Abstract
Objective
To estimate the incidence and risk factors for gastrointestinal (GI) perforation among patients with rheumatoid arthritis (RA).
Methods
Claims from employer health insurance plans were used to identify RA patients and those hospitalized for upper or lower GI perforation. GI perforation cases were identified using both a sensitive and specific definition. A Cox model using fixed and time-varying covariates was used to evaluate risk of GI perforation.
Results
Among 143,433 RA patients, and using a maximally sensitive GI perforation definition, 696 hospitalizations with perforation were identified. The rate of perforation was 1.70 per 1000 person years (PYs) [95% CI, 1.58–1.83] and most perforations (83%) occurred in the lower GI tract. The rate of perforation was lower when a more specific GI perforation definition was used (0.87, 95% CI, 0.78–0.96 per 1,000 PYs). Age and diverticulitis were among the strongest risk factors for perforation (diverticulitis hazard ratio=14.5 [95% CI, 11.8–17.7] for more sensitive definition, hazard ratio=3.9 [95% CI, 2.5–5.9] for more specific definition). Among various RA medication groups, and compared to methotrexate, the risk of GI perforation was highest among patients with exposure to concomitant non-biologic disease-modifying antirheumatic drugs and glucocorticoids. Biologics without glucocorticoid exposure was not a risk factor for perforation.
Conclusion
GI perforation is a rare but serious condition that affects patients with RA, most frequently in the lower GI tract. Clinicians should be aware of risk factors for GI perforation when managing RA patients, including age, history of diverticulitis, and use of glucocorticoids or NSAIDs.
Despite the gravity of GI perforations, little is known about the incidence and prevalence of this condition, particularly in the lower GI tract, in clinical practice among patients with rheumatoid arthritis (RA). Medications used to treat RA include systemic non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, disease-modifying antirheumatic drugs (DMARDs), and biologic response modifiers (biologics). While the expanding array of therapies has notably mitigated the disability associated with RA for many patients, all of these agents have the potential for side effects, including serious adverse events such as GI bleeding and perforation.1–4
Likewise, glucocorticoid treatment has been associated with a broad array of adverse systemic events. Even at low doses, as commonly used in RA patients, long-term glucocorticoid therapy is associated with gastritis, pancreatitis, gastric ulcers, edema, and GI bleeding when concomitantly used with NSAIDs.1, 3 Additionally, oral glucocorticoid use is associated with a three-fold increase in risk of diverticular perforation.5
Among various RA medications, aspirin and NSAIDs have been the best characterized with respect to serious GI events, including bleeding and perforation of colonic diverticula.6,7 A prospective cohort study by Strate et al. (2011) assessed the use of these medications and other risk factors biennially among 47,210 men in the United States. During the 22-year follow-up period, 256 cases of diverticular bleeding were documented. After adjustment for risk factors, men who used aspirin and nonaspirin NSAIDs regularly (≥2 times/week) had multivariable-adjusted hazard ratios (HRs) of 1.70 (95% CI, 1.21–2.39) and 1.74 (95% CI, 1.15–2.64), respectively, for diverticular bleeding, compared with men who denied use of these medications. Use of aspirin at intermediate doses (2–5.9 standard, 325-mg tablets/week) and frequency (4–6 days/week) was associated with the highest risk of bleeding (HR, 2.32; 95% CI, 1.34–4.02, and HR, 3.13; 95% CI, 1.82–5.38, respectively).7
The incidence of NSAID-associated upper GI tract complications is well recognized and described in the literature.8, 9 Although the incidence of lower GI tract complications is less well understood, an increasing body of evidence suggests NSAID-induced GI toxicity extends into the lower GI tract.10 Given this finding, an increasing number of studies are evaluating complications of both the upper and lower GI tract together.11, 12
Most studies evaluating GI events—such as ulcers, bleeding, and perforations—report data for these complications collectively, making it difficult to discern the incidence of and characterize risk factors for GI perforations alone.8, 13, 14 Few studies have evaluated either the frequency or risk of lower GI tract perforations, and fewer still have examined the impact of exposure to other medications used to treat RA, either alone or in combination, on the risk of perforation. To address these gaps in knowledge, the present study analyzed a large administrative database to estimate the incidence of GI perforation among RA patients and to identify RA and non-RA related risk factors for both upper and lower GI perforations.
METHODS
Data sources
The study analyzed data derived from the MarketScan® Commercial Claims and Encounters (Thomson Reuters; www.thomsonreuters.com) database and the Medicare Supplemental and Coordination of Benefits database for the period January 1, 2001 to June 30, 2009. These databases provide information about inpatient, outpatient, and outpatient prescription drug experience of approximately 94.1 million people of all ages covered under a variety of fee-for-service, point-of-service, and capitated benefit plans. About 7.7 million of the covered lives are from the Medicare database, which consists of persons with Medicare coverage with supplemental employer-funded coverage. These databases are derived from employer health insurance plans and have been widely used for diverse epidemiologic and health economic studies, including studies of RA.15–19
Population selection
Patients included in the analysis were 18 years of age or older and had at least two non-diagnostic inpatient or outpatient medical claims (e.g., claims from physician evaluation and management) with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for RA(714.0x or 714.3x). The RA claims had to occur between January 1, 2002 and December 31, 2008 and were required to be for services on different days—30 to 365 days apart. In addition, patients had a minimum of 12 months of continuous eligibility prior to the ‘index date,’ defined as the second date of RA diagnosis. If a patient did not have 12 months of continuous enrollment prior to their second eligible RA diagnosis, the index date was moved forward in time until the 12-month continuous enrollment requirement was met. Patients were also required to have continuous medical and pharmacy coverage for the duration of their study eligibility.
