Abstract
Background
Multiple rapid swallows (MRS) inhibit esophageal peristalsis and lower esophageal sphincter (LES) tone; a rebound excitatory response then results in an exaggerated peristaltic sequence. MRS responses are dependent on intact inhibitory and excitatory neural function and could vary by subtype in achalasia spectrum disorders.
Methods
Consecutive subjects with incomplete LES relaxation on HRM (Sierra Scientific, Los Angeles, CA) in the absence of mechanical obstruction were prospectively identified. Achalasia spectrum disorders were classified and HRM plots reviewed according to Chicago criteria. Esophageal peristaltic performance and LES function were assessed after 10 wet swallows and MRS (five 2 mL water swallows 2-3 s apart). Findings were compared to 18 healthy controls (28.5±0.6 years, 44% female).
Results
46 subjects (57.1±2.1 years, 52.2% female) met inclusion criteria. There was complete failure of peristalsis with MRS in all subjects with achalasia subtypes 1 and 2. In contrast, 80% of achalasia subtype 3 and incomplete LES relaxation (EGJ outflow obstruction) with preserved esophageal body peristalsis had a contractile response to MRS (p<0.001 compared to subtypes 1 and 2); controls demonstrated 94.4% peristalsis. Percent MRS decrease in LES residual pressure compared to wet swallows segregated achalasia subtypes; those with aperistalsis (subtypes 1 and 2) had a lesser decline (22.6%) compared to those with retained esophageal body peristalsis (40.5%) and controls (51.3%, p<0.001 across groups).
Conclusion
MRS responses segregate achalasia spectrum disorders into two patterns differentiated by presence or absence of esophageal body contraction response to wet swallows. These findings support subtyping of achalasia, with pathophysiologic implications.
Keywords: achalasia, multiple rapid swallows, high resolution manometry
BACKGROUND
Idiopathic achalasia is the best known and most studied esophageal motor disorder, representing an extreme example of abnormal deglutitive inhibition. (1) Achalasia is diagnosed when motor testing shows impaired relaxation of the lower esophageal sphincter (LES) and aperistalsis in the esophageal body.(2) Morphologically, achalasia is characterized by chronic inflammation and destruction of ganglion cells and nerves in the myenteric plexus of the esophagus.(3, 4) While impaired LES relaxation is the hallmark of symptomatic presentations, variability exists in esophageal body motor function on esophageal manometry and at the cellular level.(4-7) Early classifications of achalasia were based on the esophageal body motor response, termed classic achalasia when esophageal body peristalsis was absent, and vigorous achalasia when contractile activity was retained in the esophageal body, mainly in the form of simultaneous sequences.(8-10)
Understanding of the motor spectrum of achalasia further advanced with the introduction of high resolution esophageal pressure topography (high resolution manometry, HRM), with consequent increase in sensor density and fidelity in manometry systems.(11, 12) The esophageal body motor response is better characterized from improved spatial resolution afforded by HRM.(13, 14) Consequently, the latest classification system proposed by Pandolfino et al includes esophageal body motor responses ranging from aperistalsis at one end, to preserved peristalsis at the opposite end of the spectrum.(6, 7) These observations have led to suggestions that retained motor activity in the esophageal body may represent earlier diagnosis, or a form fruste of achalasia.(6, 7) However, controversy remains with respect to whether the achalasia subtypes represent distinct motor disorders or are simply different points in the progression from a healthy esophagus to end stage achalasia.(15)
Utilization of maneuvers that rely on physiologic inhibitory neural pathways may be one method of further evaluation of the underlying pathophysiology in achalasia subtypes.(16, 17) The best described of these is the response to multiple rapid swallows (MRS).(18) Multiple swallows in rapid sequence induce protracted central and peripheral neuronal inhibition of motor activity in the smooth muscle esophagus and the LES, thereby inhibiting peristalsis and causing profound LES relaxation.(16, 19) This is typically followed by a robust peristaltic sequence in the esophageal body and contraction of the LES. Basic and clinical studies have demonstrated MRS to be a powerful tool for investigating neural pathways in the human esophagus, and may help to further classify esophageal motility disorders.(16, 17, 20)
We hypothesize that various achalasia subtypes will demonstrate distinctive MRS responses, which might provide clues to pathophysiologic mechanisms underlying each subtype. To test this hypothesis, this study identified consecutive subjects with achalasia spectrum disorders with an adequately performed MRS maneuver, and compared responses amongst the subtypes.(21)
METHODS
Subjects
