Abstract
While it is generally accepted that women have lower pain thresholds for diverse forms of noxious stimuli, the mechanistic basis for this sexual dimorphism in nociceptive pain remains to be elucidated. We confirm, in the rat, that females have lower cutaneous mechanical nociceptive thresholds, and establish a similar sexual dimorphism in muscle. To determine if a peripheral mechanism underlies this sexual dimorphism in pain threshold, we compared biophysical properties of cultured DRG neurons that innervated the gastrocnemius muscle in female and male rats. DRG neurons from female rats, which innervated the gastrocnemius muscle, had a more hyperpolarized resting membrane potential. To determine if this was associated with a higher mechanical nociceptive threshold, opposite to our working hypothesis, we compared the function, in vivo, of nociceptive afferents innervating the gastrocnemius muscle in male and female rats. C-fiber nociceptors innervating muscle in female rats had higher mechanical thresholds than those in males. Other response characteristics of these nociceptors were not significantly different. Thus, both in vitro and in vivo electrophysiology experiments support the idea that lower mechanical nociceptive threshold in females may be due to sexual dimorphism in CNS mechanisms, a difference large enough to overcome an opposing difference in peripheral pain mechanisms.
Keywords: Sexual dimorphism, mechanical pain, threshold, peripheral, muscle
INTRODUCTION
There has been a growing recognition that there is a substantial sexual dimorphism in both acute and chronic pain, as well as response to analgesics 8, 20, 21, 26, 31, 33, 36, 40, 44, 49, 51, 54, 58. Even in terms of the most elemental pain measure, nociceptive threshold, there is an extensive literature from both humans 31, 41, 52 and animals 31, 62 demonstrating that females have lower pain thresholds across multiple sensory modalities. In experimental models of hot thermal 12, 57, 59–61, chemical 1, 7, 22 inflammatory 10, 13, 17, and mechanical 6, 9, 31 pain, the largest differences have uniformly been in studies evaluating mechanical nociceptive threshold, where effect size is moderate to large 44, 52, 54. An important unanswered question in this literature is whether these differences in pain threshold are due to sexual dimorphism in mechanisms in the peripheral and/or central nervous system.
While observations such as greater epidermal nerve fiber density in women 24, 45 have led to the suggestion that the greater sensitivity to painful stimuli in the female may have a peripheral basis 39, other observations 25, 50, 53, 55 suggest a central basis for the lower pain threshold observed in women. To address the contribution of sexual dimorphism in the peripheral nervous system, we have compared the function of nociceptive afferents in the female and male rat, using both in vivo and in vitro electrophysiology techniques.
MATERIALS AND METHODS
Animals
Experiments were performed on adult Sprague–Dawley rats (200–250 g; Charles River, Hollister, CA, USA). Animals were housed three per cage, under a 12-h light/dark cycle, in a temperature and humidity controlled environment. Food and water were available ad libitum. All experimental protocols were approved by the UCSF Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Concerted effort was made to minimize the number of animals used and their suffering.
Nociceptive testing
Experiments were primarily performed to explore the in vivo differences between male and female responses, regardless of the estrous cycle in females, and to correlate them to differences seen in vitro, where is difficult to reproduce changes induced by estrous cycle. As such, estrous cycle was not evaluated in the experiments. All behavioral nociceptive testing was performed between 10:00 am and 4:00 pm. Data were not collected blinded.
Skin
The nociceptive flexion reflex was quantified with an UgoBasileAnalgesymeter® (Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw, as described previously 17–19. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its hind paw. Hyperalgesia was defined as a decrease in mechanical nociceptive threshold, here presented as percent change from baseline (mean ± SEM of n observations). Rats were lightly restrained in cylindrical restrainers that have triangular windows on their sides, to allow extension of the hind legs from the restrainer, for nociceptive threshold testing. To acclimatize rats to the testing environment, they were brought to the experimental area in their home cages, 15–30 min prior to placing them in a restrainer. Another 15–30 min elapsed before the experiment was started. Experiments were begun only after animals appeared to be quiet in their restrainers.
Muscle
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a Chatillon digital force transducer (model DFI2; AmetekInc., Largo, FL), as described previously 2, 3. Rats were lightly restrained in the cylindrical acrylic holder that allowed access to the hind limb through the side openings. A 6-mm diameter probe attached to the force transducer was applied to the gastrocnemius muscle to deliver an increasing compression force; this probe width allows for selective evaluation of muscle pain (vis-à-vis overlying skin pain) 46, 47. The nociceptive threshold was defined as the force, in milliNewtons, at which the rat withdrew its hind leg. Nociceptive withdrawal threshold was defined as the mean of 2 readings taken at 5-minute inter-stimulus intervals. Each hind limb is treated as an independent measure.
