Abstract
Introduction
Several clinical trials are currently evaluating stem cell therapy for patients with critical limb ischemia that have no other surgical or endovascular options for revascularization. However, these trials are conducted with different protocols, including use of different stem cell populations and different injection protocols, providing little means to compare trials and guide therapy. Accordingly, we developed a murine model of severe ischemia to allow methodical testing of relevant clinical parameters.
Methods
High femoral artery ligation and total excision of the superficial femoral artery (SFA) was performed on C57BL/6 mice. MNC were isolated from the bone marrow of donor mice, characterized using FACS, and injected (5×105−2×106) into the semimembranosus (proximal) or gastrocnemius (distal) muscle. Vascular and functional outcomes were measured using invasive Doppler, laser Doppler perfusion imaging, and the Tarlov and ischemia scores. Histological analysis included quantification of muscle fiber area and number as well as capillary density.
Results
Blood flow and functional outcomes were improved in MNC-treated mice as compared to controls over 28 days (Flow: P < .0001; Tarlov: P = .0004; ischemia score: P = .0002). MNC-treated mice also showed greater gastrocnemius fiber area (P = .0053) and increased capillary density (P = .0004). Dose-response analysis showed increased angiogenesis and gastrocnemius fiber area but no changes in macroscopic vascular flow or functional scores. Mice injected proximally to the ischemic area had overall similar functional outcomes to mice injected more distally, but increased muscle flow, capillary density, and gastrocnemius fiber area (P < .05).
Conclusions
High femoral ligation with complete excision of the SFA is a reliable model of severe hind limb ischemia in C57BL/6 mice that shows a response to MNC-treatment for both functional and vascular outcomes. A dose response to MNC injection appears to be present, at least microscopically, suggesting that an optimal cell number for stem cell therapy exists and that preclinical testing needs to be performed to optimally guide human trials. Injection of MNC proximal to the site of ischemia may provide some different outcomes compared to distal injection and warrants additional study.
INTRODUCTION
The successful isolation of endothelial progenitor cells (EPC) from the peripheral circulation in 1997 transformed the field of stem cell biology and created optimism for cell-based treatment of critical limb ischemia (CLI).1 Although revascularization remains the current gold standard treatment of limb ischemia, many patients with advanced disease are not candidates for either surgical or endovascular treatment secondary to the anatomy and extent of their disease, or comorbidities.2 Despite advances in both surgical and endovascular techniques, as well as advances in anesthesia and critical care, the options for these patients remain limited with most of them ultimately requiring amputation.3–5 At present, there are no standard effective treatment strategies available for these no-option patients, and, it is precisely for these patients that stem cell therapy holds the potential to create therapeutic alternatives. Accordingly, the use of bone marrow-derived stem cells has been identified as a potential method for inducing therapeutic angiogenesis.6
The publication of the TACT trial (Therapeutic Angiogenesis using Cell Transplantation) in 2002 was the first human report describing the use of bone marrow-derived mononuclear cells (MNC) for the treatment of CLI, and several additional studies have been published since, using both MNC derived from both bone marrow7–15 or peripheral blood.7, 16–24 Recent publications have been generally positive with regard to improvements in extremity perfusion and motor function.6 However, the greatest inconsistency in these reports comes from observed variability in MNC preparation and injection. MNC injections have been intramuscular7–14, 16, 18–23 as well as intra-arterial8, 17 and anywhere between 80 to 1000mL of bone marrow has been used. 6, 10, 25 In addition, the number of MNCs used has been anywhere between 0.1×109 and as high as 100×109 per patient; the number of injections given is equally variable, with only a single study investigating the relationship between outcomes and number of injected cells.2, 6, 13, 14, 25 Although the value of human studies cannot be underestimated, the differences between these studies, including variable degrees of ischemia, small patient numbers and differing cell therapy techniques, prevents easy interpretation as to the optimal techniques to use for common practice.
Animal models are likely to play an important role in helping to answer some clinical questions to help guide both practice as well as future trials of cell-based therapy for CLI. There are several different murine hind limb ischemia models to test angiogenesis via cell based therapies. Mild ischemia models as well as severe ischemia models exist.26 Variations in the level of occlusion include iliac ligation, femoral ligation below the branches, femoral ligation with excision of all branches, artery and vein stripping, and there are operator-dependent variations in technique (e.g. suture ligature vs. electrocautery).26, 27 Variation in the type of model used results in different patterns of ischemia and reperfusion, while the level of occlusion does not.28
Among the many uncertainties in therapeutic angiogenesis are the clinical questions regarding optimal cell population, site of administration, and cell dose. In this regard, the rationale for this study was to establish an acute but reproducible murine model of severe ischemia that would allow methodical testing of such parameters.
