Abstract
Introduction
Gait impairment is a primary symptom of cervical spondylotic myelopathy (CSM); however, little is known about specific kinetic and kinematic gait parameters. The objectives of the study were: (1) to compare gait patterns of people with untreated CSM to those of age- and gender-matched healthy controls; (2) to examine the effect of gait speed on kinematic and kinetic parameters.
Materials and methods
Sixteen patients with CSM were recruited consecutively from a neurosurgery clinic, and 16 healthy controls, matched to age (±5 years) and gender, were recruited for comparison. Patients and controls underwent three-dimensional gait analysis using a Vicon® motion analysis system, at self-selected speed over a 10-m track. Controls were also assessed at the speed of their CSM match.
Results
At self-selected speed, the CSM group walked significantly more slowly, with shorter stride lengths and longer double support duration. They showed significant decreases in several kinematic and kinetic parameters, including sagittal range of motion at the hip and knee, ankle plantarflexion, anteroposterior ground reaction force (GRF) at toe-off, power absorption at the knee in loading response and terminal stance, and power generation at the ankle. At matched speed, the CSM group showed significant decreases in knee flexion during swing, total sagittal knee range of motion, peak ankle plantarflexion and anteroposterior GRF.
Conclusion and implications
The findings suggested that people with CSM have significant gait abnormalities that have not been previously reported. In particular, there are key differences in the motor strategies used in the terminal stance phase of gait that cannot be explained by speed alone.
Keywords: Cervical myelopathy, Gait, Gait analysis, Biomechanics
Introduction
Gait impairment is a primary symptom of cervical spondylotic myelopathy (CSM). In contrast to the substantial body of literature on gait patterns in neurological conditions such as cerebral palsy [1] and stroke [2], little is known about the kinematic and kinetic characteristics of gait in CSM. There is evidence that people with CSM have a slower gait speed, prolonged double support duration and reduced cadence compared to healthy controls (HCs) [3–6]. Previous studies also identified reduced knee flexion during swing in the early stages of the disease, and in more severe disease, decreased ankle plantarflexion at the terminal stance and reduced knee flexion during loading response [3, 6–8]. However, these studies evaluated a limited number of parameters. A greater insight could be attained through the systematic analysis of a larger number of discrete parameters at specific points in the gait cycle across three planes of motion [9]. Furthermore, no previous studies evaluated joint moments and powers from kinetic data, information that could significantly enhance the understanding of a gait deficit in spinal cord pathology [10].
An important consideration in the interpretation of kinematic and kinetic data is the inter-dependence of many variables. Gait speed is known to influence lower limb kinematics, with faster speeds generally associated with larger sagittal range of motion (ROM) [9]. Previous studies of the CSM gait compared patient and control groups at different speeds [6, 8, 11] or at enforced speeds [3]. It is important that participants should be assessed at self-selected gait speeds to evaluate natural walking performance; however, there is also a need to match the speeds of CSM and control participants to avoid confounding effects on kinematics [12]. Otherwise, it cannot be known whether reduced joint excursions are the cause or the effect of slower speed.
The aims of this study were:
to compare gait patterns of people with untreated CSM with age and gender-matched HCs;
to examine the effect of speed on kinematic and kinetic features of the CSM gait.
Methods
Participants
Approval was obtained from a local ethics committee. Participants with CSM were consecutively recruited from a neurosurgical clinic. The following inclusion criteria were applied: (1) aged 18 years or over; (2) able to give informed consent; (3) able to mobilise at least 10 m without assistance of another person; (4) clinical and radiological evidence of CSM. CSM was diagnosed in the presence of one or more of the symptoms such as clumsy hands, numb hands or feet, lower limb weakness, unsteady gait, and bilateral upper or lower limb paraesthesiae, and one or more of the following signs on examination: lower limb spasticity, unsteady gait, hyperreflexia, upgoing plantars, clonus, weakness in a corticospinal distribution and positive Hoffman’s sign. The diagnosis was confirmed by evidence on magnetic resonance imaging of cord compression due to spondylosis or intrinsic signal change.
Patients were excluded if they were affected by any of the following: (1) severe respiratory or cardiac disease hindering safe mobilisation; (2) history of neurological disorders with persistent deficit; (3) symptomatic musculoskeletal problems affecting gait; (4) tandem lumbar spine stenosis; (5) previous surgical decompression for CSM. Severity of myelopathy was measured using the Nurick classification [13] and the modified Japanese orthopaedic association scale [14].
Each CSM participant was matched to an HC of the same age (±5 years) and gender. HCs were recruited from a local population of colleagues and had no symptomatic lower limb injuries, neurological disorders, or cardiovascular or respiratory impairment that would hinder gait analysis. All participants gave informed consent.
Gait analysis
Three-dimensional gait analysis (3DGA) was conducted using a VICON® 250 five-camera Motion Analysis system (VICON, Oxford, UK) and an integrated Kistler multi-component force plate (Kistler, Winterthur, Switzerland). Anthropometric data were collected according to a standard protocol. Fifteen 25-mm reflective markers were applied to anatomical landmarks on the lower limbs using the modified Helen Hayes method [15]. A knee alignment device (Motion Lab Systems Inc, Baton Rouge, USA) was used to aid the identification of the knee joint axis.
Each assessment consisted of a static trial for calibration and a warm-up trial to familiarise the participant with the protocol, followed by a number of barefoot walking trials. Data were captured at a frequency of 50 Hz. Participants were instructed to walk at self-selected comfortable speed along a 12-m overground walkway. The assessment continued until ten trials, comprising five left and right force plate strikes, with good quality data had been achieved. Breaks between walking trials were provided at participants’ request to avoid fatigue.
HCs then completed a second assessment at the walking speed of the individual CSM participants to whom they were matched. Trials were timed using a stopwatch. The goal speed was indicated by verbal feedback at the end of each trial. Trials were included in the representative average if they were within 0.1 m/s of the goal speed. The assessment concluded when ten trials at goal speed were achieved.
