Abstract
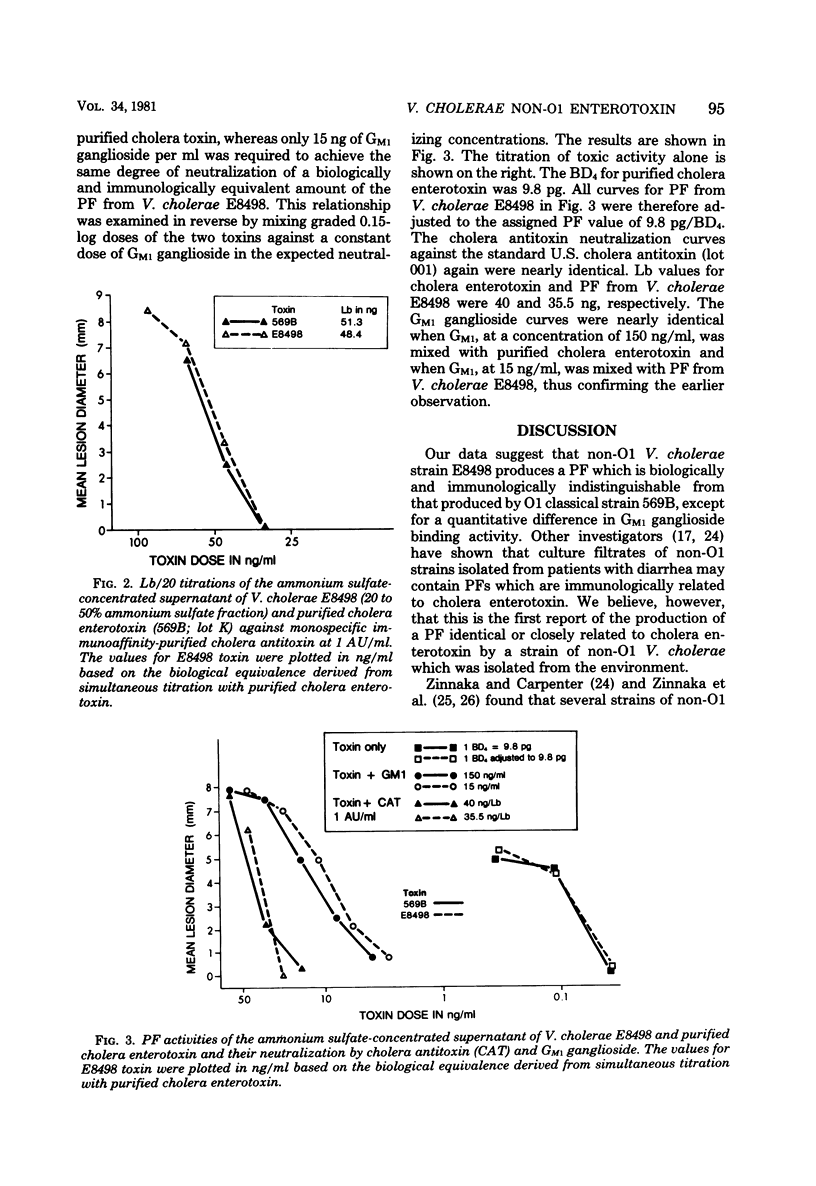
Vibrio cholerae non-O1 strain E8498, isolated in 1978 from fresh water in Louisiana, produced a vascular permeability factor when cultured in shallow resting cultures of Casamino Acids-yeast extract-glucose medium for 24 h at 30 degrees C. Undiluted resting culture filtrates contained heat-labile permeability factor activity which was only partially neutralized by cholera antitoxin and GM1 ganglioside. Supernatants concentrated with PM-10 membranes caused hemorrhage and necrosis in rabbits within 1 h after intracutaneous injection, whereas appropriate dilutions of both filtrates and concentrates demonstrated delayed permeability factor activity, without hemorrhage or necrosis, which was indistinguishable in appearance from that caused by purified cholera enterotoxin produced by V. cholerae O1 Inaba strain 569B. Crude E8498 filtrates contained the biological equivalent of about 5 ng/ml of purified enterotoxin. Permeability factor activity in the fraction obtained by 20 to 50% saturation of filtrate concentrate with ammonium sulfate could be completely neutralized by reference standard cholera antitoxin prepared against purified 569 B enterotoxin. Hemorrhagic activity was unaffected by cholera antitoxin. A 5,000-fold concentrate of the culture supernatant yielded a line of identity with purified cholera enterotoxin in an agar gel double-diffusion test against cholera antitoxin purified by affinity column chromatography with BrCN-activated Sepharose 4B-linked purified cholera enterotoxin as the adsorbent. These findings indicate that V. cholerae non-O1 E8498 produces a permeability factor which is immunologically and biologically indistinguishable from that produced by a strain of V. cholerae O1 classical biotype.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldová E., Láznicková K., Stepánková E., Lietava J. Isolation of nonagglutinable vibrios from an enteritis outbreak in Czechoslovakia. J Infect Dis. 1968 Feb;118(1):25–31. doi: 10.1093/infdis/118.1.25. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Bose A. K., Ghosh A. K. Permeability and enterotoxic factors of nonagglutinable vibrios Vibrio alcaligenes and Vibrio parahaemolyticus. Appl Microbiol. 1971 Dec;22(6):1159–1161. doi: 10.1128/am.22.6.1159-1161.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake P. A., Allegra D. T., Snyder J. D., Barrett T. J., McFarland L., Caraway C. T., Feeley J. C., Craig J. P., Lee J. V., Puhr N. D. Cholera--a possible endemic focus in the United States. N Engl J Med. 1980 Feb 7;302(6):305–309. doi: 10.1056/NEJM198002073020601. [DOI] [PubMed] [Google Scholar]
