Abstract
Purpose
To evaluate the impact of enzyme-inducing antiepileptic drugs (EI-AEDs) on serum antiretroviral (ARV) levels in patients with HIV.
Methods
Data from the U.S. Military HIV Natural History Study were screened to identify participants taking ARVs with EI-AEDs and controls taking ARVs with non enzyme inducing AEDs (NEI-AEDs). The proportion of serum ARV levels below the recommended minimum concentrations (Cmin) was compared between these groups.
Results
ARV levels were available for 10 individuals exposed to 16 intervals on combined ARVs/EI-AEDs (phenytoin and carbamazepine) and for 25 controls exposed to 30 overlap intervals on combined ARVs/NEI-AEDs. The percentage of overlap intervals with ≥1 ARV levels below Cmin was higher in the EI-AED group than in controls (37.5% vs. 23.3%; p=0.124). After excluding intervals associated with serum levels of EI-AEDs below the reference range (n=6), the proportion of intervals with ≥1 ARV level below Cmin was significantly greater among EI-AED recipients (60%) compared to controls (23.3%; p=0.008).
Conclusions
ARV levels below Cmin were more common in participants receiving EI-AEDs, the difference being statistically significant for intervals associated with EI-AED levels within the reference range. These data suggest that, in agreement with current guidelines, EI-AEDs should be avoided in patients receiving ARV therapy.
Keywords: Drug interactions, pharmacokinetics, drug resistence, epilepsy, AIDS
INTRODUCTION
Antiepileptic drugs (AEDs) are commonly used in patients with HIV for associated seizure disorders and painful peripheral neuropathies. The occurrence of seizure disorders is increased among HIV-infected patients, with an incidence of up to 11%(Kellinghaus et al., 2008, Holtzman et al., 1989, Wong et al., 1990). A distal sensory polyneuropathy can also occur in up to 57% of patients with HIV(Cornblath and McArthur, 1988, Ellis et al., McArthur et al., 2005). AEDs are also used to treat other conditions, including migraine and mood disorders(Liedtke et al., 2004).
Evidence-based guidelines for AED selection for people with HIV/AIDS(Birbeck et al., 2012b, Birbeck et al., 2012c) indicate that clinically significant drug interactions can occur when antiretroviral (ARV) agents are combined with enzyme-inducing AEDs (EI-AEDs), including carbamazepine, phenytoin, and phenobarbital. These interactions can have bi-directional effects, resulting in altered serum levels of both EI-AEDs and ARVs. Lower EI-AED levels may lead to reduced efficacy including breakthrough seizures and neuropathic pain. Conversely, a reduction in ARV levels can lead to loss of virologic control, CD4 decline, acquired immune deficiency syndrome (AIDS), and ultimately death. Additionally, reduced efficacy of ARVs can facilitate the development of resistance mutations and increase the risk of transmitting drug-resistant virus.
We previously reported that combined use of ARVs and EI-AEDs led to higher rates of HIV treatment failure compared to use of ARVs in combination with AEDs that are not enzyme-inducing (NEI-AEDs)(Okulicz et al., 2011). To further evaluate and quantify this interaction, we used stored samples from the U.S. Military HIV Natural History Study (NHS) to measure serum levels of ARVs in patients on concurrent therapy with EI-AEDs and in a control group with co-administered NEI-AEDs.
METHODS
Participants were identified from a database of over 5,300 military members, retirees, and beneficiaries 18 years or older with HIV infection enrolled in the NHS since 1986(Weintrob et al., 2008, Marconi et al., 2010). In this IRB-approved study, consented individuals are evaluated at participating United States military treatment facilities approximately every 6 months. Data including demographic characteristics, laboratory data, medication use, and clinical events with medical record confirmation are systematically collected.
As previously described(Okulicz et al., 2011), the NHS database was searched for individuals taking the EI-AEDs phenytoin, carbamazepine, or phenobarbital concurrently with a non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI)-based ARV regimen. To be included, participants were required to be taking the ARV regimen for ≥6 months and the EI-AED for ≥28 consecutive days during that period. This identified 19 participants with 53 EI-AED/ARV overlap periods, which were the focus of the previous study(Okulicz et al., 2011). For the purpose of the current study, we selected overlap periods for which frozen sera were available for drug level determination of the prescribed NNRTIs (either efavirenz or nevirapine) or PIs (lopinavir/ritonavir, atazanavir, or darunavir), and the prescribed EI-AED. Participants with EI-AED or ARV levels below the limit of detection were excluded, due to presumed non-adherence. This identified 16 overlap periods in 10 subjects for the current study, including 5 overlap periods for efavirenz, 3 for nevirapine, and 8 for lopinavir.
For the control group, we applied the same algorithm to select individuals prescribed NEI-AEDs (lamotrigine, zonisamide, levetiracetam, topiramate, gabapentin, tiagabine, or pregabalin) in combination with a NNRTI- or PI-based ARV regimen used by the EI-AED case group. Participants taking valproic acid, an enzyme inhibitor, and oxcarbazepine, a weak enzyme inducer, were excluded. Approximately two controls (as available) were randomly selected per case, within each ARV drug strata (efavirenz, nevirapine, or lopinavir/ritonavir). After exclusion of overlap periods with ≥1 ARV level below detection, a total of 30 overlap periods in 25 subjects were selected, including 12 overlap periods for efavirenz, 3 for nevirapine, and 15 for lopinavir.
