (mean age, 69.4) and 19 controls (mean age, 69), the foraminal height (FH), foraminal width (FW), posterior disc height (PDH), interval between the pedicle and superior articular process (P-SAP), and cross-sectional foraminal area (FA) were measured on reconstructed MDCT data, using image-editing software, at the entrance, minimum-area point, and exit of each foramen. The parameters of segmental deformity included the intervertebral wedging angle and anteroposterior and lateral translation rate, measured on radiographs, and the vertebral rotation angle, measured using reconstructed MDCT images.
Results
The FH, PDH, P-SAP, and FA were smaller at lower lumbar levels and on the concave side of intervertebral wedging (p < 0.05). In the DLS patients, the FH, P-SAP, and FA were significantly smaller than for the control group at all three foraminal locations and every lumbar level (p < 0.05). Intervertebral wedging strongly decreased the FA of the concave side (p < 0.05). Anteroposterior translation caused the greatest reduction in P-SAP (p < 0.05). Vertebral rotation decreased the P-SAP and FA at the minimum-area point on the same side as the rotation (p < 0.05).
Conclusion
The new analysis method proposed here is useful for understanding the pathomechanisms of foraminal stenosis in DLS patients.
Keywords: Degenerative lumbar scoliosis, Segmental deformity, Intervertebral foramina, Multidetector-row computed tomography
Introduction
Degenerative lumbar scoliosis (DLS) is common among the elderly population and often causes sciatic pain due to foraminal stenosis (FS). FS in DLS is often missed, which leads to ineffective surgical treatment for DLS [1–3]. Segmental deformities caused by wedging of the intervertebral discs, translation and rotation of the vertebral bodies, and subluxation of the superior articular process are seen in DLS patients (Fig. 1). Previous reports on FS have relied on traditional two-dimensional imaging techniques [4–9]. A cadaveric study by Stephens et al. [6] showed that on MRI images, the cross-sectional foraminal area varies from 40 to 160 mm2 in the sagittal plane. Another MRI study reported that the cross-sectional foraminal area was larger on the convex than the concave side in DLS [7]. A biomechanical study by Fujiwara et al. [8], using CT images, demonstrated that forward flexion and lateral flexion result in an increased cross-sectional foraminal area on the side opposite the flexion, but extension with lateral flexion reduces it on the same side. However, these studies used the measurements of the foramina that were performed on a single sagittal slice. Because the anatomy of the foramina in DLS patients is different from that in the normal population, due to segmental deformities that include intervertebral wedging, anteroposterior and lateral translation, and axial rotation, adjustment of the slice plane is necessary for precise evaluation of the foraminal anatomy in DLS.
Fig. 1.

Posterolateral view of foramina affected by intervertebral wedging. The posterior elements behind the superior articular process were erased. The foramen on the concave side is obviously smaller than on the convex side
In this study, we used reconstructed images obtained by multidetector-row computed tomography (MDCT) to measure the foramina. This allowed us to define each foramen three dimensionally from the entrance to the exit, with adjustment to the segmental deformities in patients with DLS.
Materials and methods
Cadaveric study
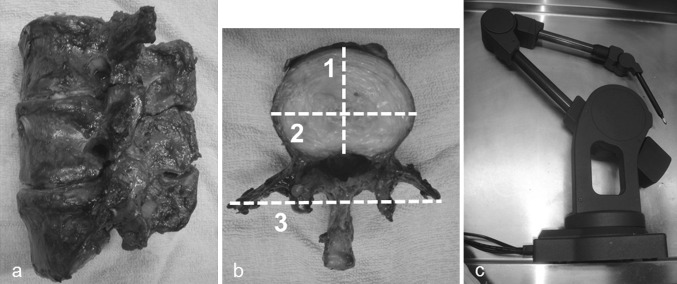
To assess the accuracy of the MDCT measurements (taken on a Lightspeed-VCT 64 MDCT scanner, GE Healthcare), we first compared them with measurements taken directly from a freshly dissected human cadaveric lumbar spine segment that included L3–L5 (Fig. 2a). Reconstructed axial images were created from DICOM-formatted MDCT data using image-editing software (Real INTAGE, KGT Inc., Tokyo, Japan). Three parameters, including (1) the anteroposterior and (2) transverse diameter of the superior edge of the vertebral bodies, and (3) the interval between the tips of the bilateral transverse processes, were measured in the axial plane using Real INTAGE (Fig. 2b). These parameters were chosen because the anatomical landmarks defining them could be clearly determined. The cadaveric spine was then dissected by total discectomy. Measurements of the same three parameters were conducted using Microscribe-3D (Immersion Corporation, California, USA, Fig. 2c). The measurements were performed twice, and the average values were calculated. The measurements from the MDCT images and those obtained by Microscribe-3D were then compared.
