Abstract
Dysfunction in noradrenergic neurotransmission has long been theorized to occur in depressive disorders. The α2 adrenergic receptor (AR) family, as a group of key players in regulating the noradrenergic system, has been investigated for involvement in the neurobiology of depression and mechanisms of antidepressant therapies. However, a clear picture of the α2ARs in depressive disorders has not been established due to the existence of apparently conflicting findings in the literature. In this article, we report that a careful accounting of methodological differences within the literature can resolve the present lack of consensus on involvement of α2ARs in depression. In particular, the pharmacological properties of the radioligand (e.g. agonist versus antagonist) utilized for determining receptor density are crucial in determining study outcome. Upregulation of α2AR density detected by radiolabeled agonists but not by antagonists in patients with depressive disorders suggests a selective increase in the density of high-affinity conformational state α2ARs, which is indicative of enhanced G protein coupling to the receptor. Importantly, this high-affinity state α2AR upregulation can be normalized with antidepressant treatments. Thus, depressive disorders appear to be associated with increased α2AR sensitivity and responsiveness, which may represent a physiological basis for the putative noradrenergic dysfunction in depressive disorders. In addition, we review changes in some key α2AR accessory proteins in depressive disorders and discuss their potential contribution to α2AR dysfunction.
Keywords: α2 adrenergic receptor, antidepressant, depressive disorder, locus coeruleus
1. Introduction
Nearly half a century ago, the classical monoamine hypothesis for depressive disorders was proposed as an explanation for the therapeutic efficacy of the first antidepressant drugs (Schildkraut, 1965). These compounds, the tricyclic antidepressants (TCAs), which inhibit monoamine reuptake, and monoamine oxidase inhibitors (MAOIs), were known to increase brain levels of the monoamine neurotransmitters norepinephrine (NE) and serotonin (5HT) (Baldessarini, 2006). It therefore seemed obvious to hypothesize that a depletion of monoamines was a causative factor in depressive disorders, although in the intervening years, efforts to provide empirical support for the monoamine hypothesis have yielded mixed results (Hindmarch, 2001). It is now generally agreed that the original hypothesis is insufficient as a neurobiological basis for depressive disorders, with the true picture likely to be much more complex and heterogeneous, involving both monoaminergic and non-monoaminergic players (Belmaker and Agam, 2008). Nevertheless, the concept of monoaminergic dysfunction remains a useful and well-regarded component of that neurobiological picture, and has stimulated an extensive and productive line of research into the function of the central noradrenergic system in depressive disorders.
The central noradrenergic system is responsible for noradrenergic neurotransmission in the brain and plays a key role in general cognitive processes (Sara, 2009). The system is anatomically based in the brainstem nucleus known as the locus coeruleus (LC) which is the primary source of central NE synthesis, and its noradrenergic projections reach virtually all areas of the brain (Sara, 2009). The actions of NE are mediated by the family of G protein-coupled receptors (GPCRs) known as the adrenergic receptors (ARs), and levels of extracellular NE are regulated by synaptic clearance via the NE transporter (NET) and modulation of NE metabolism. Almost all of these noradrenergic system components can be direct molecular targets for antidepressant drugs, including the newer 5HT/NE reuptake inhibitors (SNRIs) such as duloxetine, atypical antidepressants like mirtazepine, and the older TCAs and MAOIs mentioned above (Baldessarini, 2006). In addition, many of these noradrenergic system components have been examined for potential dysfunction in the context of depressive disorders.
The ARs, consisting of β, α1, and α2ARs, are the cellular mediators of noradrenergic neurotransmission. Arguably, α2ARs comprise the most important receptor family involved in regulation of noradrenergic transmission, and have consequently been subject to intensive study for potential roles in depressive neurobiology and antidepressant pharmacology. In this article, we will review the broad base of studies investigating α2ARs in the depressive setting and attempt to construct a summarizing model for α2AR dysfunction in depressive disorders. We contend that α2AR alterations make an important contribution to the clinical manifestation of depressive disorders and are a putative mechanistic basis for depression-related NE depletion and LC dysfunction. Additionally, we will review evidence for depression-related dysfunction of some key regulators of α2ARs, in particular GPCR kinases (GRKs), arrestins, and spinophilin.
2. The α2 adrenergic receptor family
The various physiological roles of the α2AR family in central and peripheral systems have been well-reviewed elsewhere by Hein and others (Brede et al., 2004; Kable et al., 2000; Knaus et al., 2007; Philipp et al., 2002; Wang, 2011), and so we will focus on a brief overview of the functions of these receptors in central noradrenergic neurotransmission. α2ARs impact neuronal function by classically coupling to heterotrimeric G proteins of the Gi/o subfamily upon activation by their endogenous agonists epinephrine and NE. In turn, stimulation of α2ARs leads to inhibition of adenylyl cyclase and voltage-gated Ca2+ channels and activation of inwardly rectifying K+ channels and MAPK signaling cascades (Kobilka, 1992; Limbird, 1988; Richman and Regan, 1998; Wang et al., 2006; Wang et al., 2004). Presynaptic α2AR autoreceptors are responsible for inhibition of NE synthesis and release from noradrenergic terminals as part of a negative feedback loop (Hein et al., 1999; Knaus et al., 2007), and α2ARs on non-noradrenergic terminals regulate release of other key neurotransmitters including glutamate (Shields et al., 2009). Meanwhile, activation of postsynaptic α2ARs modulates neuronal excitability via regulation of ion channels, including direct modulation of inwardly rectifying K+ channels and indirect modulation of hyperpolarization-activated channels (Gilsbach et al., 2011). The importance of postsynaptic α2ARs is increasingly becoming appreciated as their roles in mediating such classical α2AR agonist effects as sedation, analgesia, and enhancement of working memory are illuminated (Gilsbach and Hein, 2012; Gilsbach et al., 2009; Wang et al., 2007). The generally inhibitory nature of α2ARs with respect to neuronal function is also indicated by the ability of α2AR activation to decrease epileptogenesis (Wilson et al., 1998).
There are three α2AR subtypes, the α2A, α2B, and α2C, which are encoded by separate genes (Bylund et al., 1994; Cottingham et al., 2011a; Wang, 2011). Among these, the α2AAR is the predominantly expressed subtype within the central nervous system (De Vos et al., 1992; Sastre and Garcia-Sevilla, 1994; Wang et al., 1996), and is primarily responsible for the central noradrenergic functions described above (Altman et al., 1999; Franowicz et al., 2002; MacMillan et al., 1996; Stone et al., 1997). The α2CAR also has a well-appreciated role in inhibition of neurotransmitter release, although it exhibits differential responsiveness to action potential frequency compared with the α2A subtype (Hein et al., 1999). This phenomenon may be related to the unique localization of the α2C subtype in synaptic terminals of mature neurons (Brum et al., 2006). The α2BAR is mainly expressed in peripheral tissues and its physiological roles in the brain have not been clearly defined. As mentioned above, the importance of α2ARs as regulators of noradrenergic system function in general and NE levels in particular has resulted in their being the most extensively studied ARs in the context of depressive disorders. Although clinical studies in this area have generally avoided subtype specificity due to the lack of subtype-selective agents for α2ARs, genetic evidence from both human and experimental animals has clearly implicated involvement of the α2A and α2C subtypes in depressive disorder.
3. Dysregulation of α2 adrenergic receptor density and activity in depressive disorders
A wide array of different approaches has been utilized over the last few decades to directly assay both α2AR density and pharmacological properties in patients with depressive disorders. Attempts to ascertain receptor density have most commonly been made using saturation radioligand binding with radiolabeled α2AR agonists and antagonists, and less commonly using immunolabeling-based techniques and measurements of mRNA levels. These assays have been carried out directly in postmortem brain tissue largely obtained from suicide completers and using peripheral models such as platelets obtained from living depressed patients. In addition, many studies have utilized classic pharmacological methods such as competition radioligand and GTPγS binding along with other readouts to characterize receptor activity in depressed patients. Given the range of methodologies, it is unsurprising that these studies have yielded seemingly inconsistent results. In particular, variable application of radiolabeled agonists versus antagonists in α2AR binding studies has led to an apparently contradictory body of literature. As will become clear in the following sections, agonist (which leads to G protein coupling and activation) versus antagonist (which does not activate the receptor) is an important distinction in GPCR binding studies. With a careful accounting of methodological differences within the literature, consistent patterns of α2AR dysregulation in depressive disorders can be appreciated.
3.1 Platelet α2ARs
Studies on platelet α2ARs have been the most commonly-used approach to assay receptor density in patients with depressive disorders. This approach has the advantage of allowing investigators to observe receptor levels in patients with active depression and without the influence of suicidality which pervades studies in postmortem tissue. Moreover, receptor levels can be monitored in real-time during a course of treatment with an antidepressant therapy. Of course, these studies must be interpreted with a certain level of caution given that peripheral blood cell α2ARs have different physiological roles and potentially different mechanisms of regulation compared with central receptors.
The body of work investigating α2AR density in platelets obtained from major depressive disorder (MDD) patients provides a prime example of apparently contradictory findings which can be reconciled by accounting for methodological differences. Studies have variously reported increases, decreases, and no alterations in platelet α2AR density associated with MDD. However, upon closer review, the literature in this area strongly supports a selective increase in high-affinity conformational state α2AR density, which is indicative of enhanced G protein coupling and activity.
The numerous studies which have found elevated α2AR density in platelets from unmedicated MDD patients, indicated by saturation radioligand binding, have utilized radiolabeled α2AR agonists such as clonidine and UK 13,304 to detect α2ARs (Garcia-Sevilla et al., 1987; García-Sevilla et al., 2004; Gurguis et al., 1999; Healy et al., 1985; Kaneko et al., 1992; Pandey et al., 1989; Piletz et al., 1990; Piletz et al., 1991; Smith et al., 1983; Takeda et al., 1989; Werstiuk et al., 1996). In fact, only a very few studies have observed no changes (Karege et al., 1992; Werstiuk et al., 1992) or decreases (Carstens et al., 1986) in density in MDD patients when utilizing agonists as the radioligand probe, although Karege and colleagues did report a trend toward an increased density in dysthymic patients (Karege et al., 1992). Conversely, studies conducted with a radiolabeled α2AR antagonist have consistently failed to find increased platelet α2AR density in MDD patients (Bhatia et al., 1991; Katona et al., 1989; Marazziti et al., 2001; Smith et al., 1983; Stahl et al., 1983; Theodorou et al., 1991; Wolfe et al., 1987; Wolfe et al., 1989). Although Marazziti and colleagues found that platelet α2AR density overall was unaltered in their MDD patients, they did observe a significant correlation between receptor density and severity of depression symptoms (Marazziti et al., 2001), supporting a link between these two parameters.