To ensure sufficient time for drug-drug interactions to take effect, only serum specimens after 7 days of ARV/AED overlap were eligible. Two specimens from the overlap period were selected for both cases and controls when available, otherwise one specimen was selected. Two specimens were available for the majority of subjects, including 11 of 16 (69%) case periods and 21 of 30 (70%) control periods. All participants were prescribed standard daily doses of ARVs, specifically efavirenz (600mg), nevirapine (400mg), and lopinavir/ritonavir (800mg/200mg). EI-AED doses varied and were captured from the clinical database and chart review when available.
EI-AED and ARV Serum Level Testing
Serum levels of EI-AEDs and ARVs were measured in repository samples stored at -80°F. For EI-AEDs, only carbamazepine and phenytoin were prescribed in cases meeting eligibility criteria, and their levels were measured by the CEDIA Carbamazepine II assay and the ONLINE TDM Phenytoin assay (Roche Diagnostics, Indianapolis, IN). The laboratory reference ranges were 4.0 – 12.0 mcg/mL for carbamazepine and 10.0 – 20.0 mcg/mL for phenytoin. The limit of detection was 0.5 mcg/mL and 2.5 mcg/mL for carbamazepine and phenytoin, respectively.
Serum ARV levels were measured at the University of Alabama at Birmingham Antiviral Pharmacokinetic Laboratory by using a rapid, sensitive and specific high performance liquid chromatographic (HPLC) assay which permitted the simultaneous determination of the NNRTIs, efavirenz and nevirapine, and the PI lopinavir. Sample preparation involved addition of an internal standard (A86093.0) and liquid-liquid extraction with 2mL tert-butylmethylether (tBME) at basic pH, and reconstitution in 100 μL of mobile phase to concentrate the sample. Reversed phase chromatographic separation of the drugs and internal standard was performed on a YMC, C8 analytical column (100 × 4.6mm, 3μm) under isocratic conditions. A binary mobile phase was used consisting of 55% 20 mM sodium acetate buffer (pH 4.88) and 45% acetonitrile. The UV detector set to monitor the 212 nm wavelength provided adequate sensitivity with minimal interference from endogenous matrix components. The limit of detection was 50 ng/mL for each ARV analyzed, and the calibration curves were linear for all ARVs over the range of 50 to 20,000 ng/mL. The inter- and intraday variability at the low and high end of the curve were less than 10%.
Minimum recommended serum concentrations (Cmin) for each ARV were based on guidelines published by the Department of Health and Human Services (DHHS) Guidelines for the Use of ARV Agents in HIV-1-Infected Adults and Adolescents and are as follows: efavirenz (1000 ng/mL), nevirapine (3000 ng/mL), lopinavir (1000 ng/mL).
Statistical Methods
Each AED/ARV overlap period was classified by the number of serum ARV levels that were below recommended Cmin values (none, 1, or 2). The distribution of the number of low test results were compared between EI-AED cases and NEI-AED controls using the Mantel-Haenszel chi-square test, which takes into account the ordered response categories. Since the sample size was small, exact methods were used to determine the significance level. The primary analysis was performed using all patient overlap intervals and a secondary analysis was completed excluding case intervals where the EI-AED level was below the reference range.
RESULTS
Serum ARV levels were detectable in 31 of 32 (96.9%) samples in the EI-AED group and in 65 of 72 (90.3%) samples in the NEI-AED group, suggesting that adherence with ARV treatment was high in both groups. After exclusion of overlap intervals with ≥1 AED or ARV level below the limit of detection, 16 EI-AED/ ARV overlap intervals in 10 EI-AED-treated subjects and 30 NEI-AED/ARV overlap intervals in 25 controls were included in the analysis. Details of participants in the two groups are provided in Table 1. Individuals in the EI-AED group tended to be younger at the start of the overlap period, and had lower CD4 levels at both HIV diagnosis and the start of the overlap period. Ethnicity and gender distributions reflected those in the military population. Of the 10 EI-AED individuals, 6 were prescribed carbamazepine and 4 were prescribed phenytoin, in most cases because of an underlying seizure disorder (Table 2). A chart review showed that seizures were fully controlled in 3 of 5 individuals based upon neurology records during EI-AED/ARV overlap periods.
Table 1.