Gait data were processed using VICON Workstation® software. Marker trajectories were reconstructed and filtered using the Woltring routine [16] with a mean standard error of 15 mm2. VICON Plug-in Gait® was used to calculate motion at the lower limb joints in three planes. Gait cycle events (heel strike and toe-off) were identified automatically from force plate data for gait cycles where force was recorded, and by using frame-by-frame monitoring of the heel, ankle and toe trajectories, where force was not recorded. The average of ten captured trials was used to represent the gait pattern of each participant under each condition (comfortable speed only for CSM, comfortable and matched speed for HC). Data from CSM participants’ more affected lower limbs were analysed and compared to data from the same limb of their HC matches. The more affected lower limb was determined by subjective questioning. Temporal–spatial parameters and kinematic and kinetic key points, listed in Table 1, were selected based on clinical significance in a neurological population [17] and the results of a reliability study of 3DGA in CSM [18].
Table 1.
Kinematic and kinetic key points extracted from 3DGA data
| Joint | Description of key point |
|---|---|
| Pelvis | Peak pelvic tilt |
| Range of pelvic tilt | |
| Average pelvic tilt | |
| Peak pelvic obliquity | |
| Range of pelvic obliquity | |
| Range of pelvic rotation | |
| Hip | Hip position in the sagittal plane at initial contact |
| Peak hip flexion | |
| Peak hip extension | |
| Total range of hip excursion in the sagittal plane | |
| Peak hip abduction in swing | |
| Total range of hip excursion in the frontal plane | |
| Peak hip internal rotation | |
| Total range of hip rotation | |
| Knee | Knee position at initial contact |
| Peak knee flexion in stance, loading response | |
| Peak knee flexion in swing | |
| Peak knee extension in mid-stance | |
| Total range of knee excursion in the sagittal plane | |
| Ankle | Ankle position at initial contact |
| Peak ankle dorsiflexion in stance | |
| Peak ankle dorsiflexion in swing | |
| Peak ankle plantarflexion | |
| Ground reaction force (GRF) (N/kg) | Peak mediolateral GRF |
| Peak negative antero-posterior GRF (braking) | |
| Peak positive antero-posterior GRF (propulsion) | |
| First vertical GRF peak, loading | |
| Minimum value of GRF during stance | |
| Second vertical GRF peak, propulsion | |
| Moments (Nm/kg) | Peak hip flexor moment |
| Peak hip extensor moment | |
| Peak hip abductor moment | |
| Peak knee flexor moment | |
| Peak knee extensor moment | |
| Peak ankle plantarflexor moment | |
| Powers (W/kg) | Peak concentric hip extensor power during loading response (H1) |
| Peak eccentric hip flexor power during mid-stance (H2) | |
| Peak concentric hip flexor power during terminal stance (H3) | |
| Peak eccentric knee extensor power during loading response (K1) | |
| Peak concentric knee extensor power during mid-stance (K2) | |
| Peak eccentric knee extensor power in terminal stance (K3) | |
| Peak eccentric knee flexor power, terminal swing (K4) | |
| Peak eccentric ankle power in loading response through to mid-stance (A1) | |
| Peak concentric ankle plantarflexor power at terminal stance (A2) |
W watts, kg kilograms, N Newtons, Nm Newton metre
Statistical analysis
A sample size calculation was performed in Stata (StataCorp, Texas, USA). At a significance level of 0.05, 13 pairs of participants were required for 90 % power to detect a difference in gait speed of 0.1 m per second (m/s) with a standard deviation of 0.11 m/s.
Two analyses were conducted. The first analysis compared CSM with HC participants at comfortable speed, and the second compared the pairs at matched speed. The distribution of each variable was examined using quantile–quantile and box plots. Normally distributed data were analysed using two-tailed paired t tests. The Wilcoxon signed-rank test analysed variables that were not normally distributed.
Results
Sixteen participants with CSM and 16 HCs were recruited between December 2008 and December 2010. Table 2 shows their characteristics.
Table 2.
Characteristics of participants
| CSM | HC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Gender | Age (years) | Height (m) | Weight (kg) | Duration of symptoms (months) | Nurick | mJOA | MRMI | Age (years) | Height (m) | Weight (kg) |
| 02 | F | 68 | 1.52 | 85.2 | 12 | 3 | 8 | 34 | 68 | 1.69 | 74.4 |
| 03 | M | 57 | 1.66 | 80.3 | 12 | 3 | 11 | 38 | 59 | 1.76 | 78.4 |
| 04 | F | 50 | 1.61 | 57.7 | 5 | 1 | 14 | 40 | 45 | 1.67 | 62.8 |
| 05 | M | 35 | 1.93 | 100.7 | 24 | 2 | 10 | 40 | 33 | 1.76 | 85.5 |
| 06 | F | 43 | 1.46 | 80.2 | 48 | 3 | 12 | 39 | 41 | 1.73 | 67.1 |
| 07 | F | 46 | 1.5 | 56.4 | 60 | 3 | 13 | 39 | 41 | 1.67 | 61.5 |
| 08 | F | 63 | 1.6 | 70.4 | 36 | 3 | 9 | 39 | 58 | 1.71 | 71.7 |
| 11 | M | 53 | 1.56 | 54.6 | 108 | 2 | 10 | 39 | 53 | 1.73 | 76 |
| 12 | F | 51 | 1.72 | 74.8 | 48 | 2 | 10 | 40 | 55 | 1.63 | 57.5 |
| 13 | M | 74 | 1.69 | 61.5 | 420 | 3 | 12 | 38 | 73 | 1.71 | 63.9 |
| 15 | F | 73 | 1.64 | 54.1 | 10 | 2 | 10 | 40 | 68 | 1.56 | 61.3 |
| 16 | F | 48 | 1.72 | 89.8 | 48 | 1 | 13 | 40 | 50 | 1.59 | 74.5 |
| 18 | M | 47 | 1.67 | 78.7 | 12 | 3 | 11 | 39 | 52 | 1.89 | 89.5 |
| 19 | M | 64 | 1.83 | 91.1 | 180 | 2 | 14 | 40 | 65 | 1.8 | 81 |
| 20 | M | 54 | 1.79 | 96.3 | 18 | 3 | 10 | 39 | 54 | 1.79 | 69.7 |
| 22 | M | 58 | 1.66 | 75.6 | 10 | 4 | 12 | 39 | 62 | 1.66 | 94.2 |
| Mean/median | 55.25 | 1.66 | 75.5 | 36 | 3 | 11 | 39 | 54.8 | 1.71 | 73.1 | |
Italic values to indicate that the median is reported rather than the mean
mJOA modified Japanese orthopaedic association score, MRMI modified rivermead mobility index, kg kilograms, M male, F female
Temporal–spatial parameters
The mean gait speed of CSM participants was 1.12 m/s, significantly slower than HCs’ mean speed of 1.49 m/s (p < 0.0001). This slower speed resulted from a shorter mean stride length of 1.19 m compared to 1.45 m (p = 0.0001), and a lower mean cadence of 113 compared to 122 steps per min (p = 0.005). CSM participants spent a shorter proportion of the gait cycle (GC) in single limb support (36.7 % compared to 39.9 % GC duration, p < 0.0001).
