Abstract
Purpose
To investigate the incidence and risk of stroke after lumbar spinal fusion surgery.
Method
Study subjects were identified from a nationwide cohort of 1 million people from 2000 to 2005 and were divided into the lumbar spinal fusion group (n = 2,015), who received posterior lumbar spinal fusion surgery, and the comparison group (n = 16,120) composed of age-, sex-, and propensity score-matched control subjects. The matching process was intended to adjust for demographics, comorbidities, and other immeasurable covariates to minimize selection bias. All subjects were followed up for 3 years for stroke, including hemorrhagic and ischemic strokes. Kaplan–Meier and Cox regression analyses were performed.
Results
The overall incidence rate of stroke in the cohort was 9.99 per 1,000 person-year. The lumbar spinal fusion group was less likely to have any stroke (adjusted hazard ratio (HR) = 0.83, p = 0.293), hemorrhagic stroke (adjusted HR = 0.74, p = 0.739) and ischemic stroke (adjusted HR = 0.81, p = 0.250) than the comparison group, but without significance.
Conclusions
Three years post-operatively, patients who received lumbar spinal fusion had stroke incidence rates similar to those without surgery. Posterior lumbar spinal fusion surgery is not associated with increased risks for any kind of stroke.
Keywords: Cerebrovascular accident, Hemorrhagic stroke, Ischemic stroke, Lumbar fusion
Introduction
Stroke or cerebrovascular accident is a rare, but devastating complication of spinal surgery. Severe neurologic consequences include irreversible blindness, hemi-paresis, and even death. Post-operative stroke has not been addressed frequently in the literature, but remains one of the risks mentioned when obtaining informed consent from patients for spinal operations. Post-operative stroke can be related to anesthesia, intra-operative maneuvers or both [1–3]. Its incidence is seldom reported and estimates have a wide range from 2 per 10,000 to 2.5 per 1,000 depending on the complexity of the spinal surgery and the age group of the patients under investigation [4–7].
Stroke can happen not only immediately post-operation, but also long after surgery [8, 9]. Some case reports have attributed cerebrovascular accidents to durotomy and intra-operative cerebrospinal fluid (CSF) leak [10–13]. However, there are also reports of stroke without intra-operative durotomy [14, 15]. Moreover, the theory of overdraining CSF better explains hemorrhagic strokes than ischemic strokes. The rarity and unpredictability of this serious consequence may cause trouble for spine surgeons. Its true incidence is elusive and the actual causal effect of spinal surgery is questionable.
This study hypothesizes that posterior lumbar fusion surgery does not increase the incidence of stroke and instead may even be protective against stroke via increased physical activity and decreased distress of back pain. Due to the relatively low incidence of post-operative stroke, a large number of patients and high follow-up rate are necessary to ascertain the relationship between stroke and lumbar fusion. The National Health Insurance Research Database (NHIRD), a national database containing 23 million administered insurants accumulated between January 1997 and December 2007, is used here. The National Health Insurance (NHI) of Taiwan covering 99 % of the population [16] is a unique system that finances all health care for the entire population and offers unrestricted access to any health-care provider of the patient’s choice. The comprehensive data source includes all medical claims including hospitalization, medication, procedures, and outpatient clinic visits. Loss of follow-up only occurs if medical services are sought abroad. Nonetheless, the statistics gathered represent sound investigation on any subsequent stroke event after the index lumbar fusion surgery.
Materials and methods
Data source
All original claims data for 1 million beneficiaries between the years 1996 and 2008 of Taiwan’s NHI program, a single-payer payment system financing health care for all Taiwanese citizens, were available for analysis.
The NHIRD already underwent a de-identification and encryption process. Thus, this study was exempted from full review by the institutional review board. The National Health Research Institute (NHRI) re-compiled the medical claims and made the data publicly available for medical researchers in Taiwan. To protect privacy, individual and hospital identifiers were unique to the research database and could not be used to trace for individual patients or health service providers. Moreover, the Bureau of National Health Insurance (BNHI) of Taiwan performed cross-check and validation of the medical charts and claims to ensure the accuracy of diagnosis coding. A recent validation study also suggested the reliability of diagnostic codes in the NHIRD [17].