Characteristics of the Study Population
| Characteristic | EI-AED | NEI-AED | p-value |
|---|---|---|---|
| Number, n | 10 | 25 | |
| Demographics (n, % or mean ± SD) | |||
| Age at HIV diagnosis (y) | 31.8 ± 11.4 | 32.8 ± 7.7 | 0.765 |
| Age at first AED/ARV overlap (y) | 41.9 ± 11.3 | 46.8 ± 9.2 | 0.190 |
| Female | 1 (10.0) | 3 (12) | 0.869 |
| Race/ethnicity | 0.247 | ||
| European American | 7 (70) | 10 (40) | |
| African American | 3 (30) | 13 (52) | |
| Other | 0 (0) | 2 (8) | |
| Year of HIV diagnosis (median, range) | 1988 (1985 - 2000) | 1991 (1986 - 2005) | 0.541 |
| CD4 count at HIV diagnosis (cells/uL) | 376 ± 141 | 534 ± 353 | 0.344 |
| CD4 count at first AED/ARV overlap (cells/uL) | 377 ± 258 | 397 ± 226 | 0.834 |
| Viral load (log10) at first AED/ARV overlap (copies/mL) | 3.6 ± 1.4 | 3.1 ± 1.7 | 0.450 |
| AIDS-defining event prior to first AED/ARV overlap | 5 (50) | 11 (44) | 0.751 |
| Number of AED/ARV overlap periods per participant (n, %) | 0.212 | ||
| 1 | 5 (50.0) | 20 (80) | |
| 2 | 4 (40.0) | 5 (20) | |
| 3 | 1 (10.0) | 0 (0) | |
| Months of AED/ARV overlap, all participant overlaps (median, range) | 19.6 (3.8 - 155.4) | 26.8 (3.1 - 83.3) | 0.688 |
| Days from start of first AED/ARV overlap to measurement of serum drug levels (median, IQR) | 112.5 (73-261) | 126.0 (55-178) | 0.784 |
| ARV regimens (n, %) | 0.831 | ||
| PI-based | 4 (40.0) | 11 (44) | |
| NNRTI-based | 6 (60.0) | 14 (56) |
EI-AED, enzyme-inducing antiepileptic drug; NEI-AED, non enzyme-inducing antiepileptic drug (gabapentin); ARV, antiretroviral drug; NNRTI, non-nucleotide reverse transcriptase inhibitors; PIs, protease inhibitors
Table 2.
Clinical Characteristics of EI-AED Participants
| Participant | Neurologic Diagnosis | EI-AED, Dose Daily | Serum EI-AED level (mcg/mL) | ARV regimen(s) during AED/ARV overlap | Serum ARV levels (ng/mL)¶ | Seizure control during AED/ARV overlap |
|---|---|---|---|---|---|---|
| 1 | Status epilepticus secondary to Herpes Simplex Virus encephalitis | Carbamazepine 1,000 mg | 9.1 | Regimen 1: Nevirapine, Zidovudine, Didanosine |
Nevirapine: 2,071*; 4,408 | No |
| Carbamazepine 1,000 mg | 9.9 | Regimen 2: Nevirapine, Zidovudine, Abacavir |
Nevirapine: 3,231; 2,614* | Yes | ||
| 2 | Distal motor sensory neuropathy | Carbamazepine 600 mg | 0.7 | Efavirenz, Tenofovir, Lamivudine | Efavirenz: 1,379 | Not applicable |
| 3 | Focal epilepsy with complex partial seizures | Phenytoin 500-550 mg | 9 | Regimen 1: Lopinavir, Ritonavir, Didanosine, Lamivudine |
Lopinavir: 4,382; 4,360 | Yes |
| Phenytoin 500-550 mg | 15 | Regimen 2: Lopinavir. Ritonavir, Tenofovir, Lamivudine |
Lopinavir: 976*; 2,858 | Yes | ||
| 4 | Seizures secondary to progressive multifocal leukoencephalopathy | Phenytoin 600 mg | 22.2; 20 | Regimen 1: Efavirenz, Stavudine, Zalcitabine |
Efavirenz: 187*; 307* | Yes |
| Phenytoin 600 mg | 19.3; 23 | Regimen 2: Lopinavir, Ritonavir, Stavudine, Lamivudine |
Lopinavir: 1,264 | Yes | ||
| 5 | Seizure disorder, not otherwise specified | Phenytoin 300 mg | 11.7 | Efavirenz, Zidovudine, Lamivudine | Efavirenz: 200*; 7,556 | Yes |
| 6 | Seizure disorder, not otherwise specified | Carbamazepine 1,000 mg | 7.5 | Regimen 1: Efavirenz, Stavudine, Didanosine |
Efavirenz: 16,567; 13,610 | No |
| Carbamazepine 400 mg | 6.1 | Regimen 2: Efavirenz, Stavudine, Didanosine |
Efavirenz: 11,861; 16,836 | No | ||
| Carbamazepine 400 mg | 20 | Regimen 3: Lopinavir, Ritonavir, Tenofovir, Lamivudine |
Lopinavir: 1,072 | No | ||
| 7 | Seizure disorder, not otherwise specified | Carbamazepine 800 mg | 3.8 | Lopinavir, Ritonavir, Tenofovir, Didanosine, Stavudine | Lopinavir: 3,369 | Unknown |
| 8 | Seizure disorder, not otherwise specified | Phenytoin 600 mg | 15.4 | Lopinavir, Ritonavir, Didansine, Stavudine | Lopinavir: 515*; 145* | Unknown |
| 9 | Seizure disorder, not otherwise specified | Phenytoin 200-400 mg | 3.4 | Lopinavir, Ritonavir, Abacavir, Zidovudine, Lamivudine | Lopinavir: 4,890; 2,082 | Unknown |
| Phenytoin 200-400 mg | 7.6 | Lopinavir, Ritonavir, Abacavir, Zidovudine, Lamivudine | Lopinavir: 4,117; 5,810 | Unknown | ||
| 10 | Trigeminal neuralgia | Carbamazepine 400 mg | 3.1 | Nevirapine,Tenofovir, Emtricitabine | Nevirapine: 3,469 | Not applicable |
EI-AED, enzyme-inducing antiepileptic drug; ARV, antiretroviral drug;
Serum ARV level refers to efavirenz, nevirapine or lopinavir, as applicable.