Electrophysiology
In vitro electrophysiology
Labeling of DRG neurons that innervate the gastrocnemius muscle
The dorsal root ganglion (DRG) neurons innervating the gastrocnemius muscle were identified by their uptake of the retrograde tracer 1,1′-dioctadecyl-3,3,3′,3′-tertamethyllindocarbocyanine perchlorate (DiI). Rats were subjected to gaseous anesthesia (isofluorane, 3%), and incisions of the skin and b. femoris muscle made to expose the underlying gastrocnemius muscle. A 30-g needle was then inserted into the gastrocnemius muscle and 1 μl DMSO containing 2% DiI was slowly injected. The injection was performed over 2 min, followed by continued observation for 1 min to ensure that dye was not leaking from the site of injection. The skin was then sutured closed and the rats allowed to recover from the general anesthesia.
DRG cell-culture
DRGs were surgically removed from rats 5–8 days after DiI injection. Under isoflurane anesthesia, rats were decapitated and the vertebral column excised. The column was then opened on the ventral side, and L4–L5 DRGs removed and de-sheathed. Ganglia were treated with collagenase (0.125% in Neurobasal-A medium, Invitrogen, Carlsbad, CA) for 90 min at 37°C, and then treated with trypsin (0.25% in Ca2+- and Mg2+-free PBS, Invitrogen) for 10 min, followed by trituration (in Neurobasal-A) to produce a single-cell suspension. The suspension was centrifuged at 1000 RPM and re-suspended in Neurobasal-A medium supplemented with 50-ng/ml nerve growth factor, 100 U/ml penicillin/streptomycin and B-27 (Invitrogen). Cells were then plated on cover slips coated with poly-DL-ornithine (0.1 mg/ml) and laminin (5 μg/ml; Invitrogen), and incubated at 37°C in 5% CO2.
Patch clamp electrophysiology
Electrophysiology was performed using an Axopatch 200A amplifier and pCLamp 8.2 software (Molecular Devices, Sunnyvale, CA). Only DRG neurons <30μm in diameter (representing the nociceptive population) were used for the experiments, and were subjected to voltage-clamp after 2–36 hr in culture. Cover slips were incubated with FITC-conjugated Griffoniasimplicifolia isolectin B4 for 10 min before recording. Individual DRG neurons were held in the whole-cell configuration at −60 mV following seal formation (seal resistance > 1GΩ). Whole-cell capacitance and series resistance were compensated (80%) using the amplifier circuitry. Cells with a series resistance >10 MΩ were not used for experimentation.
The external bath solution was configured to minimize Na+ and Ca2+ currents. It contained (in mM): choline chloride (130); KCl (5); CaCl2 (2.5); MgCl2 (0.6); HEPES (5); glucose (10); CdCl2 (1). The solution was altered to pH 7.4 with TrisBase and 320 mOsm with sucrose. The pipette solution contained (in mM): KCl (30); K-MES (110); MgCl2 (1); HEPES (10); EGTA (0.1), altered to pH 7.2 and 310 mOsm. Current-voltage (I–V) traces were normalized to cell capacitance, as determined from the amplifier circuitry. Data were analyzed and plotted using Origin 6.1 software (OriginLab, Northampton, MA).
In vivo single fiber electrophysiology
The in vivo single fiber electrophysiology technique for studying muscle afferents has been described in detail previously 11. In brief, rats were anesthetized with sodium pentobarbital (initially 50 mg/kg, intraperitoneally, with additional doses given to maintain a reflexia throughout the experiment), their trachea cannulated to maintain patency of their upper airway, and heart rate monitored. Anesthetized animals were positioned on their right side and an incision made on the dorsal skin of the left leg, between the mid-thigh and calf. Then the b. femoris muscle was partially removed to expose the sciatic nerve and gastrocnemius muscle. The edges of the incised skin were fixed to a metal loop to provide a pool that was filled with warm mineral oil that bathed the sciatic nerve and gastrocnemius muscle.