The subjects reported in this investigation were identified from a prospectively maintained database of clinical HRM studies at Barnes-Jewish Hospital and Washington University, St. Louis, Missouri. Subjects had been referred for clinical manometric evaluation for obstructive esophageal symptoms. Included were consecutive subjects demonstrating incomplete LES relaxation on HRM in the absence of mechanical obstruction, all of whom completed a standard HRM protocol of 10 water swallows, followed by adequately performed MRS. All patients were required to have obstructive esophageal symptoms as part of their clinical presentation for inclusion. Exclusion criteria included prior foregut surgery, artifactual HRM studies, structural mechanical obstruction on endoscopy, histological evidence of esophageal hypereosinophilia (>15 eosinophils per high power field) on esophageal biopsy and inability to subtype achalasia due to incomplete swallow sequences. Esophagogastric junction outflow obstruction was defined as 4-second integrated relaxation pressure (IRP) ≥15 mmHg.(6, 7) Achalasia was subtyped according to the Chicago classification as follows: a) subtype 1 (classic achalasia) with absence of esophageal pressurization and contractile activity in the esophageal body; b) subtype 2 with ≥ 2 test swallows associated with pan-esophageal pressurization >30 mm Hg and contractile activity in the esophageal body; c) subtype 3 (spastic achalasia) with preserved contractile activity in the esophagus (≥2 spastic contractions with or without compartmentalized pressurization between the contraction front and the LES), but no normal peristalsis in the esophageal body; d) isolated EGJ outflow obstruction with preserved normal peristalsis in the distal esophagus (manifest as intact 30-mm Hg isobaric contour encompassing the distal propagated contraction), where mechanical etiologies were excluded with endoscopic, histologic and radiographic investigations (idiopathic LES relaxation error).(6, 7)
The control group was composed of healthy volunteers, with no gastrointestinal symptoms, who had undergone HRM studies at the institution's motility center as part of normative data collection. Individuals with evidence of hiatus hernia on HRM were not included in the control group. In our motility laboratory, MRS is part of the standard evaluation in all symptomatic and control patients. The review of manometric and clinical data for this study was approved by the Human Research Protection Office (institutional review board) of Washington University School of Medicine.
Esophageal Manometry
All subjects were studied following an overnight fast, and medications that can affect esophageal motor function (metoclopramide, anticholinergic medication, smooth muscle relaxants) were discontinued for 5–7 days prior to the study. Studies were performed with a 36-channel solid state catheter system with high fidelity circumferential sensors at 1 cm intervals (Sierra Scientific Instruments, Given Imaging, Los Angeles, CA). After calibration, the catheter was passed through an anesthetized nasal canal. Following this, a 20-second swallow-free period was first obtained after the subject was settled and resting quietly in the recumbent position (landmark period), from which basal LES pressures were obtained. Recordings were obtained with 10 swallows of 4-5 mL ambient temperature water spaced ≥20 seconds apart. Following completion of the routine wet swallows, MRS was performed, with the subject swallowing five 2 mL water boluses 2-3 seconds apart, administered into the mouth using a syringe. Pressure data were acquired and analyzed using dedicated computerized HRM acquisition, display and analysis systems (ManoView, Sierra Scientific Instruments, Given Imaging, Los Angeles, CA).
Analysis of Esophageal Manometry
HRM Clouse plots were evaluated to assess performance of esophageal contraction segments during routine wet swallows according to the Chicago classification.(6, 7) Normal LES function was assessed using an electronic sleeve that measured both three second nadir pressure and IRP during swallow induced relaxation. MRS-induced esophageal body inhibition was considered ‘incomplete’ if contractions measuring >30 mmHg were identified during the multiple swallows. LES pressure decrement following MRS was also assessed using the three second nadir pressure and IRP following the first swallow of the sequence, using a evaluation window that extended till the contraction sequence arrived at the LES. The percent change in LES residual pressure following MRS was calculated as follows:
The designation ‘simultaneous’ required contractile front velocity (CFV) to be >9 cm/sec. For all wet swallows that resulted in a contraction sequence, the distal latency (DL, the time interval between upper esophageal sphincter relaxation and the contractile deceleration point) was calculated according to the method previously described by Pandolfino et al. (22, 23) Contractions were considered to be premature if the DL was ≤ 4.5 seconds (only applicable to wet swallows).(23)
Statistical Analysis
Grouped values are reported as mean ± standard error of the mean unless indicated otherwise. Categorical and grouped data were compared using Fisher's exact test, χ2 test or two-tailed Student's t test as appropriate. Across group differences were evaluated with ANOVA. In each case, p<0.05 was required for statistical significance. Statistical analysis was performed using SPSS 19.1 software (IBM, Armonk, New York).