Fig. 2.
Accuracy assessment of MDCT measurements. a A fresh cadaver spine specimen including L3–L5 was scanned using an MDCT scanner (Lightspeed-VCT64, GE Healthcare). b (1) Anteroposterior and (2) transverse diameter of the superior edge of the vertebral bodies, and (3) the interval between the tips of the bilateral transverse processes were measured in the axial plane using Real INTAGE. c Dissected vertebrae were measured similarly using Microscribe (Immersion Corporation, CA, USA)
Clinical study
Between May 2006 and July 2008, 77 women of age 50 or more (mean 69.4 years; range 50–83 years) with DLS of 10 degrees or more on a standing posteroanterior radiograph participated in this study (DLS group). The curve types are shown in Table 1. Patients with a history of idiopathic scoliosis and previous lumbar surgery, and those with spondylolysis, trauma, osteoporotic vertebral fractures, tumours, rheumatoid arthritis, and infections were excluded. Nineteen women aged 50 or more without DLS but with non-specific back pain were included in this study as the control group. The DLS and control groups were matched for age, body height, and body weight (Table 2). We obtained informed consent from all subjects and approval for this study from the hospital’s ethics committee.
Table 1.
Curve type of study population
| Curve type | Number | % |
|---|---|---|
| Left lumbar | 32 | 41.6 |
| Left thoracolumbar | 9 | 11.7 |
| Right lumbar | 27 | 35.1 |
| Right thoracolumbar | 9 | 11.7 |
Table 2.
Characteristics of DLS group and control group
| DLS (n = 77) | Control (n = 19) | |
|---|---|---|
| Age (years) | 69.4 | 69.0 |
| Body height (cm) | 152.8 | 153.9 |
| Body weight (kg) | 53.3 | 51.6 |
| BMI (%) | 22.8 | 21.9 |
| Cobb angle (°)* | 12.4 ± 10.2 | 1.3 ± 7.4 |
| Lumbar lordosis (°) | 33.5 ± 14.8 | 36.9 ± 15.6 |
* Statistically significant difference
CT measurements
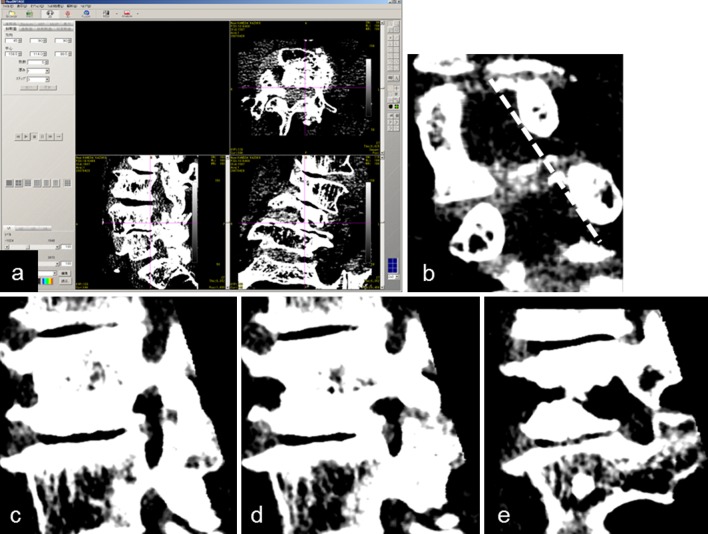
The lumbar spine was scanned using the aforementioned MDCT scanner with patients in the supine position, producing images with a 0.625-mm slice thickness and 512 × 512 pixel resolution. Three-dimensional images were reconstructed using Real INTAGE software (Fig. 3a). Because it is difficult to define the foramina anatomically in patients with DLS, due to the lateral translation and rotation of the vertebral bodies, we defined the entrance of the foramen as the line connecting the inner edges of the upper and lower pedicles on the reconstructed coronal image that was perpendicular to the median angle between the two adjacent vertebral rotational angles (Fig. 3b). The images used for the measurements were obtained by scrolling the cursor from the entrance (Fig. 3c) to the exit (Fig. 3e) via the minimum-area point of the foramen (Fig. 3d). Five parameters, including foraminal height (FH), foraminal width (FW), posterior disc height (PDH), the interval between the pedicle and superior articular process (P-SAP; which represented the subluxation of the superior articular process), and the foraminal area (FA), were measured at three points: the entrance, the minimum-area point, and the exit of the foramina, at the L3–4, L4–5, and L5–S1 levels (Fig. 4a–e). The rotation angle of the upper spine with respect to the lower spine was also measured (Fig. 4f). During the measurements, both the window level and window width were fixed at 100 to keep the ligamentum flavum and intervertebral disc differentiable from the vertebral