Abstract
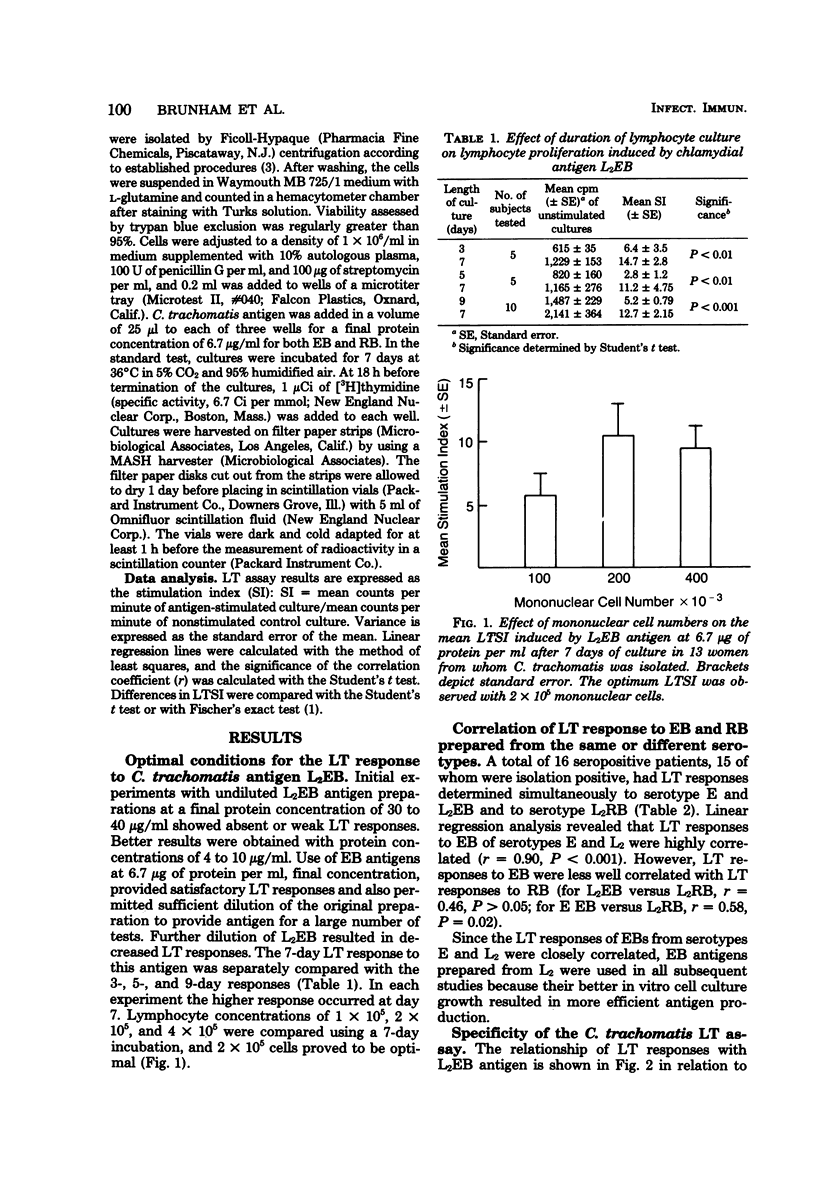
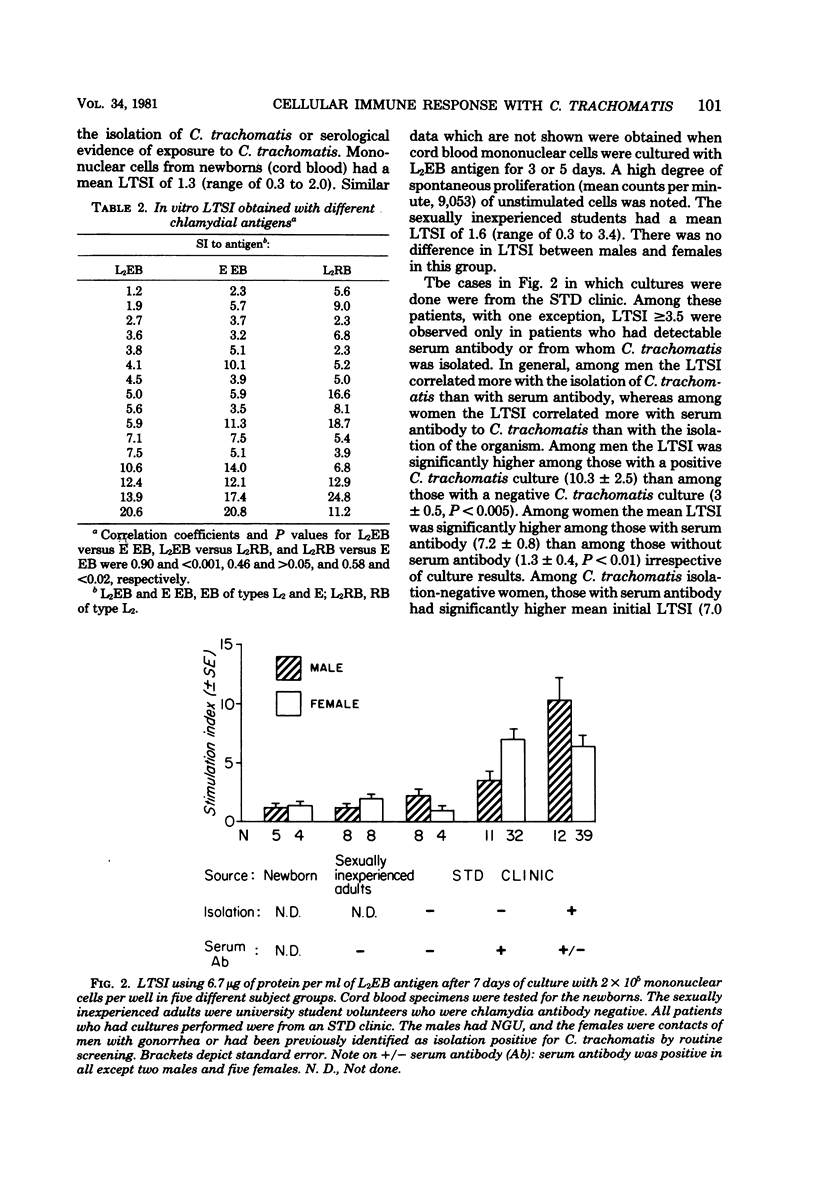
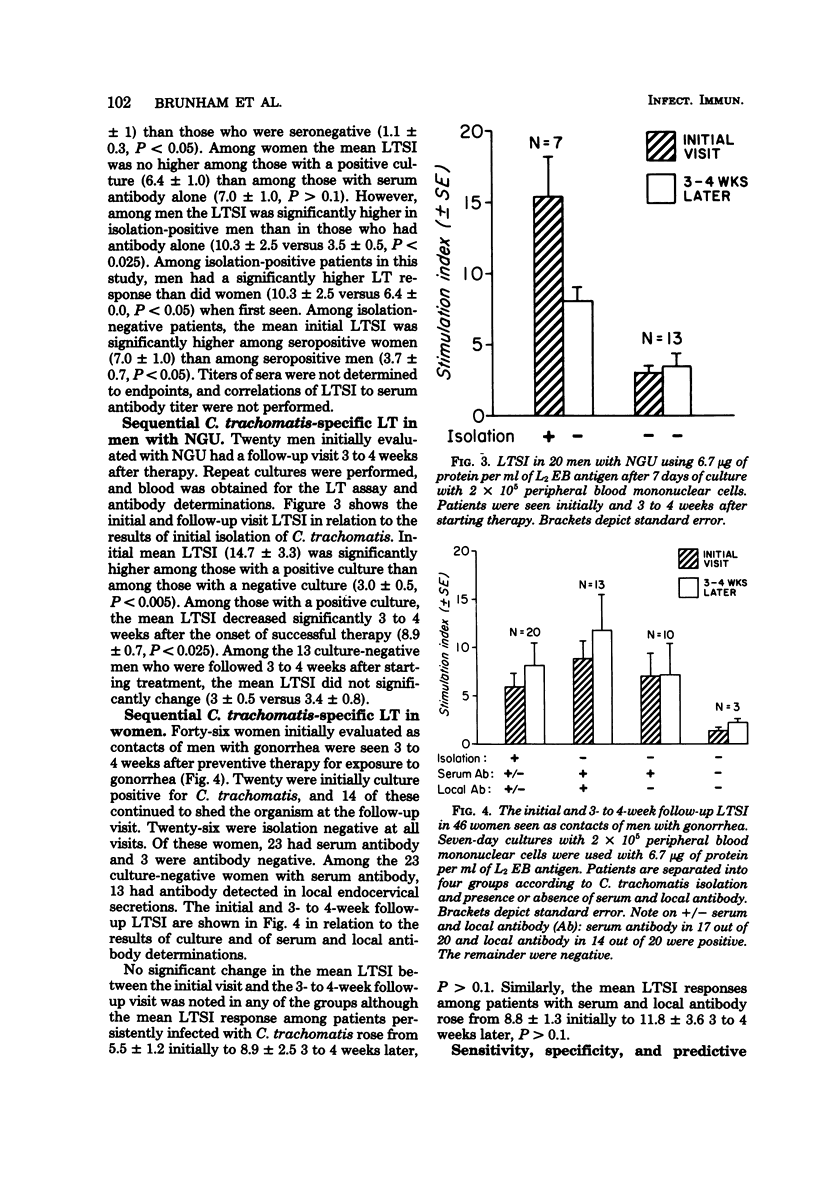
A lymphocyte transformation (LT) assay for the study of the cellular immune response to Chlamydia trachomatis in humans is described. Subjects studied included 9 newborns whose mothers were C. trachomatis culture negative, 16 seronegative, sexually inexperienced adults, and 107 patients seen at a sexually transmitted disease clinic, including 31 men presenting with acute nongonococcal urethritis and 76 women with known or suspected uncomplicated gonorrhea or with uncomplicated C. trachomatis genital infection. LT stimulation indices (SI) were less than 3.5 in newborns and normal adults, as well as 11 of 12 seronegative, isolation-negative sexually transmitted disease clinic subjects. LTSI greater than 3.5 was found only with subjects who were sero- or culture positive for C. trachomatis. Among men with nongonococcal urethritis, the LTSI correlated better with culture than with antibody. Among women, the LTSI correlated better with antibody than with culture. LTSI decline significantly 3 to 4 weeks after curative therapy in men with nongonococcal urethritis, suggesting that LT response is short-lived and that the LTSI may be an indicator of acute C. trachomatis infection. The sensitivity, specificity, and predictive value of a positive LT assay and of serum and local antibody tests, in terms of C. trachomatis infection defined as positive isolation, were also compared. The predictive value of a positive LTSI for C. trachomatis infection was generally low in the sexually transmitted disease clinic patients: 62% in men with nongonococcal urethritis and 37% in women. However, this study did show the LT assay to be a useful specific test for