RESULTS
During the study period, 46 subjects met inclusion criteria and were able to adequately tolerate the MRS procedure. Mean age was 57.1±2.13 years and 24 (52.2%) were female. The dominant presenting symptom was dysphagia in 44 (95.7%) of subjects, chest pain in 1 (2.2%) and heartburn in 1 (2.2%); all subjects had obstructive symptoms as part of their initial presentation. None of the patients had a hiatus hernia identified on endoscopy or HRM. The HRM pattern was consistent with subtype 1 or classic achalasia with aperistalsis in 9 (19.6%) subjects, subtype 2 achalasia with pan-esophageal pressurization in 17 (37.0%), subtype 3 or spastic achalasia in 16 (34.8%), and EGJ outflow obstruction with preserved peristalsis (idiopathic incomplete LES relaxation) in 4 (8.7%) subjects. The control group was composed of 18 healthy volunteers, average age 28.5±0.61 years, 8 (44.4%) were female.
Baseline esophageal body and LES parameters
A single short (<3 cm, third segment) contraction was seen in one patient with subtype 1, and none with subtype 2 had contraction sequences. In subtype 3, 91.9% of sequences demonstrated esophageal body contraction, with an average DL of 4.1 ±0.3 seconds, DCI of 5010 ±1140 mmHg.cm.second, and peak contraction amplitude of 193.0 ±9.4 mmHg. Finally, in the EGJ outflow obstruction group with idiopathic abnormal LES relaxation, 77.5% demonstrated esophageal body contraction with longer DL (5.9 ±1.7 seconds), lower DCI (2199 ±679 mmHg.cm.second), and lower peak contraction amplitude (151.6 ±8.4 mmHg). Using DL <4.5 seconds as an indicator of premature contraction in the esophageal body, 70.1% of peristaltic sequences were premature in subtype 3, while 35.5% were premature in EGJ outflow obstruction (p<0.001). Subtypes with absent and preserved esophageal body peristalsis were grouped together and compared to normal controls (Table 1). DCI in achalasia with preserved peristalsis was significantly higher than in controls, as were peak contraction amplitudes. LES basal and post swallow residual pressures were similar in all achalasia subtypes, but significantly higher when compared to controls (Figure 2).
TABLE 1.
Clinical and HRM characteristics
| No peristalsis n=26 | Preserved peristalsis n=20 | Normal controls n=18 | |
|---|---|---|---|
| Age | 56.6±3.2 | 62.1±2.2 | 28.6±0.6 |
| Female Gender | 12 (46.2%) | 12 (60%) | 9 (50%) |
| Wet swallows | |||
| Swallows evaluated | 260 | 200 | 180 |
| Swallows with contraction response | 1 (0.4%) | 178 (89.0%) | 174 (96.7%) |
| DCI (mmHg.cm.sec) | - | 4447.9 ±950.1 | 1614.4±311.3 |
| Peak amplitude (mmHg)* | - | 184.3±10.6 | 132.8±3.7 |
| LES basal pressure (mmHg) | 40.4±2.52 | 43.9±4.8 | 25.6±2.3 |
| LES residual pressure (IRP) (mmHg) | 24.1±1.7 | 22.4±1.8 | 4.6±0.5 |
| MRS response | |||
| Preserved post-MRS peristalsis | - | 80.0% | 94.4% |
| DCI (mmHg.cm.sec)** | - | 5011.7±1974.1 | 2453.7±475.3 |
| Peak amplitude (mmHg)* | - | 188.1±31.6 | 163.9±11.8 |
| LES residual pressure (IRP) (mmHg) | 18.8±1.7 | 13.1±1.2 | 2.4±0.4 |
| % Change in LES residual pressure post MRS† | 22.6±0.04 | 40.5±0.04 | 51.3±0.04 |
p=≤0.03 for control vs. preserved peristalsis
p =0.09 for control vs. preserved peristalsis
p<0.001 across groups
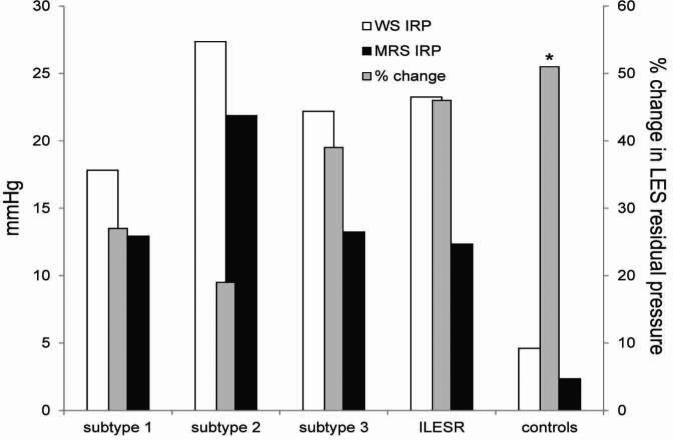
FIGURE 2.