Exclusion criteria included patients with malignant GI cancer or hospitalization for GI perforation in the 12 months preceding the index date. Patients were followed until death, occurrence of the first GI perforation, disenrollment from the study database, or study end.
Study outcome measures
The outcome of interest was hospitalization with a non-traumatic upper or lower GI perforation. The number and proportion of patients with a GI perforation, the rate per unit of person-time observed, and time to the perforation were reported. We considered upper and lower GI perforations as a single entity for two reasons: 1) the larger number of events gave us greater statistical power to identify risk factors, and 2) based on current and available evidence, both upper and lower GI complications have been linked to use of NSAIDs, aspirin, glucocorticoids, age, gender, etc.—all of which were factors of interest.
Two definitions of GI perforation were evaluated. The first definition, intended to be more sensitive, included any inpatient admission with evidence of perforation based on 1) the presence of the word perforation in the following ICD-9-CM diagnosis descriptions: esophageal rupture; gastric, duodenal, peptic or gastrojejunal ulcers; appendicitis; and GI perforation of an unspecific location in the large intestine, or 2) an ICD-9-CM diagnosis of diverticulitis, diverticulosis, or ischemic colitis plus a Current Procedural Terminology (CPT) code for suture or resection of the small or large intestine (Table 1). This sensitive GI perforation definition was our primary study outcome. A second, more specific definition was also used. This definition only included inpatient admissions with evidence of perforation based on the presence of the word perforation in ICD-9-CM diagnosis descriptions for esophageal rupture; gastric, duodenal, peptic or gastrojejunal ulcers; and unspecified GI perforation. The specific GI perforation definition did not include cases of appendicitis, diverticulitis, diverticulosis, or ischemic colitis associated with surgical GI procedures. The definitions of GI perforation were based on a validated claims-based algorithm. The validation study for the algorithm evaluated the medical charts of 92 RA patients identified by the algorithm as having a GI perforation and showed the positive predictive value (PPV) of the algorithm to be 94% (95% CI, 86–98) in identifying confirmed GI perforations.20 In the validation study, the study algorithm initially included 1) ICD-9 codes with perforation language and 2) ICD-9 codes without perforation language + a CPT code for confirmatory surgery; however, no cases in the validation sample were identified by the latter method due to limitations in the data source. Given this result, the acknowledged constraint, and additional concerns about the inclusion of perforated appendix, our study used the full study algorithm in order to provide the most sensitive perforation definition but also evaluated the more specific definition that was better supported by the methodology of the published perforation validation study.
Table 1.
Frequency1 of GI Perforation During Follow-up By Location
| Perforation Location and Subtype | N | % of Total Events | ICD-9-CM Diagnosis Code |
|---|---|---|---|
| Upper GI Tract | |||
| Esophageal Perforations | |||
| Esophageal Perforation | 11 | 1.6% | 530.4 |
| Gastroduodenal Perforations | |||
| Gastric Ulcer | 55 | 7.8% | 531.10, 531.11, 531.20, 531.21, 531.50, 531.51, 531.60, 531.61 |
| Duodenal Ulcer | 54 | 7.8% | 532.10, 532.11, 532.20, 532.21, 532.50, 532.51, 532.60, 532.61 |
| Peptic Ulcer | 17 | 2.4% | 533.10, 533.11, 533.20, 533.21, 533.50, 533.51, 533.60, 533.61 |
| Lower GI Tract | |||
| Perforations of the Small Intestine | |||
| Gastrojejunal Ulcer | 6 | 0.9% | 534.10, 534.11, 534.20, 534.21, 534.50, 534.51, 534.60, 534.61 |
| Diverticulosis2 | 3 | 0.5% | 562.00, 562.01 |
| Diverticulitis2 | 2 | 0.3% | 562.02, 562.03 |
| Perforations of the Large Intestine | |||
| Appendicitis | 140 | 19.7% | 540 |
| Ischemic Colitis3,4 | 24 | 3.4% | 557.0, 557.1, 557.9 |
| Diverticulosis2 | 94 | 13.2% | 562.10, 562.11 |
| Diverticulitis2 | 180 | 25.4% | 562.12, 562.13 |
| Unspecified Perforations | |||
| Location Unspecified | 244 | 34.4% | 569.83 |
GI, gastrointestinal; ICD-9-CM, International Classification of Diseases, Ninth Edition, Clinical Modification
Rows are not additive as patients may have multiple diagnoses on the same day (e.g., one patient with ICD-9-CM codes 530.4 and 531.10 classified as having a single ‘upper GI tract’ perforation spanning both the esophageal and gastric locations);
Requires evidence of confirmatory surgery via a professional claim with CPT code 44602, 44603, 44120, 44121, 44125, 44130, 44202, or 44203;
Requires evidence of confirmatory surgery via a professional claim with CPT code 44604, 44605, 44140, 44145, 44204, or 44205;
ICD-9-CM codes 557.0x, 557.1x and 557.9x are not specific to ischemic colitis and may include other diagnoses
Baseline characteristics and exposures of interest
Patient demographics and clinical characteristics were captured as of the study index date. Two measures of baseline comorbidity were calculated: the Deyo-adapted Charlson Comorbidity Index (CCI) and baseline medical and pharmaceutical expenditure.
Exposure to systemic medications used to treat RA was also evaluated. Medication classes analyzed include the following groups: methotrexate (MTX), anti-tumor necrosis factor (anti-TNF) biologics, other biologics (e.g., anakinra, abatacept, rituximab), glucocorticoids, prescription NSAIDs, and other DMARDs (e.g., hydroxychloroquine, gold compounds, sulfasalazine). Patients with multiple RA medication exposures were assigned a grouping based on the following mutually exclusive hierarchy: biologics (anti-TNF and other) [with or without non-biologic DMARDs] → MTX [with or without other non-biologic DMARDs] → all other DMARDs excluding MTX → none of these. Use of a medication hierarchy allowed us to explicitly evaluate possible interactions between medications. The medication hierarchy was established based upon clinical relevance in RA treatment and informed by the degree of hypothesized immunosuppression. Use of the over the counter medications, including NSAIDS, was not captured in this data source. Each of these four exposure groups was also analyzed with glucocorticoids, yielding a total of eight mutually exclusive categories. Exposure to NSAIDs was measured independently of the hierarchical RA medication groups.
