Abstract
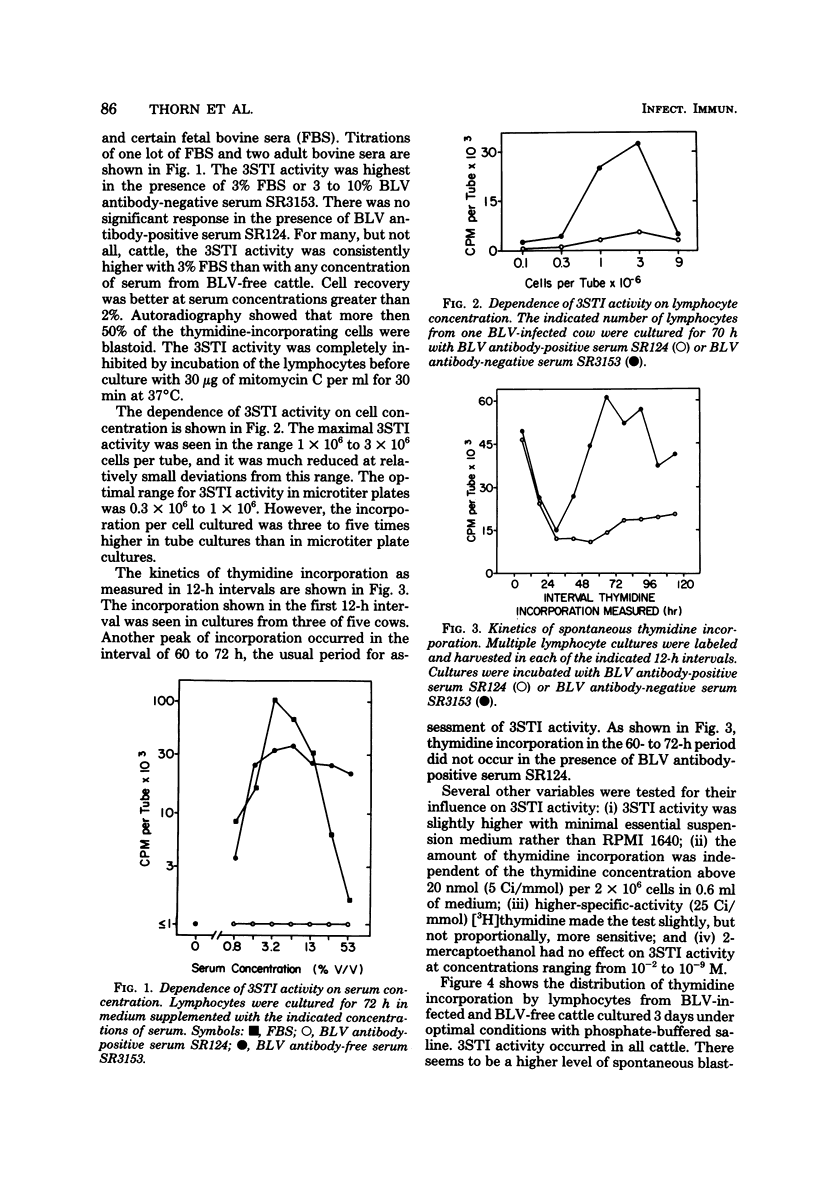
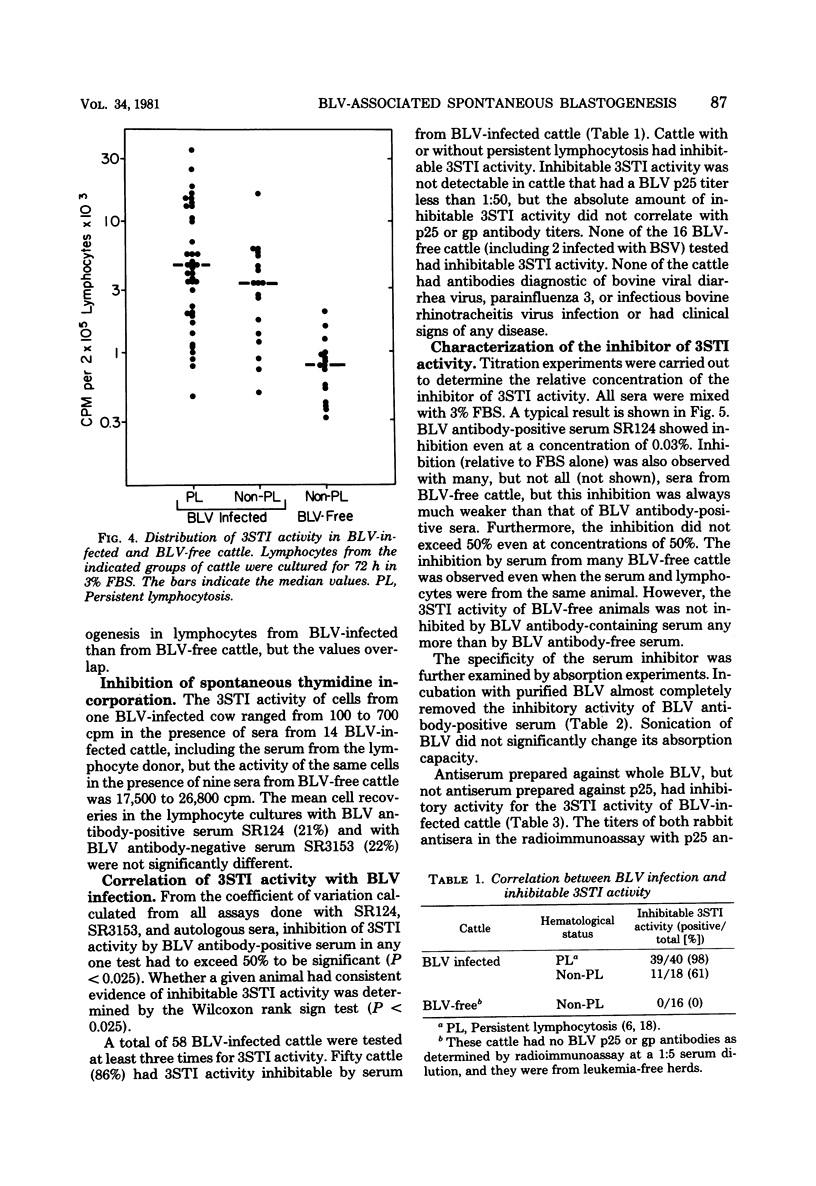
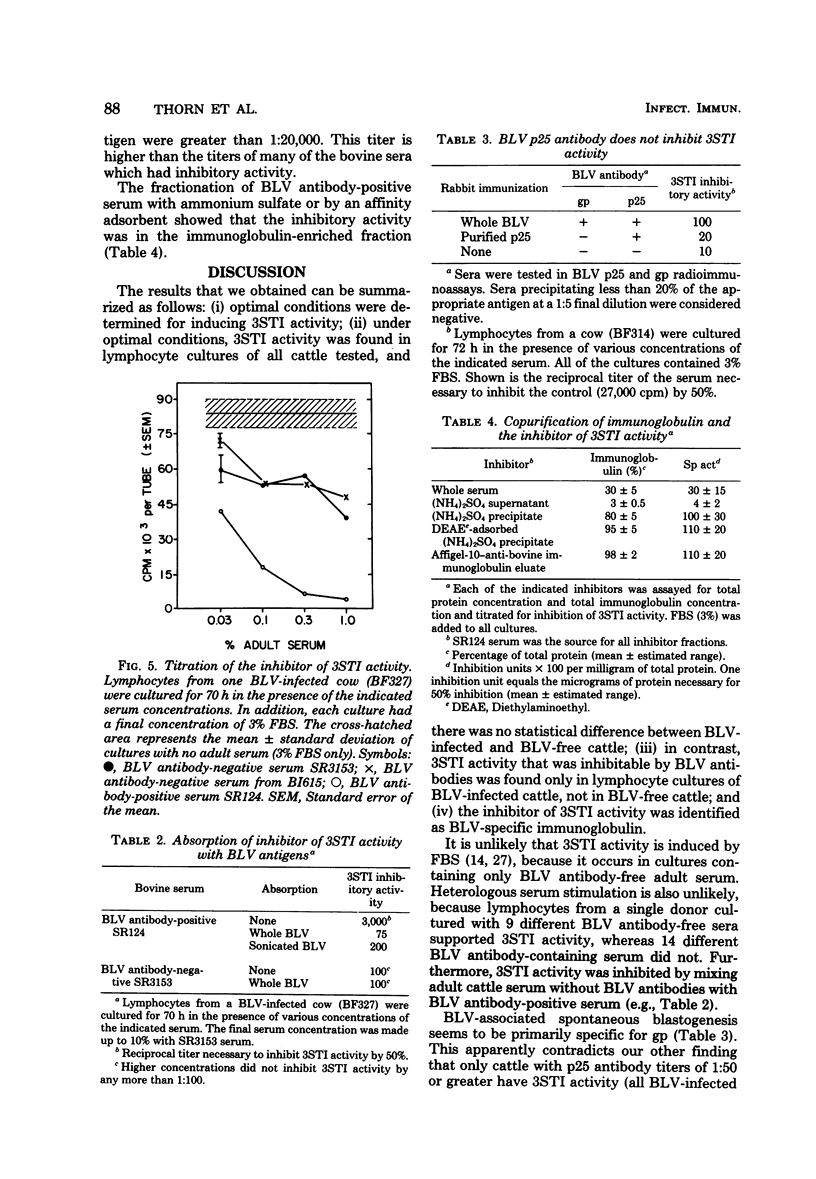
Cattle lymphocytes cultured for 3 days were found to spontaneously incorporate thymidine (3STI). Under optimal conditions of culture, the median magnitude of 3STI activity in lymphocytes from bovine leukemia virus (BLV)-infected cattle was higher than that of BLV-free cattle, but the ranges of the values overlapped. However, the 3STI activity of most BLV-infected cattle was specifically inhibited by serum containing BLV antibodies, whereas the 3STI activity of BLV-free cattle was not. The 3STI inhibitor copurified with immunoglobulin, and its activity could be absorbed with BLV. Rabbit anti-BLV serum inhibited 3STI, but rabbit anti-BLV p25 did not. These results indicate that BLV infection induces or expands a BLV-specific lymphocyte population. Spontaneous blastogenesis may be indicative of an immune response which controls virus spread.
Full text
PDF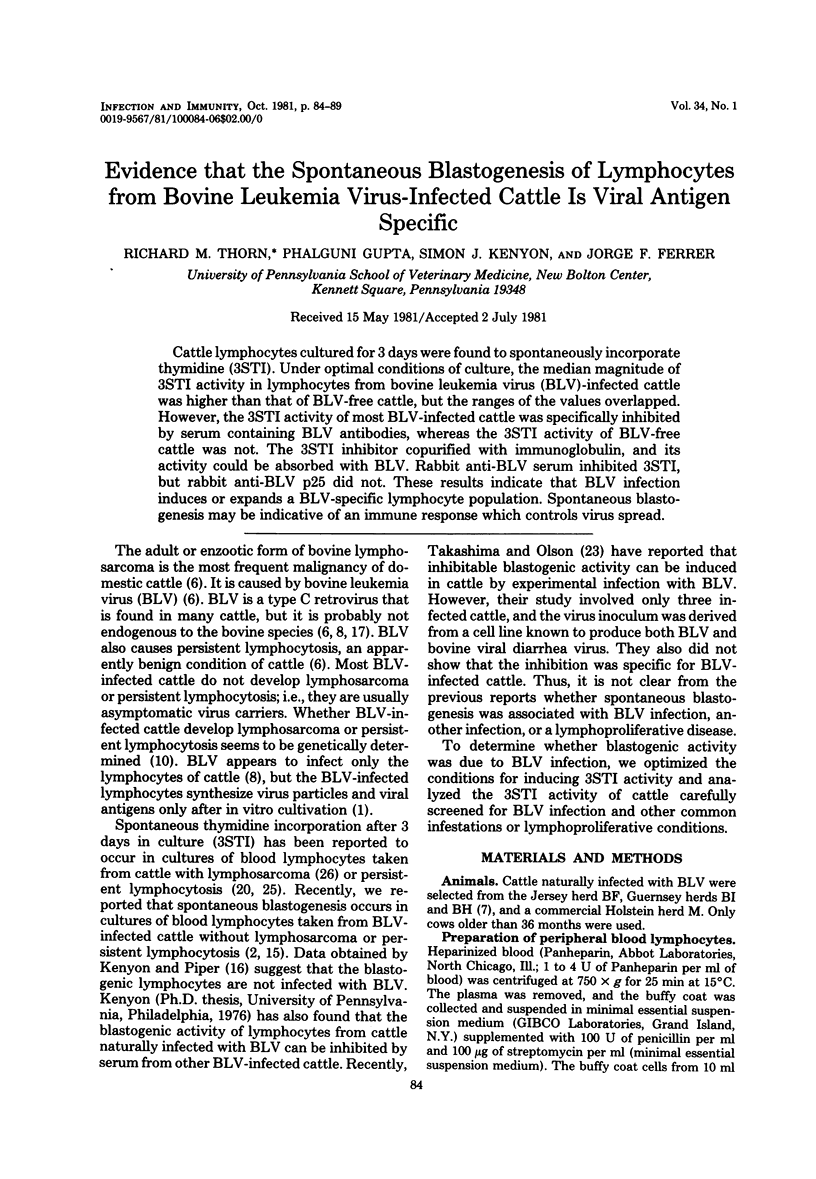


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baliga V., Ferrer J. F. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc Soc Exp Biol Med. 1977 Nov;156(2):388–391. doi: 10.3181/00379727-156-39942. [DOI] [PubMed] [Google Scholar]