METHODS
Animal Model
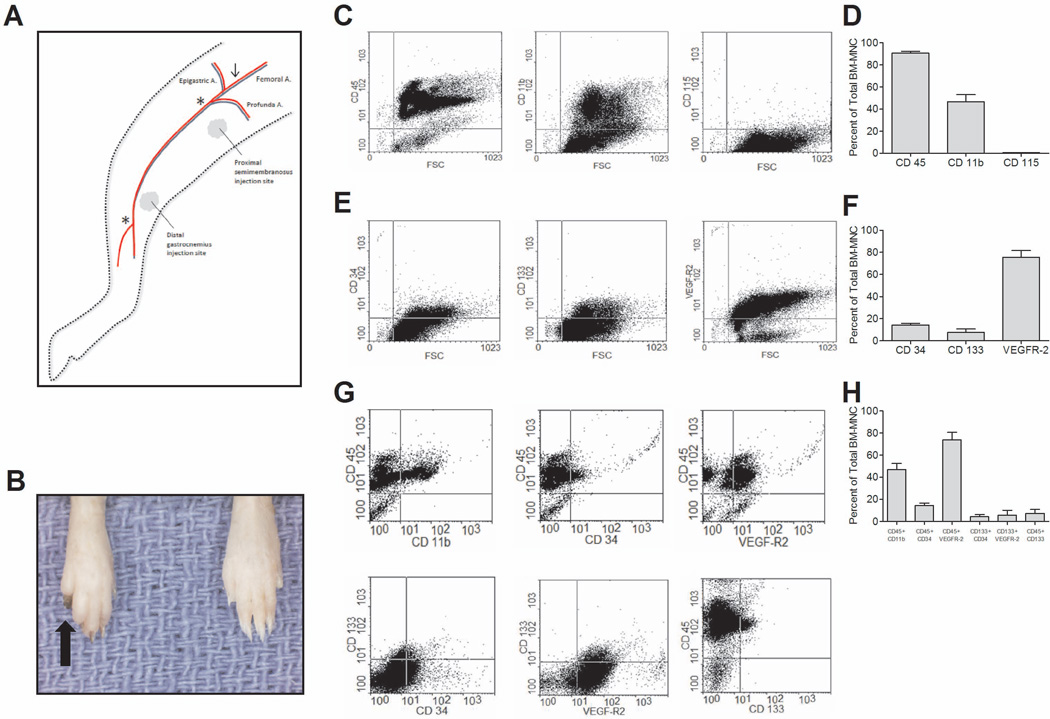
All procedures, protocols, and medications were approved by Yale University’s Institutional Animal Care and Use Committee. Unilateral high femoral artery ligation and superficial femoral artery (SFA) excision was performed on male C57BL/6 mice (6–8 week-old) (The Jackson Laboratory, Bar Harbor, ME). Intraperitoneal anesthesia was administered using ketamine (100 mg/kg) and xylazine (5 mg/kg). Mice were positioned in dorsal recumbency with their hind limbs externally rotated. A skin incision was made over the femoral artery beginning at the inguinal ligament and continued caudally to the popliteal bifurcation. The femoral artery was isolated above the level of the profunda and epigastric arterial branches, doubly ligated using 7-0 prolene suture, and transected. The SFA caudal to the major branch points was dissected, ligated and excised in its entirety. Varying concentrations of mononuclear cells (5×105, 1×106, and 2×106) or control medium, maintained at a constant volume of 0.1 ml, were injected either distally into the gastrocnemius muscle (Figures 1–5), or proximally into the semimembranosus muscle (Figure 5), immediately following femoral artery ligation (Figure 1A). A skin incision only was made on the contralateral limb for reference invasive monitoring. The incisions were closed; body temperature was maintained with heating pads until the animals recovered from surgery and were ambulatory.
Figure 1.
Methods. (A) Modified hind limb ischemia model; Arrow (↓) denotes site of femoral ligation; Asterisks (*) denote length of SFA excision. (B) Representative image of digit gangrene (black arrow). (C–D) Expression of mononuclear cell (MNC) markers. (E–F) Expression of endothelial progenitor cell (EPC) markers. (G–H) Co-expression of MNC and EPC markers.
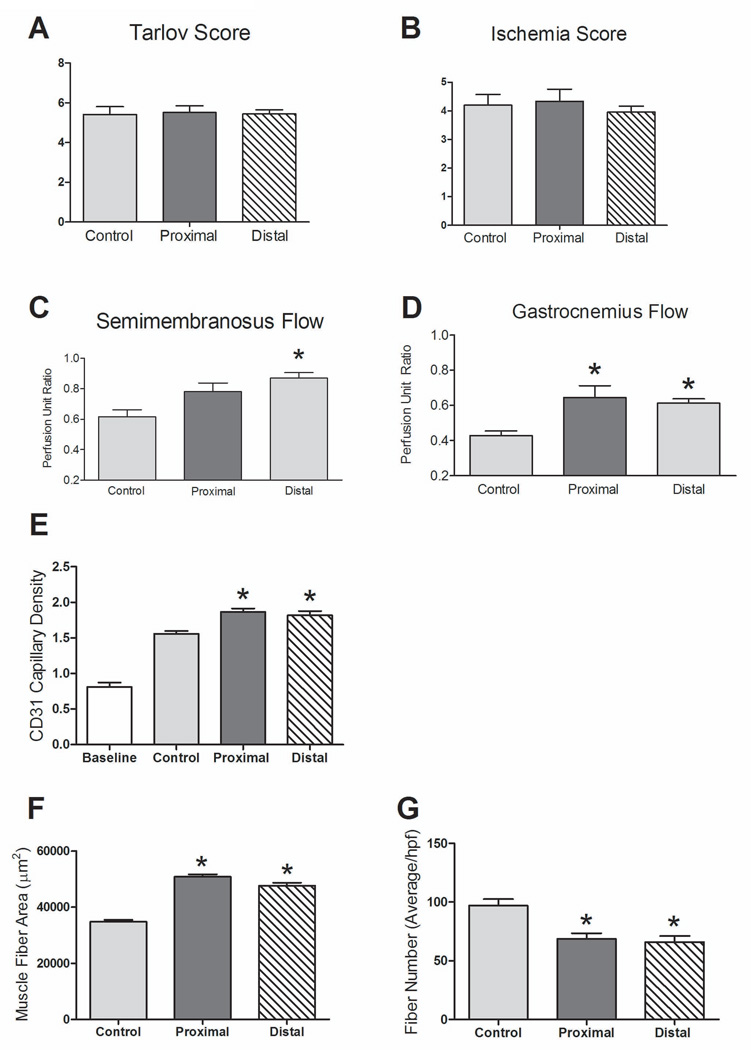
Figure 5.