The apparent discrepancies in binding data obtained with radiolabeled agonists versus antagonists can be explained by the distinct nature of these two types of ligands in binding to a GPCR. Receptors can exist in both active and inactive conformations, which are in a steady-state balance. Antagonists have equal affinity for both conformational states, and their binding does not alter the steady-state. Therefore, radiolabeled antagonist binding reflects the total density of receptors in any conformational state. By comparison, agonist binding induces a conformational change of the receptor to the active state resulting in G protein coupling to the receptor. The framework of the ternary complex model (scheme shown in Figure 1) and its extended versions describing the ternary complex of agonist, receptor, and G proteins (De Lean et al., 1980; Samama et al., 1993; Weiss et al., 1996) reveals that G protein coupling to the receptor highly impacts receptor affinity for agonists. Specifically, the receptor/G protein complex with the receptor in the high-affinity state has a much stronger interaction with agonists than the receptor alone in its low-affinity state. In a typical saturation binding assay with radiolabeled agonists, the apparent binding density primarily reflects the high-affinity state of receptor given that binding of an agonist to the low-affinity state of receptor is difficult to detect due to fast dissociation time (Limbird, 2005). Therefore, the specific increase in agonist-binding but not antagonist-binding α2AR sites in the reports outlined above suggests a selective upregulation of high-affinity state receptors with enhanced G protein coupling. Indeed, several studies have specifically identified MDD-associated increases in the density of platelet α2ARs in the high-affinity conformational state through competition binding and G protein coupling analyses (García-Sevilla et al., 1986; Garcia-Sevilla et al., 1987; García-Sevilla et al., 1981; Gurguis et al., 1999). Increases in high-affinity state α2ARs and G protein coupling to α2ARs in MDD patients are also supported by reports of depression-associated increases in platelet α2AR activity as measured by increased α2AR-mediated platelet aggregation responses (García-Sevilla et al., 1986; García-Sevilla et al., 1990; Piletz et al., 1993). Evidence from platelet α2AR studies is summarized in Table 1.
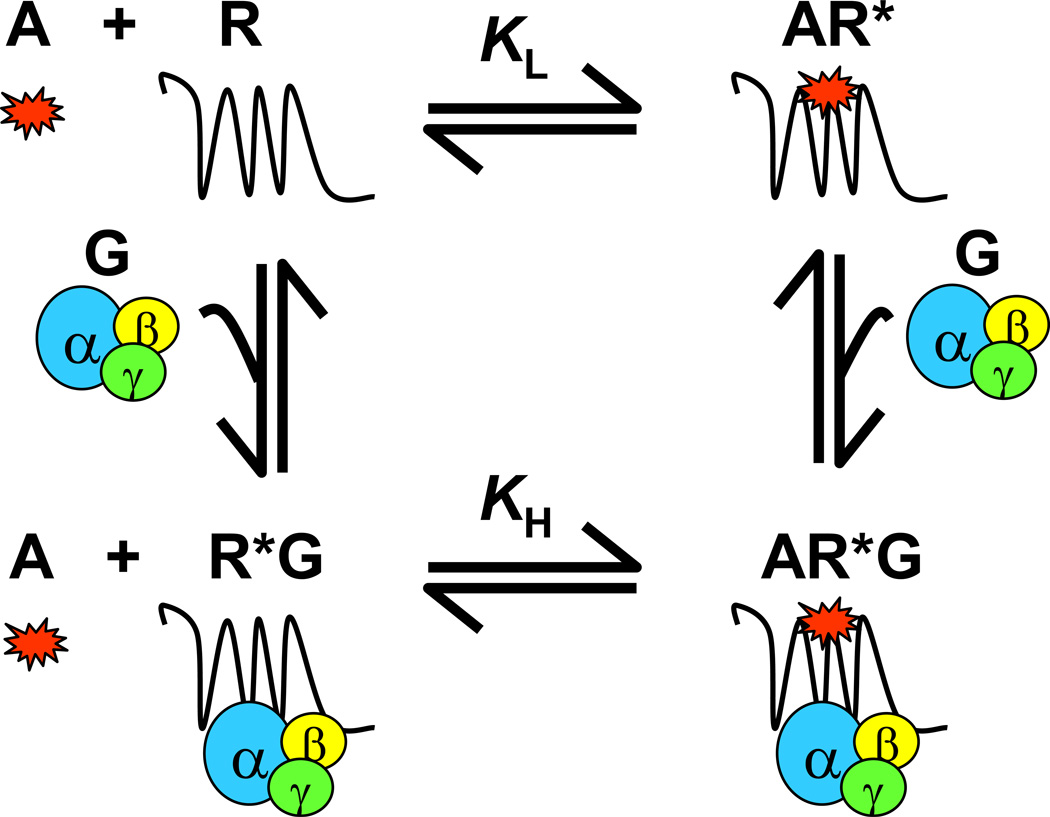
Figure 1.
Scheme of the ternary complex model for binding of agonist and G proteins to GPCRs. A receptor exists in both active and inactive conformations, with the active able to form a complex with G proteins. Agonists bind to free receptors (inactive) and receptors coupled to G protein (active) with different affinities; receptors coupled to G protein have a higher affinity than free receptors for agonists. Thus, receptor interactions with agonists as detected by radioligand binding assays are indicative of receptor-G protein coupling efficiency. A, agonist; G, G protein; R, free receptor; AR*, agonist-bound receptor (R* indicates active conformation of the receptor); R*G, G protein-bound receptor; AR*G, agonist/G protein/receptor ternary complex; KH, Kd value of receptor binding at high-affinity; KL, Kd value of receptor binding at low-affinity.
Table 1.
Summary of clinical evidence supporting upregulation of α2ARs in depressive disorders.
Abbreviations: FC, frontal cortex; PFC, prefrontal cortex; HC, hippocampus; LC, locus coeruleus.
3.2 Direct assays of central α2ARs
A number of studies investigating α2AR status in depression have been carried out using postmortem brain tissue. This tissue has been almost universally obtained from suicide completers, and so it is important to bear in mind that alterations observed in these studies may be more closely related to the pathology of suicide specifically rather than depressive disorders generally. Nevertheless, these studies have largely tended to confirm the findings of increased α2AR density in the high-affinity state from the platelet studies outlined above. A summary of these direct assays of central α2ARs can be found in Table 1.
The most consistent line of evidence demonstrating upregulated α2AR density is a series of studies from the Garcia-Sevilla group using tissue obtained from depressed suicide completers. Using radioligand-based approaches, they have shown significantly increased density of α2ARs generally (Gonzalez et al., 1994) and of the α2AAR subtype specifically (Callado et al., 1998; Meana et al., 1992; Meana and Garcia-Sevilla, 1987) in the frontal cortex, hippocampus, and hypothalamus. These findings are supported by separate studies reporting increased α2AR density by radioligand binding in temporal cortex and locus coeruleus tissue from depressed suicide completers (De Paermentier et al., 1997; Ordway et al., 2003; Ordway et al., 1994b). Given the use of radiolabeled agonists for their binding assays, these studies tend to further support an increase in the density of high-affinity state α2ARs in depressed suicide completers. In addition, the Garcia-Sevilla group has reported increased α2AAR receptor protein levels using an immunolabeling method (Garcia-Sevilla et al., 1999) and increased α2AAR mRNA levels using RT-PCR (Escriba et al., 2004) in the prefrontal cortex of depressed suicide completers.
Studies reporting no change in α2AR density associated with suicide tend to differ from those above in either lacking a requirement for depression diagnosis in their suicide subject population (Arango et al., 1993; Gross-Isseroff et al., 2000) or using an antagonist instead of an agonist as the radiolabeled probe (Sastre and Garcia-Sevilla, 1997). The first difference is supportive of increased α2AR density as an association with depressive disorders rather than suicide in general. The second provides additional support for a selective increase in high-affinity state α2AR as found with platelet α2ARs; indeed, Ordway and colleagues found increased locus coeruleus α2AR density using a radiolabeled agonist but not an antagonist in the same patient samples (Ordway et al., 1994b). Only a single study utilizing a radiolabeled agonist found no alterations in prefrontal cortical or hippocampal α2AR density in postmortem tissue from MDD patients specifically (Klimek et al., 1999). However, as the authors rightly point out, there are important differences between this study and those from the Garcia-Sevilla group. In particular, differences in the precise prefrontal cortex subregions assayed and the length of postmortem delay could most likely account for the discrepancy. Thus, these findings collectively suggest that regulation of receptors in depressive disorders is likely to vary considerably among different brain regions and even highly localized subregions.
The selective increases in the density of high-affinity state α2ARs in brain regions of depressed suicide completers are indicative of enhanced G protein coupling and receptor activity. Indeed, an investigation utilizing a GTPγS binding technique in postmortem tissue demonstrated enhanced G protein coupling to prefrontal cortex α2ARs, but not to other GPCRs including 5HT1A, μ-opioid, GABAB, and muscarinic receptors previously shown to be upregulated in depressed suicide completers (Gonzalez-Maeso et al., 2002). This finding has been characterized by the authors as “selective supersensitivity of α2ARs” (Gonzalez-Maeso et al., 2002), and has been more recently supported by a separate study through assays of α2AR-mediated G protein coupling and adenylyl cyclase inhibition in whole frontal cortex tissue (Valdizán et al., 2010).
Enhanced G protein coupling to α2ARs in depressive disorders could result from alterations in the heterotrimeric G proteins themselves. Indeed, an elevated density of Gαi2/Gαo (members of the G protein subfamily to which the α2AR classically couples) subunits has been found in platelets from MDD patients; this was subsequently normalized by antidepressant drug treatment (García-Sevilla et al., 1997). An upregulation of Gαi2 proteins has also been observed in postmortem prefrontal cortex tissue from untreated depressed suicide completers, while this alteration was not observed in patients subjected antidepressant treatment (Garcia-Sevilla et al., 1999).
Elevated G protein coupling to α2ARs in depressive disorders may also be due to changes in non-G protein interacting partners (details in section 7). It is well-appreciated that GPCR kinases (GRKs) and arrestins play a key role in terminating G protein coupling to receptors (Premont and Gainetdinov, 2007; Shenoy and Lefkowitz, 2011). Reductions in expression of both GRK2/3 (Garcia-Sevilla et al., 2010; García-Sevilla et al., 2004; Matuzany-Ruban et al., 2010) and arrestin2 (Avissar et al., 2004; Matuzany-Ruban et al., 2005) have been reported in MDD patients. Such alterations may contribute to enhanced G protein coupling to α2ARs in these patients. In addition, we have identified that binding of the scaffolding protein spinophilin to α2ARs reduces G protein coupling to the receptor (Lu et al., 2010). A downregulation of spinophilin, as reported previously in brain tissue from MDD patients (Law et al., 2004), would also result in enhanced G protein coupling to α2ARs.