- Bloom J. C., Kenyon S. J., Gabuzda T. G. Glucocorticoid effects on peripheral blood lymphocytes in cows infected with bovine leukemia virus. Blood. 1979 May;53(5):899–912. [PubMed] [Google Scholar]
- Buckley C. E., 3rd, Zitt M. J., Cate T. R. Two categories of lymphocyte unresponsiveness to phytohemagglutinin. Cell Immunol. 1973 Jan;6(1):140–148. doi: 10.1016/0008-8749(73)90014-2. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Stephenson J. R. Biochemical and immunological characterization of the major envelope glycoprotein of bovine leukemia virus. J Virol. 1977 Aug;23(2):443–447. doi: 10.1128/jvi.23.2.443-447.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diglio C. A., Hare W. C., Dodd D. C., Marshak R. R., Ferrer J. F. Cytogenetic, cytological, and virological characteristics of a bovine fibrosarcoma. Cancer Res. 1975 Dec;35(12):3628–3635. [PubMed] [Google Scholar]
- Ferrer J. F., Abt D. A., Bhatt D. M., Marshak R. R. Studies on the relationship between infection with bovine C-type virus, leukemia, and persistent lymphocytosis in cattle. Cancer Res. 1974 Apr;34(4):893–900. [PubMed] [Google Scholar]
- Ferrer J. F. Bovine lymphosarcoma. Adv Vet Sci Comp Med. 1980;24:1–68. [PubMed] [Google Scholar]