At matched speed, stride length (CSM 1.19 m, HC 1.27 m, p = 0.03) and single leg support (HC 36.7 %, CSM 37.6 % GC, p = 0.049) remained significantly lower in CSM participants. The full results are shown in Table 3.
Table 3.
Temporal–spatial parameters of CSM and HC participants
| Variable | CSM data | Comfortable speed comparison | Matched speed comparison | SEMa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSM | HC | Difference | 95 % Confidence intervals | p value | HC | Difference | 95 % Confidence intervals | p value | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||||
| Cadence (steps/min) | 113.22 | 10.40 | 122.97 | 8.62 | −9.75 | 12.05 | −16.17 | −3.33 | 0.006 | 103.91 | 12.02 | 9.31 | 9.00 | 4.52 | 14.11 | 0.0009 | 1.70 |
| Double support (s) | 0.28 | 0.06 | 0.20 | 0.03 | 0.08 | 0.06 | 0.05 | 0.11 | 0.0001 | 0.30 | 0.07 | −0.02 | 0.03 | −0.03 | 0.00 | 0.07 | 0.01 |
| Single support (s) | 0.39 | 0.04 | 0.39 | 0.02 | 0.00 | 0.04 | −0.02 | 0.02 | 0.9 | 0.44 | 0.04 | −0.05 | 0.04 | −0.07 | −0.02 | 0.0005 | 0.01 |
| Double support duration (% GC) | 26.06 | 3.82 | 20.32 | 2.03 | 5.74 | 4.14 | 3.54 | 7.95 | 0.0001 | 24.94 | 3.32 | 1.12 | 2.84 | −0.40 | 2.64 | 0.14 | |
| Single support duration (% GC) | 36.66 | 2.06 | 39.86 | 0.85 | −3.20 | 1.99 | −4.26 | −2.13 | <0.0001 | 37.56 | 1.66 | −0.90 | 1.67 | −1.79 | 0.00 | 0.049 | |
| Foot off (% GC) | 62.71 | 2.19 | 60.18 | 1.33 | 2.53 | 2.81 | 1.03 | 4.03 | 0.003 | 62.49 | 1.76 | 0.21 | 2.01 | −0.85 | 1.28 | 0.7 | 0.81 |
| Gait speed (m/s) | 1.12 | 0.24 | 1.49 | 0.18 | −0.36 | 0.24 | −0.49 | −0.24 | <0.0001 | 1.11 | 0.21 | 0.02 | 0.07 | −0.02 | 0.06 | 0.3 | 0.02 |
| Opposite foot contact (% GC) | 49.56 | 1.25 | 50.26 | 0.59 | −0.69 | 1.57 | −1.53 | 0.14 | 0.09 | 50.27 | 0.65 | −0.71 | 1.41 | −1.46 | 0.04 | 0.06 | 1.01 |
| Opposite foot off (% GC) | 12.91 | 2.14 | 10.40 | 1.13 | 2.51 | 2.33 | 1.27 | 3.75 | 0.0006 | 12.69 | 1.73 | 0.22 | 1.54 | −0.61 | 1.04 | 0.6 | 0.77 |
| Step length (m) | 0.59 | 0.10 | 0.73 | 0.06 | −0.14 | 0.11 | −0.19 | −0.08 | 0.0001 | 0.64 | 0.06 | −0.05 | 0.07 | −0.09 | −0.01 | 0.02 | 0.01 |
| Step time (s) | 0.54 | 0.05 | 0.49 | 0.03 | 0.05 | 0.06 | 0.02 | 0.08 | 0.002 | 0.58 | 0.06 | −0.04 | 0.05 | −0.07 | −0.01 | 0.007 | 0.02 |
| Step width (m) | 0.17 | 0.04 | 0.16 | 0.03 | 0.01 | 0.04 | −0.01 | 0.03 | 0.4 | 0.16 | 0.04 | 0.01 | 0.05 | −0.02 | 0.03 | 0.5 | 0.01 |
| Stride length (m) | 1.19 | 0.19 | 1.45 | 0.11 | −0.26 | 0.20 | −0.37 | −0.16 | 0.0001 | 1.27 | 0.13 | −0.08 | 0.14 | −0.16 | −0.01 | 0.03 | 0.02 |
| Stride time (s) | 1.07 | 0.11 | 0.98 | 0.06 | 0.09 | 0.11 | 0.03 | 0.15 | 0.006 | 1.17 | 0.13 | −0.10 | 0.10 | −0.15 | −0.05 | 0.0008 | 0.02 |
SD standard deviation, GC gait cycle, m metres, s seconds
aStandard error of measurement, calculated in a prior study [18]
Kinematics