Study cohort
A representative cohort composed of Taiwan’s cumulative population of 1 million covering the period from 1 January 2000 to 31 December 2005 was used. This representative cohort was randomly selected by the NHRI for scientific purposes. The NHRI reported no significant differences in age, gender or health-care costs between the representative group and all beneficiaries under the NHI program.
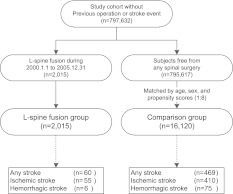
A study cohort without previous spinal surgery or history of stroke was extracted from the representative cohort. Patients who received lumbar spinal fusion surgery during the study period (1 January 2000 to 31 December 2005) were enrolled. The first day of follow-up (entry date) was defined as the date of spinal fusion surgery. The study cohort also included all enrollees unexposed to lumbar spinal fusion surgery during the study period. After matching for age, sex and years of visit dates, eight unexposed subjects were randomly assigned to one patient exposed to lumbar fusion during the study period. Each subject in the study cohort was tracked back from enrollment date to 1 January 1997 to ensure no previous spinal surgery or stroke events. The retrospective cohort study and flow of data processing are summarized in Fig. 1.
Fig. 1.
Data process flow. Study subjects were identified from a nationwide cohort of 797,632 people from 1 January 2001 to 31 December 2005 and were divided into the lumbar spinal fusion group (n = 2,806), who received posterior lumbar fusion surgery, and the comparison group (n = 16,120) composed of age-, sex-, and propensity score-matched people
Identification of the lumbar spinal fusion group
From the study cohort, all subjects who received lumbar spinal fusion between 1 January 2000 and 31 December 2005 were enrolled. First-time hospitalization with procedure codes containing the International Classification of Disease, 9th Version (ICD-9) code of lumbar and lumbosacral fusion posterior technique (81.08 and 81.38) during the study period were enrolled as the exposure group. The first date of hospitalization for the spinal fusion was designated as the first day of follow-up (entry date).
Comparison group matched for age, sex, and propensity score
To adjust for confounding factors associated with lumbar spine fusion surgery, propensity scores (i.e., the predicted probability that a person would receive lumbar spine fusion) were used to capture the comparison group who were unexposed. Propensity scores were calculated by spinal condition (i.e., inflammatory spondylopathies, spondylosis, spondylolisthesis, spinal stenosis, and inter-vertebral disc disorders), comorbidities (e.g., history of hypertension, diabetes, dyslipidemia, chronic obstructive pulmonary disease, chronic renal failure, and Parkinson’s disease), and demographic characteristics (i.e., age, sex, insurance level, geographic location of residence, and urbanization level of residence). To achieve a statistical power greater than 80 % at a 0.05 significance level in detection for a hazard ratio (HR) at 1.2, up to eight controls in the comparison group were matched to each patient in the lumbar spinal fusion group for age, sex, and propensity scores to minimize differences between groups. The first matched visit dates of subjects in the comparison group were designated as their first day of follow-up (entry date).
Ascertainment of covariates
Comorbidities, exposure to medications, and baseline demographic characteristics were included in the multiple regression model. Comorbidities included hypertension (ICD-9 code, 401-5.x), diabetes mellitus (250.x), dyslipidemia (272.0-4), chronic obstructive pulmonary diseases (COPD) (490, 491.0-1, 491.20-2, 491.8-9, 492.0, 492.8, 494, 494.0-1, and 496), chronic renal failure (585.x), Parkinson’s disease (332.0), atrial fibrillation (426-7.x), coronary heart disease (410-4.x), and valvular heart disease (394-7.x, 424.x). These were determined by the presence of either diagnostic codes of outpatient records or discharge codes of hospitalization records 6 months before the entry dates to the date of outcome event or the end of follow-up. More than 90 days cumulative exposure to aspirin, lipid-lowering drugs, nitrates, anticoagulants, and non-steroid anti-inflammatory drugs (NSAIDs) between the entry dates and end of follow-up were also identified and included as covariates.