3.3 Modeling α2AR activity through physiological responses
A final approach to studying α2ARs in depressed patients has been to assay receptor activity by measuring centrally-mediated α2AR physiological responses to the classical agonist clonidine. A selective increase of high-affinity state α2ARs in patients with depression, as discussed above, would be expected to result in enhanced sensitivity and responsiveness to α2AR agonist administration in these patients. Indeed, several studies have found enhanced physiological α2AR responses to clonidine (Coote et al., 1998; Paparrigopoulos et al., 2001), and this enhancement was normalized following antidepressant treatments (Balldin et al., 1992; Charney et al., 1981; Coote et al., 1998; Corn et al., 1984; Glass et al., 1982; Schittecatte et al., 2002). However, conflicting results of diminished (Schatzberg and Schildkraut, 1995; Schittecatte et al., 2002), and no changes to (Heninger et al., 1988; Trestman et al., 1992) α2AR activity in depressed patients have also been reported. These discrepant results are most likely explained by the diverse array of methodologies used to assay α2AR activity, which include assessment of classic α2AR agonist effects such as sedation and hypotension, measurement of peripheral NE and NE metabolite levels, measurement of hormone levels modulated by clonidine administration, and the novel clonidine REM sleep suppression test developed by Schittecatte and colleagues (Schittecatte et al., 2002). A complete understanding of the neuronal loci and signaling pathways responsible for these various responses is currently lacking and will be necessary to properly interpret these kinds of studies.
3.4 Clinical genetic studies
Not surprisingly, given the evidence outlined in the preceding sections, a number of studies have been undertaken to investigate possible genetic links between the α2AR subtypes and depressive disorders. We have recently reviewed genetic evidence for α2AR involvement in depressive disorders (Cottingham et al., 2011a), and so here we will simply emphasize a few of the most intriguing of these studies. The first comes from Sequeira and colleagues, who uncovered a possible link between the N251K variant of the α2AAR and suicide, with the mutant allele found only in the suicide group and not in matched controls (Sequeira et al., 2004). Given that the N251K variant is a gain-of-function mutant (Small et al., 2000a), this association would provide a potential genetic basis for at least some of the cases of α2AR supersensitivity reported in studies of postmortem tissue as reviewed above. Another intriguing study by Neumeister and colleagues has provided the first clinical evidence to-date for involvement of the α2CAR subtype in depressive disorders. The authors utilized a positron emission tomography (PET) imaging approach to measure neuronal activity in response to viewing of happy and sad facial expressions, finding that the Del322-325 variant of the α2CAR, a loss-of-function mutant (Small et al., 2000b), was associated with enhanced neuronal responsiveness to sad facial expressions in subjects with a history of MDD (Neumeister et al., 2006). Further investigation is necessary to elucidate the specific roles and contributions of specific α2AR subtypes in the context of depressive disorders.
4. Effects of antidepressant therapy on α2 adrenergic receptors
Given the copious evidence for altered α2AR density associated with depressive disorders, investigations into the impact of effective antidepressant therapies on α2AR density have also been undertaken within the field. Antidepressant therapies, including pharmacotherapy with antidepressants possessing noradrenergic activity (e.g. TCAs, 5HT/NE reuptake inhibitors, mirtazepine, etc.) and other treatments such as electroconvulsive therapy (ECT), have been generally associated with a normalizing effect on α2AR density (i.e. downregulation). Evidence in support of this point comes from both clinical and experimental models, and is summarized in Table 2.
Table 2.
Summary of clinical and experimental evidence for α2AR downregulation induced by antidepressant treatments.
| Model system | Treatment | References |
|---|---|---|
| Patient platelets | TCA |
Garcia-Sevilla et al., 1981, 1986, 1987 Gurguis et al., 1999 Healy et al., 1985 Karege et al., 1992 Piletz et al., 1991 Smith et al., 1983 |
| Mirtazepine ECT |
Garcia-Sevilla et al., 2004 Cooper et al., 1985 Smith et al., 1983 Werstiuk et al., 1996 |
|
| Patient brain tissue | TCA Mixed group of AD drugs |
De Paermentier et al., 1997 Garcia-Sevilla et al., 1999 |
| Rodent brain tissue | TCA |
Barturen and Garcia-Sevilla, 1992 Cottingham et al., 2011b Giaroni et al., 2008 Giralt and Garcia-Sevilla, 1989 Esteban et al., 1999 Mateo et al., 2001 Nomura et al., 1987 Smith et al, 1981 Subhash et al., 2003 |
| MAOI |
Giralt and Garcia-Sevilla, 1989 Mateo et al., 2001 |
Abbreviations: TCA, tricyclic antidepressant; ECT, electroconvulsive therapy; AD, antidepressant; MAOI, monoamine oxidase inhibitor.
4.1 Clinical evidence
Clinically, many studies have found that chronic, symptom-alleviating antidepressant therapies cause reductions in platelet α2AR density, often returning to control levels. Specifically, such an α2AR downregulation response has been associated with chronic TCA (García-Sevilla et al., 1986; Garcia-Sevilla et al., 1987; García-Sevilla et al., 1981; Gurguis et al., 1999; Healy et al., 1985; Karege et al., 1992; Piletz et al., 1991; Smith et al., 1983) and mirtazepine (García-Sevilla et al., 2004) treatment and ECT (Cooper et al., 1985; Smith et al., 1983; Werstiuk et al., 1996), including reductions in both overall receptor density and high-affinity conformational state density. Studies utilizing postmortem brain tissue have also found antidepressant treatment to be associated with a normalizing trend of decreased α2AR density in their patient populations (De Paermentier et al., 1997; Garcia-Sevilla et al., 1999), paralleling the findings on platelet α2ARs. Collectively, these findings indicate that MDD-associated increases in α2AR density are corrected over the course of therapeutically successful antidepressant treatment.
4.2 Experimental evidence
Given the clinical evidence, it is possible to draw a fairly clear connection between experimental studies investigating the phenomenon of antidepressant-induced adaptive alterations in α2AR density and the therapeutic mechanism. Indeed, several studies have reported downregulation of cortical and hippocampal α2ARs through direct assays of receptor expression levels following chronic exposure of rodents to antidepressant drugs (Barturen and Garcia-Sevilla, 1992; Cottingham et al., 2011b; Giaroni et al., 2008; Giralt and Garcia-Sevilla, 1989; Smith et al., 1981; Subhash et al., 2003). Further, studies have reported functional α2AR downregulation in the form of decreased α2AR-mediated responses following chronic exposure of rodents to antidepressant drugs (Esteban et al., 1999; Mateo et al., 2001; Menargues et al., 1990; Nomura et al., 1987). Among those studies, downregulation of both presynaptic autoreceptors (Esteban et al., 1999; Mateo et al., 2001) and postsynaptic α2ARs (Menargues et al., 1990) has been reported. Furthermore, the decreases in α2AR density are likely due to an increased turnover rate for cortical α2ARs (Barturen and Garcia-Sevilla, 1992) rather than regulation of expression at the transcriptional level (Canciani et al., 2006; Giaroni et al., 2008). This is consistent with findings that antidepressant treatment leads to increased expression of GRKs and arrestin (see sections 7.1 and 7.2), which would in turn promote α2AR turnover from the cell surface. Collectively, these findings support the notion that downregulation of central α2AR density is at least a significant component of the therapeutic antidepressant mechanism. It should be noted that of the above studies, only two (Giaroni et al., 2008; Cottingham et al., 2011b) have attempted subtype specificity, showing downregulation of the α2AAR specifically.
Mechanistically, this α2AR downregulation response has been traditionally conceived of as resulting from chronic repetitive receptor stimulation by increased levels of NE. However, our own recent work has provided new insight into the mechanism of antidepressant-induced α2AR downregulation (Cottingham et al., 2011b). We have shown that a physiologically-relevant concentration of NE, corresponding to extracellular levels reached with chronic reuptake inhibition, is in fact unable to sustain any α2AAR downregulation response. Instead, the TCA desipramine, which we identified as an arrestin-biased ligand at the receptor, directly drives reductions in α2AAR density both in vitro and in vivo through recruitment of arrestin to the α2AAR and subsequent arrestin-mediated internalization and downregulation. These findings provide a novel mechanism for therapeutic physiological antidepressant drug action.
It is important to note that antidepressant effects on α2AR expression levels may be both region- and age-dependent. There has been some variability in whether downregulation is observed in cortex, hippocampus, or both, and it has been reported that chronic NE reuptake inhibition stably downregulates presynaptic α2AR autoreceptors but not somatodendritic α2ARs in the LC (Mateo et al., 2001; Parini et al., 2005). In addition, Deupree and colleagues have reported deficits in chronic antidepressant-induced downregulation in juvenile rodents likely owing to developmental immaturity of the α2AR/noradrenergic system (Deupree et al., 2007).
5. The role of α2 adrenergic receptors in animal models of depression
Rodent models have been extensively exploited as a means to experimentally explore roles for the α2ARs in depressive disorders. Mechanistic studies in rodent models can be difficult given the limitations of currently available experimental paradigms, which often suffer from a lack of face and/or construct validity. These issues have been well-discussed by others (Nestler et al., 2002; Nestler and Hyman, 2010; Petit-Demouliere et al., 2005). For our purposes, it seems best to conceptualize the rodent studies as modeling different mechanistic aspects of depression-related neurobiology and antidepressant pharmacology rather than providing definitive answers on α2 adrenergic mechanisms in depression. Such a conceptualization can help to account for discrepancies in this area, although the relative contribution of these different putative mechanisms to the clinical therapeutic antidepressant mechanism of action remains an open question. Regardless of mechanistic complexity, animal models have provided additional confirmation of the importance of α2ARs in depressive disorders.
5.1 Rodent behavioral studies
Some rodent behavioral studies have confirmed a detrimental role for α2ARs in the context of depressive disorders. It has been recently demonstrated that α2AR antagonist treatment causes an enhancement of chronic antidepressant-induced hippocampal neurogenesis and hastens the appearance of antidepressant behavioral effects in the novelty-suppressed feeding paradigm (Yanpallewar et al., 2010). These effects have been postulated to occur through blockade of postsynaptic α2ARs. Meanwhile, in Porsolt’s forced swim test (FST) (Porsolt et al., 1977), administration of the subtype-selective α2AAR antagonist BRL44408 has been reported to exert an acute antidepressant effect (Dwyer et al., 2010). However, reports that α2AR antagonists lacking subtype-specificity do not exert antidepressant effects in the FST (Reneric et al., 2001; Zhang et al., 2009) raise the possibility that blockade of different α2AR subtypes may have opposing effects in this assay. This possibility is supported by the phenotypes of the α2AAR and α2CAR knockout models (see section 5.2 below).