bones. The standards for measurements were as follows: we defined all the parameters except FA using osseous landmarks to make reproducibility of measurements as high as possible. In patients with osteophytes making foramen irregular in shapes, we take the osteophytes into account in measurements. FA, a pathway of the nerve root, was measured as the area confined both by the osseous structures and by the intervertebral disc and ligamentum flavum. When those soft tissues contacted each other and foraminal area was divided into the upper and the lower part as Fig. 4e, we measured only the upper part as FA, which the nerve root passes through. When not divided as Fig. 3d, we measured FA including both the upper and the lower part of foramen. We compared the foraminal parameters between the control and the DLS group, both in the concave and convex sides of disc wedging. We also calculated the mean value of bilateral foramens in DLS patients, irrespective of the presence of disc wedging for gross comparison of foraminal parameters between the control and DLS groups (DLS total).
Fig. 3.
Preparation to measure MDCT data. a Original DICOM data of the lower lumbar spine of a DLS patient were displayed on Real INTAGE. bDashed line represents the entrance of the foramen. c Sagittal view of the entrance of foramen on the same plane, with the dashed line as above. d The minimum-area point of the foramen. e The exit of the foramen
Fig. 4.
Measurements on MDCT images. a Foraminal height (FH, mm); b Foraminal width (FW, mm); c Posterior disc height (PDH, mm); d Interval between the upper pedicle and superior articular process (P-SAP, mm); e Cross-sectional foraminal area (FA, mm2); f Two adjacent vertebrae can be displayed simultaneously to measure the segmental vertebral rotation angle with Real INTAGE
Radiographic measurements
The radiographic parameters of the segmental deformities measured included the intervertebral wedging angle, anteroposterior translation rate, and lateral translation rate, measured from standing radiographs. To investigate how the segmental deformities impact the foraminal morphology, multiple regression analyses were performed, in which the parameters of segmental deformity were the explanatory variables, and the parameters of the foramina confined to the PDH, P-SAP and FA were the objective variables.
All measurements were performed twice by one of the investigators (Y.K.), and the values were averaged. Intraobserver reliability was evaluated by having the same investigator repeat the measurements after 3 months. Interobserver reliability was also assessed using radiographs from 20 randomly selected patients, for which the measurements made by Y.K. were compared with the measurements made by another orthopaedic surgeon. To minimize intraobserver error, we used the averaged data measured twice by one of the investigator (Y.K.) for the analyses.
Statistical analysis
For statistical analyses, Dr. SPSS II for Windows (SPSS Japan Inc., Tokyo, Japan) was used, and a p value <0.05 was considered statistically significant. T test was used to compare the characteristics between the DLS group and the control group. ANOVA with Bonferroni correction was used to compare the MDCT measurements described in Table 3 among three lumbar levels and among the four groups including DLS total, concave, convex, and control. Interclass correlation coefficients were used for the reliabilities of measurements.
Table 3.