LES residual pressures with wet swallows (WS), multiple rapid swallows (MRS) and percent change after MRS. LES residual pressure (integreted residual pressure, IRP) was ≥15 mmHg with wet swallows in achalasia spectrum disorders. Percent change in LES residual pressure was more profound in subtype 3 and EGJ outflow obstruction with preserved peristalsis similar to controls (p=0.3 compared to controls). In contrast, this change was much less prominent in achalasia subtypes 1 and 2 (p≤0.02 compared to controls).
Esophageal body response to MRS
Deglutitive inhibition during MRS was complete in all subjects with type 1 and 2 achalasia. Esophageal pressurization during MRS was significantly more frequent in subjects with type 2 achalasia (67.7%) than in those with type 1 achalasia (18.2%), p=0.016. A complete failure of peristalsis following MRS was observed in all subjects with achalasia subtypes 1 and 2 (Figure 3).
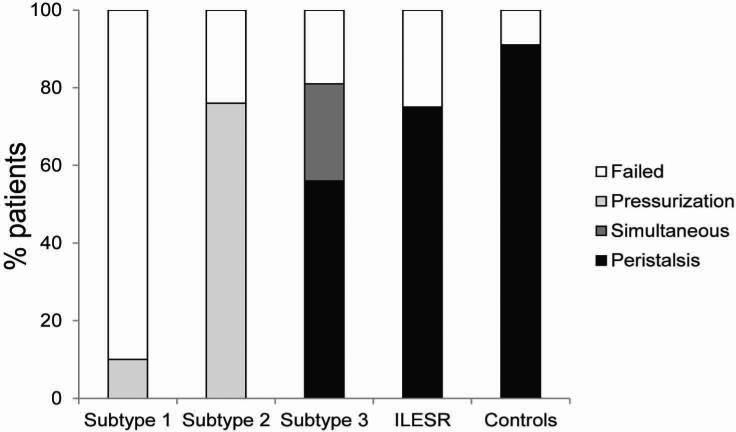
FIGURE 3.
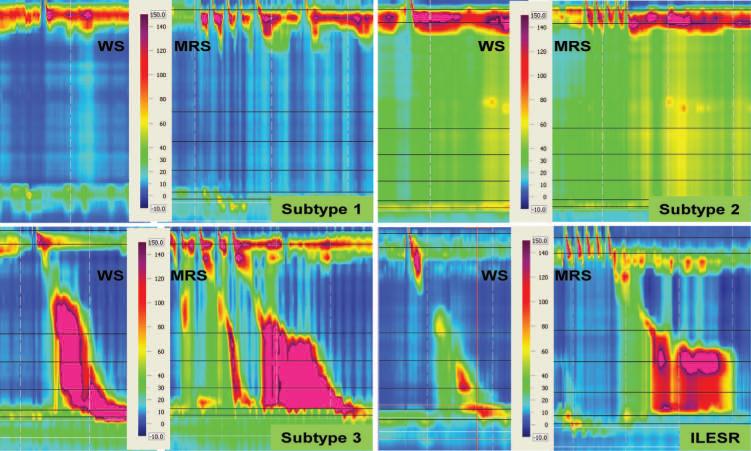
Examples of response to MRS in achalasia subtypes and incomplete LES relaxation (ILESR) with EGJ outflow obstruction and preserved esophageal body peristalsis. No esophageal body peristalsis or pressurization is seen with wet swallows (WS) and MRS in subtype 1. In subtype 2, panesophageal pressurization is seen with both WS and MRS. In subtype 3 and EGJ outflow obstruction, WS demonstrate spastic and normal esophageal body peristalsis, with compartmentalization of pressure between the contraction front and LES. With MRS, there is abnormal inhibition of esophageal body peristalsis, with a spastic and exaggerated peristaltic sequence in both instances. WS: wet swallows, MRS: multiple rapid swallows, EGJ outflow obstruction: incomplete lower esophageal sphincter relaxation.