The sciatic nerve was cut proximally to prevent stimulation of muscles in the hind limb through reflex arcs during electrical stimulation of sensory neurons. Fine fascicles of axons were then dissected from the distal stump, and placed on a recording electrode. Single units were first detected by mechanical stimulation of the gastrocnemius muscle with a small blunt-tipped glass bar, which subsequently confirmed by electrical stimulation of the mechanical receptive field according to the amplitude and duration of its action potential. Bipolar stimulating electrodes were then placed and held on the center of the receptive field of the muscle afferent by a micromanipulator (Narishige model MM-3, Tokyo, Japan). Conduction velocity of each fiber was calculated by dividing the distance between the stimulating and recording electrodes by the latency of the electrically evoked action potential. All recorded muscle afferents had conduction velocities in the range of type III (conduction velocity 2.5–30 m/s) or type IV (conduction velocity <2.5 m/s) afferent fibers 16. Mechanical threshold, determined with calibrated von Frey hairs (VFH Ainsworth, London, UK), was defined as the lowest force that elicited at least 2 spikes within 1 second, in at least 50% of trials. Sustained (60 s) suprathreshold (10 g) mechanical stimulation was accomplished by use of a mechanical stimulator that consisted of a force-measuring transducer (Entran, Fairfield, NJ, USA) with a blunt plastic tip that was applied by a micromanipulator (BC-3 and BE-8, Narishige) on the center of the afferent’s receptive field for 60 seconds. Neural activity and timing of stimulus onset and termination were monitored and stored on a computer with a Micro 1401 interface (CED, Cambridge, UK) and analyzed off-line with Spike2 software (CED).
Statistics
A t-test was employed to determine if there were significant differences between the observed experimental means of male and female populations. Statistical significance was taken as P<0.05.
RESULTS
Nociceptive threshold
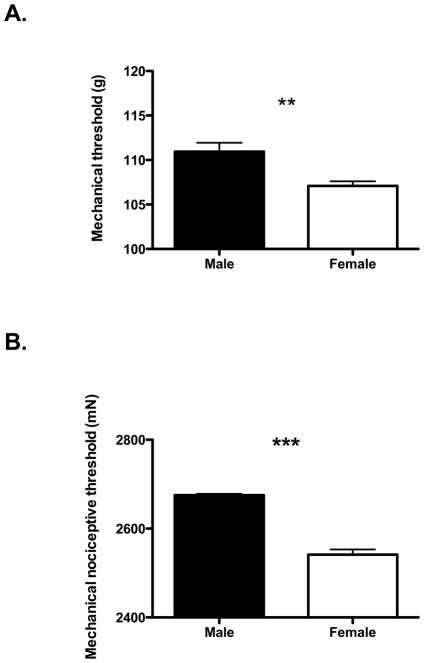
Cutaneous nociceptive threshold in the hindpaw was assessed in male and female rats. In agreement with previous experiments in the literature, we found that female rats demonstrated a significantly lower mechanical nociceptive threshold (107.16 ± 0.52 g, n = 36) compared to male rats (110.94 ± 1.00 g, n = 36, P<0.01, two-tailed t-test), as shown in Figure 1A. To further extend our understanding of the generality of sexual dimorphism in pain threshold, we also compared mechanical nociceptive threshold in skeletal muscle between female and male rats. In this experiment, we again found that mechanical nociceptive threshold in female rats was significantly lower (2.54 ± 0.00 N, n = 60) than in males (2.68 ± 0.01 mN, n = 48, P<0.001, two-tailed t-test), as shown in Fig. 1B.
Figure 1.
Behavioral experiments show a reproducibly lower mechanical nociceptive threshold in female rats. A. Cutaneous mechanical threshold in skin displays sexual dimorphism. Mechanical threshold was significantly higher in male rats compared to females B. Lower mechanical thresholds were measured in muscle of female rats compared to male. ** represents P<0.01, *** represents P<0.001 (two-tailed t-test).
In vitro electrophysiology
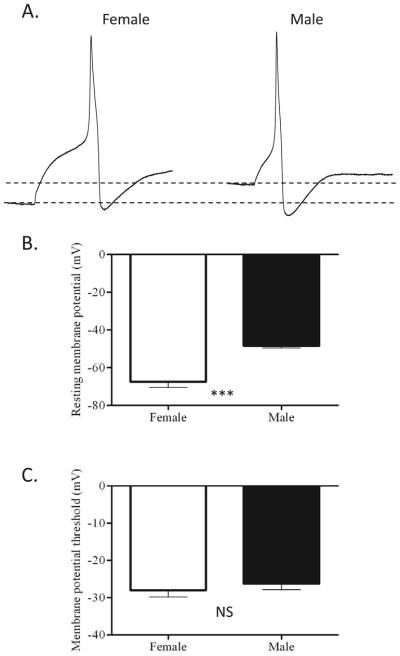
Recordings were made from acutely dissociated DRG neurons that had innervated the gastrocnemius muscle in female and male rats, in order to determine any sexual dimorphism in DRG membrane properties. IB4+ neurons were selected for study as a means of reducing heterogeneity of the population studied, as previously reported these made up roughly 70% of labeled neurons 28. All characteristics were measured from the rheobase (current threshold) action potential, with the exception of the number of action potentials fired (which was measured at 2X rheobase), and the resting membrane potential (which was measured immediately after obtaining the whole-cell configuration and switching to current clamp). The data are presented in Table 1 and Figure 2.