Proximal MNC injection response. n=21 (MNC-treated) and 6 (control). (A) Functional measurements with the Tarlov scale (day 7) showed no difference between control and MNC-treated groups (P = .9821). (B) Functional measurements with the ischemia scale (day 7) showed no difference between groups (P = .6594). (C) Increased semimembranosus flow with MNC-treated mice (P = .0041, ANOVA); there was no significant difference in flow between proximal and distal treatment (P > .05; post-hoc test). *, P < .05 vs. control, post-hoc test. (D) Distal flow was increased in proximally and distally treated mice versus control (P = .0086, ANOVA); there was no significant difference in flow between proximal and distal MNC treatment (P > .05; post-hoc test). *, P < .05 vs. control, post-hoc test. (E) MNC-treated mice showed increased capillary density compared to both control and baseline (P < .0001, ANOVA); there was no significant difference in capillary density between proximally and distally treated mice (P > .05; post-hoc test); *, P < .05 vs. control, post-hoc test. n=3. (F) MNC-treated mice showed increased gastrocnemius muscle fiber area compared to control mice (P < .0001, ANOVA); the difference between proximal and distal injection was significant (P < .05; post-hoc test). *, P < .05 vs. control, post-hoc test. n=3. (G) MNC-treated mice showed decreased gastrocnemius muscle fiber number compared to control mice (P = .002, ANOVA); the difference between proximal and distal injection was not significant (P > .05; post-hoc test). *, P < .05 vs. control, post-hoc test. n=3.
Antibodies and Reagents
Primary antibodies to the following antigens were obtained as follows: CD31 (Abcam, Cambridge, MA); VEGFR-2-FITC, CD11b-FITC, CD34-FITC, CD45-PerCP, CD133-APC and CD115-PE (eBioscience, San Diego, CA); antifade mounting reagent with DAPI, Alexa Fluor 568-conjugated IgG, and RPMI Media 1640 (Life Technologies, Carlsbad, CA); histopaque 1077 (Sigma-Aldrich, St. Louis, MO).
Mononuclear Cell Isolation
Bone marrow was collected from the long bones of euthanized C57BL/6 mice. Femurs and tibias were harvested, clipped at the metaphyses, flushed with phosphate buffered saline (PBS) using a 26-gauge needle, and then filtered through a 70-µM cell strainer. MNC were isolated by gradient centrifugation and resuspended in medium for immediate intramuscular injection. Cells isolated from one donor mouse at an injection concentration of 5×105 could be used to treat approximately 4–6 mice.
Fluorescence-Activated Cell Sorting (FACS) Analysis
MNC were isolated from bone marrow as described above and preserved with 0.5% paraformaldehyde. Aliquots were created, to which fluorescent-conjugated primary antibodies or isotype controls were added. Cells were analyzed in a flow cytometer FACSCalibur (BD Biosciences, Franklin Lakes, NJ). WinMDI 2.9 software was used to analyze data; samples were normalized to IgG isotype controls.
Cutaneous Perfusion Measurement
Laser Doppler perfusion imaging (LDPI) (Perimed, North Royalton, OH) was used to measure perfusion of the ligated and control limbs at predetermined time points. Depilatory cream was used to remove hind limb hair and the animals were kept on a heating pad at 37°C to minimize temperature variation. Consecutive measurements were obtained according to the manufacturer’s protocols and average hind limb perfusion was determined for an anatomically defined region of the lateral gastrocnemius and plantar foot by image analysis (LDPIwin 2.6). Calculated perfusion was expressed as a ratio of right (ischemic) to left (control) hind limbs.
Functional Scoring
Functional scoring was performed using the Tarlov scale,29, 30 ischemia scale30, 31 and modified ischemia scale30 (Table 1).
Table 1.
Functional Scoring
| Tarlov Score | |
| 0 | No movement |
| 1 | Barely perceptible movement, non-weight bearing |
| 2 | Frequent movement, non-weight bearing |
| 3 | Supports weight, partial weight bearing |
| 4 | Walks with mild deficit |
| 5 | Normal but slow walking |
| 6 | Full and fast walking |
| Ischemia Score | |
| 0 | Auto-amputation > half lower limb |
| 1 | Gangrenous tissue > half foot |
| 2 | Gangrenous tissue < half foot, with lower limb muscle necrosis |
| 3 | Gangrenous tissue < half foot, without lower limb muscle necrosis |
| 4 | Pale foot or gait abnormalities |
| 5 | Normal |
| Modified Ischemia Score | |
| 0 | Auto-amputation of leg |
| 1 | Leg necrosis |
| 2 | Foot necrosis |
| 3 | Discoloration of > two toes |
| 4 | Discoloration of one toe |
| 5 | Discoloration of > two nails |
| 6 | Discoloration of one nail |
| 7 | No necrosis |
Flow (invasive Doppler) measurement
In some animals, blood flow was measured in anesthetized animals, both in the ischemic leg and the control leg, preoperatively, immediately postoperatively, and at 1–4 weeks after induction of hind limb ischemia, using the PeriFlux Laser Doppler Perfusion Measurement (LDPM) unit with a “deep probe” configuration (Perimed, North Royalton, OH).30 Access to the soleus muscle was obtained through a 3 mm skin incision on each hind limb that was closed after measurement; the same incision was used for all measurements. This incision was only made for mice having flow measurements and not for any other experiments. This method allows for reproducible measurements directly in the muscle bed, avoiding cutaneous blood flow and impaired wound healing, and it is not significantly temperature dependent. Blood flow values were expressed as the ratio of ischemic to control leg perfusion.30
Histological Preparation and Analysis
At appropriate intervals after femoral artery ligation, animals were euthanized. Vessels were flushed with PBS and heparin (250 U/kg). Perfusion-fixation was performed using 10% neutral buffered formalin solution and the bilateral gastrocnemius muscles were removed. Tissue was then paraffin-embedded, cut into seven micrometer sections, and stained with hematoxylin and eosin for histology. Fiber area and number were calculated by averaging the counts of five separate fields in four distinct areas in each specimen, by a blinded observer using ImageJ software (NIH, Bethesda, MD).
Capillary Density Analysis
Tissue sections were de-paraffinized and stained using a primary antibody against CD31 followed by application of a fluorophore-conjugated secondary antibody. An anti-fade mounting medium containing DAPI was applied prior to coverslipping. Capillary density, defined as number of capillaries per muscle fiber, was counted manually by a blinded observer using ImageJ software in five different randomized fields per slide (40X).
Analysis
Statistics were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Results are expressed as the mean±SEM. Comparisons between groups were performed using analysis of variance (ANOVA) with post-hoc testing performed with Bonferroni analysis or unpaired t-tests as appropriate. P values ≤ .05 were considered statistically significant.