Several time-varying covariates were measured both during the baseline period and follow-up (updated daily), including the occurrence of diverticulitis or diverticulosis, treatment with RA medications (biologics, MTX, and other DMARDs, with or without concurrent glucocorticoids), and exposure to NSAIDs. Time on drug and the time-varying risk factors were computed for each day of the study. Patients with a history of diverticulitis or diverticulosis at baseline were flagged as having the condition on the first day of follow-up. Patients without a baseline history of these conditions but who developed them while under follow-up were identified. For these individuals, the assigned date of the diagnosis of diverticulitis and diverticulosis was ‘lagged’ by 90 days to avoid capturing these conditions only at or near the time of the perforation event.
Medication exposure was characterized as current vs. non-current on each day of the study period and determined by the prescription fill date plus the number of days’ supply of medication. A fixed extension period of 60 days was applied to all pharmacy-based prescriptions. Exposure to facility-based injectable or infused medications was based on the administration date plus a clinically relevant exposure window specific to each medication (56 days for infliximab, 30 days for abatacept, 180 days for rituximab).
Statistical analysis
Baseline characteristics of patients with and without GI perforation events during the follow-up period were compared for demographic and risk factors of clinical interest. Chi-square tests were used to assess the statistical significance of categorical variables; t-tests and ANOVA were used for continuous variables.
A Cox model with time varying covariates assessed the impact of fixed and time-varying characteristics on the risk of GI perforation. Fixed explanatory variables included age, gender, census region, population density, and the Deyo-adapted CCI score. Time-varying covariates included exposure to RA medications, diverticulitis, and diverticulosis without diverticulitis. Study RA medication categories in the model were biologics, MTX, and other DMARDs, each with and without glucocorticoids. NSAIDs were assessed independently.
Additional analyses evaluated a possible interaction between biologic use (in the absence of glucocorticoids) with both age (dichotomized as <65, 65+) and history of diverticulitis. This assessed whether the risk associated with biologics varied for these two patient subgroups. Conventional p values (< 0.05) were used to evaluate the significance of the associated interaction terms.
Finally, we performed a sensitivity analysis in which we censored unspecified and upper GI events at the perforation event date. We then used the remainder of the lower GI perforations as the outcome to see if the risk factor hazard ratios were comparable.
RESULTS
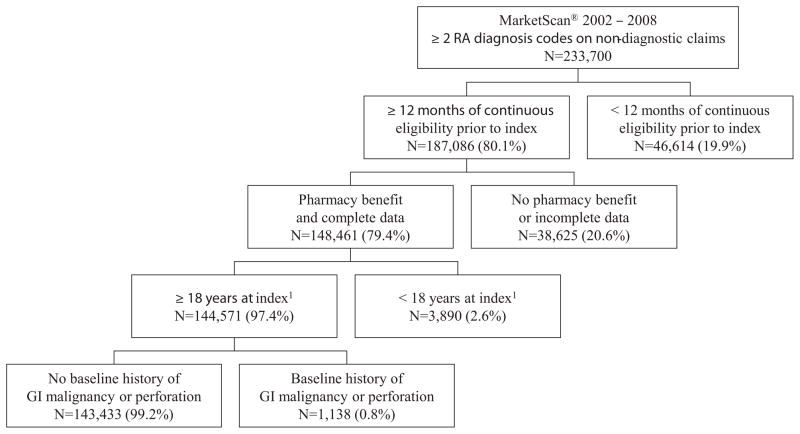
A total of 143,433 RA patients were included in the analysis (Figure 1). The most common reasons for exclusion were inadequate continuous eligibility (19.9%) or lack of data availability (16.5%) either because of incomplete data or lack of a pharmacy benefit. Patients were followed, on average (SD) for 34.8 (22.9) months, yielding 409,587 person-years (PYs) of follow-up.
Figure 1.
Study Accrual and Attrition
Perforations in the lower GI tract were most frequent, representing 83% of all cases (Table 1). The unadjusted GI perforation rate per 1000 PYs was 1.70 overall, 1.44 in the lower GI tract, and 0.30 in the upper GI tract. Upper GI tract events were largely associated with gastric and duodenal ulcers while lower GI events were mostly unspecified location perforations and those associated with diverticulitis and appendicitis.
Demographic and clinical characteristics
Approximately three-quarters of the sample population was female, with a mean (SD) age of 57.7 (14.1) years. Compared to US Census population estimates for the study period, the study population was notably concentrated in the Midwest (28.1% vs. 22.1%) and underrepresented in the Northeast (9.8% vs. 18.5%).
At baseline, 91% of patients received some form of pharmacotherapy for RA. The most commonly used medications for RA included prescription NSAIDs (56%), glucocorticoids (55%), MTX (43%), a DMARD other than MTX (34%), and biologics (18%). The study population had a median (IQR) Deyo-adapted CCI score of 1.0 (1.0–2.0), consistent with modest levels of comorbidity.