Contrastingly, other studies have indicated that α2AR activation can have antidepressant efficacy in rodents. For example, α2ARs have been consistently implicated in mediating the antidepressant behavioral effects of TCAs in the rodent FST (Cervo et al., 1990; Reneric et al., 2001; Zhang et al., 2009), with some studies demonstrating α2AAR subtype specificity (Cottingham et al., 2012; Schramm et al., 2001). Antidepressant effects of the TCA desipramine in a rodent chronic stress model were also found to be α2AR-dependent (Yalcin et al., 2005). In addition, direct α2AR activation by agonists has been shown to have antidepressant effects on behavior in the FST (Cervo and Samanin, 1991; Cottingham et al., 2012; Stone et al., 2011). These studies are consistent with a mechanism relying on a decrease in locus coeruleus firing activity mediated by somatodendritic α2ARs. Such a phenomenon has been consistently reported with antidepressant administration (Grant and Weiss, 2001; West et al., 2009) and shown to occur in an α2AR-dependent fashion (Berrocoso and Mico, 2007; Grandoso et al., 2005; Linner et al., 1999; Mateo et al., 1998). Therefore, these studies are supportive of an MDD-related increase in LC firing activity which is normalized by antidepressant treatment.
Indeed, there is evidence to directly support dysfunction of the LC in depressive disorders. In LC tissue from MDD patients, both decreased expression of NET (Klimek et al., 1997) and increased expression of tyrosine hydroxylase (Ordway et al., 1994a; Zhu et al., 1999) have been reported. Collectively, these findings are suggestive of secondary adaptive alterations in the LC compensating for a depletion of NE levels in MDD (i.e. decreased NE reuptake activity, increased NE synthesis, and increased neuronal firing activity to enhance noradrenergic transmission).
It is important to note that the above rodent studies have largely reported on acute antidepressant drug effects, and so do not directly model the full clinical therapeutic actions of these treatments. As mentioned above, these studies should be interpreted as modeling different mechanistic aspects, and are supportive of a complex and variable role for α2ARs in the neurobiology of depression and in antidepressant pharmacology. Put another way, these findings suggest that there may be more than one way to obtain an antidepressant effect by modulating noradrenergic neurotransmission.
5.2 α2AR knockout models
Studies using knockout models for the α2A and α2C subtypes have suggested opposing roles for these receptors in the FST. α2AAR-deficient mice were found to have enhanced swim stress-induced behavioral despair (Schramm et al., 2001), while the opposite was true for α2CAR-deficient mice (Sallinen et al., 1999). Although the phenotype of the α2CAR-deficient mice corresponds nicely with the aforementioned clinical genetic study of Neumeister and colleagues (see section 3.4), the phenotype of the α2AAR-deficient mice seems contradictory to the clinical findings. However, it is important to bear in mind that the FST is a pharmacological screening model and is not intended as an etiological model for depressive disorders, and so these mouse models have yet to be truly evaluated for their depressive phenotypes in behavioral paradigms with better face and/or construct validity. As well, it seems likely that global loss of either receptor subtype may have drastically different effects on behavior than the more localized alterations associated with clinical depressive disorders.
6. A working model for α2 adrenergic receptor dysfunction in depressive disorders
A schematic representation of our overall working model for α2AR dysfunction in depressive disorders is presented in Figure 2. Based upon the available evidence, it can be stated that there is clearly involvement of α2ARs in depressive disorders, and their roles certainly appear to be complex and variable. To summarize, it seems reasonable to conclude that depressive disorders are, in at least a significant proportion of cases, accompanied by a physiological upregulation of high affinity state platelet α2ARs, and so techniques geared toward detecting such receptors may have utility as both experimental and potential diagnostic tools. Further, depressive disorders seem to be accompanied by an increase in α2AR density and/or a supersensitivity of α2ARs in the central nervous system. Collectively, these alterations can be presumed to increase α2AR signaling drive in a region-specific fashion, leading to decreases in neurotransmitter release and overall neuronal activity and to corresponding compensatory changes in the LC. Accordingly, successful antidepressant therapies, including antidepressant drugs (particularly noradrenergic drugs) and ECT, are generally associated with α2AR downregulation, an effect which would serve to normalize the elevated α2AR activity. Overall, the findings reviewed here support a model whereby neuroadaptive changes to α2AR density and pharmacological properties, which normalize pathophysiological changes to these receptors, are a component of the therapeutic antidepressant mechanism of action.
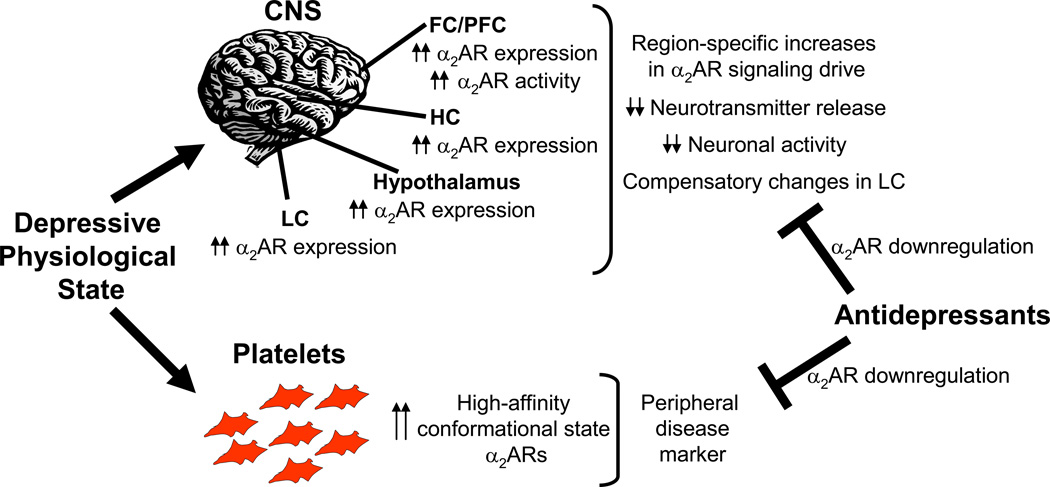
Figure 2.
Working model of α2AR dysfunction in depressive disorders. The depressive physiological state involves various alterations in α2AR expression and function, leading to abnormal α2 noradrenergic signaling activity. Antidepressant therapies (including pharmacological agents and ECT) with noradrenergic effects cause a downregulation of α2AR expression, normalizing the abnormalities.
7. Receptor accessory proteins in depressive disorders
A number of non-G protein interacting partners play important roles in regulating and mediating α2AR function. These proteins include GRKs, arrestins, and spinophilin. It is important to note, of course, that arrestins and GRKs are involved in the function of almost all GPCRs (Premont and Gainetdinov, 2007; Shenoy and Lefkowitz, 2011), while spinophilin has a number of roles in synaptic function in addition to directly regulating multiple GPCRs (Sarrouilhe et al., 2006; Wang and Limbird, 2007). Therefore, alterations in any of these players may have implications beyond α2ARs. Nevertheless, alterations in these key accessory proteins may help to provide a mechanistic basis for the α2AR dysregulation in depressive disorders in addition to potential changes in the G proteins themselves, a topic which has been well-reviewed by Gonzalez-Maeso and Meana (Gonzalez-Maeso and Meana, 2006).
7.1 GRKs
GRKs classically participate in the process of receptor desensitization by phosphorylating conformationally active receptors (Pitcher et al., 1998), which in turn leads to arrestin binding and uncoupling of G proteins, and these kinases have been implicated in a number of disease states (Gurevich et al., 2012). Reductions in GRK2/3 at the protein level (Garcia-Sevilla et al., 2010; García-Sevilla et al., 2004; Matuzany-Ruban et al., 2010) and GRK2 at the mRNA level (Matuzany-Ruban et al., 2010) have been reported in peripheral blood cells obtained from MDD patients. These levels were correspondingly normalized by antidepressant treatment. Lack of sufficient GRK phosphorylation would lead to reduced receptor desensitization and enhanced signaling responses, which may help to explain the enhanced α2AR activity in MDD. Conversely, plasma membrane-associated GRK2 (a cytosolic protein which translocates to the plasma membrane upon receptor activation) was found to be increased in PFC tissue from depressed suicide completers but not in tissue from patients subjected to antidepressant treatment (Garcia-Sevilla et al., 1999; Grange-Midroit et al., 2003). This increase in plasma membrane-associated GRK2 was correlated with the elevated level of the α2AAR and Gαi observed in the PFC of the same patients, and may be indicative of cellular efforts to compensate for elevated receptor activity. Taken together, these findings clearly support a role for GRKs both in the neurobiology of depression and in antidepressant pharmacology. However, given the large number of GPCRs that are regulated by GRKs in the central nervous system, the involvement of GRKs in depressive disorders is likely to be complicated and will require further investigation.
7.2 Arrestins
Arrestins bind to GRK-phosphorylated receptors and mediate receptor desensitization and internalization (Premont and Gainetdinov, 2007; Shenoy and Lefkowitz, 2011). The ubiquitously-expressed arrestins, arrestin2 and 3 (also called β-arrestin1 and 2), have also been investigated for possible links to depressive disorders. Much of the support for a role for arrestin in depressive disorders is indirect at this point, as recently reviewed by Golan and colleagues (Golan et al., 2009). However, direct studies have been attempted. Arrestin2 has been reported to be decreased at both the protein and mRNA levels in leukocytes obtained from MDD patients (Avissar et al., 2004; Matuzany-Ruban et al., 2005). Such a reduction may contribute to enhanced G protein coupling to α2ARs in these patients, given the classical role of arrestin in uncoupling G proteins from receptors. Correspondingly, antidepressant treatment has been shown to increase arrestin2 expression in patient leukocytes (Matuzany-Ruban et al., 2005). Experimental evidence supports antidepressant-induced increases in arrestin2 expression in rodent neural tissue (Avissar et al., 2004; Golan et al., 2011). Intriguingly, the clinical study demonstrated that during the course of antidepressant treatment, the rebound in arrestin2 density preceded the onset of symptom relief (Matuzany-Ruban et al., 2005). Such a biomarker role for arrestin is supported by a recent genome wide expression profiling study in a leukocyte cell model which identified an arrestin gene as a potential marker for the clinical response to paroxetine (Morag et al., 2011).
With regard to arrestin3, a study utilizing postmortem PFC tissue from MDD patients found no alterations in arrestin3 protein levels (Grange-Midroit et al., 2003). Experimentally, a role for arrestin3 in the antidepressant response is strongly suggested by our own recent studies, which identified the TCA desipramine as a direct arrestin3-biased ligand at the α2AAR and demonstrated that chronic desipramine exposure drove arrestin3-dependent downregulation of central α2AARs in vivo (Cottingham et al., 2011b). In addition, we have reported that the acute antidepressant response elicited by desipramine in the FST is both α2AAR- and arrestin3-dependent (Cottingham et al., 2012). Our findings indicate that the involvement of arrestin is variable in nature, as the response to the serotonergic drug fluoxetine does not require α2AARs and is actually inhibited by arrestin3. Therefore, the arrestins have specific roles in regulating the GPCRs involved in responses to differing antidepressants (i.e. α2ARs with the noradrenergic drug desipramine versus 5HT receptors with the serotonergic drug fluoxetine).