The mean DCI and peak contractile amplitude with esophageal body contraction following MRS were 5669.5±2443.9 mmHg.cm.sec and 144.8±41.7 mmHg, respectively in subtype 3. Deglutitive inhibition of peristalsis during MRS was incomplete in 31.3% of subjects with type 3 achalasia. Esophageal pressurization during MRS was seen in 23.5% of subjects with type 3 achalasia and none of the subjects with EGJ outflow obstruction or controls (p=0.69). Following MRS, contractions were seen in the esophageal body in the majority of subjects with type 3 achalasia and EGJ outflow obstruction, predominantly as peristaltic contractions; while in the control group, a peristaltic sequence followed MRS in almost all instances (Figure 4).
FIGURE 4.
Esophageal body response to MRS in achalasia spectrum disorders. In patterns with aperistalsis (subtypes 1 and 2), there was complete failure of peristalsis, with varying proportions of panesophageal pressurization. In subtype 3 and incomplete EGJ outflow obstruction, 80% of subjects had a contractile response to MRS, and proportions of peristaltic patterns were similar between the two groups. In the control group a contractile response was seen in >90% of subjects.
Although the average DCI (2380.3 ±712.4 mmHg.cm.sec) was lower in subjects with EGJ outflow obstruction and idiopathic incomplete LES relaxation than in subtype 3, peak contraction amplitude (168±29.4 mmHg) was similar. Incomplete inhibition during MRS was seen in 50.0% of cases. In contrast to subtype 3, there was no failure of peristalsis, with a normal post MRS peristaltic sequence in 75.0% (Figure 2). The differences in esophageal body response to MRS were visually apparent on inspection of Clouse plots (Figure 3).
Further comparisons were made between achalasia subtypes with and without preserved peristalsis (Table 1). Within subtypes with preserved peristalsis, 80.0% had a contractile response to MRS in the esophageal body; with simultaneous contractions (CFV>9 cm/sec) in 20.0% and a peristaltic sequence in 60.0%. When compared to wet swallows, MRS resulted in augmentation of the DCI in 40% of subjects with preserved peristalsis; the peak contractile amplitude was augmented in 46.7%.
LES response to MRS
MRS resulted in decrease of LES residual pressure in 85.1% of subjects, by an average 32.9±2.7%. There was a gradient of decline in LES residual pressure, which was less pronounced in achalasia subtypes with aperistalsis compared to those with preserved esophageal body contraction (Figure 2). Achalasia subtypes with and without preserved esophageal body motor activity could be discriminated by the percent decline in LES residual pressure in response to MRS. The decline was lower (22.0±3.9%) in those with aperistalsis (achalasia subtypes 1 & 2) compared to patterns with retained esophageal body motor activity (subtype 3 and EGJ outflow obstruction, 36.7±5.8%, p=0.002). The LES residual pressure decreased by an average of 51.3±11.8% in the control group, significantly more profound than that observed in achalasia subtypes with aperistalsis (p<0.001), but not statistically different from subtypes with preserved esophageal body contraction (p=0.325).
DISCUSSION
In the largest study to date evaluating the response to MRS in subjects with achalasia spectrum disorders, we demonstrate a dichotomous response to MRS in the esophageal body and LES, segregated by presence or absence of motor activity in the esophageal body on routine wet swallows. Subjects with achalasia subtype 3 and EGJ outflow obstruction with idiopathic incomplete LES relaxation demonstrate a physiologic response following MRS with profound EGJ relaxation and vigorous contraction of the smooth muscle esophagus. The degree of LES relaxation in these subgroups is similar to that seen in healthy controls. In contrast, those with subtypes 1 and 2 demonstrate significantly lesser degrees of LES relaxation and absent esophageal body contraction in response to MRS. These results further support the Chicago classification of achalasia subtypes and help emphasize the heterogeneity of achalasia spectrum disorders.