ARV level below minimum recommended level (Cmin) for the drug
The frequency of ARV levels below Cmin is reported in Table 3. There was a higher frequency of one or more ARV determinations below Cmin in the EI-AED group (37.5%) compared to the NEI-AED group (23.3%), but the difference was not statistically significant (p=0.124). Of the 16 EI-AED/ARV overlap periods, 4 (25%) had 1 ARV level below Cmin while 2 (12.5%) overlap periods had 2 ARV levels below Cmin. Seven (23.3%) NEI-AED/ARV overlap periods had 1 ARV level below Cmin, but no NEI-AED overlaps had 2 ARV levels below Cmin.
Table 3.
Frequency of Serum ARV Levels Below Cmin for the EI-AED Group Compared to the NEI-AED Group
| ARV levels below Cmin (analysis based on all AED/ARV overlap intervals) | EI-AED Group n (%) | NEI-AED Group n (%) | P-value |
|---|---|---|---|
| None | 10 (62.5) | 23 (76.7) | 0.124 |
| One level below Cmin | 4 (25) | 7 (23.3) | |
| Two levels below Cmin | 2 (12.5) | 0 (0) | |
| ARV levels below Cmin (analysis after exclusion of intervals with EI-AED levels below reference range) | |||
| None | 4 (40) | 23 (76.7) | 0.008 |
| One Level below Cmin | 4 (40) | 7 (23.3) | |
| Two Levels below Cmin | 2 (20) | 0 (0) | |
ARV, antiretroviral drug; Cmin, minimum recommended serum concentration; EI-AED, enzyme-inducing antiepileptic drug; n, number of AED/ARV overlaps; NEI-AED, non enzyme-inducing antiepileptic drug
Because enzyme induction is a dose-dependent process(Perucca et al., 1984), lower drug levels of EI-AEDs may have a reduced impact on ARV levels. In a subsequent analysis (Table 3) that excluded 6 EI-AED/ARV overlap periods with EI-AED levels below the reference range, the proportion of AED/ARV overlaps with one or more ARV levels below Cmin increased to 60% and was significantly higher than in the NEI-AED group (23.3%; p=0.008). All ARV determinations were above Cmin for the 6 excluded EI-AED/ARV overlap periods.
Serum levels of individual ARVs in relation to the co-administered AED are shown in Table 4. For EI-AED individuals taking carbamazepine, a high proportion of ARV levels were below Cmin, including 2 (40%) nevirapine and 2 (50%) lopinavir determinations. For phenytoin, 3 of 4 (75%) levels were below Cmin for efavirenz, but only 1 of 9 (11.1%) lopinavir determinations were below Cmin. In contrast to the EI-AED group, there was a relatively lower proportion of ARV measurements below Cmin in the NEI-AED group, with 4.3% (1 of 23 levels) for efavirenz, 16.7% (1 of 6) for nevirapine, and 22.7% (5 of 22 levels) for lopinavir. Figure 1 illustrates the specific drug levels for AED/ARV pairings in relation to Cmin for each ARV drug.
Table 4.
Frequency of Serum ARV Levels According to Co-administered AED
| EI-AED Group | NEI-AED Group | |||||
|---|---|---|---|---|---|---|
| Carbamazepine | Phenytoin | Gabapentin | ||||
| ARVs | ARV level below Cmin (n, %) | ARV level above Cmin (n, %) | ARV level below Cmin (n, %) | ARV level above Cmin (n, %) | ARV level below Cmin (n, %) | ARV level above Cmin (n, %) |
| NNRTIs | ||||||
| Efavirenz | 0 (0) | 5 (100) | 3 (75) | 1 (25) | 1 (4.3) | 22 (95.7) |
| Nevirapine | 2 (40) | 3 (60) | N/A | N/A | 1 (16.7) | 5 (83.3) |
| PIs | ||||||
| Lopinavir | 2 (50) | 2 (50) | 1 (11.1) | 8 (88.9) | 5 (22.7) | 17 (77.3) |
EI-AED, enzyme-inducing antiepileptic drug; NEI-AED, non enzyme-inducing antiepileptic drug; ARVs, antiretroviral drugs; Cmin, minimum recommended serum concentration; NNRTIs, non-nucleotide reverse transcriptase inhibitors; PIs, protease inhibitors
Figure 1.