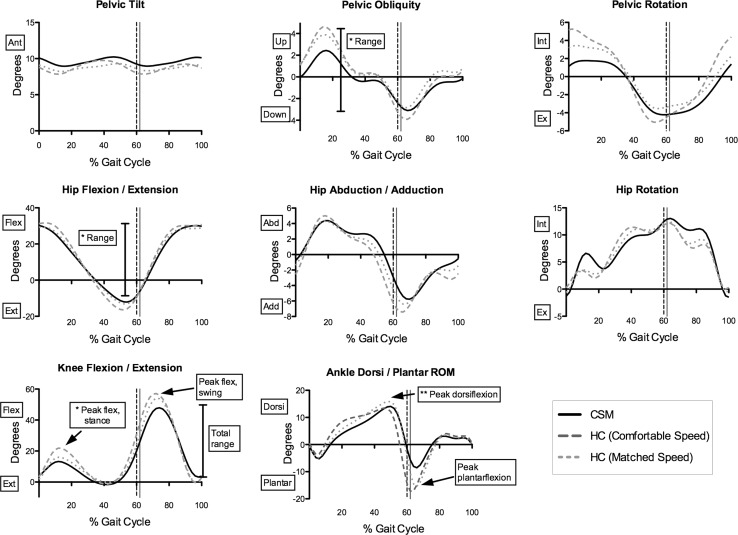
Kinematic data are provided in Fig. 1 and Table 4. At comfortable speed, CSM participants had reduced range of pelvic obliquity (CSM 6.34°, HC 8.78°, p = 0.003) and reduced total sagittal plane excursion at the hip (CSM 44.3°, HC 49.1°, p = 0.004). At the knee, CSM participants showed lower peak flexion in stance (HC 22.1°, CSM 13.7°, p = 0.0005), peak flexion in swing (HC 57.5°, CSM 48.6°, p = 0.0005), and total sagittal plane motion (HC 59.9°, CSM 51.9°, p = 0.004). Ankle plantarflexion was also reduced in CSM (11.3°, HC −18.4°, p = 0.013).
Fig. 1.
Kinematic curves of HC and CSM participants. Significant differences between HC (dashed lines) and CSM (continuous line) are indicated using boxes and arrows; asterisks denotes significant difference at comfortable speed only; double asterisks denotes significant difference at matched speed only. Ant anterior, Post posterior, Flex flexion, Ext extension, Plantar plantarflexion, Dorsi dorsiflexion, Abd abduction, Add adduction, Int internal, Ex = external. Vertical dashed lines indicate toe-off for CSM(black) and HC (grey)
Table 4.
Peak kinematic parameters of CSM and HC participants
| Variable | CSM data | Comfortable speed comparison | Matched speed comparison | SEMa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSM | HC | Difference | 95 % Confidence intervals | p value | HC | Difference | 95 % Confidence intervals | p value | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||||
| Pelvic obliquity range | 6.3 | 3.2 | 8.8 | 2.8 | −2.4 | 2.7 | −3.9 | −1.0 | 0.003 | 7.0 | 2.3 | −0.7 | 2.3 | −1.9 | 0.5 | 0.2 | 0.92 |
| Pelvic rotation range | 8.2 | 3.6 | 10.8 | 4.1 | −0.6 | 3.2 | −2.3 | 1.1 | 0.5 | 8.4 | 2.1 | 1.2 | 3.5 | −0.7 | 3.0 | 0.2 | 1.14 |
| Average pelvic tilt | 9.4 | 5.9 | 8.8 | 4.2 | 0.6 | 7.5 | −3.4 | 4.6 | 0.75 | 8.8 | 4.0 | 0.6 | 7.2 | −3.2 | 4.5 | 0.74 | 2.57 |
| Hip position at initial contact | 30.3 | 6.9 | 31.3 | 5.6 | −1.0 | 9.5 | −6.0 | 4.1 | 0.7 | 29.3 | 5.3 | 1.0 | 9.4 | −4.0 | 6.0 | 0.67 | 2.99 |
| Peak hip extension | −13.4 | 6.3 | −16.5 | 5.3 | 3.1 | 8.8 | −1.6 | 7.9 | 0.17 | −13.5 | 5.9 | 0.1 | 8.6 | −4.5 | 4.7 | 0.97 | 3.19 |
| Total sagittal plane excursion of hip | 44.3 | 5.3 | 49.1 | 4.9 | −4.8 | 5.7 | −7.8 | −1.8 | 0.004 | 43.7 | 3.2 | 0.6 | 4.2 | −1.6 | 2.9 | 0.55 | 1.28 |
| Range of hip abduction/adduction | 12.0 | 3.2 | 13.0 | 3.0 | −1.1 | 3.6 | −3.0 | 0.9 | 0.27 | 11.4 | 2.6 | 0.5 | 3.0 | −1.1 | 2.1 | 0.49 | 1.05 |
| Range of hip rotation | 19.1 | 10.1 | 16.2 | 4.8 | 2.9 | 11.6 | −3.3 | 9.1 | 0.33 | 17.0 | 4.6 | 2.1 | 10.8 | −3.6 | 7.9 | 0.45 | 3.66 |
| Knee position at initial contact | 4.2 | 6.0 | 3.3 | 3.7 | 0.9 | 6.3 | −2.4 | 4.3 | 0.57 | 1.7 | 4.5 | 2.5 | 6.9 | −1.2 | 6.1 | 0.17 | 3.11 |