Table 1.
Action potential characteristics of male and female nociceptors in vitro. No statistically significant differences were detected between male and female nociceptors, except for resting membrane potential.
| Male | (n) | Female | (n) | |
|---|---|---|---|---|
| Resting membrane potential *** | −48.44 ± 1.10 | 14 | −67.50 ± 2.93 | 12 |
| Peak (mV) | 53.90 ± 2.03 | 15 | 56.06 ± 2.74 | 14 |
| Duration (ms) | 6.60 ± 0.98 | 15 | 5.61 ± 0.57 | 14 |
| Current threshold/rheobase (pA) | 354.9 ± 50.98 | 14 | 367.4 ± 71.79 | 12 |
| Membrane potential threshold (mV) | −26.21 ± 1.67 | 14 | −28.00 ± 1.80 | 12 |
| Action potentials fired at 2x rheobase | 1.20 ± 0.14 | 15 | 1.36 ± 0.25 | 14 |
| After-hyperpolarisation (amplitude) | −61.93 ± 2.23 | 15 | −65.83 ± 1.58 | 14 |
| After-hyperpolarisation (time constant) | 16.44 ± 2.25 | 14 | 15.62 ± 2.00 | 13 |
denotes P<0.001
Figure 2.
Action potential properties of female and male nociceptors. A. Representative action potential traces, obtained at rheobase, from female (left) and male (right) nociceptors. The dotted lines represent RMP in both neurons for comparison. B. Resting membrane potential is significantly more hyperpolarized in female neurons than in male neurons. C. However, the membrane potential threshold for action potential generation was not significantly different between the two populations. *** represents P<0.001 (two-tailed t-test)
There was no significant change in action potential after-hyperpolarization between female (−65.83 ± 1.58 mV, n=14) and male(−61.93 ± 2.23 mV, n=15) neurons. There was also no significant difference between male and female neurons in either action potential peak (53.90 ± 2.03 mV in males (n=15), 56.06 ± 2.74 mV in females (n=14)) or duration (6.60 ± 0.98 ms in males (n=15), 5.61 ± 0.57 ms in females (n=14)).
In our experiments, the majority of muscle afferents fire a single action potential in response to an 800 ms step at 2X rheobase 28. A switch from phasic to repetitive firing has been observed in non-injured DRG neurons after nerve injury 64, and highlights a possible mechanism for differing mechanical thresholds in vivo. However, we found no significant difference between the number of action potentials fired (in response to an 800 ms pulse at 2X rheobase) between male (1.20 ± 0.14, n=15) and female (1.36 ± 0.25, n=14) neurons. In both male and female populations, a very small minority of neurons produced more than one action potential.
There was a marked difference between the resting membrane potential measured in DRG neurons derived from female and male neurons. Female neurons had a markedly more hyperpolarized RMP (−67.50 ± 2.93 mV, n=12) than male neurons (−48.11 ± 1.10 mV, n=14, P<0.001), as shown in Figure 2B. However, this difference did not translate into a change of electrical excitability of the neurons in vitro. The membrane potential threshold for action potential generation, as assessed by a ramp protocol, was not significantly different between male (−26.21 ± 1.67 mV, n=14) and female (−28.00 ± 1.80 mV, n=12) neurons, as shown in Figure 2C. Also, the current threshold (rheobase) was similar between male (354.93 ± 50.98 pA, n=14) and female (367.42 ± 71.79pA, n=12) neurons (see Table I).