For each subject, the outpatient and discharge diagnostic ICD-9 codes were used to calculate the Charlson’s comorbidity index, a commonly used method to adjust for differences in levels of comorbid illness in studies that use administrative data [18]. The potential range of values for this index was 0–37 with higher scores reflecting more severe comorbidities. Charlson’s comorbidity index served as an excellent prognostic value for survival and re-hospitalization [19]. Baseline demographic characteristics, including insurance level, geographic location, and urbanization level, were also included.
All subjects’ income levels were grouped into four by the premium paid (NTD$ ≥40,000, 20,000–39,999, 1–19,999, and dependents). In the Taiwan NHI, premiums were mostly determined based on the insured wage and premium rate. Thus, a higher premium implied higher income. Those without salaries such as the unemployed, students, children, or the elderly were designated as dependents by the BNHI and the government or their foster families covered their insurance premiums.
The location of NHI registration for the degree of urbanization was used as proxy parameter for socioeconomic status. According to previous reports using the NHIRD [20], urbanization levels in Taiwan were divided into seven strata. Level 1 referred to the “most urbanized” and level 7 was the “least urbanized” areas. However, given that there were few patients in levels 5–7, these three were combined into a single group and thereafter referred to as level 5.
Outcomes
All subjects were followed up for 3 years. The study end point was the event of stroke, determined by the date of hospitalization records with discharge diagnostic code of stroke (ICD-9 code, 430-435) after the entry dates. The validity of diagnosis was tested and confirmed [17]. The study censored follow-up only in the following conditions: when the subjects expired on the dates of outcome incidence or at the end of the cohort (31 December 2008). All stroke events were further categorized into hemorrhagic strokes, including sub-arachnoid hemorrhage (ICD-9 code, 430) and intra-cerebral hemorrhage (ICD-9 code, 431-432), and ischemic strokes (ICD-9 code, 432-435) for analysis of stroke characteristics.
Statistical analysis
All of the data were linked using the SQL server 2008 (Microsoft Corp.) and analyzed by the SPSS software (SPSS, Inc., Chicago, IL). Chi-square test and independent t test were used to assess differences in characteristics between the spinal fusion and comparison groups. Kaplan–Meier method and log-rank test were used to estimate and compare the incidence rates of hospitalizations for stroke. The Cox proportional hazard model with propensity scores was used to compare the incidence rates of stroke (hemorrhagic and ischemic) between the two groups after adjustment for the aforementioned covariates. A two-tailed level of 0.05 was considered statistically significant.
Results
A total of 18,135 subjects (i.e., 2,015 lumbar spinal fusion patients and 16,120 age-, sex-, and propensity score-matched controls) were followed up for 52,963 person-years. The mean ages were similar between the lumbar fusion group and the comparison group (57.90 vs. 58.04 years, p = 0.686) at the entry dates, as well as proportional gender compositions (60.7 vs. 60.7 % females, p = 0.999). The overall incidence rate of all strokes was 9.99 per 1,000 person-years.
Comparison of comorbidities and other demographics
The Charlson’s comorbidity index of the lumbar spinal fusion group was similar to that of the comparison group (2.03 vs. 2.02, p = 0.85). Despite adjustments for differences in comorbidities, several comorbidities were significantly more in the lumbar spinal fusion group including hypertension (42.8 vs. 37.4 %, p < 0.001), dyslipidemia (28.2 vs. 25.1 %, p = 0.003), COPD (30.3 vs. 27.1 %, p = 0.002), and Parkinson’s disease (1.8 vs. 1.2 %, p = 0.024). The insurance level between the two groups was not significantly different (p = 0.175), but geographic locations and urbanization levels were significantly different (p = 0.011 and p < 0.001, respectively) (Table 1). All these parameters were adjusted in the regression model.
Table 1.