| Peak knee flexion in stance | 13.7 | 6.4 | 22.1 | 6.9 | −8.3 | 7.5 | −12.3 | −4.3 | 0.0005 | 16.2 | 7.9 | −2.5 | 7.3 | −6.4 | 1.4 | 0.19 | 3.67 |
| Peak knee extension | −3.3 | 4.4 | −2.4 | 4.4 | −0.9 | 5.8 | −4.0 | 2.1 | 0.52 | −2.2 | 4.0 | −1.1 | 5.3 | −3.9 | 1.7 | 0.4 | 3.12 |
| Peak knee flexion in swing | 48.6 | 5.6 | 57.5 | 4.2 | −8.9 | 8.1 | −13.2 | −4.6 | 0.0005 | 54.6 | 5.0 | −6.0 | 7.5 | −10.0 | −2.0 | 0.006 | 3.32 |
| Total sagittal plane excursion of knee | 51.9 | 6.9 | 59.9 | 5.4 | −8.0 | 9.4 | −13.0 | −3.0 | 0.004 | 56.8 | 5.2 | −4.9 | 8.0 | −9.2 | −0.6 | 0.03 | 2.62 |
| Ankle position at initial contact | −0.6 | 3.7 | 0.5 | 3.1 | −1.1 | 4.2 | −3.4 | 1.1 | 0.3 | −0.7 | 3.5 | 0.1 | 4.0 | −2.1 | 2.2 | 0.95 | 2.35 |
| Peak ankle dorsiflexion in stance | 14.3 | 2.6 | 14.4 | 2.7 | −0.1 | 3.2 | * | * | 0.92* | 16.2 | 2.5 | −2.0 | 3.0 | −3.6 | −0.4 | 0.02 | 1.37 |
| Peak ankle plantarflexion | −11.3 | 6.9 | −18.4 | 7.6 | 7.1 | 10.0 | 1.7 | 12.4 | 0.013 | −16.6 | 6.4 | 5.3 | 8.0 | 1.1 | 9.6 | 0.02 | 2.90 |
| Peak ankle dorsiflexion in swing | 6.2 | 4.2 | 4.7 | 2.5 | 1.5 | 4.1 | −0.7 | 3.7 | 0.16 | 6.2 | 4.0 | 0.1 | 4.5 | −2.3 | 2.5 | 0.96 | 2.00 |
All variables are measured in degrees
SD standard deviation
* Denotes a non-normally distributed variable with p value calculated using Wilcoxon signed-rank test; confidence interval not calculated
aStandard error of measurement, calculated in a prior study [18]
At matched speed, CSM participants showed reduced knee flexion in swing (HC 54.6°, CSM 48.6°, p = 0.006) and total sagittal plane excursion (HC 56.8°, CSM 51.9°, p = 0.03). At the ankle, dorsiflexion in stance (HC 16.2°, CSM 14.3°, p = 0.02) and peak plantarflexion at pre-swing (HC −16.6°, CSM −11.3°, p = 0.02) were also lower in CSM participants.
Kinetics
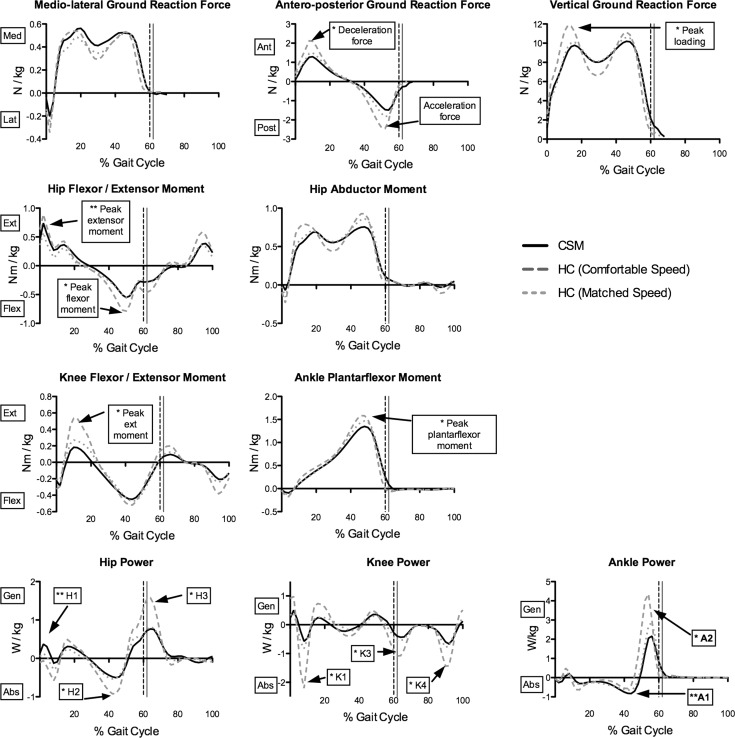
Figure 2 and Table 5 show the results of the kinetic analysis. At comfortable speed, vertical ground reaction force (GRF) during loading was significantly lower in CSM (p = 0.0004). Antero-posterior GRF was also lower in both the deceleration (CSM 1.39 N/kg, HC 2.1 N/kg, p = 0.0003) and acceleration components (CSM −1.6 N/kg, HC −2.53 N/kg, p < 0.0001). CSM participants had reduced peak hip flexor moments (HC −0.81 Nm/kg, CSM −0.62 Nm/kg, p = 0.02) and reduced peak knee extensor moments (HC 0.56 Nm/kg, CSM 0.27 Nm/kg, p = 0.0005). Peak ankle plantarflexor moments were also lower in CSM (HC 1.6 Nm/kg, CSM 1.41 Nm/kg, p = 0.0007). Analysis of net power showed reduced hip power absorption in mid-stance, H2 (HC −0.96 W/kg, CSM −0.61 W/kg, p = 0.004), and hip power generation in pre-swing, H3 (HC1.71 W/kg, CSM 1.0 W/kg, p = 0.0001). Knee power absorption peaks were also lower in CSM throughout the GC. Ankle power generation at pre-swing, A2, was higher in HC (4.85 N/kg) than in CSM (2.82 N/kg, p = 0.0001).