Table 2 shows baseline demographic and clinical characteristics of patients by perforation status. Compared to patients not experiencing a GI perforation, patients with a GI perforation were older (mean age 62.0 vs. 57.6, P<0.0001), more likely to have Medicare as a primary payer (43.0% vs. 30.4%, P<0.0001), more likely to reside in a rural area (22.8% vs. 18.3%, P=0.002), and more likely to reside in the Midwest (33.3% vs. 28.0%, P=0.002). Mean (SD) length of follow-up for patients who had a perforation was also lower (24.2 vs. 34.8 months, P<0.0001). At baseline, patients who subsequently experienced a GI perforation had an increased likelihood of exposure to glucocorticoids (without NSAIDs: 25.3% vs. 21.3%, P=0.01; with NSAIDs: 40.5% vs. 34.1%, P<0.01) and non-methotrexate DMARDs (38.5% vs. 33.7%, P<.00001), higher levels of overall comorbidity (CCI score: 1.7 vs. 1.5, P<0.001), an increased likelihood of GI conditions (diverticulitis, diverticulosis, esophageal or GI hemorrhage, and noninfectious gastroenteritis/colitis), and higher annual medical expenditures ($11,543 vs. $9,031, P<0.001) than patients not experiencing a GI perforation.
Table 2.
Baseline Demographic Characteristics by GI Perforation Status at Follow-up
| Patients without N=142,737 |
Perforation | Patients with N=696 |
Perforation | p-value | |
|---|---|---|---|---|---|
| Female Gender (n, %) | 106,714 | 74.80% | 506 | 72.70% | 0.21 |
| Age (mean, SD), years | 57.6 | 14.1 | 62 | 12.9 | < .01 |
| Insurance Plan Type (n, %) | |||||
| Commercial | 99,364 | 69.60% | 397 | 57.00% | < .01 |
| Medicare | 43,373 | 30.40% | 299 | 43.00% | < .01 |
| Geographic Region (n, %) | |||||
| Northeast | 13,935 | 9.80% | 60 | 8.60% | 0.31 |
| Midwest | 40,013 | 28.00% | 232 | 33.30% | < .01 |
| South | 56,305 | 39.40% | 262 | 37.60% | 0.33 |
| West | 31,532 | 22.10% | 137 | 19.70% | 0.13 |
| Unknown | 952 | 0.70% | 5 | 0.70% | 0.87 |
| Population Density (n, %) | |||||
| Rural | 26,111 | 18.30% | 159 | 22.80% | < .01 |
| Urban | 115,792 | 81.10% | 532 | 76.40% | < .01 |
| Unknown | 834 | 0.60% | 5 | 0.70% | 0.64 |
| Length of Follow-up [months] (mean, SD) | 34.8 | 22.9 | 24.2 | 19.2 | <0 .01 |
| Aggregate Comorbidity (mean, SD) | |||||
| Deyo-adapted Charlson Comorbidity Index | 1.5 | 1.1 | 1.7 | 1.2 | < 0.01 |
| Baseline Expenditure ($) | 9,031 | 19,856 | 11,543 | 22,212 | < 0.01 |
| RA Medication History [any use] (n, %) | |||||
| Methotrexate | 61,369 | 43.00% | 285 | 41.00% | 0.28 |
| Biologic – TNF | 24,350 | 17.10% | 119 | 17.10% | 0.98 |
| Biologic – Other | 810 | 0.60% | 3 | 0.40% | 0.63 |
| All Other DMARDs1 | 48,025 | 33.70% | 268 | 38.50% | < 0.01 |
| NSAID without Glucocorticoid | 31,581 | 22.10% | 137 | 19.70% | 0.12 |
| Glucocorticoid without NSAID | 30,413 | 21.30% | 176 | 25.30% | 0.01 |
| NSAID and Glucocorticoid | 48,632 | 34.10% | 282 | 40.50% | < 0.01 |
| No NSAID or Glucocorticoid | 32,111 | 22.50% | 101 | 14.50% | < 0.01 |
| No Prescription RA medication | 13,084 | 9.20% | 41 | 5.90% | < 0.01 |
| Any H2, PPI or Cytotec/Misoprostol (n, %) | 42,115 | 29.50% | 271 | 38.90% | < 0.01 |
| Other GI Disturbance2 (n, %) | 8,026 | 5.60% | 68 | 9.80% | < 0.01 |
| Diverticulitis | 401 | 0.30% | 20 | 2.90% | < 0.01 |
| Diverticulosis without Diverticulitis | 583 | 0.40% | 10 | 1.40% | < 0.01 |
| Esophageal or GI Hemorrhage | 1,253 | 0.90% | 13 | 1.90% | < 0.01 |
| Noninfectious Gastroenteritis/Colitis3 | 803 | 0.60% | 10 | 1.40% | < 0.01 |
includes azathioprine, chloroquine and hydroxychloroquine, cyclosporin, D-penicillamine, leflunomide, gold compounds, and sulfasalazine;
excludes perforation;
unspecified
Perforation Incidence Rates
During the follow-up period, 710 GI perforations occurred in 696 patients (14 patients had both upper and lower GI perforations). The overall incidence of GI perforation in this study was low, with 0.5% of patients hospitalized with a perforation during follow-up, yielding a rate of 1.70 events per 1000 PYs (95% CI, 1.58–1.83) [Table 3a]. This rate was 0.87 events per 1000 PYs (95% CI, 0.78–0.96) when the more specific definition of perforation was used.
Table 3a.