Summary of MDCT measurements
| Foramen region | FH (mm) | FW (mm) | PDH (mm) | P-SAP (mm) | FA (mm2) | ||
|---|---|---|---|---|---|---|---|
| L3–4 | Entrance | DLS total | 23.2 ± 3.8 | 6.8 ± 1.1 | 1.3 ± 1.3 | 13.2 ± 3.5 | 80.8 ± 25.8 |
| Concave | 20.7 ± 4.0 | 6.4 ± 1.2 | 1.1 ± 1.2 | 12.5 ± 3.2 | 62.4 ± 23.5 | ||
| Convex | 23.6 ± 3.0 | 6.7 ± 0.9 | 1.5 ± 1.3 | 13.9 ± 3.4 | 77.3 ± 27.2 | ||
| Control | 23.9 ± 2.9 | 6.6 ± 1.2 | 1.7 ± 1.2 | 14.4 ± 2.6 | 80.4 ± 27.5 | ||
| Minimum-area point | DLS total | 14.1 ± 4.3 | 7.5 ± 3.3 | 1.9 ± 1.6 | 8.1 ± 2.7 | 62.9 ± 25.7 | |
| Concave | 12.1 ± 3.3 | 7.5 ± 3.6 | 1.6 ± 1.6 | 7.6 ± 2.8 | 53.2 ± 22.1 | ||
| Convex | 14.1 ± 2.9 | 7.1 ± 2.7 | 2.3 ± 1.4 | 8.6 ± 2.7 | 58.9 ± 26.2 | ||
| Control | 14.4 ± 1.7 | 7.4 ± 1.5 | 2.5 ± 1.4 | 10.3 ± 1.8 | 64.8 ± 24.7 | ||
| Exit | DLS total | 17.9 ± 4.7 | 11.1 ± 3.7 | 2.9 ± 1.8 | 13.3 ± 3.2 | 139.1 ± 50.1 | |
| Concave | 15.7 ± 4.0 | 10.5 ± 3.2 | 2.4 ± 1.8 | 12.8 ± 3.4 | 114.0 ± 45.6 | ||
| Convex | 17.1 ± 3.2 | 10.1 ± 2.5 | 3.4 ± 1.7 | 12.9 ± 2.9 | 127.9 ± 49.8 | ||
| Control | 18.6 ± 3.2 | 11.7 ± 1.8 | 3.8 ± 2.2 | 14.8 ± 3.4 | 162.0 ± 52.4 | ||
| L4–5 | Entrance | DLS total | 22.1 ± 3.4 | 7.0 ± 2.3 | 1.6 ± 3.5 | 12.9 ± 3.6 | 67.5 ± 25.5 |
| Concave | 17.8 ± 3.5 | 6.8 ± 2.7 | 1.1 ± 1.5 | 10.7 ± 3.4 | 53.6 ± 23.3 | ||
| Convex | 20.5 ± 2.4 | 6.6 ± 3.4 | 1.6 ± 1.2 | 11.5 ± 3.1 | 59.0 ± 27.0 | ||
| Control | 22.4 ± 3.1 | 6.5 ± 1.2 | 1.8 ± 1.6 | 12.9 ± 2.5 | 67.4 ± 22.5 | ||
| Minimum-area point | DLS total | 14.5 ± 1.6 | 7.2 ± 3.4 | 2.0 ± 1.6 | 7.6 ± 3.0 | 54.8 ± 25.1 | |
| Concave | 9.3 ± 3.1 | 6.9 ± 2.8 | 1.6 ± 1.6 | 5.2 ± 2.8 | 35.9 ± 24.3 | ||
| Convex | 12.3 ± 3.0 | 7.2 ± 3.6 | 2.4 ± 1.4 | 6.5 ± 2.5 | 42.0 ± 25.9 | ||
| Control | 14.9 ± 3.2 | 7.0 ± 1.4 | 2.5 ± 1.7 | 9.7 ± 2.1 | 57.3 ± 27.3 | ||
| Exit | DLS total | 15.3 ± 4.0 | 11.6 ± 4.5 | 2.9 ± 2.3 | 11.6 ± 3.6 | 118.8 ± 51.6 | |
| Concave | 10.1 ± 4.3 | 12.0 ± 5.3 | 2.3 ± 2.1 | 10.9 ± 3.9 | 87.3 ± 56.1 | ||
| Convex | 13.1 ± 2.7 | 11.3 ± 4.7 | 3.6 ± 2.2 | 10.6 ± 2.9 | 100.8 ± 49.6 | ||
| Control | 15.7 ± 3.1 | 11.6 ± 3.1 | 3.3 ± 2.1 | 12.1 ± 3.3 | 133.2 ± 55.6 | ||
| L5–S1 | Entrance | DLS total | 21.6 ± 3.5 | 6.9 ± 4.1 | 1.2 ± 1.2 | 11.6 ± 3.5 | 73.0 ± 32.9 |
| Concave | 19.6 ± 3.5 | 6.8 ± 3.2 | 1.1 ± 1.2 | 9.5 ± 3.6 | 61.2 ± 33.0 | ||
| Convex | 20.7 ± 3.2 | 6.9 ± 4.1 | 1.3 ± 1.2 | 12.5 ± 3.2 | 73.5 ± 29.4 | ||