Comparison of demographics and comorbidities (n = 18,135)
| Comparison group | Lumbar spinal fusion group | P value | |
|---|---|---|---|
| n = 16,120 (%) | n = 2,015 (%) | ||
| Gender | |||
| Female | 6336 (39.3) | 1223 (60.7) | 0.999 |
| Male | 9784 (60.7) | 792 (39.3) | |
| Age, mean (SD) | 58.04 (15.50) | 57.90 (15.46) | 0.686 |
| Comorbidity indexa, mean (SD) | 2.02 (2.62) | 2.03 (2.13) | 0.85 |
| Comorbidities | |||
| Hypertension | 6033 (37.4) | 862 (42.8) | <0.001 |
| Diabetes | 3540 (22.0) | 445 (22.1) | 0.899 |
| Dyslipidemia | 4052 (25.1) | 569 (28.2) | 0.003 |
| COPD | 4363 (27.1) | 610 (30.3) | 0.002 |
| Chronic renal failure | 455 (2.8) | 69 (3.4) | 0.128 |
| Parkinson disease | 192 (1.2) | 36 (1.8) | 0.024 |
| Demographic characteristics | |||
| Insurance level (NTD$) | 0.175 | ||
| 40,000– | 966 (6.0) | 105 (5.2) | |
| 20,000–39,999 | 2709 (16.8) | 361 (17.9) | |
| 1–19,999 | 7257 (45.0) | 943 (46.8) | |
| Dependent | 5107 (31.7) | 606 (30.1) | |
| Geographic location | 0.011 | ||
| Northern area | 7580 (47.0) | 890 (44.2) | |
| Middle area | 3408 (21.1) | 414 (20.5) | |
| Southern area | 4729 (29.3) | 663 (32.9) | |
| Eastern area | 403 (2.5) | 48 (2.4) | |
| Urbanization level | <0.001 | ||
| 1 (most urbanization) | 3818 (23.7) | 401 (19.9) | |
| 2 | 4586 (28.4) | 602 (29.9) | |
| 3 | 2804 (17.4) | 314 (15.6) | |
| 4 | 2582 (16.0) | 327 (16.2) | |
| 5 (least urbanization) | 2330 (14.5) | 371 (18.4) | |
aComorbidity index: Charlson’s index, potential range of values for this index was 0–37 with higher the score the more severe comorbidities
Incidence of stroke
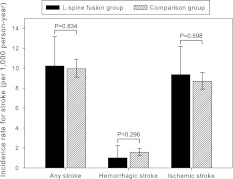
Subjects in the lumbar spinal fusion group were less likely to have stroke than the comparison group. The 2,015 patients in the lumbar spinal fusion group were followed up for 5,867.5 person-years and a total of 60 stroke events were identified including six hemorrhagic and 55 ischemic strokes. The incidence rates (95 % confidence interval) were 10.22 (7.94–13.17), 1.01 (0.45–2.25), and 9.36 (7.19–12.19) per 1,000 person-year for any stroke, hemorrhagic stroke, and ischemic strokes, respectively. In contrast, the 16,120 control subjects in the comparison group were followed up for 47,096.1 person-years. There were 469 strokes including 75 hemorrhagic, and 410 ischemic strokes for incidence rates of 9.96 (9.1–10.9), 1.57 (1.26–1.97), and 8.69 (7.9–9.58) per 1,000 person-year for any stroke, hemorrhagic stroke and ischemic stroke, respectively. Thus, there were similar incidences of stroke in the lumbar spinal fusion group and the comparison group without significance (p = 0.834, 0.296 and 0.598, respectively) (Fig. 2).
Fig. 2.
Comparison of incidence rates of strokes. The lumbar spinal fusion group had incidence rates of any stroke, hemorrhagic stroke, and ischemic stroke similar to those of the comparison group
Hazard ratios of strokes
Compared to the comparison group, the lumbar spinal fusion group had crude HRs of 1.02, 0.62, and 1.07 for any stroke, hemorrhagic stroke, and ischemic stroke, respectively (p = 0.896, 0.254 and 0.619, respectively). After adjustments for demographic characteristics, comorbidities, and medications, the adjusted HRs of the lumbar spinal fusion group were 0.83, 0.74, and 0.81, respectively (p = 0.293, 0.739 and 0.250, respectively) (Table 2). Therefore, subjects who received lumbar spinal fusion surgery were less likely to have stroke in the next 3 years, but without statistical significance.
