Summary
Systemic lupus erythematosus (SLE, lupus) is characterized by a global loss of self-tolerance with activation of autoreactive T and B cells leading to production of pathogenic autoantibodies and tissue injury. Innate immune mechanisms are necessary for the aberrant adaptive immune responses in SLE. Recent advances in basic and clinical biology have shed new light on disease mechanisms in lupus, with this review discussing the recent studies that offer valuable insights into disease-specific therapeutic targets.
Introduction
Systemic lupus erythematosus (SLE, or lupus) is a systemic autoimmune disease with multiorgan inflammation. SLE is characterized by production of pathogenic autoantibodies directed against nucleic acids and their binding proteins, reflecting a global loss of self-tolerance (reviewed in [1]). The loss of tolerance with subsequent immune dysregulation is a consequence of genetic factors, in the setting of environmental triggers and stochastic events, with recent studies implicating over 30 genetic loci in disease pathogenesis (for recent reviews, see [2-5].
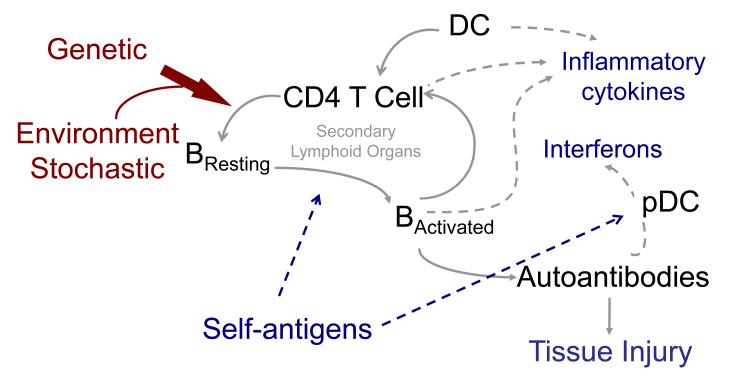
Aberrant innate immune responses play a significant role in the pathogenesis of SLE, contributing both to tissue injury via release of inflammatory cytokines as well as to aberrant activation of autoreactive T and B cells, with the latter leading to pathogenic autoantibody production and resultant end-organ injury (reviewed in [6]) (Figure). Autoantigenic nucleic acids and their binding proteins are required for self-antigen specific activation of autoreactive lymphocytes. Autoantigens complexed with their cognate autoantibodies also directly contribute to activation of innate immune cells via Fc receptor (FcR)-mediated uptake of complexes (or in the case of autoreactive B cells, initial engagement of the B cell antigen receptor by autoantigens per se), with the nucleic acid component of these complexes upon endosomal trafficking engaging intracellular Toll-like receptors (TLRs) with subsequent innate and B cell activation.
Mechanism of autoantibody production and tissue injury in lupus: A paradigm.
Self-antigen dependent activation of autoreactive B cells and CD4 T cells in secondary lymphoid organs, leads to production of pathogenic autoantibodies that, along with inflammatory cytokines, promotes tissue injury in lupus. Antigen-presenting dendritic cells are necessary for adaptive immune cell activation, and contribute to inflammatory cytokine production. Autoantibodies in complexes with autoantibodies contribute to innate immune cell activation and cytokine production. Genetic predisposition is a requisite for aberrant immune system acivation, in the setting of environmental and stochastic events. Abbreviations: DC, dendritic cells; pDC, plasmacytoid dendritic cells.
This review will focus upon recently dissected biologic events that provide insight into disease pathogenesis in three major areas, dysregulation of innate and adaptive immune responses in SLE, and the role of autoantibodies in triggering end-organ injury (Figure). We will necessarily, in the interest of space, focus upon studies that offer new paradigmatic insights into pathogenic events.