In vivo electrophysiology
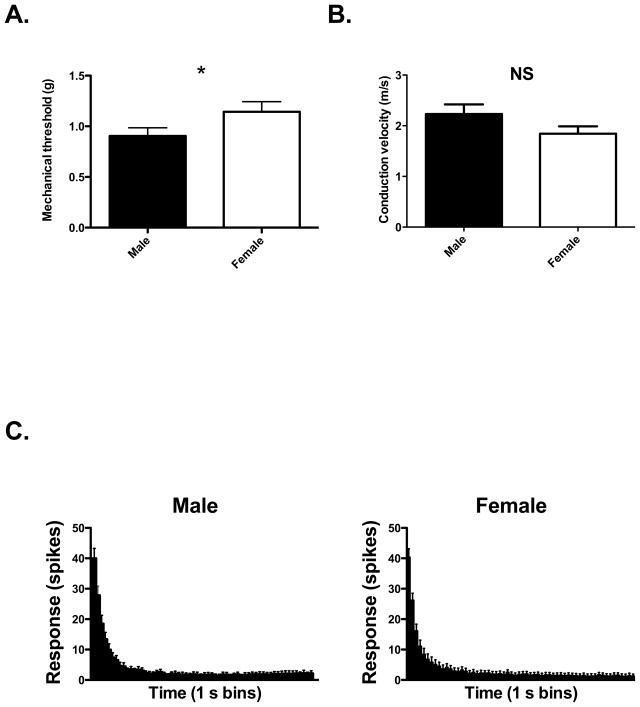
The differences in membrane potential observed in vitro between male and female DRG neurons were not accompanied by an appreciable difference in either current or voltage threshold of action potential generation. One drawback of in vitro set-up is that it represents a reduced system, involving electrical stimulation of the cell soma rather than mechanical stimulation of the peripheral terminal. We therefore wished to demonstrate that the hyperpolarized resting membrane potential observed in female DRGs in vitro might result in an increased mechanical threshold in vivo. In single fiber electrophysiological experiments we found that the mechanical threshold of muscle afferents from female rats was significantly higher than that of male rats, with thresholds of 1.14 ± 0.10 g (n = 44) and 0.90 ± 0.08 g (n = 44) respectively (P<0.05, one-tailed t-test), as shown in Figure 3A. The conduction velocity of muscle afferents from female rats was not significantly different from that of males (Figure 3B), and the response of muscle afferents to supra-threshold 10g stimulation was also similar between nociceptors in female and male rats (Figure 3C).
Figure 3.
A. The mechanical threshold of muscle afferents from male and female rats (one tailed t-test). B. The conduction velocity of muscle afferents from male and female rats. t-test, two-tailed. C. Responses of muscle afferents to 10g stimulation from male and female rats. * represents P<0.05 (one-tailed t-test).
DISCUSSION
Sexual dimorphism described for experimental and clinical pain includes both lower pain thresholds 32, 52, 63 as well as more pain with a similar degree of pathology 35 in women. Additionally, recent evidence has demonstrated a correlation between pain threshold and severity of clinical pain 55, which also showed sexual dimorphism. To identify sites in the pain pathway responsible for the sexual dimorphism in pain threshold we first confirmed that female rats had lower mechanical nociceptive thresholds in behavioral studies. As reported previously for skin 32, 52, 63 and viscera4, 43, 48, and confirmed here for the skin, we also extended these observations in experiments in which we observed a lower mechanical nociceptive threshold in skeletal muscle, of similar magnitude to skin. Thus, lower pain threshold in females observed in behavioral studies appears to be independent of body site at which it was measured.
Indirect evidence has been presented to support the suggestion that the lower nociceptive threshold in the female may be due to sexual dimorphism in the peripheral pain pathway. Thus, females have been reported to have a greater innervation density 5, 24, 45, 56. To address the question of whether sexual dimorphism (in primary afferent nociceptor function) underlies the lower pain threshold in females, we first performed in vitro patch clamp electrophysiology on cultured DRG neurons that innervated the gastrocnemius muscle of male and female rats. In this experiment we found that the resting membrane potential of DRG neurons from female rats was markedly more hyperpolarized than those harvested from males; a singular difference between female and male sensory neurons, since other biophysical properties of the muscle afferents were not sexually dimorphic. While a more hyperpolarized resting potential might underlie a higher mechanical threshold, in vivo, we did not see a difference in action potential threshold when cultured DRG neurons were exposed to a depolarizing current ramp. Given that these experiments were performed on the cell bodies of the DRG neurons, and the stimulus was electrical rather than mechanical, it is possible that the appropriate mechanical transduction mechanisms were not activated to allow us to observe a difference.
To determine if primary afferent nociceptors in female rats actually had a higher mechanical nociceptive threshold, we performed in vivo single fiber electrophysiology experiments on primary afferent nociceptors innervating the gastrocnemius muscle. We confirmed that nociceptors in the female rat did have a higher mechanical threshold than nociceptors in male rats. Other aspects of the response of nociceptors in the female, such as the number of action potentials generated in response to prolonged threshold and suprathreshold stimulation did not differ between nociceptors in male and female rats. Thus, our data support the suggestion that sexual dimorphism in the central rather than the peripheral nervous system accounts for the lower nociceptive threshold in the female, a difference that must be able to more than compensate for an opposing sexual dimorphism in the primary afferent nociceptor.
In the peripheral nervous system, the scope for a difference between male and female nociceptors is vast, with exposure to circulating sex hormones an obvious consideration. Indeed, estrogen (or estradiol as used in many experiments) can cause both pro- and anti-nociceptive effects 14, and may target a multitude of cellular processes such as intracellular nociceptive signaling pathways 30 and ion channel expression 29, 37, 38.