monitoring the cellular immune response to C. trachomatis infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barwell C. F., Dunlop E. M., Race J. W. Results of complement-fixation and intradermal tests for Bedsoniae in genital infection, disease of the eye and Reiter's disease. Am J Ophthalmol. 1967 May;63(5 Suppl):1527–1534. doi: 10.1016/0002-9394(67)94142-6. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. I. Antigenic heterogeneity between C. trachomatis and C. psittaci. J Immunol. 1975 Oct;115(4):963–968. [PubMed] [Google Scholar]
- Hanna L., Schmidt L., Sharp M., Stites D. P., Jawetz E. Human cell-mediated immune responses to chlamydial antigens. Infect Immun. 1979 Feb;23(2):412–417. doi: 10.1128/iai.23.2.412-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Wentworth B. B., Grayston J. T. Primary isolation of TRIC organisms in HeLa 229 cells treated with DEAE-dextran. J Infect Dis. 1972 Jun;125(6):665–668. doi: 10.1093/infdis/125.6.665. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McComb D. E., Nichols R. L., Semine D. Z., Evrard J. R., Alpert S., Crockett V. A., Rosner B., Zinner S. H., McCormack W. M. Chlamydia trachomatis in women: antibody in cervical secretions as a possible indicator of genital infection. J Infect Dis. 1979 Jun;139(6):628–633. doi: 10.1093/infdis/139.6.628. [DOI] [PubMed] [Google Scholar]
- Møller B. R., Weström L., Ahrons S., Ripa K. T., Svensson L., von Mecklenburg C., Henrikson H., Mårdh P. A. Chlamydia trachomatis infection of the Fallopian tubes. Histological findings in two patients. Br J Vener Dis. 1979 Dec;55(6):422–428. doi: 10.1136/sti.55.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Schoop J. W., Wang S. P., Munzinger J., Schläpfer H. U., Knoblauch M., Tammann R. W. Chlamydia trachomatis as possible cause of peritonitis and perihepatitis in young women. Br Med J. 1978 Apr 22;1(6119):1022–1024. doi: 10.1136/bmj.1.6119.