Table 2.
Hazard ratios for subsequent strokes after lumbar spinal fusion surgery (n = 18,135, 2005–2008)
| Stroke during 3-year follow-up | Comparison group | Lumbar spinal fusion group | (95 % CI) | P value |
|---|---|---|---|---|
| Any stroke | ||||
| Crude hazard ratio | 1.00 | 1.02 | (0.78–1.33) | 0.896 |
| Adjusted hazard ratioa | 1.00 | 0.83 | (0.59–1.17) | 0.293 |
| Hemorrhagic stroke | ||||
| Crude hazard ratio | 1.00 | 0.62 | (0.27–1.42) | 0.254 |
| Adjusted hazard ratioa | 1.00 | 0.74 | (0.27–2.04) | 0.739 |
| Ischemic stroke | ||||
| Crude hazard ratio | 1.00 | 1.07 | (0.81–1.42) | 0.619 |
| Adjusted hazard ratioa | 1.00 | 0.81 | (0.56–1.16) | 0.250 |
aAdjustment was made for demographic characteristics (e.g., age, sex, insurance level, geographic location, and urbanization level), comorbidities (i.e., hypertension, diabetes, valvular heart disease, arrhythmia, cardiovascular disease, and Charlson’s comorbidity index), medications (i.e., aspirin, nitrates, lipid-lowering drugs, anticoagulants, and NSAIDs), and baseline propensity scores
Discussion
This study used a comprehensive national representative cohort to investigate the incidence of stroke in 2,015 patients receiving posterior lumbar spinal fusion surgery. Compared to 16,120 controls from the same cohort with similar comorbidity characteristics, the surgically treated patients were less likely to have subsequent stroke without significance. The adjusted HR for any stroke, hemorrhagic stroke, and ischemic stroke were 0.83, 0.74, and 0.81, respectively. This study is the first in literature that specifically investigated post-operative stroke events after a common spinal procedure posterior lumbar spinal fusion. Furthermore the age-, sex-, and comorbidity-matched 1–8 comparison cohort, combined with complete follow-up for 3 years, yielded a sound estimation of the stroke incidences in patients with lumbar spinal diseases, whether or not they received surgical treatment. Regarding subsequent stroke events, this study demonstrated that lumbar spinal fusion surgery was a safe procedure with protection even though there was no statistical significance. This information might be useful for spine surgeons and anesthesiologists.
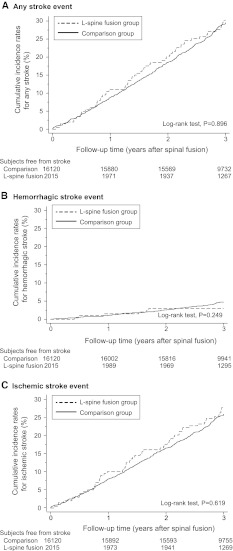
Although post-operative stroke rarely happens in lumbar spinal surgery, it is repeatedly reported with various kinds of manifestations including blindness, weakness, impaired cognitive function, or even death [6–8, 21–26]. Patients with spinal diseases usually need to pass a series of cardiac and pulmonary evaluations before being considered as candidates for spinal surgery, especially if they are old or have risk factors for cerebrovascular or heart diseases. Screening may sometimes preclude these high risk patients for spinal surgery. If the planned spinal surgery is complex, surgeons or anesthesiologists hold more stringent screening. But despite the precautions taken, post-operative stroke is never eliminated. Whether or not stroke develops because of the spinal fusion surgery per se or procedure-related maneuvers predispose to stroke remains elusive. On the other hand, this rare catastrophic neurologic event happens by itself, regardless of spinal or any other surgery. Although definitive conclusions cannot be drawn from the results, some findings may shed light on this issue. The Kaplan–Meier analysis in the present study demonstrates that strokes happen at a steady rate from immediately post-operation to 3 years after (Fig. 3). This observation is valid for all strokes, hemorrhagic stroke and ischemic stroke.
Fig. 3.