RESULTS
Mononuclear and endothelial progenitor cells are present in bone marrow isolates
MNC were isolated using a protocol similar to one used in clinical practice.32 Flow cytometry showed that the majority of murine bone marrow cells (90.74%±1.67%) isolated with this protocol were CD45+ leukocytes. Further characterization of this isolate found it to be CD115− and CD11b+ (46.65%±6.41%; Figures 1C, 1D). EPC markers were found at varying amounts, with high levels of expression of VEGFR2 (75.55%±6.10%), but lower levels of both CD34 (14.29%±1.32%) and CD133 (7.48%±3.10%; Figures 1E, 1F); cells were most frequently dual-positive for CD45 and VEGFR2 (Figures 1G, 1H). These results suggest that MNC derived from a clinically-used protocol have a large number of monocytes with varying quantities of EPC.
MNC treatment improves outcomes in the murine hind limb ischemia model
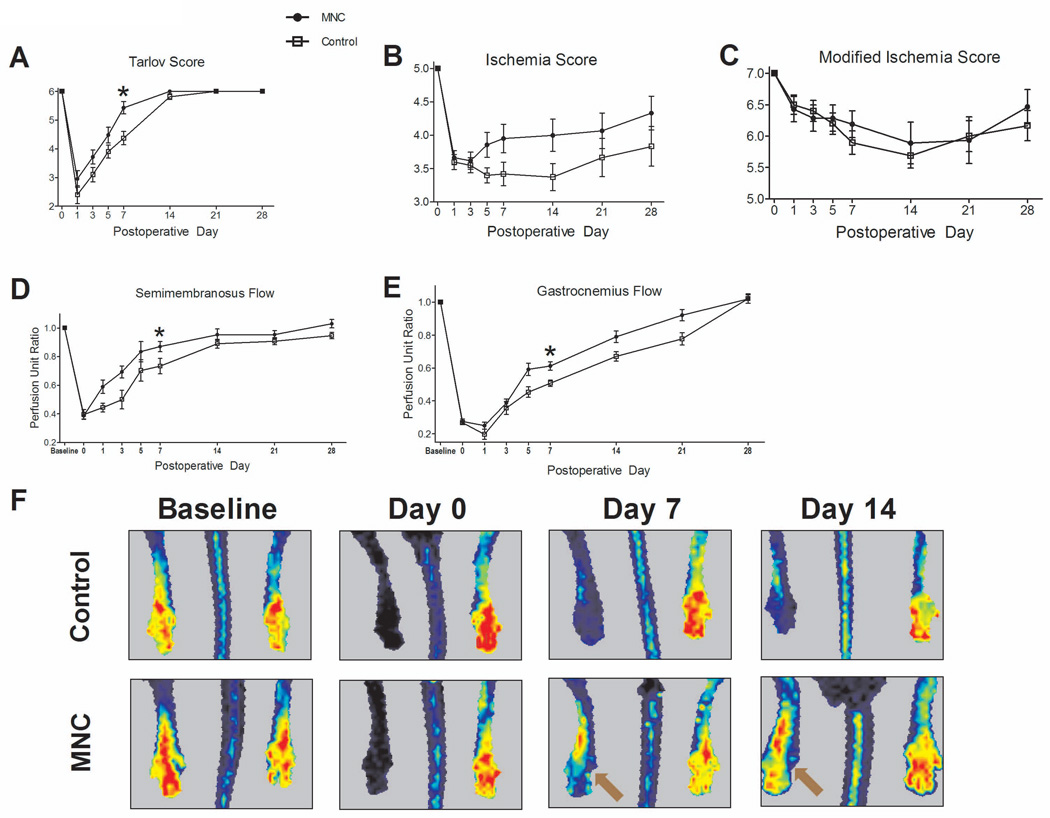
Hind limb ischemia was induced acutely in mice using common femoral artery ligation and superficial femoral artery excision (Figure 1A), producing reproducible toe gangrene (Figure 1B); mice were treated immediately afterwards with either control vehicle without any MNC, or 5×105 MNC injected into the gastrocnemius muscle. Both mice treated with MNC and control mice showed equally diminished proximal and distal flow on day 0 following femoral artery ligation, compared to the contralateral leg (Proximal: 0.39±0.02 vs. 0.40±0.03, P = .8305; Distal: 0.28±0.01 vs. 0.27±0.01, P = .6904, t-test). Over 28 days, MNC-treated mice showed improved functional outcomes compared to control mice, with accelerated improvement in the Tarlov score (Day 7: 5.43±0.21 vs. 4.37±0.23, P = .0017,t-test; Figure 2A). The grade of ischemia improved in MNC-treated mice using the ischemia score (P = .0002) but not the modified ischemia score (P = .456; Figures 2B, 2C). Mice treated with MNC showed significantly augmented perfusion, both proximally and distally as measured with invasive Doppler (Proximal semimembranosus flow: 0.87±0.04 vs. 0.73±0.05, P = .0429; Distal gastrocnemius flow: 0.61±0.03 vs. 0.51±0.02, P = .0024,t-test; day 7; Figure 2D–E) or with noninvasive laser Doppler (Figure 2F).
Figure 2.
MNC treatment (5×105) improves hind limb ischemia. n=21 (MNC-treated) and 20 (control). (A) Tarlov score: MNC-treated mice show improved functional outcomes (*, P = .0004). (B) Ischemia score: MNC-treated mice had greater improvement of ischemia (P = .0002). (C) Modified ischemia score: no significant differences were observed (P = .4587). (D–E) Invasive Doppler measurements show MNC-treated mice to have improved (D) proximal flow (*, P < .0001) and (E) distal flow (*, P < .0001). (F) Representative laser Doppler images show improved perfusion in MNC-treated mice. Arrows show improved perfusion in MNC-treated mice at days 7 and 14. P vales represent statistical analysis by ANOVA.