Incidence of GI perforation, by medication exposure, in total study population
| Medication Exposure Group | Sensitive GI Perforation Definition n=143,433 |
Specific GI Perforation Definition n=143,433 |
||
|---|---|---|---|---|
| IR/1000 PYs | 95% CI | IR/1000 PYs | 95% CI | |
| Biologics with glucocorticoids | 1.87 | 1.46 – 2.35 | 0.91 | 0.63 – 1.26 |
| Biologics without glucocorticoids | 1.02 | 0.80 – 1.29 | 0.47 | 0.32 – 0.66 |
| Methotrexate with glucocorticoids | 2.24 | 1.82 – 2.74 | 1.25 | 0.94 – 1.63 |
| Methotrexate without glucocorticoids | 1.08 | 0.86 – 1.35 | 0.47 | 0.33 – 0.66 |
| All other DMARDs1 with glucocorticoids | 3.03 | 2.34 – 3.85 | 1.65 | 1.16 – 2.29 |
| All other DMARDs1 without glucocorticoids | 1.71 | 1.34 – 2.16 | 0.66 | 0.44 – 0.96 |
| Glucocorticoids without DMARDs or biologics | 2.86 | 2.27 – 3.56 | 2.15 | 1.64 – 2.76 |
| No DMARDs, biologics or glucocorticoids | 1.68 | 1.44 – 1.96 | 0.81 | 0.64 – 1.01 |
| Overall | 1.70 | 1.58 – 1.83 | 0.87 | 0.78 – 0.96 |
DMARD, disease modifying antirheumatic drug; IR, incidence rate; NSAID, non-steroid anti-inflammatory drug; PYs, person-years; Rx, prescription; 95% CI, 95% confidence interval;
azathioprine, chloroquine, hydroxychloroquine, cyclosporine, D-penicillamine, leflunomide, sulfasalazine, gold compounds
The unadjusted rate of GI perforation per 1000 PYs in patients with diverticulitis was 22.98 (95% CI, 19.16–27.35) compared to 1.41 (95% CI, 1.29–1.53) in those without such history [Table 3b]. Conversely, the unadjusted rate of GI perforation per 1000 PYs in patients with diverticulosis was 1.30 (95% CI, 0.65–2.33), which was not higher than the rate among patients without the disorder (data not shown). The highest rates of GI perforation (per 1000 PYs) were observed in patients with a history of diverticulitis and exposure to DMARDs (other than MTX) and glucocorticoids (41.0; 95% CI, 22.9–67.6), exposure to other DMARDs without glucocorticoids (33.1; 95% CI, 18.5–54.6), and exposure to biologics with glucocorticoids (32.0; 95% CI, 18.64–51.22). The addition of glucocorticoids to each RA medication combination, with the exception of MTX, was associated with a higher incidence of perforation ranging from 24% (during exposure to other DMARDs) to 95% (during exposure to biologics) among patients with a history of diverticulitis.
Table 3b.
Incidence of GI perforation, by medication exposure, in patients with and without history of diverticulitis and exposure to prescription NSAIDs
| Medication Exposure Group | (+) History of Diverticulitis n=1,802 |
(−) History of Diverticulitis n=141,631 |
(+) Rx NSAID Exposure n=88,899 |
(−) Rx NSAID Exposure n=54,534 |
||||
|---|---|---|---|---|---|---|---|---|
| IR/1000 PYs | 95% CI | IR/1000 PYs | 95% CI | IR/1000 PYs | 95% CI | IR/1000 PYs | 95% CI | |
| Biologics w/glucocorticoids | 31.99 | 18.64 – 51.22 | 1.44 | 1.09 – 1.88 | 2.19 | 1.52 – 3.04 | 1.64 | 1.15 – 2.26 |
| Biologics w/o glucocorticoids | 16.42 | 8.19 – 29.37 | 0.87 | 0.66 – 1.12 | 1.28 | 0.88 – 1.79 | 0.87 | 0.61 – 1.20 |
| MTX w/glucocorticoids | 21.77 | 11.90 – 36.52 | 1.95 | 1.55 – 2.42 | 2.73 | 2.01 – 3.62 | 1.91 | 1.41 – 2.52 |
| MTX w/o glucocorticoids | 23.92 | 13.67 – 38.84 | 0.87 | 0.67 – 1.11 | 1.41 | 1.03 – 1.89 | 0.83 | 0.58 – 1.16 |
| All other DMARDs1 w/glucocorticoids | 40.99 | 22.94 – 67.61 | 2.38 | 1.77 – 3.13 | 3.57 | 2.43 – 5.07 | 2.67 | 1.86 – 3.71 |
| All other DMARDs1 w/o glucocorticoids | 33.12 | 18.53 – 54.62 | 1.36 | 1.03 – 1.77 | 1.75 | 1.19 – 2.47 | 1.69 | 1.19 – 2.31 |
| Glucocorticoids w/o DMARDs, biologics | 20.04 | 10.67 – 34.27 | 2.45 | 1.90 – 3.12 | 3.37 | 2.25 – 4.83 | 2.64 | 1.96 – 3.47 |
| No DMARDs, biologics, glucocorticoids | 16.83 | 11.00 – 24.67 | 1.44 | 1.21 – 1.70 | 1.56 | 1.09 – 2.15 | 1.73 | 1.44 – 2.05 |
| Overall | 22.98 | 19.16 – 27.35 | 1.41 | 1.29 – 1.53 | 1.93 | 1.71 – 2.16 | 1.57 | 1.42 – 1.73 |
DMARD, disease modifying antirheumatic drug; IR, incidence rate; MTX, methotrexate; NSAID, non-steroid anti-inflammatory drug; PYs, person-years; Rx, prescription; 95% CI, 95% confidence interval;
azathioprine, chloroquine, hydroxychloroquine, cyclosporine, D-penicillamine, leflunomide, sulfasalazine, gold compounds
The rate of GI perforation during exposure to NSAIDs, independent of exposure to other study RA medications, was 1.93 (95% CI, 1.71–2.16) per 1000 PYs. When NSAIDs were added to most other study RA medication groups, there was an increased rate of GI perforation of approximately 0.4–0.9 per 1000 PYs across the various rows in Table 3b (P=0.007 comparing overall rate with and without NSAID exposure).