Cumulative incidence of stroke. The lumbar spinal fusion group had similar cumulative incidence of a any stroke, b hemorrhagic stroke, and c ischemic stroke than the comparison group from immediately post-surgery up to 3 years
The explanation of the insignificantly smaller HRs for all kinds of stroke in the lumbar spinal fusion group is obscure. Perhaps, reduction in some of the risks factors for stroke after lumbar spinal fusion surgery can be taken into account. For example, many reports correlate increased physical activity to lower risk for strokes [27–29]. A meta-analysis has concluded that moderate or high levels of physical activity are associated with reduced risk of strokes [30]. It also demonstrates reduced risk of total and ischemic stroke in a dose–response manner by moderate-intensity physical activities [31]. Walking reportedly decreases risks of total, ischemic, and hemorrhagic strokes in women [32]. Even milder physical activities, like daily commuting and leisure-time activities, reduce the risk of stroke [33, 34]. Physical activity is now a well-documented modifiable risk factor of first stroke and is listed in the American Heart Association (AHA) and American Stroke Association (ASA) guidelines for primary prevention of ischemic stroke [35, 36]. Theoretically, patients might have less pain and improved ambulatory function after lumbar spinal fusion surgery. It can be inferred that there is likelihood of increased physical activity after lumbar spinal surgery, which diminishes the risk factor of stroke. However, this implication is beyond the scope of this study and requires further corroboration. On the other hand, for these patients undergoing lumbar spinal fusion, there might be limitations in physical exertion imposed by their surgeons in the short term post-operative period. Therefore, the association between stroke and the effect on physical activity after lumbar spinal fusion surgery remains uncertain.
A merit of this study is the stratification of stroke types into hemorrhagic and ischemic strokes. The true incidence of stroke in such a group of patients with lumbar spinal diseases requiring fusion is reportedly approximately 10 per 1,000 person-year with a 1:9 ratio between hemorrhagic and ischemic strokes. Such data not only provide references in spinal surgery, but is also valuable for future stroke prevention in the given patient population regardless of medical or surgical managements. This epidemiologic estimation came from a national representative cohort of Taiwan composed of a health-care system with universal coverage. Therefore, the reported incidence of stroke is highly accurate, because every subject in the study has been followed up, unless they sought medical services in a foreign country not reimbursed by the insurance which is extremely unlikely.
The strength of this study is its design. In this cohort, a well-matched comparison group with demographics and comorbidities that risk stroke is deliberately used. Most of the stroke confounders, identifiable or unidentified, are controlled or adjusted by Cox regression model and propensity score. Stroke events after lumbar spinal fusion surgery are observed based on the single-payer health insurance system with universal coverage in a nationwide scale, allowing more accurate estimation than institution-based studies. Furthermore, Kaplan–Meier analysis with complete follow-up improved the censored data. The lack of association between lumbar fusion and stroke established hereby is therefore sound. Perhaps, a study with larger number of cases and similar design or a randomized control trial can validate the current results.
The study has several limitations. First, the current study includes a mixture of different levels of lumbar spinal fusion with various surgical indications, operation time, blood loss, and anesthesiology. Due to database limits, details of every operative note have not been investigated or traced back for each individual’s medical charts. A single-level lumbar fusion for isthmic spondylolisthesis in a young man apparently is of lower risk for stroke than an old patient with degenerative scoliosis requiring a long construct of spinal fusion. However, the propensity score-matched comparison with comorbidity adjustment in the current study design compensates for this intra-group heterogeneity. Second, iatrogenic stroke happening immediately after lumbar spinal fusion may be less frequently coded than in reality. Nonetheless, the NHIRD medical records also serve for billing and thereby under prudent internal monitor. Fraudulent coding that is heavily penalized promotes the accuracy of coding. A recent validation study also suggests the reliability of diagnostic codes in NHIRD [17]. Hence, the number of stroke events in the current study is valid. Lastly, there might be a selection of lower risk patients for spinal surgery. Patients who undergo lumbar spinal fusion surgery may be more scrutinizing about health issues, thus modifying stroke risk factors such as cessation of smoking or better control of medical diseases (e.g., hypertension and diabetes). Risk factors that are coded in NHIRD such as comorbidities (e.g., hypertension, diabetes, dyslipidemia, chronic obstructive pulmonary disease, chronic renal failure, and Parkinson’s disease) were adjusted. However, there are factors (i.e., cigarette smoking) not applicable due to database limitation. Therefore, this issue could be partially addressed by the propensity score matching in the current study design.