While the specific differences in female and male nociceptors that selectively produced a difference in mechanical threshold, and resting membrane potential, without affecting other properties of their response to mechanical stimulation, is unknown, a candidate mechanism would be action at two-pore potassium (K2P) channels. These channels have been demonstrated in human, rat and mouse sensory neurons, and their passive potassium leak conductance contributes to the resting membrane potential in these neurons, thus helping define their excitability 42. Various members of the K2P family are also mechanosensitive, and inhibiting their function has been linked to increased mechanosensitivity in inflammatory bowel disease 34, a painful condition more common in women 27. Interestingly, genistein (an isoflavone which has effects at estrogen receptors) has been shown to inhibit K2P channels, although the study focused on its tyrosine kinase inhibition rather than estrogen receptor activity 23.
Contrary to our initial hypothesis, we found that nociceptors in females have a higher mechanical threshold for action potential generation than those in males. Thus, our studies provide evidence that CNS rather than PNS mechanisms account for the sexual dimorphism in mechanical pain threshold observed in multiple human and animal behavioral studies. In support of this alternative hypothesis, it has been shown that electrical stimulation, which bypasses the transduction process in the sensory neuron also produces pain in females requiring greater current stimulation 15, 39, 52, in spite of the fact that women have greater subcutaneous adipose tissue, which would be expected to elevate the electrical stimulus threshold needed to activate cutaneous nociceptors. Other evidence in support of a central nervous system mechanism underlying the lower pain threshold in females includes: possible hypoactivity in inhibitory systems of pain in females 25, 50 and, findings indicating that temporal summation of mechanically evoked pain is higher in females compared to males, is stimulation frequency dependent, and is centrally-mediated 55.
The present experiments do not exclude the presence of other forms of sexual dimorphism in the primary afferent nociceptors that could contribute, independent of mechanical nociceptive threshold, to sexual dimorphism in acute and chronic pain syndromes. Thus, we have shown an estrogen dependent sexual dimorphism in the second messenger mediating nociceptor sensitization 17, the clinical ramifications of which remain to be evaluated. Going forward, greater attention needs to be given to comparisons of central and peripheral mechanisms for pain and analgesia, in order to unravel the fundamental aspects of sexual dimorphism responsible for differences between females and males in acute and chronic pain syndromes.
PERSPECTIVE.
This article unifies in vitro and in vitro electrophysiology with behavioral data examining the differences in mechanical nociceptive threshold between male and female rats. The data provide a novel perspective on the peripheral and behavioral outcomes of noxious mechanical stimulation.
Footnotes
DISCLOSURES
All research was funded by the NIH. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aloisi AM, Albonetti ME, Carli G. Sex differences in the behavioural response to persistent pain in rats. Neurosci Lett. 1994;179:79–82. doi: 10.1016/0304-3940(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez P, Gear RW, Green PG, Levine JD. IB4-saporin attenuates acute and eliminates chronic muscle pain in the rat. Exp Neurol. 2012;233:859–65. doi: 10.1016/j.expneurol.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci. 2010;32:819–25. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L, Bajaj P, Drewes AM. Visceral pain: gender differences in response to experimental and clinical pain. Eur J Pain. 2004;8:465–72. doi: 10.1016/j.ejpain.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Bakkers M, Merkies IS, Lauria G, Devigili G, Penza P, Lombardi R, Hermans MC, van Nes SI, De Baets M, Faber CG. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology. 2009;73:1142–8. doi: 10.1212/WNL.0b013e3181bacf05. [DOI] [PubMed] [Google Scholar]
- 6.Barrett AC, Smith ES, Picker MJ. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur J Pharmacol. 2002;452:163–73. doi: 10.1016/s0014-2999(02)02274-4. [DOI] [PubMed] [Google Scholar]