Multivariate analysis results
Results of the Cox model indicated that patients with a history of diverticulitis were at the greatest risk of GI perforation (HR, 14.5; 95% CI, 11.8–17.7; P<0.0001; Table 4). Although the magnitude of the risk decreased, diverticulitis remained a significant risk factor when the specific GI perforation definition was used (HR 3.9; 95% CI, 2.5–5.9; P<0.0001). Other clinically significant factors with both GI perforation definitions were use of glucocorticoids, use of NSAIDs, increasing age, and higher levels of comorbidity. The HR for diverticulosis without diverticulitis was not significant for either of the outcome definitions. There were no significant interactions between biologic use without steroids and age or a history of diverticulitis.
Table 4.
Relative Risk of GI Perforation during Follow-up
| Risk Factor | Sensitive GI Perforation Definition | Specific GI Perforation Definition | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Age | ||||
| Age 18 to 39 | -- | -- | -- | -- |
| Age 40 to 64 | 1.6 | 1.1 – 2.4 | 2.1 | 1.1 – 3.9 |
| Age 65 or older | 2.1 | 1.4 – 3.1 | 3.6 | 1.9 – 6.9 |
| Gender | ||||
| Male | -- | -- | -- | -- |
| Female | 0.9 | 0.8 – 1.1 | 1.0 | 0.8 – 1.2 |
| Population Density | ||||
| Rural or Suburban | -- | -- | -- | -- |
| Urban | 0.8 | 0.7 – 0.9 | 0.9 | 0.7 – 1.1 |
| Census Region | ||||
| Northeast | -- | -- | -- | -- |
| Midwest | 1.0 | 0.8 – 1.4 | 1.0 | 0.6 – 1.4 |
| Southern | 1.1 | 0.8 – 1.4 | 1.1 | 0.7 – 1.7 |
| Western | 1.1 | 0.8 – 1.4 | 1.2 | 0.8 – 1.8 |
| History of Diverticulitis | ||||
| No | -- | -- | -- | -- |
| Yes | 14.5 | 11.8 – 17.7 | 3.9 | 2.5 – 5.9 |
| History of Diverticulosis | ||||
| No | -- | -- | -- | -- |
| Yes | 0.8 | 0.5 – 1.5 | 0.8 | 0.4 – 1.6 |
| Deyo-adapted Charlson Comorbidity Index Score | ||||
| 0 | -- | -- | -- | -- |
| Score [n] | 1.1 | 1.0 – 1.2 | 1.2 | 1.1 – 1.3 |
| During RA medication exposure (time-varying)2 | ||||
| Methotrexate without glucocorticoids | -- | -- | -- | -- |
| Glucocorticoids w/o DMARDs or biologics | 2.2 | 1.6 – 3.1 | 4.0 | 2.6 – 6.2 |
| Other DMARDs1 with glucocorticoids | 2.5 | 1.8 – 3.5 | 3.2 | 2.0 – 5.2 |
| MTX with glucocorticoids | 1.9 | 1.4 – 2.5 | 2.5 | 1 6 – 3.8 |
| Biologics with glucocorticoids | 1.8 | 1.3 – 2.4 | 2.1 | 1.3 – 3.3 |
| No DMARDs, glucocorticoids, biologics | 1.5 | 1.2 – 2.0 | 1.8 | 1.2 – 2.7 |
| Other DMARDs1 w/o glucocorticoids | 1.6 | 1.1 – 2.2 | 1.4 | 0.8 – 2.3 |
| Biologics w/o glucocorticoids | 1.0 | 0.8 – 1.5 | 1.1 | 0.7 – 1.8 |
| During NSAID exposure (time-varying)3 | ||||
| No | -- | -- | -- | -- |
| Yes | 1.4 | 1.2 – 1.6 | 1.8 | 1.4 – 2.2 |
CI, confidence interval; DMARD, disease modifying antirheumatic drug; GI, gastrointestinal; HR, hazard ratio; MTX, methotrexate; NSAID, non-steroid anti-inflammatory drug;
azathioprine, chloroquine, hydroxychloroquine, cyclosporine, D-penicillamine, leflunomide, sulfasalazine, gold compounds;
With or without NSAIDs;
With or without DMARDs, glucocorticoids, biologics
Patients with no exposure to DMARDs, glucocorticoids, or biologics had a higher risk for GI perforation than patients with exposure to methotrexate alone. We conducted additional analysis on those patients (n=8,032, 6%) who had no medical or pharmacy claim evidence of any RA specific prescription medication during follow-up and found they were significantly older (p<0.0001), more likely to be 75+ years of age, to have higher CCI scores, to have each of the comorbid conditions evaluated during baseline, to have shorter follow-up windows, and to have higher baseline expenditures. These findings are consistent with channelling of older, higher risk patients away from methotrexate and other commonly used RA therapies.
In the sensitivity analysis, the exclusion of upper GI perforations and perforations with an unspecified location decreased the number of GI perforation events from 696 to 374 and the rate of GI perforations from 1.70 to 0.91 (95% CI, 0.82–1.01). Although the statistical significance of several variables was no longer present in this analysis, use of other DMARDs with glucocorticoids remained significant (HR, 2.0; 95% CI, 1.3–3.1) and the risk associated with history of diverticulitis increased (HR, 32.4; 95% CI, 25.5–41.2). The HR for diverticulosis without diverticulitis was not significant.
DISCUSSION
Findings from this study suggest that the risk of GI perforation is low in RA patients and perforation occurs more frequently in the lower GI tract than in the upper GI tract. Results of the multivariate analysis showed that the most significant factors associated with increased risk of GI perforation were history of diverticulitis, use of glucocorticoids, exposure to NSAIDs, increasing age, and higher levels of comorbidity. Diverticulosis, without diverticulitis, was not a risk factor for perforation, nor was use of the biologic agents that were studied (predominantly anti-TNF therapy).