- Ferrer J. F., Cabradilla C., Gupta P. Use of a feline cell line in the syncytia infectivity assay for the detection of bovine leukemia virus infection in cattle. Am J Vet Res. 1981 Jan;42(1):9–14. [PubMed] [Google Scholar]
- Ferrer J. F., Marshak R. R., Abt D. A., Kenyon S. J. Relationship between lymphosarcoma and persistent lymphocytosis in cattle: a review. J Am Vet Med Assoc. 1979 Oct 1;175(7):705–708. [PubMed] [Google Scholar]
- Finkel A., Dent P. B. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol. 1973 Jan;6(1):41–48. doi: 10.1016/0008-8749(73)90004-x. [DOI] [PubMed] [Google Scholar]
- Gupta P., Ferrer J. F. Detection of a precursor-like protein of bovine leukaemia virus structural polypeptides in purified virions. J Gen Virol. 1980 Apr;47(2):311–322. doi: 10.1099/0022-1317-47-2-311. [DOI] [PubMed] [Google Scholar]
- Johnson G. J., Russell P. S. Reaction of human lymphocytes in culture to components of the medium. Nature. 1965 Oct 23;208(5008):343–345. doi: 10.1038/208343a0. [DOI] [PubMed] [Google Scholar]
- Kenyon S. J., Piper C. E. Cellular basis of persistent lymphocytosis in cattle infected with bovine leukemia virus. Infect Immun. 1977 Jun;16(3):891–897. doi: 10.1128/iai.16.3.891-897.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon S. J., Piper C. E. Properties of density gradient-fractionated peripheral blood leukocytes from cattle infected with bovine leukemia virus. Infect Immun. 