Fig. 2.
Kinetic curves of CSM and HC participants. Significant differences between HC (dashed lines) and CSM (continuous line) are indicated using boxes and arrows; asterisks denotes significant difference at comfortable speed only; double asterisks denotes significant difference at matched speed only. Med medial, Lat lateral, N Newtons, kg kilograms, Nm Newton metres, Ant anterior, Post posterior, Ext extensor, Flex flexor, W Watts, kg kilograms, Gen generation, Abs absorption, H Hip power peak, K knee power peak, A ankle power peak. Vertical dashed line indicates toe-off for both groups
Table 5.
Peak kinetic parameters of CSM and HC participants
| Variable | CSM data | Comfortable speed comparison | Matched speed comparison | SEMa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSM | HC | Difference | 95 % Confidence intervals | p value | HC | Difference | 95 % Confidence intervals | p value | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||||
| Medio-lateral GRF | 0.66 | 0.19 | 0.59 | 0.19 | 0.07 | 0.27 | −0.07 | 0.21 | 0.3 | 0.59 | 0.19 | 0.07 | 0.27 | −0.07 | 0.21 | 0.3 | 0.05 |
| AP GRF, deceleration | 1.39 | 0.46 | 2.10 | 0.53 | −0.71 | 0.61 | −1.03 | −0.38 | 0.0003 | 1.53 | 0.45 | −0.13 | 0.44 | −0.37 | 0.10 | 0.24 | 0.16 |
| AP GRF, acceleration | −1.60 | 0.52 | −2.53 | 0.41 | 0.93 | 0.59 | 0.61 | 1.24 | <0.0001 | −1.90 | 0.45 | 0.30 | 0.45 | 0.05 | 0.54 | 0.02 | 0.10 |
| Vertical GRF | 10.58 | 0.74 | 12.23 | 1.42 | −1.65 | 1.57 | b | b | 0.0004b | 10.82 | 0.57 | −0.24 | 0.60 | −0.56 | 0.08 | 0.12 | 0.40 |
| Hip extensor moment | 0.78 | 0.20 | 0.88 | 0.22 | −0.10 | 0.21 | −0.21 | 0.01 | 0.075 | 0.61 | 0.23 | 0.16 | 0.23 | 0.04 | 0.29 | 0.01 | 0.122 |
| Hip flexor moment | −0.62 | 0.27 | −0.81 | 0.23 | 0.19 | 0.28 | 0.04 | 0.34 | 0.02 | −0.58 | 0.27 | −0.04 | 0.26 | −0.18 | 0.10 | 0.5 | 0.088 |
| Hip abductor moment | 0.84 | 0.18 | 0.98 | 0.22 | −0.14 | 0.30 | −0.30 | 0.02 | 0.08 | 0.90 | 0.21 | −0.06 | 0.25 | −0.19 | 0.07 | 0.3 | 0.069 |
| Knee extensor moment | 0.27 | 0.18 | 0.56 | 0.26 | −0.30 | 0.27 | −0.44 | −0.15 | 0.0005 | 0.33 | 0.22 | −0.06 | 0.21 | −0.18 | 0.05 | 0.25 | 0.056 |
| Knee flexor moment | −0.52 | 0.11 | −0.57 | 0.14 | 0.06 | 0.17 | −0.03 | 0.14 | 0.2 | −0.51 | 0.16 | 0.00 | 0.18 | −0.10 | 0.09 | 0.95 | 0.058 |
| Ankle plantarflexor moment | 1.41 | 0.21 | 1.60 | 0.12 | −0.18 | 0.18 | −0.28 | −0.09 | 0.0007 | 1.48 | 0.13 | −0.07 | 0.17 | −0.16 | 0.02 | 0.12 | 0.053 |
| Hip power generation, H1 | 0.51 | 0.29 | 0.58 | 0.41 | −0.07 | 0.45 | −0.31 | 0.17 | 0.5 | 0.32 | 0.25 | 0.19 | 0.36 | 0.00 | 0.38 | 0.05 | 0.19 |
| Hip power absorption, H2 | −0.61 | 0.27 | −0.96 | 0.39 | 0.35 | 0.41 | 0.13 | 0.56 | 0.004 | −0.57 | 0.31 | −0.05 | 0.28 | −0.20 | 0.10 | 0.5 | 0.10 |
| Hip power generation, H3 | 1.00 | 0.45 | 1.71 | 0.47 | −0.71 | 0.54 | −1.00 | −0.42 | 0.0001 | 0.94 | 0.45 | 0.06 | 0.40 | −0.15 | 0.28 | 0.54 | 0.12 |
| Knee power absorption, K1 | −0.70 | 0.87 | −2.10 | 1.36 | 1.40 | 1.47 | 0.62 | 2.18 | 0.002 | −0.84 | 0.56 | 0.15 | 0.73 | −0.24 | 0.53 | 0.43 | 0.42 |
| Knee power generation, K2 | 0.59 | 0.28 | 0.97 | 0.33 | −0.38 | 0.39 | −0.58 | −0.17 | 0.0015 | 0.56 | 0.25 | 0.03 | 0.31 | −0.13 | 0.19 | 0.7 | 0.17 |
| Knee power absorption, K3 | −0.68 | 0.42 | −1.21 | 0.50 | 0.53 | 0.63 | 0.20 | 0.87 | 0.004 | −0.66 | 0.44 | −0.02 | 0.50 | −0.28 | 0.25 | 0.9 | 0.11 |
| Knee power absorption, K4 | −0.70 | 0.27 | −1.56 | 0.63 | 0.86 | 0.69 | 0.49 | 1.22 | 0.0002 | −0.84 | 0.44 | 0.14 | 0.41 | −0.08 | 0.36 | 0.19 | 0.12 |
| Ankle power absorption, A1 | −0.99 | 0.34 | −0.92 | 0.24 | −0.07 | 0.29 | −0.22 | 0.09 | 0.37 | −0.80 | 0.24 | −0.19 | 0.31 | −0.36 | −0.02 | 0.03 | 0.12 |
| Ankle power generation, A2 | 2.82 | 1.34 | 4.85 | 1.19 | −2.03 | 1.56 | −2.86 | −1.20 | 0.0001 | 3.32 | 1.09 | −0.50 | 1.04 | −1.06 | 0.06 | 0.075 | 0.36 |
GRFs are reported in Newtons per kilogram, moments in Newton metres per kilogram, and powers in watts per kilogram
SD standard deviation, AP antero-posterior, GRF ground reaction force, SEM standard error of measurement
aSEM calculated in a previous study [18]
bDenotes non-normally distributed data tested with a Wilcoxon signed-rank test; confidence intervals were not calculated in this case
There were fewer differences between CSM and HC participants at matched speed; however, some findings persisted. The acceleration component of antero-posterior GRF remained reduced in CSM (HC −1.9 N/kg, CSM −1.6 N/kg, p = 0.02). CSM participants generated higher peak hip extensor moments (CSM 0.78 Nm/kg, HC 0.61 Nm/kg, p = 0.013), a parameter that was not different at comfortable speed. In keeping with this finding, peak hip power generation during loading response (H1) was higher in CSM (0.51 W/kg) compared to HCs (0.25 W/kg, p = 0.05). There were no differences in power generation and absorption at the knee, although there was a non-significant tendency for lower absorption peaks at K1 and K4 in CSM. At the ankle, there was a non-significant trend towards higher power generation at the ankle at toe-off in HC (3.32 W/kg) compared to CSM (2.82 W/kg) (p = 0.075). All significant differences in temporal–spatial, kinematic and kinetic parameters exceeded previously calculated SEM values [18].