- 7.Barrett AC, Smith ES, Picker MJ. Capsaicin-induced hyperalgesia and mu-opioid-induced antihyperalgesia in male and female Fischer 344 rats. J Pharmacol Exp Ther. 2003;307:237–45. doi: 10.1124/jpet.103.054478. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar RJ, Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav. 2010;58:72–81. doi: 10.1016/j.yhbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Bourquin AF, Suveges M, Pertin M, Gilliard N, Sardy S, Davison AC, Spahn DR, Decosterd I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14, e1. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain. 2000;85:93–9. doi: 10.1016/s0304-3959(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Green PG, Levine JD. Neuropathic pain-like alterations in muscle nociceptor function associated with vibration-induced muscle pain. Pain. 2010;151:460–6. doi: 10.1016/j.pain.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26:907–23. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 13.Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund’s adjuvant-induced arthritic male and female rats. J Pharmacol Exp Ther. 2005;313:449–59. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- 14.Craft RM. Modulation of pain by estrogens. Pain. 2007;132 (Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Dawson A, List T. Comparison of pain thresholds and pain tolerance levels between Middle Easterners and Swedes and between genders. J Oral Rehabil. 2009;36:271–8. doi: 10.1111/j.1365-2842.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- 16.Diehl B, Hoheisel U, Mense S. The influence of mechanical stimuli and of acetylsalicylic acid on the discharges of slowly conducting afferent units from normal and inflamed muscle in the rat. Exp Brain Res. 1993;92:431–40. doi: 10.1007/BF00229031. [DOI] [PubMed] [Google Scholar]
- 17.Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–33. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- 18.Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci. 2000;20:8614–9. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–5. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–25. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–45. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- 23.Gierten J, Ficker E, Bloehs R, Schlomer K, Kathofer S, Scholz E, Zitron E, Kiesecker C, Bauer A, Becker R, Katus HA, Karle CA, Thomas D. Regulation of two-pore-domain (K2P) potassium leak channels by the tyrosine kinase inhibitor genistein. Br J Pharmacol. 2008;154:1680–90. doi: 10.1038/bjp.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goransson LG, Mellgren SI, Lindal S, Omdal R. The effect of age and gender on epidermal nerve fiber density. Neurology. 2004;62:774–7. doi: 10.1212/01.wnl.0000113732.41127.8f. [DOI] [PubMed] [Google Scholar]
- 25.Graven-Nielsen T, Arendt-Nielsen L. Gender differences in response to pain. Ugeskr Laeger. 2007;169:2425–7. [PubMed] [Google Scholar]
- 26.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 (Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heitkemper M, Jarrett M. Irritable bowel syndrome: does gender matter? J Psychosom Res. 2008;64:583–7. doi: 10.1016/j.jpsychores.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Hendrich J, Alvarez P, Chen X, Levine JD. GDNF induces mechanical hyperalgesia in muscle by reducing I(BK) in isolectin B4-positive nociceptors. Neuroscience. 2012;219:204–13. doi: 10.1016/j.neuroscience.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu F, Wang Q, Wang P, Wang W, Qian W, Xiao H, Wang L. 17beta-Estradiol regulates the gene expression of voltage-gated sodium channels: role of estrogen receptor alpha and estrogen receptor beta. Endocrine. 2012;41:274–80. doi: 10.1007/s12020-011-9573-z. [DOI] [PubMed] [Google Scholar]
- 30.Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci. 2006;24:527–34. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- 31.Hurley RW, Adams MC. Sex, gender, and pain: an overview of a complex field. Anesth Analg. 2008;107:309–17. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen R, Rasmussen BK, Pedersen B, Lous I, Olesen J. Cephalic muscle tenderness and pressure pain threshold in a general population. Pain. 1992;48:197–203. doi: 10.1016/0304-3959(92)90059-K. [DOI] [PubMed] [Google Scholar]
- 33.Kuba T, Quinones-Jenab V. The role of female gonadal hormones in behavioral sex differences in persistent and chronic pain: clinical versus preclinical studies. Brain Res Bull. 2005;66:179–88. doi: 10.1016/j.brainresbull.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 34.La JH, Gebhart GF. Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2011;301:G165–74. doi: 10.1152/ajpgi.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine JD, Gordon NC, Smith R, Fields HL. Post-operative pain: effect of extent of injury and attention. Brain Res. 1982;234:500–4. doi: 10.1016/0006-8993(82)90894-0. [DOI] [PubMed] [Google Scholar]
- 36.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–38. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu YC, Chen CW, Wang SY, Wu FS. 17Beta-estradiol mediates the sex difference in capsaicin-induced nociception in rats. J Pharmacol Exp Ther. 2009;331:1104–10. doi: 10.1124/jpet.109.158402. [DOI] [PubMed] [Google Scholar]
- 38.Ma B, Yu LH, Fan J, Cong B, He P, Ni X, Burnstock G. Estrogen modulation of peripheral pain signal transduction: involvement of P2X(3) receptors. Purinergic Signal. 2011;7:73–83. doi: 10.1007/s11302-010-9212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maffiuletti NA, Herrero AJ, Jubeau M, Impellizzeri FM, Bizzini M. Differences in electrical stimulation thresholds between men and women. Ann Neurol. 2008;63:507–12. doi: 10.1002/ana.21346. [DOI] [PubMed] [Google Scholar]