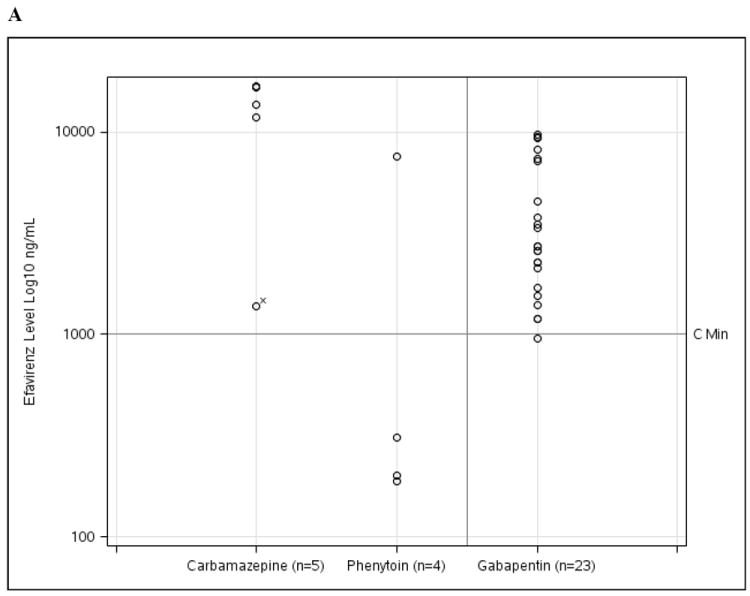
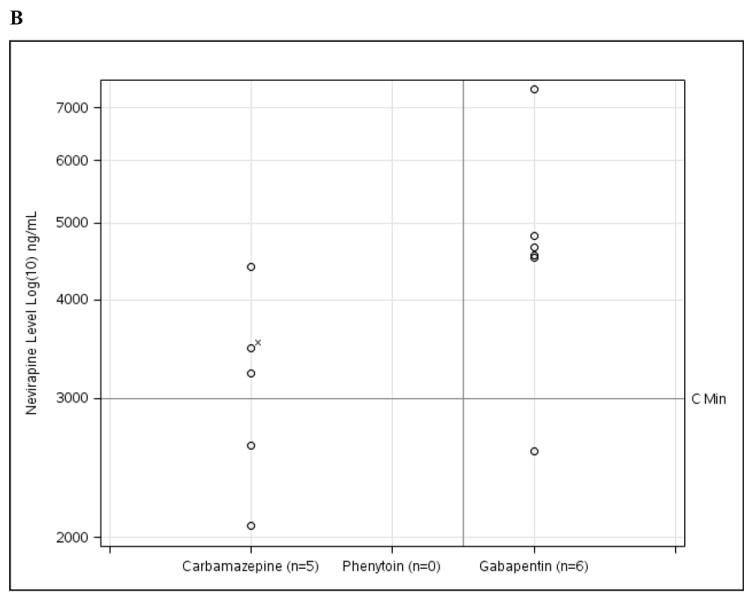
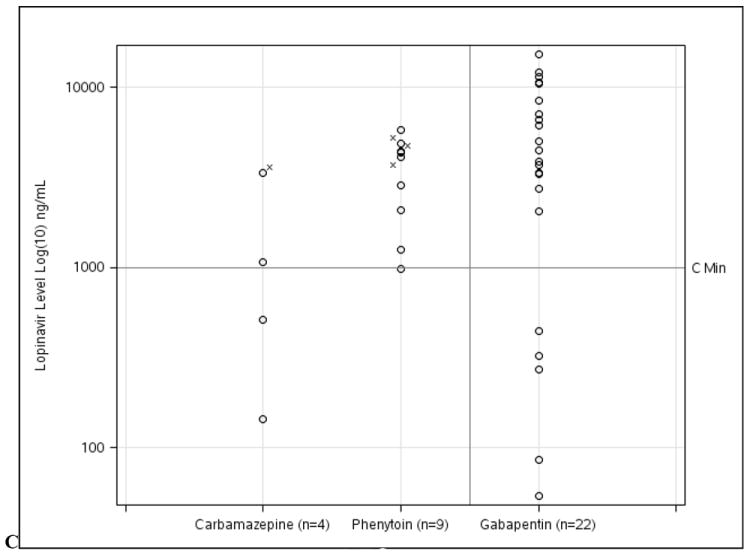
ARV drug levels for (A) efavirenz, (B) nevirapine, and (C) lopinavir for EI-AED and NEI-AED participants based on co-administered EI-AED (carbamazepine or phenytoin) or NEI-AED (gabapentin). For the cases where EI-AED levels were below the reference range (n=6), ARV levels are notated with (x).
DISCUSSION
Because ARVs are metabolized by inducible cytochrome P450 (CYP) and UDP-glucuronsyltransferase (UGT) enzymes, the possibility of these agents being subject to clinically important interactions with EI-AEDs needs to be taken into consideration(Patsalos et al., 2008). In a previous study, we demonstrated that viral load suppression during ARV treatment was inferior in patients who were co-administered EI-AEDs compared to patients who were co-administered NEI-AEDs, a finding which was assumed to be due to a reduction in ARV levels by EI-AEDs.10 The current study was designed to test this hypothesis by investigating the frequency of ARV levels below the recommended Cmin in participants taking concurrent EI-AEDs compared with controls receiving NEI-AEDs. We found that ARV levels tended to be lower in participants taking EI-AEDs, with the difference being statistically significant for participants in whom EI-AEDs were used at doses associated with serum drug levels within the reference range.