1977 Jun;16(3):898–903. doi: 10.1128/iai.16.3.898-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Portetelle D., Mammerickx M., Cleuter Y., Dekegel D., Galoux M., Ghysdael J., Burny A., Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHAK R. R., HARE W. C., ABT D. A., CROSHAW J. E., Jr, SWITZER J. W., IPSEN I., DUTCHER R. M., MARTIN J. E. OCCURRENCE OF LYMPHOCYTOSIS IN DAIRY CATTLE HERDS WITH HIGH INCIDENCE OF LYMPHOSARCOMA. Ann N Y Acad Sci. 1963 Nov 4;108:1284–1301. doi: 10.1111/j.1749-6632.1963.tb13451.x. [DOI] [PubMed] [Google Scholar]
- McDonald H. C., Ferrer J. F. Detection, quantitation, and characterization of the major internal virion antigen of the bovine leukemia virus by radioimmunoassay. J Natl Cancer Inst. 1976 Oct;57(4):875–882. doi: 10.1093/jnci/57.4.875. [DOI] [PubMed] [Google Scholar]
- Muscoplat C. C., Alhaji I., Johnson D. W., Pomeroy K. A., Olson J. M., Larson V. L., Stevens J. B., Sorensen D. K. Characteristics of lymphocyte responses to phytomitogens: comparison of responses of lymphocytes from normal and lymphocytotic cows. Am J Vet Res. 1974 Aug;35(8):1053–1055. [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel C. M., Philipson J., Arthur E., Gardiner S. E., Newton M. S., McIntosh R. V. Possibility of EB virus preferentially transforming a subpopulation of human B lymphocytes. Nature. 