Innate immunity in SLE
Dendritic cells (DCs) play a central role in adaptive immunity by activating B and T cells, with the presumption that they are similarly required for the activation of autoreactive T and B cells. But their precise involvement in autoimmunity, and the effects of their selective subsets in autoreactive lymphocyte activation, are less clearly understood. A recent study addressed this question by adapting a DC-depletion model (CD11c-diptheria toxin A; CD11c-DTA) to the widely used MRL.Faslpr mouse model of lupus [7]. These mice offered the unique opportunity to study the natural onset and progression of disease in lupus-prone animals in the absence of DCs, with the demonstration that the latter are crucial in regulating the magnitude of spontaneously arising systemic autoimmunity in that DC-deficient mice exhibited less severe disease than DC-intact controls. In particular, expansion of T cells and plasmablasts with autoantibody production depended on DCs, indicating their previously unrecognized role in promoting extrafollicular (EF) humoral responses in SLE. Previous work by the same group and others has shown that EF sites in murine lupus are critical for continued activation of and autoantibody production by short-lived plasmablasts [8,9] (more about this later; see Adaptive Immunity in SLE), with their activation dependent upon autoreactive B cell receptor (BCR) and subsequent TLR engagement by lupus autoantigens [10] [11]. Although the role of EF responses in promoting human SLE is unknown, in part due to the general lack of access of lymphoid tissues from patients, the finding of increased numbers of circulating plasmablasts in patients with active SLE suggests such responses may be operative [12,13]. DC promotion of plasmablast function in EF sites is appealing, given the role of BAFF in B cell survival with myeloid cells potentially potent producers of this and other soluble and contact-dependent factors that promote B cell maturation [14].
Other data suggesting that DCs can initiate EF humoral responses comes from slightly older intravital imaging studies, revealing engagement of the B cell receptor by DC-associated antigen, with B cell activation EF occurring before entry into B cell follicles [15]. More recent work links DCs to EF B cell maturation with the finding that a splenic DC subset found in the marginal zone, those expressing the DC-inhibitory receptor 2 (DCIR2), has the unique capacity to initiate T-cell dependent extrafollicular B cell responses [16]. Although the implications of these findings for SLE are uncertain at this time, it is clear that further exploration of the role of DC-driven T and B cell maturation in EF sites as well as in germinal center (GC) responses in SLE is warranted, with DCs a tempting therapeutic target in SLE.
In lupus-prone CD11c-DTA animals, both conventional DC (cDC) and plasmacytoid DC (pDC) are efficiently depleted, underscoring the potential role of both subsets in disease. Depletion of the latter with disease amelioration recalls earlier findings that these cells produce large amounts of type I interferons (IFNs) in response to nucleic acid-containing immune complexes [17,18], with an increase in this cytokine paralleling activity and severity of SLE in humans [19]. Recent studies support this idea with the finding that interferon regulatory factors (IRFs), including IRF5, are strongly associated with higher serum IFNα levels or IFNa signaling and autoantibody titers in patients with SLE [20](for review, see [21]. Thus, blockade of IFNs is an appealing therapeutic strategy in SLE [22], with early results demonstrating some promise [23-25].
New work has now provided a pathogenic link between the heightened IFN production in SLE and dysregulation of another innate immune cell, the neutrophil. Activation of the latter has long been found in SLE, including in association with accelerated vascular disease [26,27]; however, the precise role of neutrophils in global disease pathogenesis has been less clear. Activated neutrophils die in a unique process, NETosis that is distinct from necrosis and apoptosis [28]. Dying neutrophils extrude a large amount of DNA in the form of web-like structures (neutrophil extracellular traps, NETs) that are associated with antimicrobial cationic peptides LL37 (also known as calthelicidin) and that promote bacteria entrapment and efficient killing [28]. Immune complexes of autoantibodies and nucleic acids, abundantly circulating in the plasma of patients with SLE, engage TLRs in neutrophils after FcR-mediated uptake with resultant activation and death by NETosis. NET DNA is protected from nuclease degradation and is available as an autoantigen for TLR-directed pDC activation and IFN release [29,30]. The latter cytokines further prime additional neutrophils for NETosis while also aiding cDC maturation with subsequent autoreactive T cell activation [17](reviewed in [6]). These findings indicate a feed forward loop among cytokines and neutrophils, resulting in adaptive immune cell activation that amplifies chronic inflammation with resultant tissue damage. Although it is crucial to determine whether this amplification mechanism operates in vivo and whether NET formation is required for disease progression, these data add weight to the argument that blockade of interferon and/or TLR signaling may be therapeutically beneficial in SLE [6,31].