Despite widespread concerns about potential adverse consequences resulting from simultaneous use of EI-AEDs and ARVs, studies investigating the interactions between these agents have been sparse. For nevirapine, a small study of 4 healthy HIV-uninfected women reported shortening of the mean half life of nevirapine (from 52 to 33 hours) after a single 400mg dose of carbamazepine(L’Homme R et al., 2006), an observation that needs to be interpreted cautiously because a single carbamazepine dose is insufficient to produce a steady level of enzyme induction(Birbeck et al., 2012a). Interestingly, a recent study utilized this drug interaction in a positive manner by co-administering single-dose carbamazepine with nevirapine at the onset of labor in pregnant HIV-infected women in an attempt to reduce post-partum nevirapine resistance due to the long half-life of the ARV(Muro et al.). In our study, we found that nevirapine levels were below Cmin in 2 of 5 (40%) tests, a particularly important finding for the developing world where nevirapine is often the preferred NNRTI used in an HIV treatment regimen.
Serum levels of the NNRTI efavirenz showed contrasting results when co-administered with phenytoin compared to carbamazepine. Efavirenz levels were below Cmin in 3 of 4 (75%) tests when taken with phenytoin, while no efavirenz levels (n=5) were below Cmin when co-administered with carbamazepine. The combination of phenytoin and efavirenz has not been formally studied, but DHHS HIV treatment guidelines recommend monitoring of efavirenz levels or using an alternative AED in efavirenz-treated patients. Even though efavirenz levels were not reduced in our study when carbamazepine was co-administered, a randomized crossover study in 18 healthy subjects showed a 36% reduction in efavirenz area under the curve (AUC) with concurrent use of 400mg/day carbamazepine compared to efavirenz alone(Ji et al., 2008). Due to the CYP inducing effect of efavirenz, carbamazepine AUC was lowered by 27% in the same study, whereas the levels of the active metabolite carbamazepine-10,11 epoxide were unaffected.
For the PI lopinavir, concurrent use of carbamazepine and phenytoin resulted in 50% (2 of 4) and 11% (1 of 9) of lopinavir levels below Cmin, respectively. DHHS HIV treatment guidelines recommend avoidance of once-daily dosing for lopinavir/ritonavir when used with carbamazepine. With respect to the effect of phenytoin on lopinavir, a study in 12 subjects showed that coadministration of phenytoin 300mg/day led to a mean 33% reduction in lopinavir AUC compared to use of lopinavir alone(Lim et al., 2004). The recent evidence-based guidelines for AED selection for people with HIV/AIDS state that patients receiving phenytoin may require a 50% increase in lopinavir dose to maintain adequate serum concentrations(Birbeck et al., 2012a). Bidirectional effects should also be considered because lopinavir/ritonavir was shown to reduce mean phenytoin AUC by 31%(Lim et al., 2004). Unfortunately, EI-AED levels were not routinely captured in the NHS cohort and therefore the longitudinal impact of ARVs on EI-AED levels could not be assessed in the present investigation.
The finding that the frequency of at least one ARV level below Cmin increased in our study from 37.5% to 60% after excluding the 6 EI-AED/ARV periods in which EI-AED levels were below reference ranges (Table 3) is consistent with the dose-dependent nature of enzyme induction(Perucca et al., 1984), and suggests that the magnitude of interaction may be more prominent when EI-AEDs are used at dosages which produce serum levels above the lower limit of the reference range. Another valuable aspect of our study is that data were derived from a setting which fully reflects clinical practice. However, our study also has limitations, including the small number of participants for each EI-AED/ARV pairing and the lack of standardization in time of sampling. Despite these limitations, our findings are consistent with other studies and support the recommendations listed in recent guidelines(Birbeck et al., 2012a). Our estimate of low ART levels in the EI-AED group (38%) and in the subgroup with therapeutic levels of EI-AED (60%) suggest that the effect size, or impact of EI-AEDs on drug levels of the ARVs studied is likely quite substantial.
Metabolic drug interactions between EI-AEDs and ARVs are highly complex(Birbeck et al., 2012a). Therefore, if anticonvulsant therapy is indicated in ARV-treated individuals, an AED devoid of effects on drug metabolizing enzymes should be used preferentially. If this is not possible, then more frequent clinical and laboratory monitoring, including therapeutic drug monitoring, should be considered whenever feasible. Larger prospective studies are needed to further investigate the clinical impact of AED/ARV interactions in HIV-infected patients.
Acknowledgments
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
Support for this work (IDCRP-000-03) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072.
Footnotes
Statistical analysis completed by Greg Grandits
Authors Contributions:
Jason Okulicz participated in study design, data analysis, and development of the manuscript.
Greg Grandits participated in study design and performed the statistical analysis.