| Control | 21.7 ± 4.0 | 6.8 ± 1.5 | 1.4 ± 1.6 | 11.8 ± 2.6 | 85.6 ± 29.4 | ||
| Minimum-area point | DLS total | 13.2 ± 3.6 | 7.6 ± 4.4 | 1.4 ± 1.4 | 7.5 ± 2.9 | 63.8 ± 35.2 | |
| Concave | 11.4 ± 3.4 | 7.3 ± 3.9 | 1.2 ± 1.2 | 6.8 ± 3.0 | 49.3 ± 36.1 | ||
| Convex | 12.5 ± 3.4 | 7.5 ± 4.5 | 1.6 ± 1.5 | 7.6 ± 2.6 | 59.0 ± 33.1 | ||
| Control | 14.8 ± 3.1 | 8.1 ± 2.5 | 1.3 ± 1.7 | 10.2 ± 2.4 | 80.8 ± 38.2 | ||
| Exit | DLS total | 12.3 ± 3.4 | 12.1 ± 6.5 | 1.9 ± 1.8 | 10.4 ± 3.5 | 102.3 ± 49.3 | |
| Concave | 9.9 ± 3.1 | 11.7 ± 5.8 | 1.6 ± 1.6 | 9.7 ± 3.7 | 76.4 ± 53.7 | ||
| Convex | 11.3 ± 3.2 | 11.5 ± 6.4 | 2.1 ± 1.8 | 10.7 ± 3.1 | 102.4 ± 44.7 | ||
| Control | 12.2 ± 3.7 | 12.3 ± 2.3 | 1.5 ± 1.9 | 13.5 ± 3.4 | 112.8 ± 49.6 |
Values are mean ± SD
FH foraminal height, FW foraminal width, PDH posterior disc height, P-SAP interval between the upper pedicle and superior articular process, FA cross-sectional foraminal area
DLS total the mean value of the bilateral foraminal parameters
Concave the mean value of the foraminal parameters on the concave side at the segments with intervertebral wedging
Convex the mean value of the foraminal parameters on the convex side at the segments with intervertebral wedging
Results
Reliabilities of measurements
The interclass correlation coefficient was 0.99 for MDCT images compared with the actual measurements made directly from the cadaveric lumbar spine. The absolute errors in these measurements ranged 0.7–4.8 mm, which were considered to be small. The correlation coefficients were 0.84–0.91 for intraobserver reliability and 0.71–0.89 for interobserver reliability. Together, these data show that the measurements made from the MDCT images were consistent and reliable.
Measurements of foramina on MDCT
A total of 462 intervertebral foramina on both sides, at L3–4, L4–5, and L5–S1, were measured in the DLS group; 114 were measured in the control group. The mean ± SD of the foraminal parameters are shown in Table 3. FH, PDH, P-SAP, and FA were larger at the upper lumbar levels, and smaller at the lower levels, especially at the exit of the foramina (p < 0.01), but there was no significant difference in FW at the different lumbar levels. In the three groups including the DLS total, the concave, and the convex, FH, P-SAP, and FA were significantly smaller than for the control group at all three foraminal locations and every lumbar level (p < 0.05).