Dissection of immune-complex driven production of IFNs by pDCs has also shed light on the role of glucocorticoids in the treatment of SLE. These drugs are widely used to treat autoimmune diseases and are a mainstay for induction of disease remission and maintenance in SLE via inhibition of the transcription factor NFκB [32], with subsequent pDC death and consequently reduced IFN production. Yet, lupus patients often require higher therapeutic doses of steroids to relieve inflammatory symptoms than other related conditions, such as rheumatoid arthritis, with toxic side effects including immune suppression, weight gain, and osteoporosis. Recent work has demonstrated how the therapeutic potency of glucocorticoids may be dampened in SLE via disease-associated resistance to their immune modulatory effects [33]. Engagement of TLR7 and 9 after endosomal uptake of nucleic acid containing immune complexes promotes pDC survival and IFN production via activation of NFκB, overcoming the glucocorticoid inhibitory effect. In addition to providing novel insights into the mechanisms whereby autoantibody-immune complexes amplify inflammation and induce drug resistance in SLE [10], this work further suggests TLR7/9 targeting may be importantly therapeutically in SLE, in addition to providing a means to utilize lower, and therefore less toxic, doses of glucocorticoids.
Adaptive Immunity in SLE
Given the roles of autoantibodies and B cells in disease pathogenesis [14,34], a number of studies have been devoted to analysis of the function of autoreactive B and T cells in SLE (for reviews, see [35,36]). B cell tolerance is defective at several levels in SLE, including both abnormalities in central and peripheral selection responsible for removal of self-reactive immature B cells [37-39]. Aberrant tolerance, combined with enhanced BCR [40], TLR [41], and BAFF receptor signaling operative in lupus (reviewed in [42]) ultimately promotes activation and survival of autoreactive B cells. CD4 T cells are critical players in the pathogenesis of lupus as they regulate B cell responses and also infiltrate target tissues with effector function, leading to tissue damage (reviewed in [43]), with genetically determined defects in tolerance regulation and receptor signaling also contributing to their activation.
The combined T and B cell abnormalities in SLE result in production of pathogenic autoantibodies. The latter are high-affinity, somatically mutated, and Ig-switched, supporting the idea that they are the product of GC responses [44-46], with defects in GC selection operative in human SLE [37,47]. Autoreactive B cells differentiate into pathogenic memory and plasma cells via the GC response [37], with lupus nephritis patients exhibiting abnormalities in the peripheral B cell compartment with increased autoantibody titers that can be attributed to intensive GC activity [47].
Although the role of B cells in disease promotion in lupus has been well established [48], the precise nature of the CD4 T cells that promote autoreactive B cell maturation has been less clear. New data suggest that follicular helper T (Tfh) cells [49,50], which reside in GCs and provide essential signals for B cell maturation and Ig production after immunization with thymus-dependent antigens, are crucial to the pathogenesis of lupus in mice. Dysregulation of Tfh cells that promote B cell differentiation in GCs is associated with the development of SLE in the Roquinsan/san mouse model [51]. In addition, abundant Tfh-like cells are located outside the GC where they support EF B cell differentiation in mouse models of SLE [52,53], with this site an important one for maturation of plasmablasts that contribute to the ongoing pathogenic production of autoantibodes in murine SLE models [9,54] as outlined above (Innate Immunity in SLE). Although data supporting the involvement of Tfh cells in human SLE remains relatively limited, expansion of a circulating Tfh (cTfh) cell population in patients with active SLE has been reported [55,56], with such expansion and dysregulation also occurring in patients with other systemic autoimmune disease [57]. Reassessment of past clinical trials in light of this newer data provides valuable insights into the potential involvement of Tfh cells in the pathogenesis of human lupus [58]. Treatment of lupus nephritis patients with anti-CD40L antibodies caused disappearance of circulating CD38bright plasma cells with decrease in anti-dsDNA titers, indicating these autoantibodies are a product of CD154-CD40 interactions, likely arising via the Tfh-driven GC response [47]. In murine lupus, at least, Tfh-like cells in the EF focus also express CD40L that is critical for B cell maturation [52,59]; thus, blockade of CD154-CD40 would likely also diminish EF plasmablast maturation. While anti-CD154 therapy in humans resulted in unexpected thromboembolic events, most likely a consequence of Fc-receptor mediated platelet activation [60], such data nonetheless establish a role for Tfh cells in SLE pathogenesis, providing crucial insights into the role of effectors of Tfh cell function as potential therapeutic targets in disease [61].