Considering the limitations in the design of the study retrospective cohort, selection bias, analysis of administrative database, lack of direct clinical follow-up of patients, and statistical power of 99 %, it seems unlikely that posterior lumbar fusion increases the risk of stroke compared to matched patients. Exclusion of patients as candidates for lumbar fusion due to fear of post-operative stroke is not supported by our data.
Acknowledgments
This study was based partly on data from the NHRI database provided by the BNHI, Department of Health, and managed by NHRI in Taiwan. The interpretation and conclusions contained herein do not represent those of the BNHI, the Department of Health, or NHRI.
Conflict of interest
None.
Glossary
- ICD-9
International Classification of Disease, 9th Version
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
References
- 1.Larsen SF, Zaric D, Boysen G. Postoperative cerebrovascular accidents in general surgery. Acta Anaesthesiol Scand. 1988;32(8):698–701. doi: 10.1111/j.1399-6576.1988.tb02811.x. [DOI] [PubMed] [Google Scholar]
- 2.Newman NJ. Perioperative visual loss after nonocular surgeries. Am J ophthalmol. 2008;145(4):604–610. doi: 10.1016/j.ajo.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carl A, Kaufman E, Lawrence J. Complications in spinal deformity surgery: issues unrelated directly to intraoperative technical skills. Spine (Phila Pa 1976) 2010;35(25):2215–2223. doi: 10.1097/BRS.0b013e3181fd591f. [DOI] [PubMed] [Google Scholar]
- 4.Faciszewski T, Winter RB, Lonstein JE, Denis F, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. A review of 1,223 procedures. Spine (Phila Pa 1976) 1995;20(14):1592–1599. doi: 10.1097/00007632-199507150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez LF, Thisted R. Complications and demographic characteristics of patients undergoing lumbar discectomy in community hospitals. Neurosurgery. 1989;25(2):226–230. doi: 10.1227/00006123-198908000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Raffo CS, Lauerman WC. Predicting morbidity and mortality of lumbar spine arthrodesis in patients in their ninth decade. Spine (Phila Pa 1976) 2006;31(1):99–103. doi: 10.1097/01.brs.0000192678.25586.e5. [DOI] [PubMed] [Google Scholar]
- 7.Shen Y, Silverstein JC, Roth S. In-hospital complications and mortality after elective spinal fusion surgery in the United States: a study of the nationwide inpatient sample from 2001 to 2005. J Neurosurg Anesthesiol. 2009;21(1):21–30. doi: 10.1097/ANA.0b013e31818b47e9. [DOI] [PubMed] [Google Scholar]
- 8.Katz JN, Lipson SJ, Chang LC, Levine SA, Fossel AH, Liang MH. Seven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosis. Spine (Phila Pa 1976) 1996;21(1):92–98. doi: 10.1097/00007632-199601010-00022. [DOI] [PubMed] [Google Scholar]
- 9.Fokter SK, Yerby SA. Patient-based outcomes for the operative treatment of degenerative lumbar spinal stenosis. Eur Spine J. 2006;15(11):1661–1669. doi: 10.1007/s00586-005-0033-4. [DOI] [PubMed] [Google Scholar]
- 10.Khong P, Jerry Day M. Spontaneous cerebellar haemorrhage following lumbar fusion. J Clin Neurosci. 2009;16(12):1673–1675. doi: 10.1016/j.jocn.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman RM, Kebaish KM. Intracranial hemorrhage following incidental durotomy during spinal surgery. A report of four patients. J Bone Joint Surg Am. 2007;89(10):2275–2279. doi: 10.2106/JBJS.F.01550. [DOI] [PubMed] [Google Scholar]
- 12.Andrews RT, Koci TM. Cerebellar herniation and infarction as a complication of an occult postoperative lumbar dural defect. AJNR Am J Neuroradiol. 1995;16(6):1312–1315. [PMC free article] [PubMed] [Google Scholar]