7.3 Spinophilin
Spinophilin is a dendritic spine-enriched scaffolding protein (Allen et al., 1997; Satoh et al., 1998) which regulates the activity of multiple GPCRs (Sarrouilhe et al., 2006; Wang and Limbird, 2007). We have previously reported that spinophilin interferes with coupling of the α2AAR to cognate G proteins in the mouse brain (Lu et al., 2010). In the context of depressive disorders, reduced spinophilin expression has been reported in hippocampal tissue obtained from MDD patients (Law et al., 2004) and in cortical tissue from animal models of stress-induced depressive behavior (Law et al., 2009; Leussis and Andersen, 2008). Such alterations in spinophilin would result in enhanced G protein coupling to the α2AAR, which may contribute to enhanced high-affinity state α2AR density in depressive patients. Furthermore, our laboratory has established spinophilin as a functional antagonist of arrestin functions at activated α2ARs regulating in vivo response sensitivity to α2AR agonists (Wang et al., 2004). Consistent with this finding, we have recently demonstrated that the acute α2AAR- and arrestin3-dependent antidepressant response to desipramine is enhanced in spinophilin-deficient mice (Cottingham et al., 2012), indicating a role for spinophilin and this α2AR regulatory system in antidepressant pharmacology. In addition, an association between decreased dendritic spine density and depression has been suggested both clinically (Soetanto et al., 2010) and experimentally (Hajszan et al., 2009), further implicating spinophilin given its importance to spine formation and function (Feng et al., 2000). Collectively, these findings strongly suggest that dysregulation of spinophilin may make a contribution to depressive disorders, potentially related to both its α2AR and synaptic regulatory functions.
8. Conclusions and perspectives
Based upon the evidence presented here, there is clear support for dysfunction of α2ARs and some key α2AR regulators in depressive disorders. As summarized in Tables 1 and 2 and Figure 2, available evidence indicates that an upregulation of α2ARs, either in terms of absolute expression level or overall receptor activity, represents a valid component of the physiological state of depressive disorders. This α2AR dysregulation would have clear consequences to noradrenergic neurotransmission in the brain, given the important role for α2ARs in regulating the noradrenergic system. Alterations in other components of the receptor system, including heterotrimeric G proteins, GRKs, arrestin, and spinophilin may contribute to α2AR dysfunction. In fact, some evidence is suggestive of coordinated changes in both the receptor and its partner proteins in the neurobiology of depression and in response to antidepressant therapy. Future investigations examining the full receptor system may be particularly useful in elucidating the relationship between alterations in the receptor and alterations in its interacting partners.
While our review of the literature has clarified and underscored the importance of α2AR dysregulation in depressive disorders, this is most likely not the sole causative factor in depressive disorders, given that the neurobiology of depressive disorders is clearly complex and multifactorial. There is almost certainly some etiological heterogeneity in this class of disorders, with α2AR dysfunction being of great importance in some cases but less so in others. Further, although the alterations in the α2AR system outlined in this review carry significant consequences for central nervous system function, it is presently unclear if they are symptomatic or in fact causative in the putative noradrenergic pathobiology of depression. In other words, α2AR alterations could certainly cause LC dysfunction, but they could also be adaptive changes secondary to LC dysfunction with some other root cause. Even as a secondary change, α2AR alterations could certainly exacerbate the pathobiological changes. These issues remain to be resolved.
Although strongly implicating α2ARs in depressive disorders, the current body of literature on this subject has several drawbacks. One such drawback is a dearth of subtype-selective studies. Although the α2A subtype seems a likely culprit in most of the reports on α2ARs given its predominance in the central nervous system, the α2C subtype should certainly not be ignored. Future studies should be aimed at identifying potential subtype-selective roles within the α2AR family. Another drawback is the extreme methodological variability. Methodology plays an important role in influencing the outcomes of these studies, especially with regard to the identity of the radioligand in the binding assays most commonly used to assay receptor density. Although at first glance there appear to be great contradictions among these studies, accounting for methodological differences reveals a convincing case for upregulation of high-affinity conformational state α2ARs in depressive disorders. Assaying for this parameter in platelet samples from depressed patients may have use as a diagnostic tool in directing antidepressant therapy. For example, patients with this symptom may benefit more strongly from antidepressant drugs such as desipramine with noradrenergic specificity and which can drive robust α2AR downregulation.
Finally, it is important to note that our present knowledge on the state of central α2ARs is limited to studies which have in turn been limited by the almost exclusive use of brain tissue from suicide completers. Suicidality is not a universal feature of depressive disorders, and so it is possible that α2AR abnormalities observed in these studies apply more specifically to depressive suicidality. At any rate, these findings indicate that depression with suicidality may respond particularly well to strongly noradrenergic antidepressants.
The ability to directly study central α2ARs in living patients suffering from depressive disorders is currently lacking. However, the advent of PET methodology raises the possibility of being able to do this in the future. PET has already shown promise for assaying central protein levels in living patients, with the Pittsburgh compound B agent for labeling β-amyloid plaques in Alzheimer’s disease a conspicuous example (Sweatt, 2010). Indeed, there has been some progress, albeit uneven, in designing labeled α2AR ligands for PET studies (Jakobsen et al., 2006; Marthi et al., 2004; Prabhakaran et al., 2010). It will be important, of course, to bear in mind the choice of agonist versus antagonist for PET ligands. This methodology would allow investigators to scan depressive patients for central α2AR density, and then monitor changes to that density over a course of antidepressant treatment. The information that can be obtained from such studies would be invaluable in advancing our understanding of noradrenergic dysfunction in depressive disorders, building upon the existing knowledge base which we have reviewed here.
Highlights.
-
>
Evidence for α2 adrenergic receptor dysregulation in depression is reviewed.
-
>
The state of key receptor accessory proteins in depression is also appraised.
-
>
Major depression is associated with α2 adrenergic receptor elevation.
-
>
Antidepressant therapies normalize upregulated α2 adrenergic receptor levels.
-
>
New insights with clinical and basic science implications are uncovered.
Acknowledgments
This work has been supported by the UAB Training Program in Neurobiology of Cognition and Cognitive Disorders [National Institutes of Health T32 grant NS061788-03, CC] and the National Institute of Mental Health [grant MH081917, QW].
Abbreviations
- AD
antidepressant drug
- AR
adrenergic receptor
- ECT
electroconvulsive therapy
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- LC
locus coeruleus
- MAOI
monoamine oxidase inhibitor
- MDD
major depressive disorder
- NE
norepinephrine
- NET
norepinephrine transporter
- PET
positron emission tomography
- TCA
tricyclic antidepressant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc.Natl.Acad.Sci.U.S.A. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, Kobilka BK, Hein L. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol.Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Sved AF, Mann JJ. Quantitative autoradiography of alpha 1- and alpha 2-adrenergic receptors in the cerebral cortex of controls and suicide victims. Brain Res. 1993;630:271–282. doi: 10.1016/0006-8993(93)90666-b. [DOI] [PubMed] [Google Scholar]
- Avissar S, Matuzany-Ruban A, Tzukert K, Schreiber G. Beta-arrestin-1 levels: reduced in leukocytes of patients with depression and elevated by antidepressants in rat brain. Am J Psychiatry. 2004;161:2066–2072. doi: 10.1176/appi.ajp.161.11.2066. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ. Drug therapy of depression and anxiety disorders. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, Inc.; 2006. pp. 429–460. [Google Scholar]
- Balldin J, Berggren U, Lindstedt G, Modigh K. Neuroendocrine evidence for decreased function of alpha 2-adrenergic receptor after electroconvulsive therapy. Psychiatry Res. 1992;41:257–265. doi: 10.1016/0165-1781(92)90007-p. [DOI] [PubMed] [Google Scholar]
- Barturen F, Garcia-Sevilla JA. Long term treatment with desipramine increases the turnover of alpha 2-adrenoceptors in the rat brain. Mol.Pharmacol. 1992;42:846–855. [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N.Engl.J.Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Berrocoso E, Mico JA. In vivo effect of venlafaxine on locus coeruleus neurons: role of opioid, alpha(2)-adrenergic, and 5-hydroxytryptamine(1A) receptors. J Pharmacol Exp Ther. 2007;322:101–107. doi: 10.1124/jpet.107.120915. [DOI] [PubMed] [Google Scholar]
- Bhatia SC, Hsieh HH, Theesen KA, Townley RG, Andersen JM, Weiss S, Agrawal DK. Platelet alpha-2 adrenoreceptor activity pre-treatment and post-treatment in major depressive disorder with melancholia. Res Commun Chem Pathol Pharmacol. 1991;74:47–57. [PubMed] [Google Scholar]
- Brede M, Philipp M, Knaus A, Muthig V, Hein L. alpha2-adrenergic receptor subtypes - novel functions uncovered in gene-targeted mouse models. Biol Cell. 2004;96:343–348. doi: 10.1016/j.biolcel.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Brum PC, Hurt CM, Shcherbakova OG, Kobilka B, Angelotti T. Differential targeting and function of alpha2A and alpha2C adrenergic receptor subtypes in cultured sympathetic neurons. Neuropharmacology. 2006;51:397–413. doi: 10.1016/j.neuropharm.2006.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Callado LF, Meana JJ, Grijalba B, Pazos A, Sastre M, Garcia-Sevilla JA. Selective increase of alpha2A-adrenoceptor agonist binding sites in brains of depressed suicide victims. J.Neurochem. 1998;70:1114–1123. doi: 10.1046/j.1471-4159.1998.70031114.x. [DOI] [PubMed] [Google Scholar]