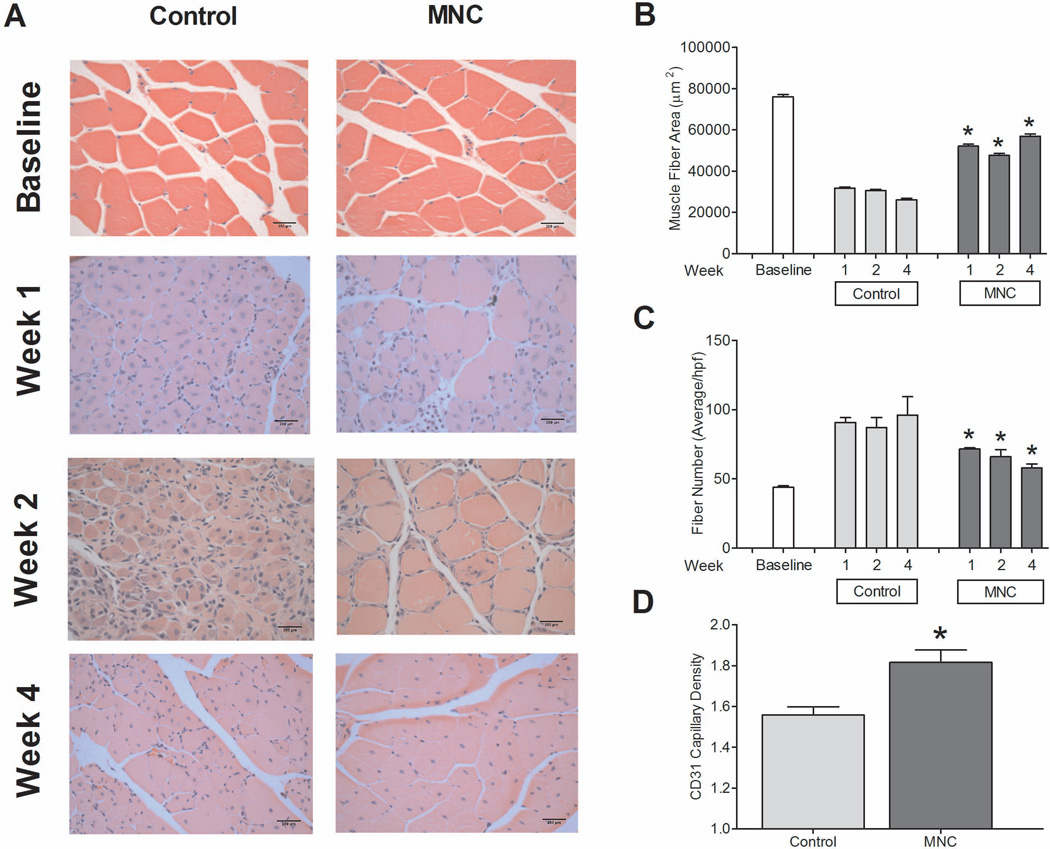
Histological findings correlated with vascular and functional outcomes (Figure 3A). MNC-treated mice showed increased gastrocnemius muscle fiber area compared to control mice (P = .0053; Figure 3B). In addition, MNC-treated mice showed fewer muscle fibers per high power field compared to control mice (P < .0001; Figure 3C). Angiogenesis was measured in histological sections by direct assessment of capillary density; at day 14, MNC-treated mice showed increased gastrocnemius capillary density compared to control mice (P = .0004,t-test; Figure 3D). These data show increased angiogenesis in MNC-treated mice compared to control mice, consistent with the data showing increased limb perfusion (Figures 2B, 2D, 2E, and 2F).
Figure 3.
Gastrocnemius muscle histology. n=3 (MNC-treated) and 3 (control). (A) Histological comparison of MNC treated and control mice over 28 days. (B) MNC-treated mice demonstrated increased muscle fiber area at day 7, 14 and 28 (*, P = 0.0053). (C) MNC-treated mice showed decreased number of muscle fibers (*, P < .0001). (D) MNC-treated mice demonstrated higher capillary density at day 14(*, P < .0001). P values represent statistical analysis by ANOVA.
Increased MNC dose affects outcomes
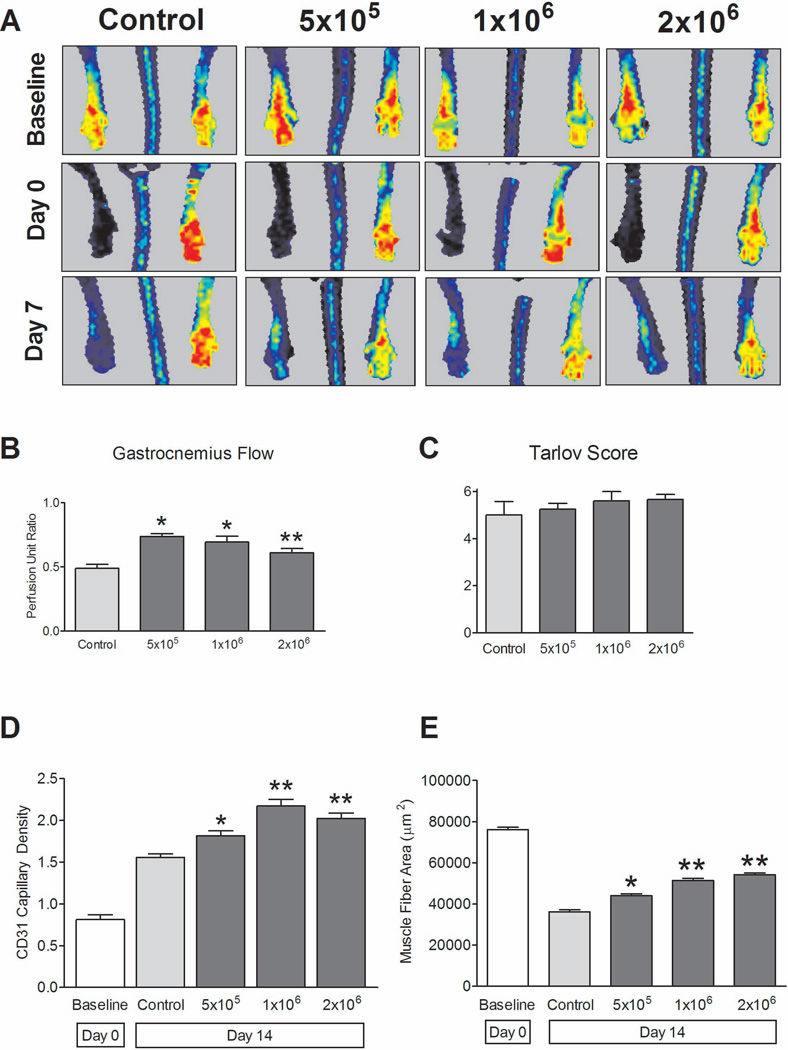
To demonstrate the relevance of the animal model, we evaluated a question of clinical significance, i.e. does the number of MNC injected make a difference in outcome; accordingly, dose-response groups were evaluated. MNC-treated mice received gastrocnemius muscle injections with doses of either 5×105, 1×106 or 2×106 MNC, with control mice receiving no cells. At day 7, laser Doppler perfusion imaging of the distal foot showed increased perfusion in the MNC-treated mice compared to control mice, but without any significant differences between the treatment groups (P = .7501; Figure 4A). Using invasive Doppler flow for quantification, all MNC treatment groups (5×105, 1×106 and 2×106) displayed increased distal flow compared to control mice (P = 0.0018; Figure 4B), similar to the findings with noninvasive laser Doppler (Figure 4A). Functional scores showed no differences between the groups (Tarlov: P = .5520; ischemia score: P = .4444; Figure 4C).