Variability in esophageal body motor patterns has long been recognized within achalasia, predating HRM. Conventional manometric criteria subdivided achalasia into classic achalasia with complete aperistalsis and ‘vigorous’ achalasia if simultaneous post-deglutitive contractile activity was seen.(4, 10) With HRM, Pandolfino et al were able to provide further breakdown of achalasia spectrum disorders, with implications on management and outcome.(6, 7) For instance, patients with achalasia subtype 2 (panesophageal pressurization) demonstrate the best response to treatments directed at disrupting the LES, while those with subtype 3 and EGJ outflow obstruction respond suboptimally with persistent symptoms despite such therapy.(6, 7, 24) These differences in HRM patterns and outcome have led several authors to speculate that the pathophysiologic mechanism may differ to some degree between achalasia patients with aperistalsis (subtype 1 and 2) and those with preserved esophageal body motor activity (subtype 3 and EGJ outflow obstruction).(15, 25)
The MRS responses we observed in achalasia spectrum disorders lend further support to the hypothesis that two pathophysiologic patterns exist. In the esophageal body, we observed that patients with achalasia subtype 3 and EGJ outflow obstruction exhibited predominantly peristaltic rather than simultaneous or failed contraction following MRS, suggesting that a physiologically significant portion of the inhibitory neural network is preserved in these patients. Conversely, those with subtypes 1 and 2 did not demonstrate any discernible motor activity in the esophageal body following MRS, implying that the inhibitory neuron network had been disrupted beyond a critical threshold. The MRS results reported by Savojardo et al, who observed that 40% of achalasia subjects have motor activity in the esophageal body, are also supportive of our findings.(17) At the LES, in stark contrast to subtypes without peristalsis (subtypes 1 and 2), the degree of LES relaxation following MRS in subtype 3 and EGJ outflow obstruction is in line with that seen in healthy controls. The conclusion is that these findings reflect the physiologic expression of the degree of inhibitory neuronal loss, but only longitudinal studies can ascertain if achalasia subtype 3 and EGJ outflow obstruction represent an earlier phase in the spectrum of achalasia; none currently exist. The finding of incomplete inhibition during MRS in patients with EGJ outflow obstruction also supports abnormal esophageal inhibition, albeit not to the extent as in subtypes 1 and 2. This conclusion is in line with observations made by Sifrim et al in 1994, when they described a spectrum of inhibitory neuronal disorders of the esophagus, with differing motor patterns being observed at different stages of inhibitory nerve failure, ranging from symptomatic diffuse esophageal spasm to classic achalasia on the two extremes.(26) Additional evidence supporting this hypothesis comes from a clinicopathologic study by Goldblum et al, who observed that patients with vigorous achalasia (conventional manometry equivalent of subtype 3) had distinct histological features from classic achalasia. Vigorous achalasia was characterized by myenteric inflammation with injury, but preservation of ganglion cells, while aganglionosis was observed in patients with classic achalasia.(4)
The MRS responses provide more than an interesting sidebar discussion on the pathophysiology and perhaps the natural history of achalasia spectrum disorders - there may be value in clarifying subtypes when the motor pattern on routine wet swallows does not lead to clear identification of the Chicago classification subtype. For instance, if the proportion of peristaltic sequences is very limited, or the presence of contractile activity is not clearly recognized because of prominent compartmentalization of pressure, one can resort to the MRS response for further direction. We and others have previously reported suboptimal symptomatic responses in subjects with subtype 3 and EGJ outflow obstruction with vigorous distal esophageal contraction amplitudes (exceeding 180 mmHg).(6, 24, 25) Heightened esophageal symptom perception is associated with disorders that are believed to involve deranged inhibitory function.(27-29) Indeed, peripheral inhibitory pathways have been identified as an integral to endogenous analgesia; thus abnormal function of esophageal inhibitory nerves may lead to a decreased threshold for the development of esophageal symptoms.(30, 31) Additional evidence supporting this comes from both clinical and basic studies, in which the administration of adenosine (a vagolytic neurotransmitter) increases esophageal sensitivity, while pharmacologic blockade of adenosine receptors with theophylline decreases esophageal sensitivity and improves symptoms in patients with functional symptoms.(32, 33) Therefore, more complete inhibitory neuronal loss in subtypes 1 and 2 likely leads to decreased esophageal perception, with less perceptive symptoms following medical and surgical therapy aimed at disrupting the LES to improve obstructive features. Further, longitudinal esophageal muscle function is preserved subtype 2, leading to more rapid bolus clearance and thus decreased transit symptoms after disruption of the LES.(34) Conversely, in patients with subtype 3 achalasia and EGJ outflow obstruction, aberrant inhibitory influences contribute to esophageal hypersensitivity, the end result being ongoing perceptive symptoms despite LES disruption and relief of obstruction. Our anecdotal experience is that many subjects with continuing perceptive symptoms (e.g. chest pain) in this setting respond to neuromodulators such as tricyclic antidepressants.(35) The distinction between subtype 3 and EGJ outflow obstruction may be further refined with continued use of DL as a metric for designation into subtype 3 as the new Chicago classification suggests, and indeed, some of the latter category may fit subtype 3 better (6, 7, 22, 23).