- Canciani L, Giaroni C, Zanetti E, Giuliani D, Pisani R, Moro E, Trinchera M, Crema F, Lecchini S, Frigo G. Functional interaction between alpha2-adrenoceptors, mu- and kappa-opioid receptors in the guinea pig myenteric plexus: effect of chronic desipramine treatment. Eur J Pharmacol. 2006;553:269–279. doi: 10.1016/j.ejphar.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Carstens ME, Engelbrecht AH, Russell VA, Aalbers C, Gagiano CA, Chalton DO, Taljaard JJ. Alpha 2-adrenoceptor levels on platelets of patients with major depressive disorders. Psychiatry Res. 1986;18:321–331. doi: 10.1016/0165-1781(86)90017-x. [DOI] [PubMed] [Google Scholar]
- Cervo L, Grignaschi G, Samanin R. Alpha 2-adrenoceptor blockade prevents the effect of desipramine in the forced swimming test. Eur.J.Pharmacol. 1990;175:301–307. doi: 10.1016/0014-2999(90)90568-q. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Clonidine causes antidepressant-like effects in rats by activating alpha 2-adrenoceptors outside the locus coeruleus. Eur.J.Pharmacol. 1991;193:309–313. doi: 10.1016/0014-2999(91)90144-f. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Sternberg DE, Redmond DE, Leckman JF, Maas JW, Roth RH. Presynaptic adrenergic receptor sensitivity in depression. The effect of long-term desipramine treatment. Arch Gen Psychiatry. 1981;38:1334–1340. doi: 10.1001/archpsyc.1981.01780370036004. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Kelly JG, King DJ. Adrenergic receptors in depression. Effects of electroconvulsive therapy. Br J Psychiatry. 1985;147:23–29. doi: 10.1192/bjp.147.1.23. [DOI] [PubMed] [Google Scholar]
- Coote M, Wilkins A, Werstiuk ES, Steiner M. Effects of electroconvulsive therapy and desipramine on neuroendocrine responses to the clonidine challenge test. J Psychiatry Neurosci. 1998;23:172–178. [PMC free article] [PubMed] [Google Scholar]
- Corn TH, Thompson C, Checkley SA. Effects of desipramine treatment upon central adrenoceptor function in normal subjects. Br J Psychiatry. 1984;145:139–145. doi: 10.1192/bjp.145.2.139. [DOI] [PubMed] [Google Scholar]
- Cottingham C, Chen H, Chen Y, Peng Y, Wang Q. Genetic variations of alpha2-adrenergic receptors illuminate the diversity of receptor functions. In: Wang Q, editor. Current Topics in Membranes. Volume 67. Amsterdam: Elsevier Inc.; 2011a. [DOI] [PubMed] [Google Scholar]
- Cottingham C, Chen Y, Jiao K, Wang Q. The antidepressant desipramine is an arrestin-biased ligand at the alpha2A adrenergic receptor driving receptor downregulation in vitro and in vivo. J.Biol.Chem. 2011b;286:36063–36075. doi: 10.1074/jbc.M111.261578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham C, Li X, Wang Q. Noradrenergic antidepressant responses to desipramine in vivo are reciprocally regulated by arrestin3 and spinophilin. Neuropharmacology. 2012;62:2353–2361. doi: 10.1016/j.neuropharm.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- De Paermentier F, Mauger JM, Lowther S, Crompton MR, Katona CL, Horton RW. Brain alpha-adrenoceptors in depressed suicides. Brain Res. 1997;757:60–68. doi: 10.1016/s0006-8993(97)00138-8. [DOI] [PubMed] [Google Scholar]
- De Vos H, Vauquelin G, De KJ, De Backer JP, Van L, I Regional distribution of alpha 2A- and alpha 2B-adrenoceptor subtypes in postmortem human brain. J.Neurochem. 1992;58:1555–1560. doi: 10.1111/j.1471-4159.1992.tb11378.x. [DOI] [PubMed] [Google Scholar]
- Deupree JD, Reed AL, Bylund DB. Differential effects of the tricyclic antidepressant desipramine on the density of adrenergic receptors in juvenile and adult rats. J Pharmacol Exp Ther. 2007;321:770–776. doi: 10.1124/jpet.106.118935. [DOI] [PubMed] [Google Scholar]
- Dwyer JM, Platt BJ, Rizzo SJ, Pulicicchio CM, Wantuch C, Zhang MY, Cummons T, Leventhal L, Bender CN, Zhang J, Kowal D, Lu S, Rajarao SJ, Smith DL, Shilling AD, Wang J, Butera J, Resnick L, Rosenzweig-Lipson S, Schechter LE, Beyer CE. Preclinical characterization of BRL 44408: antidepressant- and analgesic-like activity through selective alpha2A-adrenoceptor antagonism. Int J Neuropsychopharmacol. 2010;13:1193–1205. doi: 10.1017/S1461145709991088. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ozaita A, Garcia-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- Esteban S, Llado J, Sastre-Coll A, Garcia-Sevilla JA. Activation and desensitization by cyclic antidepressant drugs of alpha2-autoreceptors, alpha2-heteroreceptors and 5-HT1A-autoreceptors regulating monamine synthesis in the rat brain in vivo. Naunyn Schmiedebergs Arch.Pharmacol. 1999;360:135–143. doi: 10.1007/s002109900045. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc.Natl.Acad.Sci.U.S.A. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Alvaro-Bartolome M, Diez-Alarcia R, Ramos-Miguel A, Puigdemont D, Perez V, Alvarez E, Meana JJ. Reduced platelet G protein-coupled receptor kinase 2 in major depressive disorder: antidepressant treatment-induced upregulation of GRK2 protein discriminates between responder and non-responder patients. Eur Neuropsychopharmacol. 2010;20:721–730. doi: 10.1016/j.euroneuro.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Escriba PV, Ozaita A, La HR, Walzer C, Eytan A, Guimon J. Up-regulation of immunolabeled alpha2A-adrenoceptors, Gi coupling proteins, and regulatory receptor kinases in the prefrontal cortex of depressed suicides. J.Neurochem. 1999;72:282–291. doi: 10.1046/j.1471-4159.1999.0720282.x. [DOI] [PubMed] [Google Scholar]
- García-Sevilla JA, Guimón J, García-Vallejo P, Fuster MJ. Biochemical and functional evidence of supersensitive platelet alpha 2-adrenoceptors in major affective disorder. Effect of long-term lithium carbonate treatment. Arch Gen Psychiatry. 1986;43:51–57. doi: 10.1001/archpsyc.1986.01800010053007. [DOI] [PubMed] [Google Scholar]
- García-Sevilla JA, Padró D, Giralt MT, Guimón J, Areso P. Alpha 2-adrenoceptor-mediated inhibition of platelet adenylate cyclase and induction of aggregation in major depression. Effect of long-term cyclic antidepressant drug treatment. Arch Gen Psychiatry. 1990;47:125–132. doi: 10.1001/archpsyc.1990.01810140025005. [DOI] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Udina C, Fuster MJ, Alvarez E, Casas M. Enhanced binding of [3H] (-) adrenaline to platelets of depressed patients with melancholia: effect of long-term clomipramine treatment. Acta Psychiatr Scand. 1987;75:150–157. doi: 10.1111/j.1600-0447.1987.tb02767.x. [DOI] [PubMed] [Google Scholar]
- García-Sevilla JA, Ventayol P, Pérez V, Rubovszky G, Puigdemont D, Ferrer-Alcón M, Andreoli A, Guimón J, Alvarez E. Regulation of platelet alpha 2A-adrenoceptors, Gi proteins and receptor kinases in major depression: effects of mirtazapine treatment. Neuropsychopharmacology. 2004;29:580–588. doi: 10.1038/sj.npp.1300356. [DOI] [PubMed] [Google Scholar]
- García-Sevilla JA, Walzer C, Busquets X, Escribá PV, Balant L, Guimón J. Density of guanine nucleotide-binding proteins in platelets of patients with major depression: increased abundance of the G alpha i2 subunit and down-regulation by antidepressant drug treatment. Biol Psychiatry. 1997;42:704–712. doi: 10.1016/s0006-3223(96)00493-3. [DOI] [PubMed] [Google Scholar]
- García-Sevilla JA, Zis AP, Hollingsworth PJ, Greden JF, Smith CB. Platelet alpha 2-adrenergic receptors in major depressive disorder. Binding of tritiated clonidine before and after tricyclic antidepressant drug treatment. Arch Gen Psychiatry. 1981;38:1327–1333. doi: 10.1001/archpsyc.1981.01780370029003. [DOI] [PubMed] [Google Scholar]
- Giaroni C, Canciani L, Zanetti E, Giuliani D, Pisani R, Oldrini R, Moro E, Trinchera M, Crema F, Lecchini S, Frigo G. Effects of chronic desipramine treatment on alpha2-adrenoceptors and mu-opioid receptors in the guinea pig cortex and hippocampus. Eur J Pharmacol. 2008;579:116–125. doi: 10.1016/j.ejphar.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Albarran-Juarez J, Hein L. Pre- versus postsynaptic signaling by alpha2-adrenoceptors. In: Wang Q, editor. Current Topics in Membranes. Volume 67. Amsterdam: Elsevier, Inc.; 2011. pp. 139–160. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Hein L. Are the pharmacology and physiology of alpha adrenoceptors determined by alpha-heteroreceptors and autoreceptors respectively? Br J Pharmacol. 2012;165:90–102. doi: 10.1111/j.1476-5381.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsbach R, Roser C, Beetz N, Brede M, Hadamek K, Haubold M, Leemhuis J, Philipp M, Schneider J, Urbanski M, Szabo B, Weinshenker D, Hein L. Genetic dissection of alpha2-adrenoceptor functions in adrenergic versus nonadrenergic cells. Mol.Pharmacol. 2009;75:1160–1170. doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- Giralt MT, Garcia-Sevilla JA. Acute and long-term regulation of brain alpha 2-adrenoceptors after manipulation of noradrenergic transmission in the rat. Eur.J.Pharmacol. 1989;164:455–466. doi: 10.1016/0014-2999(89)90253-7. [DOI] [PubMed] [Google Scholar]
- Glass IB, Checkley SA, Shur E, Dawling S. The effect of desipramine upon central adrenergic function in depressed patients. Br J Psychiatry. 1982;141:372–376. doi: 10.1192/bjp.141.4.372. [DOI] [PubMed] [Google Scholar]
- Golan M, Schreiber G, Avissar S. Antidepressants, beta-arrestins and GRKs: from regulation of signal desensitization to intracellular multifunctional adaptor functions. Curr Pharm Des. 2009;15:1699–1708. doi: 10.2174/138161209788168038. [DOI] [PubMed] [Google Scholar]
- Golan M, Schreiber G, Avissar S. Antidepressants elevate GDNF expression and release from C glioma cells in a beta-arrestin1-dependent, CREB interactive pathway. Int J Neuropsychopharmacol. 2011;14:1289–1300. doi: 10.1017/S1461145710001550. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Meana JJ. Heterotrimeric g proteins: insights into the neurobiology of mood disorders. Curr Neuropharmacol. 2006;4:127–138. doi: 10.2174/157015906776359586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Rodriguez-Puertas R, Meana JJ, Garcia-Sevilla JA, Guimon J. Neurotransmitter receptor-mediated activation of G-proteins in brains of suicide victims with mood disorders: selective supersensitivity of alpha(2A)-adrenoceptors. Mol.Psychiatry. 2002;7:755–767. doi: 10.1038/sj.mp.4001067. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Pascual J, Meana JJ, Barturen F, del Arco C, Pazos A, Garcia-Sevilla JA. Autoradiographic demonstration of increased alpha 2-adrenoceptor agonist binding sites in the hippocampus and frontal cortex of depressed suicide victims. J Neurochem. 1994;63:256–265. doi: 10.1046/j.1471-4159.1994.63010256.x. [DOI] [PubMed] [Google Scholar]