Figure 4.
MNC dose-response. n=4–6 per group. (A) Representative laser Doppler perfusion images; quantification showed no differences between MNC-treated groups at day 7 (P = .7501, ANOVA). (B) MNC-treated mice show increased perfusion (day 7) compared to control mice (P = .0018, ANOVA); 5×105 and 1×106 MNC groups showed increased distal flow versus control (*, P < .05; post-hoc test). **, P > .05 compared to other groups. (C) Functional outcomes: No significant differences between MNC treatment groups and control (P = .5520, ANOVA). (D) Increased capillary density (day 14) in MNC-treated mice compared to control mice (P < .0001, ANOVA). *, P < .05 vs. control, post-hoc test. **, P < .05 vs. control, post-hoc test; P > .05 vs. each other, post-hoc test). n=3. (E) Increased gastrocnemius muscle fiber area (day 14) in MNC-treated mice compared to control mice (P < .0001, ANOVA). *, P < .05 vs. control, post-hoc test. **, P < .05 vs. control, post-hoc test; P > .05 vs. each other, post-hoc test). n=3.
Despite the lack of gross functional responses with higher MNC doses, there was evidence of response on the microscopic level (Figures 4D, 4E). At day 14, all MNC-treated mice demonstrated increased capillary density as compared to control mice (P < .0001; Figure 4D). The highest capillary density was observed in the 1×106 MNC treatment group, with no significant difference in capillary density observed between the 1×106 and 2×106 treatment groups (P > .05; Figure 4D).
Histological evaluation of gastrocnemius muscle fiber area suggested a correlation between improved gastrocnemius muscle architecture and treatment with increased MNC concentrations. At day 14, all MNC-treated mice showed increased gastrocnemius fiber area as compared to control mice (P < .0001; Figure 4E). Gastrocnemius fiber area was statistically increased in both the 1×106 and 2×106 treatment groups in comparison to both the 5×105 treatment group and control mice (P < .05), but without difference between the 1×106 and 2×106 treatment groups (P > .05). Similar outcomes were seen between the MNC-treated mice and control mice with regard to gastrocnemius muscle fiber number, where higher dose MNC-treated groups showed diminished fiber numbers as compared to control mice (P = .0002). These results suggest that increased dose of injected MNC have effects on the microcirculation, but, at the doses examined, may be inadequate to affect functional scores or gross perfusion.
Proximal MNC injections influence distal ischemia outcomes
We also examined another clinical question, e.g. does a more proximal injection site, proximal to the ischemic area, affect the response? Treatment groups received 5×105 MNC cells that were injected proximally into the semimembranosus muscle (Figure 1A), whereas control mice received no cells. There were no statistically significant differences observed in the functional or ischemic scores between the proximally-injected and distally-injected mice (Figures 5A, 5B). At day 7, flow in the proximal muscles was higher in the MNC-treated mice compared to control mice (P = .0041) and there was no difference between proximal and distal injection (P > .05; Figure 5C). However, flow in the distal muscles was increased in both proximally-injected and distally-injected mice compared to control mice (P = .0086), without difference between proximal and distal injections (P > .05; Figure 5D). Similar to this data, the proximally-injected MNC-treated mice showed higher capillary density at day 14 as compared to control mice (P < .0001), but without any significant differences in capillary density compared to the distally-injected group (P > .05; Figure 5E). Histological evaluation of the gastrocnemius muscle at day 14 demonstrated increased fiber area in MNC-treated mice as compared to control mice (P < .0001); there was increased fiber area in proximally-injected mice compared to distally-injected mice (P < .05; Figure 5F). Similarly, gastrocnemius fiber number was decreased at day 14 in MNC-treated mice as compared to control mice (P = .002), without any significant differences in fiber number between proximally-injected and distally-injected mice (P > .05; Figure 5G). These data are consistent with stem cell injections improving the kinetics of recovery from hind limb ischemia, despite the limitations of our mouse model.
DISCUSSION
New clinical studies suggest that stem cell injection for CLI is a valid therapeutic option in selected patients. Despite supporting clinical evidence, conflicting clinical protocols as well as ignorance regarding stem cell biology have tempered clinical adoption of stem cell therapy. We describe an easy to perform preclinical model to testing different parameters of stem cell therapy. We show that MNC-treatment provided a more rapid improvement in perfusion in this ischemic model, that a dose-response curve is likely to exist for stem cells, and that the site of stem cell injection may have functional consequences.