MDCT measurements and intervertebral wedging
In this study, we defined observable intervertebral wedging angle of 3° or more on a standing posteroanterior radiograph, with consideration for the variation of X-ray incidence angle and the error of measurement on image-editing software. On radiographs, intervertebral wedging was observed in 85.7 % of the DLS patients at L3–4, 84.4 % at L4–5, and 58.4 % at L5–S1; the respective average wedging angles were 4.6°, 5.4°, and 3.2°. We compared the MDCT parameters between the concave and convex sides of the intervertebral wedging (Table 3). FH was significantly smaller on the concave side at the entrance, minimum-area point, and exit, at L3–4 and L4–5 (p < 0.05), whereas there was no significant difference at L5–S1. Also, no significant differences were found for FW at any lumbar level. P-SAP was significantly smaller on the concave side at all three points of the foramina and at all lumbar levels, except for the exit at L3–4 and L4–5 (p < 0.05). PDH and FA were significantly smaller on the concave side at all lumbar levels (p < 0.05). Thus, although all the parameters tended to be smaller in the DLS groups including DLS total, the concave side, and the convex side than the control group, the difference was significant for FH, P-SAP, and FA (p < 0.05). No significant difference was found in PDH between DLS total and control group except for the entrance at L3–4. PDH in concave side in DLS was significantly smaller than in the control group at all the three points of the foramina at L3–4 and L4–5, and the entrance at L5–S1 (p < 0.05). There was no significant difference in PDH between the convex side in DLS and the control group.
Relationships between radiographic and MDCT parameters
The parameters representing segmental deformity are shown in Table 4. For the multiple regression analysis, we excluded lateral translation, which was correlated with the vertebral rotation, from the explanatory variables, to avoid multi-collinearity (Pearson r = 0.844 at L3–4, 0.747 at L4–5). Similarly, we excluded PDH, which was moderately correlated with the intervertebral wedging angle (Pearson r = 0.39–0.68).
Table 4.
Parameters of segmental deformities
| Intervertebral wedging angle (°) | Anteroposterior translation rate (%) | Lateral translation rate (%) | Vertebral rotation angle (°) | |
|---|---|---|---|---|
| L3–4 | 4.5 ± 3.3 (0–14.5) | −1.2 ± 7.7 (−29.7–16.7) | 6.6 ± 5.0 (0–25.4) | 4.2 ± 3.3 (0–15.5) |
| L4–5 | 4.8 ± 3.8 (0–15.2) | 1.7 ± 7.4 (−12.4–27) | 6.1 ± 5.1 (0–13.3) | 3.4 ± 2.8 (0–12.6) |
| L5–S1 | 2.8 ± 2.8 (0–8.7) | −0.9 ± 6.7 (−13.4–35) | 0.2 ± 1.2 (0–1.7) | 1.2 ± 1.4 (0–6.6) |
Values are mean ± SD (minimum–maximum)
In anteroposterior translation, positive/negative value indicates anterior/posterior translation
The results of the multiple regression analysis for P-SAP are shown in Table 5. The intervertebral wedging angle was significantly correlated with P-SAP at the entrance and the minimum-area points of the foramina at L3–4 and L4–5, and at the entrance of the L5–S1 foramina (p < 0.05). The anteroposterior translation rate was significantly correlated with P-SAP at all three points at each lumbar level (p < 0.05). The vertebral rotation angle was significantly correlated with P-SAP at the minimum-area points of the foramina at L3–4 and L4–5, and at all three points at L5–S1 (p < 0.05).
Table 5.