1977 Dec 22;270(5639):729–731. doi: 10.1038/270729a0. [DOI] [PubMed] [Google Scholar]
- Takashima I., Olson C. Effect of mitogens and anti-bovine leukosis virus serums on DNA synthesis of lymphocytes from cattle. Eur J Cancer. 1980 May;16(5):639–645. doi: 10.1016/0014-2964(80)90204-2. [DOI] [PubMed] [Google Scholar]
- Thorn R. M., Palmer J. C., Manson L. A. A simplified 51Cr-release assay for killer cells. J Immunol Methods. 1974 Mar;4(2):301–315. doi: 10.1016/0022-1759(74)90073-8. [DOI] [PubMed] [Google Scholar]
- Weiland F., Straub O. C. Differences in the in vitro response of lymphocytes from leukotic and normal cattle to concanavalin A. Res Vet Sci. 1976 May;20(3):340–341. [PubMed] [Google Scholar]
- Yang T. J., Hare W. C., Abt D. A. DNA and RNA synthesis in cultures of peripheral blood mononuclear leucocytes from normal and lymphosarcomatous cows. Br J Haematol. 1967 Nov;13(6):855–861. doi: 10.1111/j.1365-2141.1967.tb08856.x. [DOI] [PubMed] [Google Scholar]
- Zielske J. V., Golub S. H. Fetal calf serum-induced blastogenic and cytotoxic responses of human lymphocytes. Cancer Res. 1976 Oct;36(10):3842–3846. [PubMed] [Google Scholar]