The rationale for this study was to establish an acute murine model of severe ischemia that could accommodate the testing of several clinical parameters important to clinical trials of cell therapy. A study demonstrating six different ischemia models concluded that simple ligation of the femoral artery at the level below the deep femoral (profunda) artery is most suitable for studying chronic mild ischemia.26 This model resulted in a range from no necrosis to toe necrosis. Many articles in the literature use the severe ischemia model which consists of femoral artery ligation with excision of all side branches.26, 33–39 This model results in severe ischemia with profound necrosis, ranging from toe necrosis to auto-amputation of limbs. Stripping the femoral artery from the distal site of bifurcation of the deep femoral artery to saphenous artery is most suitable for severe ischemia.26 However, the deep arterial system remains intact and blood flow is redirected through this route.
We believe that our model closer resembles diseases in human population where arteries are occluded but still present. This can result in arteriogenesis as well as angiogenesis. We adopted a variation of the arterial stripping model as well as interrupting collateral flow via the deep system by ligation above the branches. Through the use of this modified model, we were able to consistently achieve distal hind limb tissue necrosis and thus more accurately represent chronic manifestations of atherosclerotic disease (Figure 1B). However, small technical variations in this ischemia model have physiological consequences.28 With excision of all branches, arteriogenesis can not be studied because all pre-existing connections of arterioles to the vascular tree are disrupted and can not be repaired.
The lack of an in-depth understanding regarding EPCs and their lineage continues to be problematic. Clinically, it has been observed that cell therapies based on whole bone marrow appear to be more successful as compared to isolated cell subpopulations.6, 23 This observation has led to the idea that several different bone marrow cell types, all sharing a common monocytic phenotype, are necessary for successful angiogenesis. Clinical studies have traditionally relied on the use of a density gradient centrifugation to isolate MNC fractions from whole bone marrow.40 Therefore we used this method to isolate murine MNC to be tested in this study. Prior human studies have documented the composition of the clinically used bone marrow-derived MNC isolate and have found it to contain a heterogenous population of cell types that includes hematopoietic stem cells (CD34+), mononuclear cells (CD14+, CD11b, CD115), and endothelial progenitor cells (CD133+, VEGFR2+).40, 41 Using C57BL/6 bone marrow, we were able to closely replicate clinical cell therapy marrow isolates. Analysis of our marrow isolate found it to be primarily mononuclear in composition (CD45+, CD11b+) with elimination of granulocytes achieved through gradient centrifugation. Similar to clinical studies, we found varying numbers of endothelial progenitor cells (CD34+, CD133+, VEGFR2+) as well as colocalization of the leukocyte-common antigen CD45+ with progenitor cell markers (CD34+, CD133+, VEGFR2+). The ability to closely replicate human cell therapy using C57BL/6 bone marrow enhances the clinical utility of this mouse model.
Our results are similar to those reported in several clinical trials.7, 9, 10, 42, 43 We observed improvements in function as well as limb perfusion with MNC treatment (Figure 2), as well as augmented muscle fiber regeneration with evidence of angiogenesis (Figure 3). In previous reports the number of injected MNC used to treat CLI has been variable, and interestingly, positive effects on perfusion have been observed even when low cell numbers were used.6 To date there has only been one study attempting to establish an association between clinical response and cell number; the authors observed a correlation between the number of CD34+ cells injected and improvements in ABI and concluded that the number of injected CD34+ cells was a primary factor influencing the clinical efficacy of cell therapy.13 Our findings show a similar correlation, with the 5×105 MNC treatment group showing improved flow over control and the higher 1×106 group showing improved flow over both the control as well as the 5×105 MNC treatment group (Figure 4). This positive correlation, however, appears to plateau at a certain cell number, perhaps secondary to a heightened inflammatory response that interferes with the angiogenic potential of progenitor cells, although the exact mechanism is unclear.
Clinical trials using intramuscular and intravascular injections, or a combination of both, have produced favorable results, however, intramuscular injection into the gastrocnemius muscle has been the preferred application in most trials.43–46 In our study, more proximally injected MNC showed similar increase in muscle flow and capillary density compared to the more distal MNC injection sites (Figure 5). Interestingly, proximal injection did result in increased significantly improved muscle area compared to both control and distal injection (Figure 5F). However, there was no overall increase in limb ischemia or function (Figures 5A, 5B). Therefore we believe that more proximal injection of MNC has the potential for greater therapeutic effect, but it is likely that the numbers of MNC need to be increased to achieve greater functional outcomes; this may be tempered by the plateau in effect with increased doses of cells (Figure 4), suggesting that both cell dose and injection location need to be optimized simultaneously.
Although we believe that our model of murine hind limb ischemia has clinical value, we likewise recognize its limitations. Despite the added modification of total SFA excision, this model continues to be acute in nature. In addition, without additional treatment, C57BL/6 mice spontaneously regain near maximal flow within several weeks post-ligation. This quick recovery from ischemia makes it more difficult to quantify and observe subtle flow and functional recovery as a result of cell therapy; it is precisely this inherent ability for spontaneous angiogenesis that may mask improvements in some of our treatment groups. Other improvements that could be made to our model include use of animal models of disease, such as ApoE-knockout mice, use of aged animals to mimic aged human patients, and use of other mouse strains that have different features of human disease.30 The mouse demonstrates spontaneous recovery from hind limb ischemia, limiting the ability to discriminate between changes in the final endpoint, and thus the ability to measure gross changes in tester parameters. However, we believe that our model shows that cellular therapy provides a kinetic advantage, with faster recovery from ischemia with parameters measured between 7 and 14 days. Finally, ex vivo treatment of the isolated MNC with cell culture conditions or drugs, such as statins or growth factors, may mimic other clinical protocols used in some trials.