Our study does have some limitations. Firstly, the sample size was limited, particularly in the EGJ outflow obstruction group, which limits the generalizability of our findings. Further, subtle mechanical obstruction could have been missed, particularly in this group, despite careful and exhaustive evaluation. Nonetheless, we believe the clear distinction seen between subjects with and without motor activity in the esophageal body has clinical and pathophysiologic relevance. Moreover, as this study was not geared to address histopathology, we can only speculate on the pathophysiologic mechanisms responsible for the MRS differences. Further studies involving histopathology and pharmacologic provocative maneuvers are needed to definitively evaluate the underlying pathophysiologic differences within achalasia spectrum disorders. Our study potentially reflects tertiary institution bias in the range of achalasia subtypes evaluated, and may not be representative of primary gastroenterologic practice. We did not assess the response to free drinking of a volume of water, mainly because achalasia subjects have trouble handling a large fluid volume while supine. While the full clinical relevance of our findings is difficult to determine with our limited numbers, we believe that the information gained by adding MRS to HRM studies is sufficient to justify the adoption of this maneuver to standard clinical manometry protocols. Finally, our control subjects were significantly older then the control; this is due to the fact that subjects had to be free of systemic illness or esophageal symptoms. While it is possible that the range of normal findings on HRM may change with age, we believe that our group of asymptomatic control subjects is representative of the range of MRS responses in healthy individuals.
In conclusion, response to MRS in achalasia spectrum disorders suggests two pathophysiologic patterns. Subtypes of achalasia with preserved esophageal body peristalsis respond physiologically to MRS comparable to controls, indicating partial retention of neural networks and inhibitory nerve function. When aperistalsis ensues, the esophageal body and LES lose the physiologic MRS response. Our results support the performance of MRS in HRM clinical protocols. Further prospective and blinded studies are needed to replicate our results in larger patient cohorts, and to determine if treatment outcome can be predicted by MRS response in achalasia spectrum disorders.
FIGURE 1.
Normal response to MRS in the esophageal body and LES in a healthy control subject. Note the profound inhibition of LES tone and esophageal body peristalsis during repetitive swallows, followed by a robust contraction sequence and return of LES tone.
ACKNOWLEDGEMENTS
VMK collected data from high resolution studies in patients and controls, analyzed data and prepared the manuscript; GSS analyzed data and provide critical review of the manuscript; CPG conceived and designed the study, analyzed data, prepared and revised the manuscript.
Supported by National Institutes of Health (NIH) National Research Service Award 5-T32-DK07301-35 (VMK), and NIH K23DK84413-2 (GSS), from the National Institute of Digestive Diseases and Kidney (NIDDK). CPG has received teaching honoraria and research funding from Given Imaging.
The study was not funded.
Footnotes
No competing interests exist. No writing assistance was obtained.
REFERENCES
- 1.Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369–74. doi: 10.1053/j.gastro.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Richter JE. The Diagnosis and Misdiagnosis of Achalasia: It Does Not Have to Be so Difficult. Clin Gastroenterol Hepatol. 2011;9:1010–1. doi: 10.1016/j.cgh.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327–37. [PubMed] [Google Scholar]
- 4.Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology. 1996;111:648–54. doi: 10.1053/gast.1996.v111.pm8780569. [DOI] [PubMed] [Google Scholar]
- 5.Hirano I, Tatum RP, Shi G, Sang Q, Joehl RJ, Kahrilas PJ. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology. 2001;120:789–98. doi: 10.1053/gast.2001.22539. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–33. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCord GS, Staiano A, Clouse RE. Achalasia, diffuse spasm and non-specific motor disorders. Baillieres Clin Gastroenterol. 1991;5:307–35. doi: 10.1016/0950-3528(91)90032-v. [DOI] [PubMed] [Google Scholar]
- 9.Todorczuk JR, Aliperti G, Staiano A, Clouse RE. Reevaluation of manometric criteria for vigorous achalasia. Is this a distinct clinical disorder? Dig Dis Sci. 1991;36:274–8. doi: 10.1007/BF01318195. [DOI] [PubMed] [Google Scholar]