Autoantibodies as Initiators of Tissue Injury in SLE
The kidney is a primary site of tissue injury in murine and human lupus. Nephritis results from glomerular deposition of immune complexes of autoantibodies and autoantigens, with engagement of FcRs on immune cells along with complement fixation [62]. These effector mechanisms initiate infiltration and activation of tissue-infiltrating macrophages that promote the inflammatory response with resultant tissue injury [63,64]. The contributions of autoantibody isotypes to tissue injury in the kidney has not been well understood, although those associated with Th1 responses are thought to predominate in the human and murine diseases [65-67]. More recent data has shown that a subset of SLE patients have high titers of circulating IgE autoantibodies, without associated allergy [68], raising the question that Th2 cytokines (IL-4) or IgE per se contributes to lupus nephritis. Recent analysis of mice deficient in the Src family tyrosine kinase Lyn, a defect that leads to intrinsic B cell hyperactivation with autoantibody production and subsequent mild immune-complex nephritis, supports this notion [69]. These lupus-prone mice contain elevated serum titers of IL-4 with immune complexes containing IgE autoantibodies capable of basophil activation with promotion of tissue injury. Of particular relevance, this study also found that patients with SLE had both serum IgE autoantibodies and activated circulating basophils that could migrate to the spleens and lymph nodes [69]. If these mechanisms are operative in human lupus nephritis, targeting basophils may effective in those patients with high concentrations of IgE antinuclear antibodies [68].
As autoantibodies are critical for the pathogenesis of SLE and resultant tissue injury, B cell depletion is an attractive therapeutic option in disease. Early studies in lupus-prone mice revealed that B cells are absolutely essential for disease induction [48] via both autoantibody-dependent and –independent pathways [70], suggesting a role for B cell depletion as a remittive agent. Indeed, targeting CD20, which is expressed on almost all lineages of B cells except early pro B cells and plasmablast and plasma cells, is therapeutically beneficial in murine lupus [71,72], albeit at high doses as elevated plasma antibody levels in disease block FcR-mediated uptake and elimination of anti-CD20 coated B cells [73]. Rituximab, an anti-human CD20 monoclonal antibody, has now been shown to have clinical benefits in almost 200 off-label trials in autoimmune diseases [74]; however, results of two randomized clinical trials in lupus were disappointing. In the Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial, 257 patients with moderate SLE without renal involvement were randomized to treatment with rituximab or placebo, in both cases with background immunosuppressant therapy and steroids. After 52 weeks of therapy, rituximab-treated patients did not show significant improvement in disease activity compared to the placebo (background immunosuppressant therapy) group [75]. A second randomized controlled study, The LUpus Nephritis Assessment With Rituximab Study (LUNAR) trial, compared therapy with rituximab plus standard therapy for lupus nephritis, mycophenolate mofetil and steroids, to standard therapy alone [76]. After one year of treatment, the addition of rituximab did not result in enhanced clinical effectiveness over standard therapy, although benefits were present with subset analyses. Why did these trials not show substantive clinical benefit in light of the data from murine studies and observational data in humans supporting the efficacy of B cell depletion in lupus? Questions have been raised about both trial design and clinical outcome measures [36]. Thus, while disappointing, the results of these two studies have not scuttled the idea that targeting B cells in SLE may be therapeutically beneficial. Indeed, therapy with belimumab, a fully humanized monoclonal antibody directed against B-lymphocyte stimulator (BLyS), proved beneficial in recent clinical trials. Upon binding to its receptors, TACI, BAFF-R, and B cell maturation antigen (BCMA), BLyS activates signals for B cell survival and maturation. Circulating levels of BLyS mRNA and BLyS are elevated in lupus patients and are correlated with disease activity [77,78], with the belimumab effect primarily mediated by depletion of recently formed, rather than memory, B cells or long-lived plasma cells [79]. Two large, randomized controlled trials involving more than 800 SLE patients treated with intravenous belimumab or placebo have recently been completed (BLISS-52, BLISS-76) [80,81]. At 52 weeks follow up in both trials, patients who received belimumab had improvement of lupus activity and serological parameters with less disease progression compared to patients in the placebo group, with comparable, typically mild, adverse events. While these clinical benefits were lost at 76 weeks of follow up for reasons that are unclear, belimumab was recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of autoantibody-positive adult patients with active SLE who are receiving standard therapies.