- Grandoso L, Torrecilla M, Pineda J, Ugedo L. alpha(2)-Adrenoceptor involvement in the in vitro inhibitory effect of citalopram on a subpopulation of rat locus coeruleus neurons. Eur J Pharmacol. 2005;517:51–58. doi: 10.1016/j.ejphar.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Grange-Midroit M, García-Sevilla JA, Ferrer-Alcón M, La Harpe R, Huguelet P, Guimón J. Regulation of GRK 2 and 6, beta-arrestin-2 and associated proteins in the prefrontal cortex of drug-free and antidepressant drug-treated subjects with major depression. Brain Res Mol Brain Res. 2003;111:31–41. doi: 10.1016/s0169-328x(02)00667-8. [DOI] [PubMed] [Google Scholar]
- Grant MM, Weiss JM. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biol Psychiatry. 2001;49:117–129. doi: 10.1016/s0006-3223(00)00936-7. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Weizman A, Fieldust SJ, Israeli M, Biegon A. Unaltered alpha(2)-noradrenergic/imidazoline receptors in suicide victims: a postmortem brain autoradiographic analysis. Eur Neuropsychopharmacol. 2000;10:265–271. doi: 10.1016/s0924-977x(00)00075-4. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurguis GN, Vo SP, Griffith JM, Rush AJ. Platelet alpha2A-adrenoceptor function in major depression: Gi coupling, effects of imipramine and relationship to treatment outcome. Psychiatry Res. 1999;89:73–95. doi: 10.1016/s0165-1781(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D, Carney PA, O'Halloran A, Leonard BE. Peripheral adrenoceptors and serotonin receptors in depression. Changes associated with response to treatment with trazodone or amitriptyline. J Affect Disord. 1985;9:285–296. doi: 10.1016/0165-0327(85)90059-x. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Charney DS, Price LH. alpha 2-Adrenergic receptor sensitivity in depression. The plasma MHPG, behavioral, and cardiovascular responses to yohimbine. Arch Gen Psychiatry. 1988;45:718–726. doi: 10.1001/archpsyc.1988.01800320028003. [DOI] [PubMed] [Google Scholar]
- Hindmarch I. Expanding the horizons of depression: beyond the monoamine hypothesis. Hum Psychopharmacol. 2001;16:203–218. doi: 10.1002/hup.288. [DOI] [PubMed] [Google Scholar]
- Jakobsen S, Pedersen K, Smith DF, Jensen SB, Munk OL, Cumming P. Detection of alpha2-adrenergic receptors in brain of living pig with 11C-yohimbine. J Nucl Med. 2006;47:2008–2015. [PubMed] [Google Scholar]
- Kable JW, Murrin LC, Bylund DB. In vivo gene modification elucidates subtype-specific functions of alpha(2)-adrenergic receptors. J Pharmacol Exp Ther. 2000;293:1–7. [PubMed] [Google Scholar]
- Kaneko M, Kanno T, Honda K, Mashiko H, Oosuga N, Watanabe A, Kumashiro H. Platelet alpha-2 adrenergic receptor binding and plasma free 3-methoxy-4-hydroxyphenylethylene glycol in depressed patients before and after treatment with mianserin. Neuropsychobiology. 1992;25:14–19. doi: 10.1159/000118803. [DOI] [PubMed] [Google Scholar]
- Karege F, Bovier P, Widmer J, Gaillard JM, Tissot R. Platelet membrane alpha 2-adrenergic receptors in depression. Psychiatry Res. 1992;43:243–252. doi: 10.1016/0165-1781(92)90057-a. [DOI] [PubMed] [Google Scholar]
- Katona CL, Theodorou AE, Davies SL, Hale AS, Kerry SM, Horton RW, Kelly JS, Paykel ES. [3H]yohimbine binding to platelet alpha 2-adrenoceptors in depression. J Affect Disord. 1989;17:219–228. doi: 10.1016/0165-0327(89)90003-7. [DOI] [PubMed] [Google Scholar]
- Klimek V, Rajkowska G, Luker SN, Dilley G, Meltzer HY, Overholser JC, Stockmeier CA, Ordway GA. Brain noradrenergic receptors in major depression and schizophrenia. Neuropsychopharmacology. 1999;21:69–81. doi: 10.1016/S0893-133X(98)00134-1. [DOI] [PubMed] [Google Scholar]
- Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus AE, Muthig V, Schickinger S, Moura E, Beetz N, Gilsbach R, Hein L. Alpha2-adrenoceptor subtypes--unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem.Int. 2007;51:277–281. doi: 10.1016/j.neuint.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Feldon J, Pryce CR, Harrison PJ. Gene expression in the anterior cingulate cortex and amygdala of adolescent marmoset monkeys following parental separations in infancy. Int J Neuropsychopharmacol. 2009;12:761–772. doi: 10.1017/S1461145708009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am.J.Psychiatry. 2004;161:1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Limbird LE. Receptors linked to inhibition of adenylate cyclase: additional signaling mechanisms. FASEB J. 1988;2:2686–2695. doi: 10.1096/fasebj.2.11.2840317. [DOI] [PubMed] [Google Scholar]
- Limbird LE. Cell Surface Receptors: A Short Course on Theory and Methods. New York: Springer Science+Business Media, Inc.; 2005. [Google Scholar]
- Linner L, Arborelius L, Nomikos GG, Bertilsson L, Svensson TH. Locus coeruleus neuronal activity and noradrenaline availability in the frontal cortex of rats chronically treated with imipramine: effect of alpha 2-adrenoceptor blockade. Biol Psychiatry. 1999;46:766–774. doi: 10.1016/s0006-3223(99)00126-2. [DOI] [PubMed] [Google Scholar]
- Lu R, Chen Y, Cottingham C, Peng N, Jiao K, Limbird LE, Wyss JM, Wang Q. Enhanced hypotensive, bradycardic, and hypnotic responses to alpha2-adrenergic agonists in spinophilin-null mice are accompanied by increased G protein coupling to the alpha2A-adrenergic receptor. Mol.Pharmacol. 2010;78:279–286. doi: 10.1124/mol.110.065300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Masala I, Di Nasso E, Giannaccini G, Betti L, Lucacchini A, Cassano GB. Correlation between platelet alpha(2)-adrenoreceptors and symptom severity in major depression. Neuropsychobiology. 2001;44:122–125. doi: 10.1159/000054930. [DOI] [PubMed] [Google Scholar]
- Marthi K, Jakobsen S, Bender D, Hansen SB, Smith SB, Hermansen F, Rosenberg R, Smith DF. [N-methyl-11C]Mirtazapine for positron emission tomography neuroimaging of antidepressant actions in humans. Psychopharmacology (Berl) 2004;174:260–265. doi: 10.1007/s00213-003-1754-x. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Fernandez-Pastor B, Meana JJ. Acute and chronic effects of desipramine and clorgyline on alpha(2)-adrenoceptors regulating noradrenergic transmission in the rat brain: a dual-probe microdialysis study. Br.J.Pharmacol. 2001;133:1362–1370. doi: 10.1038/sj.bjp.0704196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Pineda J, Meana JJ. Somatodendritic alpha2-adrenoceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J.Neurochem. 1998;71:790–798. doi: 10.1046/j.1471-4159.1998.71020790.x. [DOI] [PubMed] [Google Scholar]
- Matuzany-Ruban A, Avissar S, Schreiber G. Dynamics of beta-arrestin1 protein and mRNA levels elevation by antidepressants in mononuclear leukocytes of patients with depression. J Affect Disord. 2005;88:307–312. doi: 10.1016/j.jad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Matuzany-Ruban A, Golan M, Miroshnik N, Schreiber G, Avissar S. Normalization of GRK2 protein and mRNA measures in patients with depression predict response to antidepressants. Int J Neuropsychopharmacol. 2010;13:83–91. doi: 10.1017/S1461145709000364. [DOI] [PubMed] [Google Scholar]
- Meana JJ, Barturen F, Garcia-Sevilla JA. Alpha 2-adrenoceptors in the brain of suicide victims: increased receptor density associated with major depression. Biol.Psychiatry. 1992;31:471–490. doi: 10.1016/0006-3223(92)90259-3. [DOI] [PubMed] [Google Scholar]
- Meana JJ, Garcia-Sevilla JA. Increased alpha 2-adrenoceptor density in the frontal cortex of depressed suicide victims. J Neural Transm. 1987;70:377–381. doi: 10.1007/BF01253612. [DOI] [PubMed] [Google Scholar]
- Menargues A, Obach R, Garcia-Sevilla JA. Modulation by antidepressant drugs of CNS postsynaptic alpha 2-adrenoceptors mediating mydriasis in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:101–107. doi: 10.1007/BF00195065. [DOI] [PubMed] [Google Scholar]
- Morag A, Pasmanik-Chor M, Oron-Karni V, Rehavi M, Stingl JC, Gurwitz D. Genome-wide expression profiling of human lymphoblastoid cell lines identifies CHL1 as a putative SSRI antidepressant response biomarker. Pharmacogenomics. 2011;12:171–184. doi: 10.2217/pgs.10.185. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol.Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat.Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O, Herscovitch P, Goldman D, Charney DS. Effects of a alpha 2C-adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology. 2006;31:1750–1756. doi: 10.1038/sj.npp.1301010. [DOI] [PubMed] [Google Scholar]
- Nomura S, Duman RS, Enna SJ. In vivo or in vitro exposure to imipramine reduces alpha 2-adrenoceptor-mediated inhibition of cyclic AMP production in rat brain cerebral cortical slices. Brain Res. 1987;410:195–198. doi: 10.1016/s0006-8993(87)80046-x. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Schenk J, Stockmeier CA, May W, Klimek V. Elevated agonist binding to alpha2-adrenoceptors in the locus coeruleus in major depression. Biol.Psychiatry. 2003;53:315–323. doi: 10.1016/s0006-3223(02)01728-6. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Smith KS, Haycock JW. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J Neurochem. 1994a;62:680–685. doi: 10.1046/j.1471-4159.1994.62020680.x. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Widdowson PS, Smith KS, Halaris A. Agonist binding to alpha 2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem. 1994b;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Janicak PG, Javaid JI, Davis JM. Increased 3H-clonidine binding in the platelets of patients with depressive and schizophrenic disorders. Psychiatry Res. 1989;28:73–88. doi: 10.1016/0165-1781(89)90199-6. [DOI] [PubMed] [Google Scholar]
- Paparrigopoulos T, Psarros C, Bergiannaki JD, Varsou E, Dafni U, Stefanis C. Melatonin response to clonidine administration in depression: indication of presynaptic alpha2- adrenoceptor dysfunction. J Affect Disord. 2001;65:307–313. doi: 10.1016/s0165-0327(00)00270-6. [DOI] [PubMed] [Google Scholar]