Jacqueline French participated in study design and manuscript development including providing substantive feedback on early versions of this manuscript.
Emilio Perucca participated in study design and manuscript development including providing substantive feedback on early versions of this manuscript.
Jomy George participated in study design and manuscript development including providing substantive feedback on early versions of this manuscript.
Michael Landrum participated in study design and manuscript development including providing substantive feedback on early versions of this manuscript.
Edward Acosta participated in study design and performed serum level analyses for antiretroviral agents.
Gretchen Birbeck participated in study design and manuscript development including providing substantive feedback on early versions of this manuscript.
Disclosures:
Jason Okulicz- Nothing to disclose
Greg Grandits Mr. Grandits receives support through a University of Minnesota contract with the Henry Jackson Foundation, which funds analysis of projects of the HIV Natural History Study. Jacqueline French Jacqueline French serves as the president of The Epilepsy Study Consortium, a non-profit organization. NYU, where Dr. French is employed, receives a fixed amount from the Epilepsy Study Consortium towards Dr French1s salary. The money is for work performed by Dr. French on behalf of The Epilepsy Study Consortium, for consulting and clinical trial related activities. Dr French receives no personal income for these activities. Within the past year, The Epilepsy Study Consortium received paymentsfrom: Cyberonics, Eisai Medical Research,EntraPharmaceuticals,GlaxoSmithKline, Icagen, Inc.,Johnson & Johnson,Marinus,Neurotherapeutics,NeuroVista Corporation,Ono Pharma USA, Inc., Lundbeck, Pfizer, Sepracor,Sunovion, SK Life Science, Supernus Pharmaceuticals, UCB Inc/SchwarzPharma, Upsher Smith, Valeant, and Vertex.
Dr. French as also received funding from NINDS, Milken Foundation and the Epilepsy Therapy Project.
Emilio Perucca Emilio Perucca received research grants from the European Union, the Italian Medicines Agency, the Italian Ministry of Health, and the Italian Ministry for Education, University and Research. He also received speaker’s or consultancy fees and/or research grants from Bial, Eisai, GSK, Novartis, Ortho-McNeil Janssen, Pfizer, SK Life Sciences, Supernus, UCB Pharma, Upsher Smith, Valeant, Vertex.
Jomy George-Nothing to disclose
Michael Landrum –Nothing to disclose
Edward Acosta –Nothing to disclose
Gretchen Birbeck receives research funding from the US NIH for projects in Zambia and Malawi related to epilepsy, HIV and cerebral malaria.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason F Okulicz, Email: jason.okulicz@amedd.army.mil.
Greg A Grandits, Email: greg-g@ccbr.umn.edu.
Jacqueline A French, Email: Jacqueline.French@nyumc.org.
Emilio Perucca, Email: perucca@unipv.it.
Jomy M George, Email: joseph-jomy@cooperhealth.edu.
Michael L Landrum, Email: mlandrum@idcrp.org.
Edward P Acosta, Email: Edward.Acosta@ccc.uab.edu.
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Jan 10, 2011. pp. 1–161. [Google Scholar]
- BIRBECK G, CLIFFORD D, FRAIMOW H, FRENCH J, HACHAD H, GEORGE J, LEVY R, OKULICZ J, PERUCCA E, SIMPSON J. Antiepileptic drug selection for people with HIV/AIDS: Evidence-based guidelines for the Quality Standards subcommittee of the American Academy of Neurology and the ad hoc task force of the Commission on Therapeutic Strategies of the International League Against Epilepsy. Epilepsia. 2012a;53:207–214. doi: 10.1212/WNL.0b013e31823efcf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRBECK GL, FRENCH JA, PERUCCA E, SIMPSON DM, FRAIMOW H, GEORGE JM, OKULICZ JF, CLIFFORD DB, HACHAD H LEVY FOR THE QUALITY STANDARDS SUBCOMMITTEE OF THE AMERICAN ACADEMY OF NEUROLOGY AND THE AD HOC TASK FORCE OF THE COMMISSION ON THERAPEUTIC STRATEGIES OF THE INTERNATIONAL LEAGUE AGAINST EPILEPSY, R. Antiepileptic drug selection for people with HIV/AIDS: Evidence-based guidelines from the ILAE and AAN. Epilepsia. 2012b;53:207–214. doi: 10.1111/j.1528-1167.2011.03335.x. [DOI] [PubMed] [Google Scholar]