Conclusions
The pathogenic mechanisms that lead to the clinical lupus phenotype are becoming clear, with genetic predisposition in the setting of environmental and/or stochastic triggers leading to innate immune system activation associated with pathological T-B cell collaboration and subsequent inflammation and tissue injury. These interactions are critical to understand, as their interruption is important therapeutically, as demonstrated by clinical studies in patients. Since there have been, and will undoubtedly continue to be, therapeutic toxicities or failures along the way, efforts to refine the mechanistic basis of these aberrant immune interactions are necessary. Their dissection offers a means to better understand disease biology and to maintain the pipeline of disease targets and ultimately that of therapeutic agents.
Highlights.
Innate effectors are critical for the lupus phenotype
Aberrant adaptive immune responses promote disease progression in SLE
Dissection of pathogenic events in SLE offers new therapeutic targets in SLE
Acknowledgments
This work was supported in part by NIH grants AR40072, AR44076, AI075157, and AR053495, and by the Alliance for Lupus Research. S. Kim was supported by a Research Scientist Development Award from the American College of Rheumatology Research and Education Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 2.Delgado-Vega Al, S√°nchez E, L√∂fgren S, Castillejo-L√≥pez C, Alarc√≥n-Riquelme ME. Recent findings on genetics of systemic autoimmune diseases. Current Opinion in Immunology. 2010;22:698–705. doi: 10.1016/j.coi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng Y, Tsao BP. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol. 2010;6:683–692. doi: 10.1038/nrrheum.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor KE, Chung SA, Graham RR, Ortmann WA, Lee AT, Langefeld CD, Jacob CO, Kamboh MI, Alarcon-Riquelme ME, Tsao BP, et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 2011;7:e1001311. doi: 10.1371/journal.pgen.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronson PG, Chaivorapol C, Ortmann W, Behrens TW, Graham RR. The genetics of type I interferon in systemic lupus erythematosus. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Craft JE. Dissecting the immune cell mayhem that drives lupus pathogenesis. Sci Transl Med. 2011;3:73ps79. doi: 10.1126/scitranslmed.3002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. Dendritic cells surprisingly play a major role in expansion of both autoreactive T and B cells in murine lupus.
- 8.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 11.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Zou Y, Davidson A. Plasma cells in systemic lupus erythematosus: the long and short of it all. Eur J Immunol. 2011;41:588–591. doi: 10.1002/eji.201041354. [DOI] [PubMed] [Google Scholar]
- 13.Mathian A, Gallegos M, Pascual V, Banchereau J, Koutouzov S. Interferon-alpha induces unabated production of short-lived plasma cells in pre-autoimmune lupus-prone (NZBxNZW)F1 mice but not in BALB/c mice. Eur J Immunol. 2011;41:863–872. doi: 10.1002/eji.201040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlomchik MJ. Sites and Stages of Autoreactive B Cell Activation and Regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 16.Chappell CP, Draves KE, Giltiay NV, Clark EA. Extrafollicular B cell activation by marginal zone dendritic cells drives T cell-dependent antibody responses. J Exp Med. 2012 doi: 10.1084/jem.20120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 18.Ronnblom L, Alm GV. The natural interferon-alpha producing cells in systemic lupus erythematosus. Hum Immunol. 2002;63:1181–1193. doi: 10.1016/s0198-8859(02)00757-7. [DOI] [PubMed] [Google Scholar]
- 19.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Niewold TB, Kelly JA, Kariuki SN, Franek BS, Kumar AA, Kaufman KM, Thomas K, Walker D, Kamp S, Frost JM, et al. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann Rheum Dis. 2012;71:463–468. doi: 10.1136/annrheumdis-2011-200463. Demonstration that certain autoantibodies are associated with genetic polymorphisms in type I interferon signaling, with the latter appearing to play a major role in the genesis of SLE in humans.