- 10.Camacho-Lobato L, Katz PO, Eveland J, Vela M, Castell DO. Vigorous achalasia: original description requires minor change. J Clin Gastroenterol. 2001;33:375–7. doi: 10.1097/00004836-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Clouse RE, Staiano A, Alrakawi A, Haroian L. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95:2720–30. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 14.Fox M, Hebbard G, Janiak P, Brasseur JG, Ghosh S, Thumshirn M, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533–42. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 15.Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13:2219–25. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fornari F, Bravi I, Penagini R, Tack J, Sifrim D. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil. 2009;21:718–e41. doi: 10.1111/j.1365-2982.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- 17.Savojardo D, Mangano M, Cantu P, Penagini R. Multiple rapid swallowing in idiopathic achalasia: evidence for patients’ heterogeneity. Neurogastroenterol Motil. 2007;19:263–9. doi: 10.1111/j.1365-2982.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 18.Ask P, Tibbling L. Effect of time interval between swallows on esophageal peristalsis. Am J Physiol. 1980;238:G485–90. doi: 10.1152/ajpgi.1980.238.6.G485. [DOI] [PubMed] [Google Scholar]
- 19.Meyer GW, Gerhardt DC, Castell DO. Human esophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol. 1981;241:G129–36. doi: 10.1152/ajpgi.1981.241.2.G129. [DOI] [PubMed] [Google Scholar]
- 20.Patuto N, Pohl D, Castell DO, Tutuian R. Multiple Rapid Swallowing Testing in Patients With Esophageal Symptoms: A Study Using High-Resolution Manometry. Gastroenterology. 2010;138:S-341–2. [Google Scholar]
- 21.Kushnir VM, Gyawali CP, Sayuk GS. Differing multiple rapid swallow responses in Achalasia spectrum disorders on high resolution manometry (HRM). Neurogastroenterol Motil. 2011;23(S1):33. doi: 10.1111/j.1365-2982.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roman S, Lin Z, Pandolfino JE, Kahrilas PJ. Distal contraction latency: a measure of propagation velocity optimized for esophageal pressure topography studies. Am J Gastroenterol. 2011;106:443–51. doi: 10.1038/ajg.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandolfino JE, Roman S, Carlson D, Luger D, Bidari K, Boris L, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology. 2011;141:469–75. doi: 10.1053/j.gastro.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratap N, Kalapala R, Darisetty S, Joshi N, Ramchandani M, Banerjee R, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil. 2011;17:48–53. doi: 10.5056/jnm.2011.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter RF, Gyawali CP. Botulinum toxin injection in dysphagia syndromes with preserved esophageal peristalsis and incomplete lower esophageal sphincter relaxation. Neurogastroenterol Motil. 2011;23:139–44. doi: 10.1111/j.1365-2982.2010.01604.x. [DOI] [PubMed] [Google Scholar]
- 26.Sifrim D, Janssens J, Vantrappen G. Failing deglutitive inhibition in primary esophageal motility disorders. Gastroenterology. 1994;106:875–82. doi: 10.1016/0016-5085(94)90745-5. [DOI] [PubMed] [Google Scholar]
- 27.Katz PO, Dalton CB, Richter JE, Wu WC, Castell DO. Esophageal testing of patients with noncardiac chest pain or dysphagia. Results of three years’ experience with 1161 patients. Ann Intern Med. 1987;106:593–7. doi: 10.7326/0003-4819-106-4-593. [DOI] [PubMed] [Google Scholar]
- 28.Gyawali CP, Kushnir VM. High-resolution manometric characteristics help differentiate types of distal esophageal obstruction in patients with peristalsis. Neurogastroenterol Motil. 2011;23:502–e197. doi: 10.1111/j.1365-2982.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kushnir VM, Prakash Gyawali C. High resolution manometry patterns distinguish acid sensitivity in non-cardiac chest pain. Neurogastroenterol Motil. 2011;23:1066–72. doi: 10.1111/j.1365-2982.2011.01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remes-Troche JM. The hypersensitive esophagus: pathophysiology, evaluation, and treatment options. Curr Gastroenterol Rep. 2010;12:417–26. doi: 10.1007/s11894-010-0122-3. [DOI] [PubMed] [Google Scholar]
- 31.Cervero F, Laird JM, Garcia-Nicas E. Secondary hyperalgesia and presynaptic inhibition: an update. Eur J Pain. 2003;7:345–51. doi: 10.1016/s1090-3801(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 32.Rao SS, Mudipalli RS, Remes-Troche JM, Utech CL, Zimmerman B. Theophylline improves esophageal chest pain--a randomized, placebo-controlled study. Am J Gastroenterol. 2007;102:930–8. doi: 10.1111/j.1572-0241.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- 33.Ru F, Surdenikova L, Brozmanova M, Kollarik M. Adenosine-induced activation of esophageal nociceptors. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G485–93. doi: 10.1152/ajpgi.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong SJ, Bhargava V, Jiang Y, Denboer D, Mittal RK. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology. 2010;139:102–11. doi: 10.1053/j.gastro.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clouse RE, Lustman PJ. Tricyclic antidepressants for chest pain from achalasia: Adjuvants to conventional therapy. Gastroenterology. 2003;2003(124):A 257. [Google Scholar]