- BIRBECK GL, FRENCH JA, PERUCCA E, SIMPSON DM, FRAIMOW H, GEORGE JM, OKULICZ JF, CLIFFORD DB, HACHAD H, LEVY RH. Evidence-based guideline: Antiepileptic drug selection for people with HIV/AIDS: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Ad Hoc Task Force of the Commission on Therapeutic Strategies of the International League Against Epilepsy. Neurology. 2012c;78:139–45. doi: 10.1212/WNL.0b013e31823efcf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNBLATH DR, MCARTHUR JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38:794–6. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- ELLIS RJ, ROSARIO D, CLIFFORD DB, MCARTHUR JC, SIMPSON D, ALEXANDER T, GELMAN BB, VAIDA F, COLLIER A, MARRA CM, ANCES B, ATKINSON JH, DWORKIN RH, MORGELLO S, GRANT I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 67:552–8. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTZMAN DM, KAKU DA, SO YT. New-onset seizures associated with human immunodeficiency virus infection: causation and clinical features in 100 cases. Am J Med. 1989;87:173–7. doi: 10.1016/s0002-9343(89)80693-x. [DOI] [PubMed] [Google Scholar]
- JI P, DAMLE B, XIE J, UNGER SE, GRASELA DM, KAUL S. Pharmacokinetic interaction between efavirenz and carbamazepine after multiple-dose administration in healthy subjects. J Clin Pharmacol. 2008;48:948–56. doi: 10.1177/0091270008319792. [DOI] [PubMed] [Google Scholar]
- KELLINGHAUS C, ENGBRING C, KOVAC S, MODDEL G, BOESEBECK F, FISCHERA M, ANNEKEN K, KLONNE K, REICHELT D, EVERS S, HUSSTEDT IW. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure. 2008;17:27–33. doi: 10.1016/j.seizure.2007.05.017. [DOI] [PubMed] [Google Scholar]
- L’HOMME RF, DIJKEMA T, VAN DER VEN AJ, BURGER DM. Brief report: enzyme inducers reduce elimination half-life after a single dose of nevirapine in healthy women. J Acquir Immune Defic Syndr. 2006;43:193–6. doi: 10.1097/01.qai.0000234089.41785.c8. [DOI] [PubMed] [Google Scholar]
- LIEDTKE MD, LOCKHART SM, RATHBUN RC. Anticonvulsant and antiretroviral interactions. Ann Pharmacother. 2004;38:482–9. doi: 10.1345/aph.1D309. [DOI] [PubMed] [Google Scholar]
- LIM ML, MIN SS, ERON JJ, BERTZ RJ, ROBINSON M, GAEDIGK A, KASHUBA AD. Coadministration of lopinavir/ritonavir and phenytoin results in two-way drug interaction through cytochrome P-450 induction. J Acquir Immune Defic Syndr. 2004;36:1034–40. doi: 10.1097/00126334-200408150-00006. [DOI] [PubMed] [Google Scholar]
- MARCONI VC, GRANDITS GA, WEINTROB AC, CHUN H, LANDRUM ML, GANESAN A, OKULICZ JF, CRUM-CIANFLONE N, O’CONNELL RJ, LIFSON A, WORTMANN GW, AGAN BK. Outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the U.S. Military HIV Natural History Study. AIDS Res Ther. 2010;7:14. doi: 10.1186/1742-6405-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCARTHUR JC, BREW BJ, NATH A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–55. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- MURO EP, FILLEKES Q, KISANGA ER, L’HOMME R, AITKEN SC, MARIKI G, VAN DER VEN AJ, DOLMANS W, SCHUURMAN R, WALKER AS, GIBB DM, BURGER DM. Intrapartum single-dose carbamazepine reduces nevirapine levels faster and may decrease resistance after a single dose of nevirapine for perinatal HIV prevention. J Acquir Immune Defic Syndr. 59:266–73. doi: 10.1097/QAI.0b013e31824234d8. [DOI] [PubMed] [Google Scholar]
- OKULICZ JF, GRANDITS GA, FRENCH JA, GEORGE JM, SIMPSON DM, BIRBECK GL, GANESAN A, WEINTROB AC, CRUM-CIANFLONE N, LALANI T, LANDRUM ML. Virologic outcomes of HAART with concurrent use of cytochrome P450 enzyme-inducing antiepileptics: a retrospective case control study. AIDS Res Ther. 2011;8:18. doi: 10.1186/1742-6405-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATSALOS PN, BERRY DJ, BOURGEOIS BF, CLOYD JC, GLAUSER TA, JOHANNESSEN SI, LEPPIK IE, TOMSON T, PERUCCA E. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49:1239–76. doi: 10.1111/j.1528-1167.2008.01561.x. [DOI] [PubMed] [Google Scholar]
- PERUCCA E, HEDGES A, MAKKI KA, RUPRAH M, WILSON JF, RICHENS A. A comparative study of the relative enzyme inducing properties of anticonvulsant drugs in epileptic patients. Br J Clin Pharmacol. 1984;18:401–10. doi: 10.1111/j.1365-2125.1984.tb02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINTROB AC, FIEBERG AM, AGAN BK, GANESAN A, CRUM-CIANFLONE NF, MARCONI VC, ROEDIGER M, FRASER SL, WEGNER SA, WORTMANN GW. Increasing age at HIV seroconversion from 18 to 40 years is associated with favorable virologic and immunologic responses to HAART. J Acquir Immune Defic Syndr. 2008;49:40–7. doi: 10.1097/QAI.0b013e31817bec05. [DOI] [PubMed] [Google Scholar]
- WONG MC, SUITE ND, LABAR DR. Seizures in human immunodeficiency virus infection. Arch Neurol. 1990;47:640–2. doi: 10.1001/archneur.1990.00530060048015. [DOI] [PubMed] [Google Scholar]