- 13.Konya D, Ozgen S, Pamir MN. Cerebellar hemorrhage after spinal surgery: case report and review of the literature. Eur Spine J. 2006;15(1):95–99. doi: 10.1007/s00586-005-0987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beier AD, Soo TM, Claybrooks R. Subdural hematoma after microdiscectomy: a case report and review of the literature. Spine J. 2009;9(10):e9–e12. doi: 10.1016/j.spinee.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Huber JF, Grob D. Bilateral cortical blindness after lumbar spine surgery. A case report. Spine (Phila Pa 1976) 1998;23(16):1807–1809. doi: 10.1097/00007632-199808150-00017. [DOI] [PubMed] [Google Scholar]
- 16.Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med. 2008;148(4):258–267. doi: 10.7326/0003-4819-148-4-200802190-00004. [DOI] [PubMed] [Google Scholar]
- 17.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 18.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Halfon P, Eggli Y, Melle G, Chevalier J, Wasserfallen JB, Burnand B. Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–587. doi: 10.1016/S0895-4356(01)00521-2. [DOI] [PubMed] [Google Scholar]
- 20.Lin HC, Chao PZ, Lee HC. Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke. 2008;39(10):2744–2748. doi: 10.1161/STROKEAHA.108.519090. [DOI] [PubMed] [Google Scholar]
- 21.Baig MN, Lubow M, Immesoete P, Bergese SD, Hamdy EA, Mendel E. Vision loss after spine surgery: review of the literature and recommendations. Neurosurg Focus. 2007;23(5):E15. doi: 10.3171/FOC-07/11/15. [DOI] [PubMed] [Google Scholar]
- 22.Cheng MA, Sigurdson W, Tempelhoff R, Lauryssen C. Visual loss after spine surgery: a survey. Neurosurgery. 2000;46(3):625–631. doi: 10.1097/00006123-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976) 2005;30(3):E83. doi: 10.1097/01.brs.0000152169.48117.c7. [DOI] [PubMed] [Google Scholar]
- 24.Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 25.Winter RB. Neurologic safety in spinal deformity surgery. Spine (Phila Pa 1976) 1997;22(13):1527–1533. doi: 10.1097/00007632-199707010-00022. [DOI] [PubMed] [Google Scholar]
- 26.Castro FP., Jr Role of activated growth factors in lumbar spinal fusions. J Spinal Disord Tech. 2004;17(5):380–384. doi: 10.1097/01.bsd.0000110342.54707.19. [DOI] [PubMed] [Google Scholar]
- 27.Lee IM, Paffenbarger RS., Jr Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke. 1998;29(10):2049–2054. doi: 10.1161/01.STR.29.10.2049. [DOI] [PubMed] [Google Scholar]
- 28.Wannamethee G, Shaper AG. Physical activity and stroke in British middle aged men. BMJ. 1992;304(6827):597–601. doi: 10.1136/bmj.304.6827.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendel-Vos GC, Schuit AJ, Feskens EJ, Boshuizen HC, Verschuren WM, Saris WH, Kromhout D. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33(4):787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 30.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34(10):2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 31.Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;283(22):2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 32.Sattelmair JR, Kurth T, Buring JE, Lee IM. Physical activity and risk of stroke in women. Stroke. 2010;41(6):1243–1250. doi: 10.1161/STROKEAHA.110.584300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agnarsson U, Thorgeirsson G, Sigvaldason H, Sigfusson N. Effects of leisure-time physical activity and ventilatory function on risk for stroke in men: the Reykjavik Study. Ann Intern Med. 1999;130(12):987–990. doi: 10.7326/0003-4819-130-12-199906150-00006. [DOI] [PubMed] [Google Scholar]
- 34.Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. 2005;36(9):1994–1999. doi: 10.1161/01.STR.0000177868.89946.0c. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke. 2006;37(6):1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]