Abstract
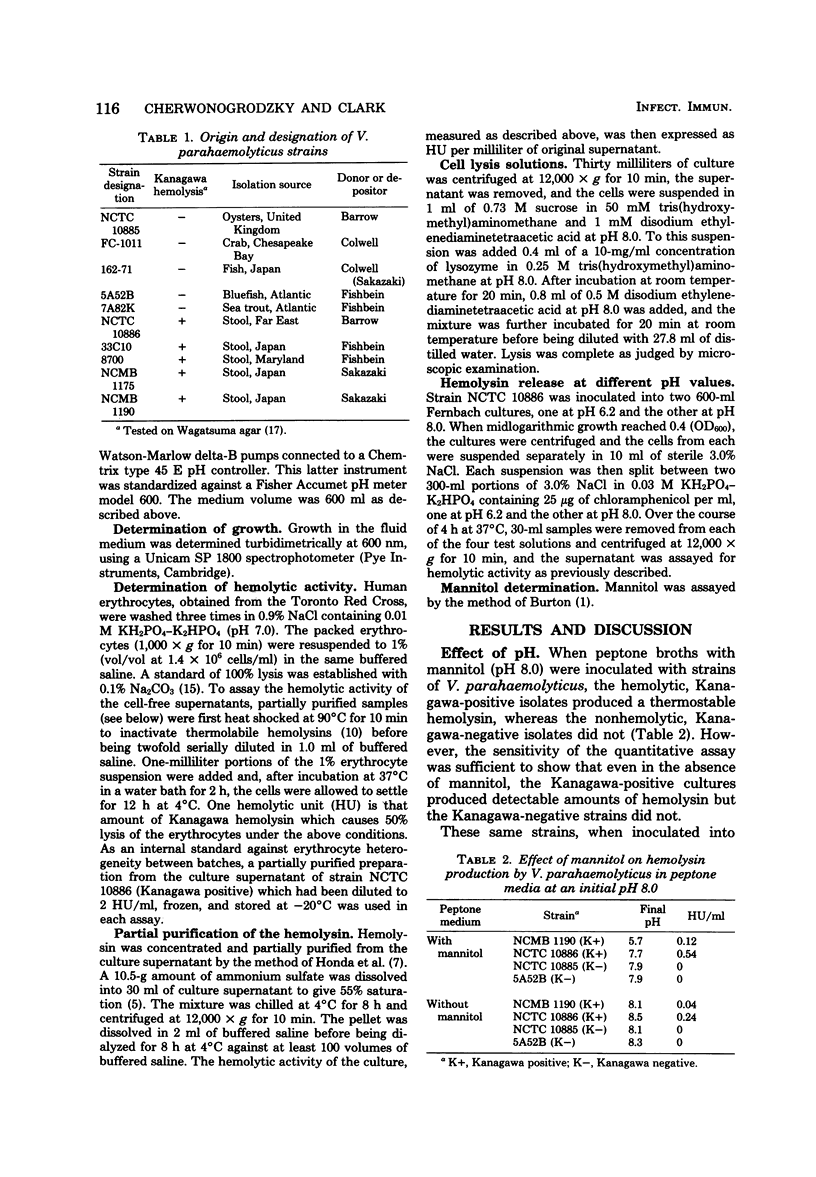
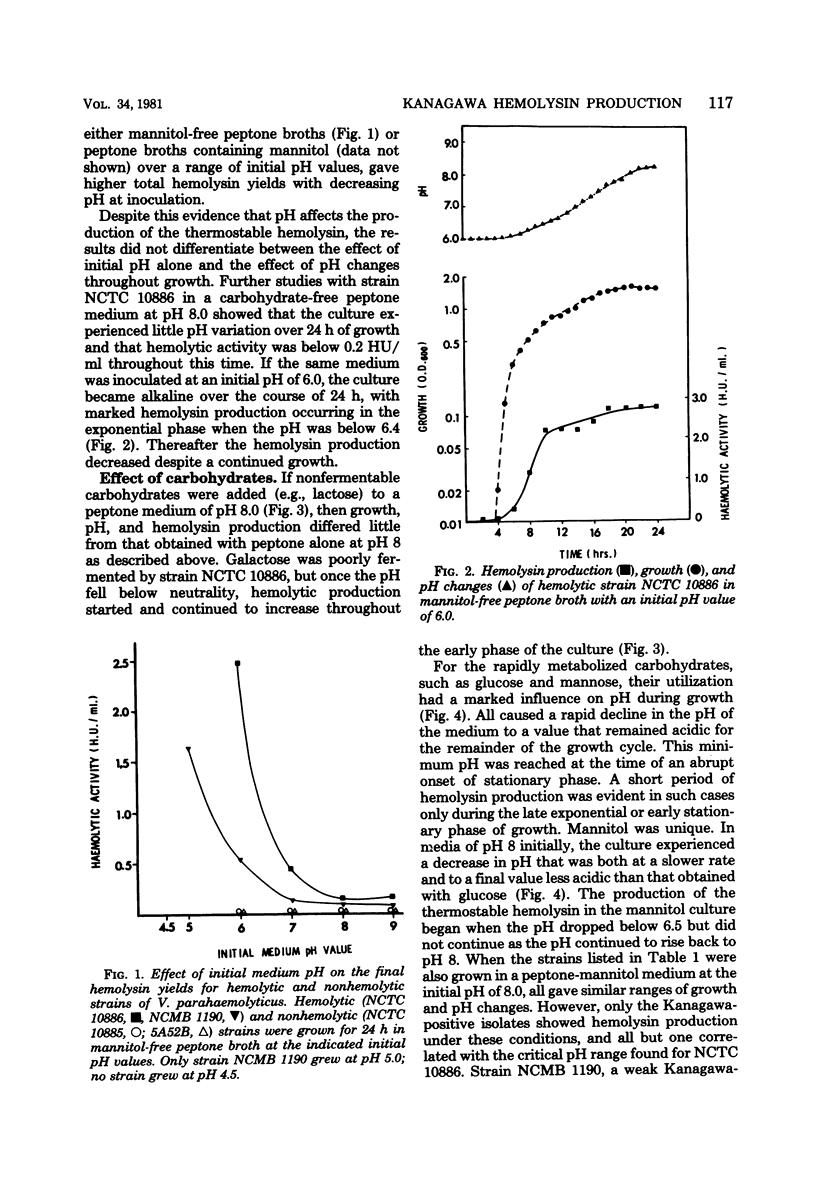
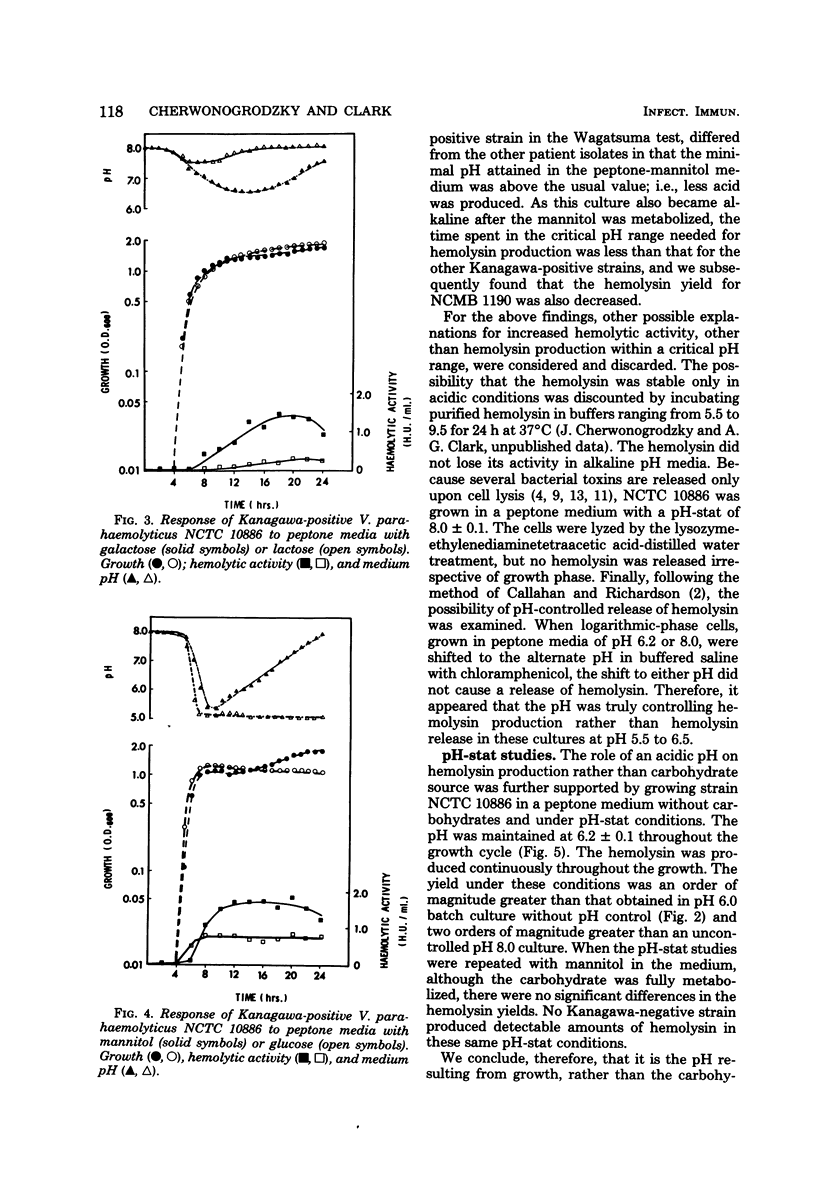
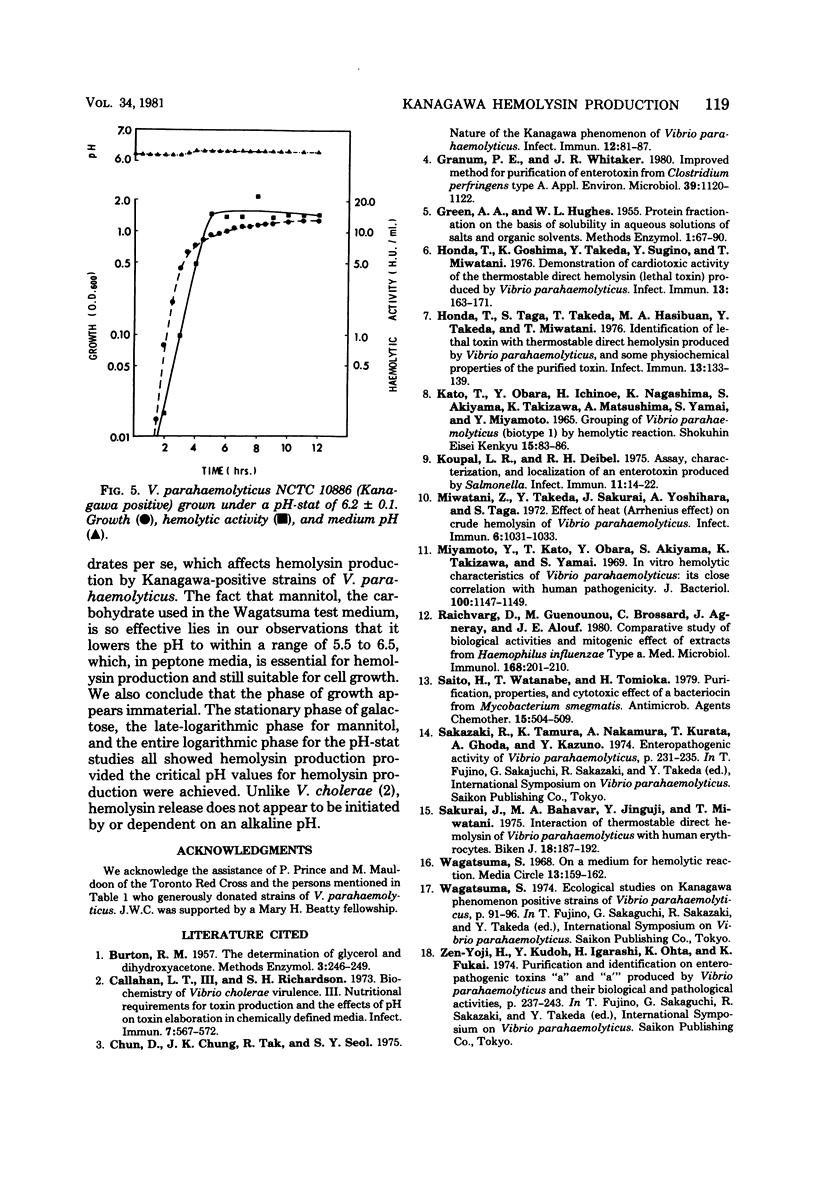
Production of the Kanagawa hemolysin by patient strains of Vibrio parahaemolyticus was found to respond to the pH rather than to the type of carbohydrate present in the growth medium. Regardless of the carbohydrate present, hemolysin production in peptone broth cultures occurred only when the pH was between 6.5 and 5.5. Mannitol, the sugar used in the Wagatsuma agar, lowered the pH to within this range, thus providing optimal conditions for hemolysin production. Glucose and mannose, although readily metabolized, lowered the pH below this range, inhibiting growth and hemolysin production. Alkaline cultures either without carbohydrates or containing non-metabolizable sugars showed little hemolytic activity because the pH always remained alkaline. In pH-stat cultures maintained at pH 6.2, higher hemolysin yields were produced irrespective of the presence or absence of mannitol. We conclude that the production of the Kanagawa hemolysin