Multiple regression analysis of segmental deformities and P-SAP (mm)
| Entrance | Minimum-area point | Exit | |
|---|---|---|---|
| IW (°) | |||
| L3–4 | 0.178 (0.09–0.27)* | 0.099 (0.03–0.17)* | 0.083 (−0.002–0.168) |
| L4–5 | 0.193 (0.11–0.28)* | 0.155 (0.09–0.22)* | 0.085 (−0.01–0.18) |
| L5–S1 | 0.154 (0.03–0.28)* | 0.055 (−0.05–0.16) | 0.056 (−0.07–0.19) |
| APT (%) | |||
| L3–4 | 0.110 (0.05–0.17)* | 0.132 (0.08–0.18)* | 0.126 (0.06–0.19)* |
| L4–5 | 0.086 (0.02–0.15)* | 0.116 (0.07–0.16)* | 0.122 (0.05–0.20)* |
| L5–S1 | 0.221 (0.15–0.29)* | 0.195 (0.14–0.25)* | 0.203 (0.13–0.28)* |
| VR (°) | |||
| L3–4 | −0.053 (−0.14–0.04) | 0.095 (0.02–0.17)* | 0.081 (−0.01–0.17) |
| L4–5 | 0.116 (−0.003–0.23) | 0.220 (0.14–0.31)* | 0.075 (−0.05–0.20) |
| L5–S1 | 0.365 (0.15–0.29)* | 0.195 (0.14–0.25)* | 0.203 (0.13–0.28)* |
Values show regression coefficient (95 % confidence interval)
IW, APT, VR are explanatory variables. P-SAP is the objective variable
P-SAP interval between pedicle and superior articular process, IW intervertebral wedging angle, APT anteroposterior translation rate, VR vertebral rotation angle
* Statistically significant differences
The results of the multiple regression analysis for FA are shown in Table 6. Significant correlations were found between the FA and the intervertebral wedging angle at all three points and at each lumbar level (p < 0.05) and between FA and the anteroposterior translation rate at only the minimum-area point and the exit of the L3–4 foramen (p < 0.05). The vertebral rotation angle was significantly correlated with FA at the minimum-area point of the foramina at L3–4 and L4–5 and at all three points of the L5–S1 foramina (p < 0.05). The results of the multiple regression analyses for FH and FW have been omitted, because there was little significant correlation with segmental deformities.
Table 6.
Multiple regression analysis of segmental deformities and FA (mm2)
| Entrance | Minimum-area point | Exit | |
|---|---|---|---|
| IW (°) | |||
| L3–4 | 1.898 (1.20–2.59)* | 1.242 (0.57–1.91)* | 1.986 (0.65–3.32)* |
| L4–5 | 1.749 (1.09–2.40)* | 1.742 (0.80–2.08)* | 2.871 (1.47–4.27)* |
| L5–S1 | 1.492 (0.23–2.76)* | 1.363 (0.10–2.56)* | 2.593 (0.63–4.56)* |
| APT (%) | |||
| L3–4 | 0.458 (−0.04–0.96) | 0.526 (0.04–1.01)* | 1.214 (0.25–2.18)* |
| L4–5 | 0.250 (−0.28–0.78) | 0.405 (−0.11–0.92) | 0.792 (−0.32–1.91) |
| L5–S1 | −0.146 (−0.87–0.58) | −0.011 (−0.82–0.80) | 0.543 (−0.58–1.62) |
| VR (°) | |||
| L3–4 | −0.106 (−0.83–0.62) | 0.786 (0.09–1.49)* | −0.374 (−1.77–1.02) |
| L4–5 | 0.783 (−0.14–1.71) | 1.415 (0.51–2.32)* | −1.455 (−3.42–0.51) |
| L5–S1 | 3.777 (1.01–6.54)* | 3.756 (0.68–6.84)* | 6.302 (2.01–10.59)* |
Values show the regression coefficient (95 % confidence interval)
IW, APT, VR are explanatory variables. FA is objective variable
FA Cross-sectional foraminal area, IW intervertebral wedging angle, APT anteroposterior translation rate, VR vertebral rotation angle
* Statistically significant difference
Discussion
The progression of foraminal stenosis seen in DLS is related to the narrowing of the intervertebral space, growth of the superior articular processes, thickening of the ligamentum flavum, and degenerative changes in the bone spurs on the vertebral body endplate, which are associated with intervertebral disc bulging [10]. Here, we proposed a new method for evaluating the foramina in DLS patients, in which the measurements took account of the oblique alignment of the foramina, frequently seen with this disease. The results of this study demonstrated that the FH, PDH, P-SAP, and FA were smaller at the lower lumbar levels than at the upper levels, but the FW values were similar for all three spinal levels, i.e., smallest at the entrance of the foramina and largest at the exit. In cases of vertebral wedging, FH, P-SAP, and particularly PDH and FA, were significantly smaller on the concave than the convex side. In a comparison between the DLS group and the control group, each parameter was smaller in the DLS group, with the greatest difference in FA at L5–S1. These radio-anatomical results supported the clinical findings that lumbar radiculopathy in DLS patients is more frequently seen on the concave side and at the lower lumbar levels, especially at L5–S1.