- 21.Niewold TB. Interferon alpha as a primary pathogenic factor in human lupus. J Interferon Cytokine Res. 2011;31:887–892. doi: 10.1089/jir.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo MS, Tsokos GC. Treatment of systemic lupus erythematosus: new advances in targeted therapy. Ann N Y Acad Sci. 2012;1247:138–152. doi: 10.1111/j.1749-6632.2011.06263.x. [DOI] [PubMed] [Google Scholar]
- 23.Merrill JT, Wallace DJ, Petri M, Kirou KA, Yao Y, White WI, Robbie G, Levin R, Berney SM, Chindalore V, et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon alpha monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann Rheum Dis. 2011;70:1905–1913. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- 24.McBride JM, Jiang J, Abbas AR, Morimoto A, Li J, Maciuca R, Townsend M, Wallace DJ, Kennedy WP, Drappa J. Safety and pharmacodynamic results of rontalizumab in a phase i, placebo controlled, double blind, dose escalation study in systemic lupus erythematosus. Arthritis Rheum. 2012 doi: 10.1002/art.34632. [DOI] [PubMed] [Google Scholar]
- 25.Mathian A, Amoura Z, Adam E, Colaone F, Hoekman MF, Dhellin O, Vandepapeliere P, Haroche J, Piette JC, Lebon P, et al. Active immunisation of human interferon alpha transgenic mice with a human interferon alpha Kinoid induces antibodies that neutralise interferon alpha in sera from patients with systemic lupus erythematosus. Ann Rheum Dis. 2011;70:1138–1143. doi: 10.1136/ard.2010.141101. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol. 2011;7:691–699. doi: 10.1038/nrrheum.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- **29.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. Two studies using human samples that suggest that NETs are an important source of autoantigens in SLE, with uptake by plasmacytoid dendritic cells and subsequent production of the pathogenic type I interferon.
- 31.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 32.De Bosscher K, Vanden Berghe W, Haegeman G. The Interplay between the Glucocorticoid Receptor and Nuclear Factor-κB or Activator Protein-1: Molecular Mechanisms for Gene Repression. Endocrine Reviews. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- **33.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. This work demonstrates how nucleic acids in circulating immune complexes in lupus acting via Toll-like receptors protect plasmacytoid dendritic cells from glucocorticoid-induced death, blocking the effect of this therapeutic mainstay on down regulation of type I interferon production.
- 34.Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 35.Crispin JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:317–325. doi: 10.1038/nrrheum.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coca A, Sanz I. Updates on B-cell immunotherapies for systemic lupus erythematosus and Sjogren’s syndrome. Curr Opin Rheumatol. 2012;24:451–456. doi: 10.1097/BOR.0b013e32835707e4. [DOI] [PubMed] [Google Scholar]
- 37.Cappione A, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005 doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 39.Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, Price C, Abraham C, Motaghedi R, Buckner JH, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenks SA, Sanz I. Altered B cell receptor signaling in human systemic lupus erythematosus. Autoimmun Rev. 2009;8:209–213. doi: 10.1016/j.autrev.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anders HJ. A Toll for lupus. Lupus. 2005;14:417–422. doi: 10.1191/0961203305lu2102rr. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Davidson A. BAFF and selection of autoreactive B cells. Trends Immunol. 2011;32:388–394. doi: 10.1016/j.it.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moulton VR, Tsokos GC. Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:207. doi: 10.1186/ar3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravirajan CT, Rahman MA, Papadaki L, Griffiths MH, Kalsi J, Martin AC, Ehrenstein MR, Latchman DS, Isenberg DA. Genetic, structural and functional properties of an IgG DNA-binding monoclonal antibody from a lupus patient with nephritis. Eur J Immunol. 1998;28:339–350. doi: 10.1002/(SICI)1521-4141(199801)28:01<339::AID-IMMU339>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 47.Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, Illei GG, Lipsky PE. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003;112:1506–1520. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 51.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- **55.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- **56.Terrier B, Costedoat-Chalumeau N, Garrido M, Geri G, Rosenzwajg M, Musset L, Klatzmann D, Saadoun D, Cacoub P. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J Rheumatol. 2012;39:1819–1828. doi: 10.3899/jrheum.120468. [DOI] [PubMed] [Google Scholar]
- **57.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. Three papers that demonstrate a likely role of follicular helper T cells in the genesis of human systemic autoimmunity.