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattin S., Durosoir J. L., Thabaut A., Doury P. Le test de transformation lymphoblastique avec l'antigène bedsonien (TTL bedsonien) dans les syndromes de Fiessinger-Leroy-Reiter anciens et récents et dans les spondylarthrites ankylosantes Etude complémentaire. Rev Rhum Mal Osteoartic. 1976 Jun;43(6):407–410. [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Schachter J., Smith D. E., Dawson C. R., Anderson W. R., Deller J. J., Jr, Hoke A. W., Smartt W. H., Meyer K. F. Lymphogranuloma venereum. I. Comparison of the Frei test, complement fixation test, and isolation of the agent. J Infect Dis. 1969 Sep;120(3):372–375. doi: 10.1093/infdis/120.3.372. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Alexander E. R., Holmes K. K. Simplified microimmunofluorescence test with trachoma-lymphogranuloma venereum (Chlamydia trachomatis) antigens for use as a screening test for antibody. J Clin Microbiol. 1975 Mar;1(3):250–255. doi: 10.1128/jcm.1.3.250-255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis. 1974 Oct;130(4):388–397. doi: 10.1093/infdis/130.4.388. [DOI] [PubMed] [Google Scholar]
- Wentworth B. B., Alexander E. R. Isolation of Chlamydia trachomatis by use of 5-iodo-2-deoxyuridine-treated cells. Appl Microbiol. 1974 May;27(5):912–916. doi: 10.1128/am.27.5.912-916.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. C., Chinn J. S., Caldwell H. D., Kuo C. C. Reticulate bodies as single antigen in Chlamydia trachomatis serology with microimmunofluorescence. J Clin Microbiol. 1979 Sep;10(3):351–356. doi: 10.1128/jcm.10.3.351-356.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