- 40.Manson JE. Pain: sex differences and implications for treatment. Metabolism. 2010;59 (Suppl 1):S16–20. doi: 10.1016/j.metabol.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Maquet D, Croisier JL, Demoulin C, Crielaard JM. Pressure pain thresholds of tender point sites in patients with fibromyalgia and in healthy controls. Eur J Pain. 2004;8:111–7. doi: 10.1016/S1090-3801(03)00082-X. [DOI] [PubMed] [Google Scholar]
- 42.Marsh B, Acosta C, Djouhri L, Lawson SN. Leak K(+) channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol Cell Neurosci. 2012;49:375–86. doi: 10.1016/j.mcn.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8:451–63. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res. 2010;186:141–57. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- 45.Mowlavi A, Cooney D, Febus L, Khosraviani A, Wilhelmi BJ, Akers G. Increased cutaneous nerve fibers in female specimens. Plast Reconstr Surg. 2005;116:1407–10. doi: 10.1097/01.prs.0000182339.83156.06. [DOI] [PubMed] [Google Scholar]
- 46.Murase S, Terazawa E, Queme F, Ota H, Matsuda T, Hirate K, Kozaki Y, Katanosaka K, Taguchi T, Urai H, Mizumura K. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness) J Neurosci. 2010;30:3752–61. doi: 10.1523/JNEUROSCI.3803-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nasu T, Taguchi T, Mizumura K. Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. Eur J Pain. 2010;14:236–44. doi: 10.1016/j.ejpain.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen P, Lee SD, Castell DO. Evidence of gender differences in esophageal pain threshold. Am J Gastroenterol. 1995;90:901–5. [PubMed] [Google Scholar]
- 49.Paller CJ, Campbell CM, Edwards RR, Dobs AS. Sex-based differences in pain perception and treatment. Pain Med. 2009;10:289–99. doi: 10.1111/j.1526-4637.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmeira CC, Ashmawi HA, de Posso IP. Sex and pain perception and analgesia. Rev Bras Anestesiol. 2011;61:814–28. doi: 10.1016/S0034-7094(11)70091-5. [DOI] [PubMed] [Google Scholar]
- 51.Rasakham K, Liu-Chen LY. Sex differences in kappa opioid pharmacology. Life Sci. 2011;88:2–16. doi: 10.1016/j.lfs.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–7. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 53.Roehr B. The puzzle of gender and pain. J Int Assoc Physicians AIDS Care. 1998;4:23–7. [PubMed] [Google Scholar]
- 54.Rollman GB, Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–4. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Sarlani E, Greenspan JD. Gender differences in temporal summation of mechanically evoked pain. Pain. 2002;97:163–9. doi: 10.1016/s0304-3959(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 56.Selim MM, Wendelschafer-Crabb G, Hodges JS, Simone DA, Foster SX, Vanhove GF, Kennedy WR. Variation in quantitative sensory testing and epidermal nerve fiber density in repeated measurements. Pain. 2010;151:575–81. doi: 10.1016/j.pain.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 57.Sternberg WF, Smith L, Scorr L. Nociception and antinociception during the first week of life in mice: sex differences and test dependence. J Pain. 2004;5:420–6. doi: 10.1016/j.jpain.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Sun LS. Gender differences in pain sensitivity and responses to analgesia. J Gend Specif Med. 1998;1:28–30. [PubMed] [Google Scholar]
- 59.Terner JM, Barrett AC, Cook CD, Picker MJ. Sex differences in (−)-pentazocine antinociception: comparison to morphine and spiradoline in four rat strains using a thermal nociceptive assay. Behav Pharmacol. 2003;14:77–85. doi: 10.1097/00008877-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Terner JM, Lomas LM, Smith ES, Barrett AC, Picker MJ. Pharmacogenetic analysis of sex differences in opioid antinociception in rats. Pain. 2003;106:381–91. doi: 10.1016/j.pain.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 61.Wala EP, Sloan JW, Jing X, Holtman JR. The effects of diazepam dependence and withdrawal on morphine-induced antinociception and changes in locomotion in male and female rats. Pharmacol Biochem Behav. 2001;69:475–84. doi: 10.1016/s0091-3057(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 62.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–45. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 63.Woodrow KM, Friedman GD, Siegelaub AB, Collen MF. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548–56. doi: 10.1097/00006842-197211000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Zhang XF, Zhu CZ, Thimmapaya R, Choi WS, Honore P, Scott VE, Kroeger PE, Sullivan JP, Faltynek CR, Gopalakrishnan M, Shieh CC. Differential action potentials and firing patterns in injured and uninjured small dorsal root ganglion neurons after nerve injury. Brain Res. 2004;1009:147–58. doi: 10.1016/j.brainres.2004.02.057. [DOI] [PubMed] [Google Scholar]