Discussion
This study was the first to examine a wide range of kinematic key points, moments and powers during gait in the CSM population, and to control for the confounding effects of gait speed when comparing to healthy individuals. The reliability of the primary outcome measure, three-dimensional gait analysis, was evaluated in a previous paper, ensuring that all statistically significant differences could be interpreted within the context of prior known measurement error [18]. In keeping with other studies [3, 4, 6–8], the current study found that CSM participants walked at significantly slower gait speeds, demonstrated difficulty in generating adequate stride length and spent less time in single support, suggesting either a lack of stability in single leg stance, weak contralateral push off at pre-swing, premature cessation of swing due to hyperactivity of the hamstrings or limited hip extension on the stance leg [19].
Kinematic and kinetic analyses provided further insight into the underlying movement patterns. Peak hip extension showed a mean reduction of 3° in CSM compared to HC. This did not reach statistical significance; however, it caused a significant reduction in total sagittal plane range at the hip. Stimulation of afferent receptors in the hip joint occurs during hip extension and signals the transition from stance to swing, contributing to appropriate muscle activation. If the hip is prevented from reaching an extended position, the generation of the flexor burst and the onset of swing may be inhibited [20]. This may have contributed to the shorter step lengths and reduced single support duration of the CSM cohort.
CSM participants showed reduced knee flexion in both stance and swing. Power absorption peaks at loading response and initial swing were also reduced, confirming that the losses of knee flexion in stance and swing were associated with reduced eccentric activity.
Peak ankle plantarflexion at the pre-swing phase was lower in CSM, and associated with reduced plantarflexor moments and peak ankle power generation. The plantarflexors provide most of the body’s support during pre-swing [21]. The loss of their concentric power burst at this point would have limited the propulsion of the leg into swing [22]. This lack of propulsion was not compensated by an increase in the H3 hip power generation peak, as noted in other populations [23]. An effective swing phase, and therefore stride length, depends on the generation of sufficient momentum at the toe-off phase of gait [22], and the lack thereof could have contributed to the CSM participants’ shorter stride lengths and reduced single support times.
The matched speed condition was designed to distinguish true differences between CSM and HC gait patterns from speed-dependent differences. Without controlling for speed, a difference in a kinematic or kinetic parameter could simply restate the fact that people with CSM walk more slowly, rather than providing insight into the causes of the slower gait [2]. The abnormal features at matched speed indicated that there were fundamental changes in the biomechanical strategies of gait in CSM. For example, single support time was significantly shorter in CSM than in HC participants at matched speed, supporting the hypothesis that people with CSM lacked either stability in stance or adequate propulsive power in pre-swing [22].
Reductions in peak ankle dorsiflexion, plantarflexion and knee flexion in swing further supported the hypothesis of inadequate propulsion [24]. Similar patterns have been reported in the paretic limbs of people with stroke [2]. Furthermore, the increase in hip power generation and extensor moment at loading response in CSM is a known compensatory mechanism for a disruption of forward progression during stance [22]. It indicated that hip extensors were activated more intensely to transfer the trunk over the supporting limb, compensating for a lack of momentum that would usually be present from pre-swing of the previous stride.
This study identified the key biomechanical strategies underlying gait impairment in CSM. Clinically, 3DGA offers the potential for sensitive, detailed and reliable evaluation of change following surgery for CSM, as well as detailed monitoring of disease progression and the opportunity to develop rehabilitation strategies based on key gait impairments identified by this study. Our study included only participants with clear clinical and radiological evidence of CSM. Given the difficulties in diagnosing early-stage or uncertain CSM [25], 3DGA could play a role in informing the diagnostic process through a detailed evaluation of gait in people with suspected, but unconfirmed disease.