- 58.Grammer AC, Lipsky PE. B cell abnormalities in systemic lupus erythematosus. Arthritis Res Ther. 2003;5(Suppl 4):S22–27. doi: 10.1186/ar1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In Vivo Regulation of Bcl6 and T Follicular Helper Cell Development. J Immunol. 2010 doi: 10.4049/jimmunol.0904023. jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robles-Carrillo L, Meyer T, Hatfield M, Desai H, Davila M, Langer F, Amaya M, Garber E, Francis JL, Hsu YM, et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185:1577–1583. doi: 10.4049/jimmunol.0903888. [DOI] [PubMed] [Google Scholar]
- 61.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 62.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171:489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 63.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 64.Bergtold A, Gavhane A, D’Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol. 2006;177:7287–7295. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 65.Masutani K, Akahoshi M, Tsuruya K, Tokumoto M, Ninomiya T, Kohsaka T, Fukuda K, Kanai H, Nakashima H, Otsuka T, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44:2097–2106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 68.Atta AM, Santiago MB, Guerra FG, Pereira MM, Sousa Atta ML. Autoimmune response of IgE antibodies to cellular self-antigens in systemic Lupus Erythematosus. Int Arch Allergy Immunol. 2010;152:401–406. doi: 10.1159/000288293. [DOI] [PubMed] [Google Scholar]
- **69.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–707. doi: 10.1038/nm.2159. Th2 responses and IgE promote immune-complex mediated glomerulonephritis in lupus-prone mice.
- 70.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B Cells in Murine Lupus: Efficacy and Resistance. The Journal of Immunology. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 72.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis & Rheumatism. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahuja A, Teichmann LL, Wang H, Dunn R, Kehry MR, Shlomchik MJ. An Acquired Defect in IgG-Dependent Phagocytosis Explains the Impairment in Antibody-Mediated Cellular Depletion in Lupus. The Journal of Immunology. 2011;187:3888–3894. doi: 10.4049/jimmunol.1101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramos-Casals M, Roberto Perez A, Diaz-Lagares C, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188–193. doi: 10.1016/j.autrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- **75.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **76.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64:1215–1226. doi: 10.1002/art.34359. Two randomized clinical trials suggesting rituximab does not substantially improve clinical outcome when layered on standard immunosuppressive therapy in SLE. These were unexpected results, raising concerns about appropriate trial design.
- 77.Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, Recta V, Zhong J, Freimuth W. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58:2453–2459. doi: 10.1002/art.23678. [DOI] [PubMed] [Google Scholar]
- 78.Collins CE, Gavin AL, Migone TS, Hilbert DM, Nemazee D, Stohl W. B lymphocyte stimulator (BLyS) isoforms in systemic lupus erythematosus: disease activity correlates better with blood leukocyte BLyS mRNA levels than with plasma BLyS protein levels. Arthritis Res Ther. 2006;8:R6. doi: 10.1186/ar1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **79.Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, Mackay M, Aranow C, Diamond B, Davidson A. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62:201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **80.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- **81.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. Belimumab is effective in SLE, likely via depletion of newly formed B cells.