- Craig J. B. Toward the development of a standard reference cholera antitoxin. Dev Biol Stand. 1978;41:415–422. [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Draskovicová M., Karolcek J., Winkler Experimental Toxigenicity of NAG Vibrios. Zentralbl Bakteriol Orig A. 1977 Feb;237(1):65–71. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Taga S., Takeda Y., Miwatani T. Modified Elek test for detection of heat-labile enterotoxin of enterotoxigenic Escherichia coli. J Clin Microbiol. 1981 Jan;13(1):1–5. doi: 10.1128/jcm.13.1.1-5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Hollis D. G., Gangarosa E. J., Weaver R. E. Non-cholera vibrio infections in the United States. Clinical, epidemiologic, and laboratory features. Ann Intern Med. 1978 May;88(5):602–606. doi: 10.7326/0003-4819-88-5-602. [DOI] [PubMed] [Google Scholar]
- Kaper J., Lockman H., Colwell R. R., Joseph S. W. Ecology, serology, and enterotoxin production of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol. 1979 Jan;37(1):91–103. doi: 10.1128/aem.37.1.91-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama H., Craig J. P. Production of Biologically Active Substances by Two Strains of Vibrio cholerae. Infect Immun. 1970 Jan;1(1):80–87. doi: 10.1128/iai.1.1.80-87.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCINTYRE O. R., FEELEY J. C., GREENOUGH W. B., 3rd, BENENSON A. S., HASSAN S. I., SAAD A. DIARRHEA CAUSED BY NON-CHOLERA VIBRIOS. Am J Trop Med Hyg. 1965 May;14:412–418. doi: 10.4269/ajtmh.1965.14.412. [DOI] [PubMed] [Google Scholar]
- Oashi M., Shimada T., Fukumi H. In vitro production of enterotoxin and hemorrhagic principle by Vibrio cholerae, NAG. Jpn J Med Sci Biol. 1972 Jun;25(3):179–194. doi: 10.7883/yoken1952.25.179. [DOI] [PubMed] [Google Scholar]
- Singh S. J., Sanyal S. C. Enterotoxicity of the so-called NAG vibrios. Ann Soc Belg Med Trop. 1978;58(2):133–140. [PubMed] [Google Scholar]
- Smith H. L., Jr Serotyping of non-cholera vibrios. J Clin Microbiol. 1979 Jul;10(1):85–90. doi: 10.1128/jcm.10.1.85-90.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhariev Z., Tyufekchiev T., Valkov V., Todeva M. Food poisoning caused by parahaemolytic and NAG vibrios after eating meat products. J Hyg Epidemiol Microbiol Immunol. 1976;20(2):150–156. [PubMed] [Google Scholar]
- Zinnaka Y., Carpenter C. C., Jr An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972 Dec;131(6):403–411. [PubMed] [Google Scholar]