- Parini S, Renoldi G, Battaglia A, Invernizzi RW. Chronic reboxetine desensitizes terminal but not somatodendritic alpha2-adrenoceptors controlling noradrenaline release in the rat dorsal hippocampus. Neuropsychopharmacology. 2005;30:1048–1055. doi: 10.1038/sj.npp.1300661. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–R295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Chikkala D, Khaitan L, Jackson K, Qu Y. Delayed desensitization of alpha 2-adrenoceptor-mediated platelet aggregation in depressed patients. Neuropsychopharmacology. 1993;9:55–66. doi: 10.1038/npp.1993.43. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Saran A, Marler M. Elevated 3H-para-aminoclonidine binding to platelet purified plasma membranes from depressed patients. Neuropsychopharmacology. 1990;3:201–210. [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Saran A, Marler MR. Desipramine lowers tritiated para-aminoclonidine binding in platelets of depressed patients. Arch Gen Psychiatry. 1991;48:813–820. doi: 10.1001/archpsyc.1991.01810330037006. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le PM, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prabhakaran J, Majo VJ, Milak MS, Mali P, Savenkova L, Mann JJ, Parsey RV, Kumar JS. Synthesis and in vivo evaluation of [11C]MPTQ: a potential PET tracer for alpha2A-adrenergic receptors. Bioorg Med Chem Lett. 2010;20:3654–3657. doi: 10.1016/j.bmcl.2010.04.099. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Reneric JP, Bouvard M, Stinus L. Idazoxan and 8-OH-DPAT modify the behavioral effects induced by either NA, or 5-HT, or dual NA/5-HT reuptake inhibition in the rat forced swimming test. Neuropsychopharmacology. 2001;24:379–390. doi: 10.1016/S0893-133X(00)00214-1. [DOI] [PubMed] [Google Scholar]
- Richman JG, Regan JW. Alpha 2-adrenergic receptors increase cell migration and decrease F-actin labeling in rat aortic smooth muscle cells. Am J Physiol. 1998;274:C654–C662. doi: 10.1152/ajpcell.1998.274.3.C654. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, MacDonald E, Viitamaa T, Lähdesmäki J, Rybnikova E, Pelto-Huikko M, Kobilka BK, Scheinin M. Genetic alteration of the alpha2-adrenoceptor subtype c in mice affects the development of behavioral despair and stress-induced increases in plasma corticosterone levels. Mol Psychiatry. 1999;4:443–452. doi: 10.1038/sj.mp.4000543. [DOI] [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sarrouilhe D, di Tommaso A, Metaye T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88:1099–1113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Sastre M, Garcia-Sevilla JA. Alpha 2-adrenoceptor subtypes identified by [3H]RX821002 binding in the human brain: the agonist guanoxabenz does not discriminate different forms of the predominant alpha 2A subtype. J.Neurochem. 1994;63:1077–1085. doi: 10.1046/j.1471-4159.1994.63031077.x. [DOI] [PubMed] [Google Scholar]
- Sastre M, Garcia-Sevilla JA. Densities of I2-imidazoline receptors, alpha 2-adrenoceptors and monoamine oxidase B in brains of suicide victims. Neurochem Int. 1997;30:63–72. doi: 10.1016/s0197-0186(96)00032-0. [DOI] [PubMed] [Google Scholar]
- Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y. Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites. J.Biol.Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Schildkraut JJ. Recent studies on norepinephrine systems in mood disorders. In: Kupfer FEBDJ, editor. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 911–920. [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schittecatte M, Dumont F, Machowski R, Fontaine E, Cornil C, Mendlewicz J, Wilmotte J. Mirtazapine, but not fluvoxamine, normalizes the blunted REM sleep response to clonidine in depressed patients: implications for subsensitivity of alpha(2)-adrenergic receptors in depression. Psychiatry Res. 2002;109:1–8. doi: 10.1016/s0165-1781(01)00362-6. [DOI] [PubMed] [Google Scholar]
- Schramm NL, McDonald MP, Limbird LE. The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J.Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Lalovic A, Anguelova M, Lesage A, Seguin M, Chawky N, Desautels A, Turecki G. Alpha 2A adrenergic receptor gene and suicide. Psychiatry Res. 2004;125:87–93. doi: 10.1016/j.psychres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AD, Wang Q, Winder DG. alpha2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163:339–351. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, Forbes SL, Brown KM, Liggett SB. An asn to lys polymorphism in the third intracellular loop of the human alpha 2A-adrenergic receptor imparts enhanced agonist-promoted Gi coupling. J Biol Chem. 2000a;275:38518–38523. doi: 10.1074/jbc.M004550200. [DOI] [PubMed] [Google Scholar]
- Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB. A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem. 2000b;275:23059–23064. doi: 10.1074/jbc.M000796200. [DOI] [PubMed] [Google Scholar]
- Smith CB, Garcia-Sevilla JA, Hollingsworth PJ. alpha 2-Adrenoreceptors in rat brain are decreased after long-term tricyclic antidepressant drug treatment. Brain Res. 1981;210:413–418. doi: 10.1016/0006-8993(81)90919-7. [DOI] [PubMed] [Google Scholar]
- Smith CB, Hollingsworth PJ, Garcia-Sevilla JA, Zis AP. Platelet alpha 2 adrenoreceptors are decreased in number after antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:241–247. doi: 10.1016/0278-5846(83)90114-8. [DOI] [PubMed] [Google Scholar]
- Soetanto A, Wilson RS, Talbot K, Un A, Schneider JA, Sobiesk M, Kelly J, Leurgans S, Bennett DA, Arnold SE. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry. 2010;67:448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Lemoine PM, Ciaranello RD, Berger PA. Platelet alpha 2-adrenergic receptor sensitivity in major depressive disorder. Psychiatry Res. 1983;10:157–164. doi: 10.1016/0165-1781(83)90051-3. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. Antidepressant-like action of intracerebral 6-fluoronorepinephrine, a selective full alpha-adrenoceptor agonist. Int J Neuropsychopharmacol. 2011;14:319–331. doi: 10.1017/S1461145710000507. [DOI] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhash MN, Nagaraja MR, Sharada S, Vinod KY. Cortical alpha-adrenoceptor downregulation by tricyclic antidepressants in the rat brain. Neurochem.Int. 2003;43:603–609. doi: 10.1016/s0197-0186(03)00097-4. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mechanisms of Memory. London: Academic Press; 2010. [Google Scholar]
- Takeda T, Harada T, Otsuki S. Platelet 3H-clonidine and 3H-imipramine binding and plasma cortisol level in depression. Biol Psychiatry. 1989;26:52–60. doi: 10.1016/0006-3223(89)90007-3. [DOI] [PubMed] [Google Scholar]
- Theodorou AE, Lawrence KM, Healy D, Whitehouse AM, White W, Wilton-Cox H, Kerry SM, Horton RW, Paykel ES. Platelet alpha 2-adrenoceptors, defined with agonist and antagonist ligands, in depressed patients, prior to and following treatment. J Affect Disord. 1991;23:99–106. doi: 10.1016/0165-0327(91)90021-j. [DOI] [PubMed] [Google Scholar]
- Trestman RL, Lawrence TL, Coccaro EF, Harvey P, Bernstein D, Lawrence EK, Condello V, Mahon T, Yang RK, Knott P. Noradrenergic responses to clonidine in acute and remitted depressed male patients. Psychiatry Res. 1992;43:199–213. doi: 10.1016/0165-1781(92)90053-6. [DOI] [PubMed] [Google Scholar]
- Valdizán EM, Díez-Alarcia R, González-Maeso J, Pilar-Cuéllar F, García-Sevilla JA, Meana JJ, Pazos A. α2-Adrenoceptor functionality in postmortem frontal cortex of depressed suicide victims. Biol Psychiatry. 2010;68:869–872. doi: 10.1016/j.biopsych.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wang Q. Alpha2-adrenergic receptors. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, editors. Primer on the Autonomic Nervous System. Elsevier, Ltd.; 2011. [Google Scholar]
- Wang Q, Limbird LE. Regulation of alpha2AR trafficking and signaling by interacting proteins. Biochem Pharmacol. 2007;73:1135–1145. doi: 10.1016/j.bcp.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Lu R, Zhao J, Limbird LE. Arrestin serves as a molecular switch, linking endogenous alpha2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J.Biol.Chem. 2006;281:25948–25955. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- Wang R, Macmillan LB, Fremeau RT, Jr, Magnuson MA, Lindner J, Limbird LE. Expression of alpha 2-adrenergic receptor subtypes in the mouse brain: evaluation of spatial and temporal information imparted by 3 kb of 5' regulatory sequence for the alpha 2A AR-receptor gene in transgenic animals. Neuroscience. 1996;74:199–218. doi: 10.1016/0306-4522(96)00116-9. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Morgan PH, Lutz MW, Kenakin TP. The cubic ternary complex receptor-occupancy model. III. resurrecting efficacy. J Theor Biol. 1996;181:381–397. doi: 10.1006/jtbi.1996.0139. [DOI] [PubMed] [Google Scholar]
- Werstiuk ES, Auffarth SE, Coote M, Gupta RN, Steiner M. Platelet alpha 2-adrenergic receptors in depressed patients and healthy volunteers: the effects of desipramine. Pharmacopsychiatry. 1992;25:199–206. doi: 10.1055/s-2007-1014406. [DOI] [PubMed] [Google Scholar]
- Werstiuk ES, Coote M, Griffith L, Shannon H, Steiner M. Effects of electroconvulsive therapy on peripheral adrenoceptors, plasma, noradrenaline, MHPG and cortisol in depressed patients. Br J Psychiatry. 1996;169:758–765. doi: 10.1192/bjp.169.6.758. [DOI] [PubMed] [Google Scholar]
- West CH, Ritchie JC, Boss-Williams KA, Weiss JM. Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol. 2009;12:627–641. doi: 10.1017/S1461145708009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MH, Puranam RS, Ottman R, Gilliam C, Limbird LE, George AL, Jr, McNamara JO. Evaluation of the alpha(2A)-adrenergic receptor gene in a heritable form of temporal lobe epilepsy. Neurology. 1998;51:1730–1731. doi: 10.1212/wnl.51.6.1730. [DOI] [PubMed] [Google Scholar]
- Wolfe N, Cohen BM, Gelenberg AJ. Alpha 2-adrenergic receptors in platelet membranes of depressed patients: increased affinity for 3H-yohimbine. Psychiatry Res. 1987;20:107–116. doi: 10.1016/0165-1781(87)90003-5. [DOI] [PubMed] [Google Scholar]
- Wolfe N, Gelenberg AJ, Lydiard RB. Alpha 2-adrenergic receptor sensitivity in depressed patients: relation between 3H-yohimbine binding to platelet membranes and clonidine-induced hypotension. Biol Psychiatry. 1989;25:382–392. doi: 10.1016/0006-3223(89)90191-1. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Aksu F, Belzung C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol. 2005;514:165–174. doi: 10.1016/j.ejphar.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Yanpallewar SU, Fernandes K, Marathe SV, Vadodaria KC, Jhaveri D, Rommelfanger K, Ladiwala U, Jha S, Muthig V, Hein L, Bartlett P, Weinshenker D, Vaidya VA. Alpha2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J.Neurosci. 2010;30:1096–1109. doi: 10.1523/JNEUROSCI.2309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, Whisler LR, Huang Y, Xiang Y, O'Donnell JM. Postsynaptic alpha-2 adrenergic receptors are critical for the antidepressant-like effects of desipramine on behavior. Neuropsychopharmacology. 2009;34:1067–1077. doi: 10.1038/npp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, Meltzer HY, Ordway GA. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry. 1999;46:1275–1286. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]