Conclusion
This study identified and described key features of gait in CSM that were independent of speed and could be attributed to the biomechanical effects of this neurological impairment. Limited propulsion, possibly due to paresis of the distal lower limb muscles with some compensation by the proximal hip musculature, was hypothesised as a likely impairment. Future studies should examine the effect of decompressive surgery on these gait features. The role of 3DGA in identifying sub-clinical changes in gait in early or uncertain CSM should also be determined.
Acknowledgments
This work was funded by the Health Research Board of Ireland under grant number CTPF/2008/2.
Conflict of interest
None.
References
- 1.Gough M, Shortland AP. Can clinical gait analysis guide the management of ambulant children with bilateral spastic cerebral palsy? J Pediatr Orthop. 2008;28(8):879–883. doi: 10.1097/BPO.0b013e31818e197c. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22(1):51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Kuhtz-Buschbeck JP, Johnk K, Muder S, Stolze H, Mehdorn M. Analysis of gait in cervical myelopathy. Gait Posture. 1999;9(3):184–189. doi: 10.1016/S0966-6362(99)00015-6. [DOI] [PubMed] [Google Scholar]
- 4.Singh A, Crockard HA. Quantitative assessment of cervical spondylotic myelopathy by a simple walking test. Lancet. 1999;354(9176):370–373. doi: 10.1016/S0140-6736(98)10199-X. [DOI] [PubMed] [Google Scholar]
- 5.Singh A, Choi D, Crockard A. Use of walking data in assessing operative results for cervical spondylotic myelopathy: long-term follow-up and comparison with controls. Spine (Phila Pa 1976) 2009;34(12):1296–1300. doi: 10.1097/BRS.0b013e3181a09796. [DOI] [PubMed] [Google Scholar]
- 6.Maezawa Y, Uchida K, Baba H. Gait analysis of spastic walking in patients with cervical compressive myelopathy. J Orthop Sci Off J Jpn Orthop Assoc. 2001;6(5):378–384. doi: 10.1007/s007760170002. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Lee SH, Seo IS. The characteristics of gait disturbance and its relationship with posterior tibial somatosensory evoked potentials in patients with cervical myelopathy. Spine (Phila Pa 1976) 2011;36(8):E524–E530. doi: 10.1097/BRS.0b013e3181f412d9. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki E, Nakamura H, Konishi S, Yamano Y. Analysis of the spastic gait caused by cervical compression myelopathy. J Spinal Disord Tech. 2002;15(6):519–522. doi: 10.1097/00024720-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Hanlon M, Anderson R. Prediction methods to account for the effect of gait speed on lower limb angular kinematics. Gait Posture. 2006;24(3):280–287. doi: 10.1016/j.gaitpost.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Patrick JH. Case for gait analysis as part of the management of incomplete spinal cord injury. Spinal Cord. 2003;41(9):479–482. doi: 10.1038/sj.sc.3101524. [DOI] [PubMed] [Google Scholar]
- 11.Moorthy RK, Bhattacharji S, Thayumanasamy G, Rajshekhar V. Quantitative changes in gait parameters after central corpectomy for cervical spondylotic myelopathy. J Neurosurg Spine. 2005;2(4):418–424. doi: 10.3171/spi.2005.2.4.0418. [DOI] [PubMed] [Google Scholar]
- 12.Roislien J, Skare O, Gustavsen M, Broch NL, Rennie L, Opheim A. Simultaneous estimation of effects of gender, age and walking speed on kinematic gait data. Gait Posture. 2009;30(4):441–445. doi: 10.1016/j.gaitpost.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Nurick S. The Pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100. doi: 10.1093/brain/95.1.87. [DOI] [PubMed] [Google Scholar]
- 14.Japanese Orthopaedic Association Scoring system (17–2) for cervical myelopathy. J Jpn Orthop Assoc. 1994;68:490–503. [Google Scholar]
- 15.Davis R, Ounpuu S, Tyburski D, Gage J. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–587. doi: 10.1016/0167-9457(91)90046-Z. [DOI] [Google Scholar]
- 16.Woltring HJ. A fortran package for generalized, cross-validatory spline smoothing and differentiation. Adv Eng Softw UK. 1986;8(2):104–113. doi: 10.1016/0141-1195(86)90098-7. [DOI] [Google Scholar]
- 17.Williams G, Morris ME, Schache A, McCrory PR. Incidence of gait abnormalities after traumatic brain injury. Arch Phys Med Rehabil. 2009;90(4):587–593. doi: 10.1016/j.apmr.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 18.McDermott A, Bolger C, Keating L, McEvoy L, Meldrum D. Reliability of three-dimensional gait analysis in cervical spondylotic myelopathy. Gait Posture. 2010;32(4):552–558. doi: 10.1016/j.gaitpost.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Winter DA. Concerning the scientific basis for the diagnosis of pathological gait and for rehabilitation protocols. Physiother Can. 1985;37(4):245–252. [Google Scholar]
- 20.Dietz V. Proprioception and locomotor disorders. Nat Rev Neurosci. 2002;3(10):781–790. doi: 10.1038/nrn939. [DOI] [PubMed] [Google Scholar]
- 21.Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture. 2003;17(2):159–169. doi: 10.1016/S0966-6362(02)00073-5. [DOI] [PubMed] [Google Scholar]
- 22.Kirtley C. Clinical gait analysis: theory and practice. London: Churchill Livingstone; 2006. [Google Scholar]
- 23.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clinical Biomechanics (Bristol, Avon) 1999;14(2):125–135. doi: 10.1016/S0268-0033(98)00062-X. [DOI] [PubMed] [Google Scholar]
- 24.Gage J. Gait analysis in cerebral palsy. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- 25.Cook C, Brown C, Isaacs R, Roman M, Davis S, Richardson W. Clustered clinical findings for diagnosis of cervical spine myelopathy. J Man Manip Ther. 2010;18(4):175–180. doi: 10.1179/106698110X12804993427045. [DOI] [PMC free article] [PubMed] [Google Scholar]