Our findings are consistent with results from other studies reporting perforation rates among RA patients. A similar but smaller administrative database analysis involving 40,841 RA patients found among the 37 patients hospitalized for GI perforation, 19 (51.4%) were currently being treated with glucocorticoids at the time of the event.21 Similar to our findings, risk factors for GI perforation included diverticulitis and current treatment with glucocorticoids, with or without NSAIDs. The MEDAL (Multinational Etoricoxib and Diclofenac Arthritis Long-term) program, comparing use of etoricoxib or diclofenac in >34,000 patients with OA or RA, reported upper GI perforation rates of 0.2–0.4 per 1000 PYs—similar to our finding of 0.30 upper GI perforations per 1000 PYs.22
We tested the sensitivity of these results to our definition of GI perforation, acknowledging some clinicians would exclude GI perforation events associated with appendicitis and those with diverticulitis/diverticulosis or ischemic colitis diagnoses and GI surgery but without explicit mention of perforation. Removal of these types of events resulted in a reduction of the unadjusted GI perforation rate (1.70 vs. 0.87 per 1000 PYs), a reduction in the number of GI perforation events (696 vs. 356), and a lower HR for GI perforation associated with diverticulitis (14.5 vs. 3.9).
Given the majority of events were lower GI perforations, we felt the addition of upper GI perforations and unspecified perforations should not change, but may dilute, our study findings. To test this, we performed a sensitivity analysis in which we censored unspecified and upper GI events as of the event dates. With this most stringent GI perforation definition, statistical significance was lost for many variables. This may have been a function of the decreasing number of events as this definition was restrictive (n=374) or due to better precision in evaluating risk factors for lower GI perforations. Variables that remained significant included history of diverticulitis; other DMARDs with glucocorticoids, biologics with glucocorticoids, methotrexate with glucocorticoids; and other DMARDs without glucocorticoids.
Among the key strengths of this study is the data source. Large claims databases provide insight into the routine practice of medical care and allow for the analysis of relatively uncommon adverse events in specific patient populations. Furthermore, this particular database had a relatively large proportion of older patients. Of the 696 RA patients who experienced a GI perforation, 294 (42.2%) were > 65 years of age. Finally, although we did not have access to medical records, our case definition for GI perforation was based on a validated and high performing claims algorithm, shown to have a PPV of 94% among RA patients.20
In interpreting our findings, several factors should be considered. While the present study was based on a large and diverse sample of patients with RA, it is not a random sample and medical records were not available to supplement or validate information. The MarketScan Databases comprise employer-sponsored coverage for active employees, dependents, and retirees, and therefore did not include patients with other forms of insurance (Medicaid, military, self-insured) or the uninsured. Thus, the generalizability of these findings may be limited to relatively healthier patients, although this does not compromise the internal validity of our results.
Another limitation is that we did not conduct a new user design and follow-up time began based upon the first date that patients were observable in the health plan and met eligibility criteria. We used a validated algorithm to identify GI perforation cases, although it is possible that cases were misclassified, perhaps more likely for outcomes using our more sensitive definition that were associated with nonspecific ICD-9-CM diagnosis codes (e.g., diverticulitis) with a CPT code for suture or resection. In the construction of medication exposure windows, prescription medications were presumed to be taken based upon their pharmacy fill dates. We assigned clinically relevant exposure windows or a grace period to all medications. To the extent that this window could have been too short or long, we may have under- or overstated exposure. Also, information was not available for over the counter (OTC) medications such as OTC NSAIDs and OTC GI medications (e.g., histamine receptor blockers and proton pump inhibitors). Furthermore, glucocorticoid use was characterized only as current or not current in this study, not by daily dose or cumulative exposure. Finally, we had an insufficient sample size to be able to compare the risks for perforation between anti-TNF biologics versus those biologics with different mechanisms of action.
CONCLUSION
GI perforation is a rare but serious condition that affects patients with RA, most frequently in the lower GI tract, and was observed among patients exposed to every combination of RA therapies. Our retrospective analysis of a large US database showed that the main risk factors for GI perforations in RA patients include age, history of diverticulitis, and use of glucocorticoids and prescription NSAIDs. Reassuringly, diverticulosis in the absence of diverticulitis and biologic use (predominantly anti-TNF therapy, in this population) did not appear to be risk factors for GI perforation. Clinicians should be aware of risk factors for GI perforation when managing patients with RA, especially when prescribing medications such as systemic glucocorticoids or NSAIDs to older patients and those with a history of diverticulitis.
SIGNIFICANCE AND INNOVATION.
Our retrospective analysis of a large US health plan database showed that the main risk factors for GI perforation in RA patients included age, history of diverticulitis, and use of glucocorticoids and prescription NSAIDs.
Using a sensitive definition for GI perforation, the overall rate of perforation was 1.70 per 1000 PYs and was 0.87 per 1000 PYs when a more specific definition of perforation was used.
Biologic use (predominantly anti-TNF therapy) was not a significant risk factor for perforation.
Acknowledgments
The authors thank Kristin A. Hanson, PharmD, MS and Jyoti S. Nandi, MD, PhD who provided medical writing services on behalf of United BioSource Corporation, Bethesda, Maryland, USA.
FUNDING
Funding for this study, analysis, and manuscript preparation was provided by Genentech, Inc. The sponsor had no role in the study design, the collection, analysis, or interpretation of data, or in writing the report or deciding to submit the report for publication. The statements contained in this paper are solely those of the authors and no endorsement by Genentech should be inferred or implied. No authors received funding for preparation of the manuscript.