Jenis and An [10] reported in their review article that the most common roots involved in foraminal stenosis are the L5 nerve root (75 %), followed by L4 (15 %) and L3 (5.3 %). A cadaveric study by Stephens et al. [6] showed that FA in the sagittal plane ranged from 40 to 160 mm2 when measured on MRI images. Here, the maximum value of FA at the minimum-area point of the foramen was 160 mm2, consistent with the findings from previous studies, and the minimum value of FA associated with intervertebral wedging at L5–S1 was 7.7 mm2. An MRI study by Ploumis et al. [7] reported that FA was larger on the convex than the concave side in DLS, which is partially consistent with the results of our study.
The segmental deformities in DLS patients correlated with the foraminal morphology to varying degrees. Intervertebral wedging significantly reduced the FA, and it reduced the P-SAP at the entrance and the minimum-area point of the foramina on the concave side. Posterior translation was associated with a significant reduction in P-SAP, but not in FA. Vertebral rotation reduced P-SAP and FA significantly at the minimum-area point of the foramina at each lumbar level, on the side of the rotation.
There were several limitations of this study. First, we were not able to investigate nerve compression within the intervertebral foramina and associated clinical symptoms, because the MDCT images did not show the nerve roots, which are visible by MRI and in cadaveric specimens. The correlation between these morphological data and patients’ symptoms should be conducted by further well-designed prospective study in which meticulous surgical explorations of the foramen, clinical symptoms, and pedicle morphologies are correlated. Second, we could not evaluate the dynamic changes in morphology of the intervertebral foramina according to patient postures. A dynamic study may be conducted by open MRI machines or low-dose radiation CT scanning techniques in the future.
Conclusion
With the new analysis method described here, using three-dimensional reconstructed images from MDCT data, we clarified the morphologies of the foramina in DLS patients in more detail than in previous studies, and we elucidated their association with radiological segmental deformities. The anatomical parameters were impacted differently by the different deformities. These radio-anatomical data are useful for understanding the pathomechanisms of foraminal stenosis in DLS patients.
Conflict of interest
None.
References
- 1.Saint-Louis LA. Lumbar spinal stenosis assessment with computed tomography, magnetic resonance imaging, and myelography. Clin Orthop. 2001;384:122–136. doi: 10.1097/00003086-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Burton R, Kirkaldy-Willis W, Yong-Hing K, et al. Cases of failure of surgery on the lumbar spine. Clin Orthop. 1981;157:191–197. [PubMed] [Google Scholar]
- 3.MacNab I. Negative disc exploration: an analysis of the causes of nerve root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53:891–903. [PubMed] [Google Scholar]
- 4.Schlegel JD, Champine J, Taylor MS, et al. The role of distraction in improving the space available in the lumbar stenotic canal and foramen. Spine. 1994;18:2041–2047. doi: 10.1097/00007632-199409150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Inufusa A, An HS, Lim TH, et al. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine. 1996;21:2412–2420. doi: 10.1097/00007632-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Stephens MM, Evans JH. Lumbar intervertebral foramens: an in vitro study of their shape in relation to intervertebral disc pathology. Spine. 1991;16:525–529. doi: 10.1097/00007632-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ploumis A, Transfeldt EE, Gilbert TJ, et al. Degenerative lumbar scoliosis: radiographic correlation of lateral rotatory olisthesis with neural canal dimensions. Spine. 2006;31:2353–2358. doi: 10.1097/01.brs.0000240206.00747.cb. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara A, An HS, Lim TH, et al. Morphologic changes in the lumbar intervertebral foramen due to flexion-extension, lateral bending, and axial rotation: an in vitro anatomic and biomechanical study. Spine. 2001;26:876–882. doi: 10.1097/00007632-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 9.Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine. 1991;16:1312–1320. doi: 10.1097/00007632-199111000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Jenis LG, An HS. Spine update: lumbar foraminal stenosis. Spine. 2000;25:389–394. doi: 10.1097/00007632-200002010-00022. [DOI] [PubMed] [Google Scholar]