is under pH control. Marine strains of V. parahaemolyticus, which are Kanagawa negative, did not express detectable amounts of hemolysin under those conditions shown to stimulate hemolysin production by Kanagawa-positive strains.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan L. T., 3rd, Richardson S. H. Biochemistry of Vibrio cholerae virulence. 3. Nutritional requirements for toxin production and the effects of pH on toxin elaboration in chemically defined media. Infect Immun. 1973 Apr;7(4):567–572. doi: 10.1128/iai.7.4.567-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun D., Chung J. K., Tak R., Seol S. Y. Nature of the Kanagawa phenomenon of Vibrio parahaemolyticus. Infect Immun. 1975 Jul;12(1):81–87. doi: 10.1128/iai.12.1.81-87.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum P. E., Whitaker J. R. Improved method for purification of enterotoxin from Clostridium perfringens type A. Appl Environ Microbiol. 1980 Jun;39(6):1120–1122. doi: 10.1128/aem.39.6.1120-1122.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Goshima K., Takeda Y., Sugino Y., Miwatani T. Demonstration of the cardiotoxicity of the thermostable direct hemolysin (lethal toxin) produced by Vibrio parahaemolyticus. Infect Immun. 1976 Jan;13(1):163–171. doi: 10.1128/iai.13.1.163-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Taga S., Takeda T., Hasibuan M. A., Takeda Y., Miwatani T. Identification of lethal toxin with the thermostable direct hemolysin produced by Vibrio parahaemolyticus, and some physicochemical properties of the purified toxin. Infect Immun. 1976 Jan;13(1):133–139. doi: 10.1128/iai.13.1.133-139.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Arai K., Wagatsuma T., Tsuji A., Yo M. Clinical evaluation of the lippes loop. J Jpn Obstet Gynecol Soc. 1968 Jul;15(3):159–162. [PubMed] [Google Scholar]
- Koupal L. R., Deibel R. H. Assay, characterization, and localization of an enterotoxin produced by Salmonella. Infect Immun. 1975 Jan;11(1):14–22. doi: 10.1128/iai.11.1.14-22.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwatani T., Takeda Y., Sakurai J., Yoshihara A., Taga S. Effect of heat (Arrhenius effect) on crude hemolysin of Vibrio parahaemolyticus. Infect Immun. 1972 Dec;6(6):1031–1033. doi: 10.1128/iai.6.6.1031-1033.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Kato T., Obara Y., Akiyama S., Takizawa K., Yamai S. In vitro hemolytic characteristic of Vibrio parahaemolyticus: its close correlation with human pathogenicity. J Bacteriol. 1969 Nov;100(2):1147–1149. doi: 10.1128/jb.100.2.1147-1149.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichvarg D., Guenounou M., Brossard C., Agneray J., Alouf J. E. Comparative study of biological activities and mitogenic effect of extracts from Haemophilus influenzae type a. Med Microbiol Immunol. 1980;168(3):201–210. doi: 10.1007/BF02122854. [DOI] [PubMed] [Google Scholar]
- Saito H., Watanabe T., Tomioka H. Purification, properties, and cytotoxic effect of a bacteriocin from Mycobacterium smegmatis. Antimicrob Agents Chemother. 1979 Apr;15(4):504–509. doi: 10.1128/aac.15.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J., Bahavar M. A., Jinguji Y., Miwatani T. Interaction of thermostable direct hemolysin of Vibrio parahaemolyticus with human erythrocytes. Biken J. 1975 Dec;18(4):187–192. [PubMed] [Google Scholar]