Footnotes
COMPETING INTERESTS
Dr. Curtis receives support from the NIH (AR053351) and AHRQ (R01HS018517). He has received grant/research support and been a consultant for UCB, Centocor, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abbott, and Genentech, a member of the Roche group. Angel Lanas has been a member of the Adjudication Committee of the international multicenter CONDOR trial sponsored by Pfizer and a consultant to AstraZeneca and Genentech, a member of the Roche group. Ani John is an employee of Genentech, a member of the Roche group. David Johnson and Kathy Schulman are consultants to Genentech, a member of the Roche group.
References
- 1.Cafardi J, Rakatansky H, Alarcón G. Gastrointestinal manifestations of rheumatoid arthritis. In: Asherson R, Font J, Ramos-Casals M, Rodés J, editors. Handbook of Systemic Autoimmune Diseases. Vol. 8. Amsterdam: Elsevier BV; 2008. pp. 109–117. [Google Scholar]
- 2.Masso Gonzalez EL, Patrignani P, Tacconelli S, Garcia Rodriguez LA. Variability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis Rheum. 2010;62(6):1592–1601. doi: 10.1002/art.27412. [DOI] [PubMed] [Google Scholar]
- 3.Saag KG, Koehnke R, Caldwell JR, Brasington R, Burmeister LF, Zimmerman B, Kohler JA, Furst DE. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96(2):115–123. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 4.Straube S, Tramer MR, Moore RA, Derry S, McQuay HJ. Mortality with upper gastrointestinal bleeding and perforation: effects of time and NSAID use. BMC Gastroenterol. 2009;9:41. doi: 10.1186/1471-230X-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humes DJ, Fleming KM, Spiller RC, West J. Concurrent drug use and the risk of perforated colonic diverticular disease: a population-based case-control study. Gut. 2011;60(2):219–224. doi: 10.1136/gut.2010.217281. [DOI] [PubMed] [Google Scholar]
- 6.Goh H, Bourne R. Non-steroidal anti-inflammatory drugs and perforated diverticular disease: a case-control study. Ann R Coll Surg Engl. 2002;84(2):93–96. [PMC free article] [PubMed] [Google Scholar]
- 7.Strate LL, Liu YL, Huang ES, Giovannucci EL, Chan AT. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140(5):1427–1433. doi: 10.1053/j.gastro.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet. 2010;376(9736):173–179. doi: 10.1016/S0140-6736(10)60673-3. [DOI] [PubMed] [Google Scholar]
- 9.Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective--1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol Suppl. 1998;51:8–16. [PubMed] [Google Scholar]
- 10.Chan FK, Cryer B, Goldstein JL, Lanas A, Peura DA, Scheiman JM, Simon LS, Singh G, Stillman MJ, Wilcox CM, Berger MF, Breazna A, Dodge W. A novel composite endpoint to evaluate the gastrointestinal (GI) effects of nonsteroidal antiinflammatory drugs through the entire GI tract. J Rheumatol. 2010;37(1):167–174. doi: 10.3899/jrheum.090168. [DOI] [PubMed] [Google Scholar]
- 11.Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, Bujanda L, Castro M, Munoz M, Rodrigo L, Calvet X, Del-Pino D, Garcia S. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104(7):1633–1641. doi: 10.1038/ajg.2009.164. [DOI] [PubMed] [Google Scholar]
- 12.Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, Ponce M, Quintero E, Perez-Aisa MA, Gisbert JP, Bujanda L, Castro M, Munoz M, Del-Pino MD, Garcia S, Calvet X. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33(5):585–591. doi: 10.1111/j.1365-2036.2010.04563.x. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284(10):1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, Geis GS. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123(4):241–249. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Gu N, Yan, Huang X-y, Fox KM, Patel VD, Baumgartner SW, Chiou C-F. Claims Data Analysis of Dosing and Cost of TNF Antagonists. American Journal of Pharmacy Benefits. 2010;2(6):351–359. [Google Scholar]
- 16.Briesacher B, Kamal-Bahl S, Hochberg M, Orwig D, Kahler KH. Three-tiered-copayment drug coverage and use of nonsteroidal anti-inflammatory drugs. Arch Intern Med. 2004;164(15):1679–1684. doi: 10.1001/archinte.164.15.1679. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Carls GS, Panopalis P, Wang S, Gibson TB, Goetzel RZ. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large medicaid population. Arthritis Rheum. 2009;61(6):755–763. doi: 10.1002/art.24545. [DOI] [PubMed] [Google Scholar]
- 18.Nair KV, Tang B, Van Den Bos J, Zhang V, Saseen JJ, Naim A, Rahman M. Categorization of infliximab dose changes and healthcare utilization and expenditures for patients with rheumatoid arthritis in commercially insured and Medicare-eligible populations. Curr Med Res Opin. 2009;25(2):303–314. doi: 10.1185/03007990802598736. [DOI] [PubMed] [Google Scholar]
- 19.Petri H, Maldonato D, Robinson NJ. Data-driven identification of co-morbidities associated with rheumatoid arthritis in a large US health plan claims database. BMC Musculoskelet Disord. 2010;11:247. doi: 10.1186/1471-2474-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis JR, Chen S, Werther W, John A, Johnson D. Validation of ICD-9-CM codes to identify GI perforation events in administrative claims data among hospitalized rheumatoid arthritis patients. Pharmacoepidemiology and Drug Safety. 2011;20:1150–1158. doi: 10.1002/pds.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis JR, Xie F, Chen L, Spettell C, McMahan RM, Fernandes J, Delzell E. The incidence of gastrointestinal perforations among rheumatoid arthritis patients. Arthritis Rheum. 2011;63(2):346–351. doi: 10.1002/art.30107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2007;369(9560):465–473. doi: 10.1016/S0140-6736(07)60234-7. [DOI] [PubMed] [Google Scholar]