In conclusion, our study shows that high femoral ligation with complete excision of the SFA is a reproducible model of hind limb ischemia in C57BL/6 mice. Similar to human trials, MNC injection into murine ischemic limbs improves vascular and functional outcomes. Our data suggests that cell number, type, and location of injection can be optimized. As such, it is likely that preclinical animal models will continue to serve an important role in comparing factors of clinical utility.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health (R01-HL095498-01 to A.D.); the American Vascular Association William J. von Liebig Award; as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Sprengers RW, Lips DJ, Moll FL, et al. Progenitor cell therapy in patients with critical limb ischemia without surgical options. Ann Surg. 2008;247(3):411–420. doi: 10.1097/SLA.0b013e318153fdcb. [DOI] [PubMed] [Google Scholar]
- 3.Minar E. Critical limb ischaemia. Hamostaseologie. 2009;29(1):102–109. [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 5.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Lawall H, Bramlage P, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost. 103(4):696–709. doi: 10.1160/TH09-10-0688. [DOI] [PubMed] [Google Scholar]
- 7.Kajiguchi M, Kondo T, Izawa H, et al. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007;71(2):196–201. doi: 10.1253/circj.71.196. [DOI] [PubMed] [Google Scholar]
- 8.Bartsch T, Falke T, Brehm M, et al. [Intra-arterial and intramuscular transplantation of adult, autologous bone marrow stem cells. Novel treatment for therapy-refractory peripheral arterial occlusive disease] Dtsch Med Wochenschr. 2006;131(3):79–83. doi: 10.1055/s-2006-924928. [DOI] [PubMed] [Google Scholar]
- 9.Esato K, Hamano K, Li TS, et al. Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transplant. 2002;11(8):747–752. [PubMed] [Google Scholar]
- 10.Higashi Y, Kimura M, Hara K, et al. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109(10):1215–1218. doi: 10.1161/01.CIR.0000121427.53291.78. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto K, Nishigami K, Nagaya N, et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114(24):2679–2684. doi: 10.1161/CIRCULATIONAHA.106.644203. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto M, Yasutake M, Takano H, et al. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transplant. 2004;13(4):429–437. doi: 10.3727/000000004783983837. [DOI] [PubMed] [Google Scholar]
- 13.Saigawa T, Kato K, Ozawa T, et al. Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J. 2004;68(12):1189–1193. doi: 10.1253/circj.68.1189. [DOI] [PubMed] [Google Scholar]
- 14.Durdu S, Akar AR, Arat M, et al. Autologous bone-marrow mononuclear cell implantation for patients with Rutherford grade II–III thromboangiitis obliterans. J Vasc Surg. 2006;44(4):732–739. doi: 10.1016/j.jvs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Franz RW, Parks A, Shah KJ, et al. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. J Vasc Surg. 2009;50(6):1378–1390. doi: 10.1016/j.jvs.2009.07.113. [DOI] [PubMed] [Google Scholar]
- 16.Kudo FA, Nishibe T, Nishibe M, et al. Autologous transplantation of peripheral blood endothelial progenitor cells (CD34+) for therapeutic angiogenesis in patients with critical limb ischemia. Int Angiol. 2003;22(4):344–348. [PubMed] [Google Scholar]
- 17.Lenk K, Adams V, Lurz P, et al. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. Eur Heart J. 2005;26(18):1903–1909. doi: 10.1093/eurheartj/ehi285. [DOI] [PubMed] [Google Scholar]
- 18.Huang PP, Li SZ, Han MZ, et al. Autologous transplantation of peripheral blood stem cells as an effective therapeutic approach for severe arteriosclerosis obliterans of lower extremities. Thromb Haemost. 2004;91(3):606–609. doi: 10.1160/TH03-06-0343. [DOI] [PubMed] [Google Scholar]
- 19.Huang P, Li S, Han M, et al. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28(9):2155–2160. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura A, Horie T, Tsuda I, et al. Prevention of limb amputation in patients with limbs ulcers by autologous peripheral blood mononuclear cell implantation. Ther Apher Dial. 2005;9(1):59–63. doi: 10.1111/j.1774-9987.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura A, Horie T, Tsuda I, et al. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. J Artif Organs. 2006;9(4):226–233. doi: 10.1007/s10047-006-0351-2. [DOI] [PubMed] [Google Scholar]
- 22.Ishida A, Ohya Y, Sakuda H, et al. Autologous peripheral blood mononuclear cell implantation for patients with peripheral arterial disease improves limb ischemia. Circ J. 2005;69(10):1260–1265. doi: 10.1253/circj.69.1260. [DOI] [PubMed] [Google Scholar]
- 23.Canizo MC, Lozano F, Gonzalez-Porras JR, et al. Peripheral endothelial progenitor cells (CD133 +) for therapeutic vasculogenesis in a patient with critical limb ischemia. One year follow-up. Cytotherapy. 2007;9(1):99–102. doi: 10.1080/14653240601034708. [DOI] [PubMed] [Google Scholar]
- 24.Burt RK, Testori A, Oyama Y, et al. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone Marrow Transplant. 45(1):111–116. doi: 10.1038/bmt.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartsch T, Falke T, Brehm M, et al. [Transplantation of autologous adult bone marrow stem cells in patients with severe peripheral arterial occlusion disease] Med Klin (Munich) 2006;101(Suppl 1):195–197. [PubMed] [Google Scholar]
- 26.Goto T, Fukuyama N, Aki A, et al. Search for appropriate experimental methods to create stable hind-limb ischemia in mouse. Tokai J Exp Clin Med. 2006;31(3):128–132. [PubMed] [Google Scholar]
- 27.Masaki I, Yonemitsu Y, Yamashita A, et al. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res. 2002;90(9):966–973. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
- 28.Hellingman AA, Bastiaansen AJ, de Vries MR, et al. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg. 40(6):796–803. doi: 10.1016/j.ejvs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Tarlov IM. Spinal cord compression studies. III. Time limits for recovery after gradual compression in dogs. AMA Arch Neurol Psychiatry. 1954;71(5):588–597. [PubMed] [Google Scholar]
- 30.Westvik TS, Fitzgerald TN, Muto A, et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg. 2009;49(2):464–473. doi: 10.1016/j.jvs.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, deMuinck ED, Zhuang Z, et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102(31):10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limbourg A, Korff T, Napp LC, et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4(12):1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Yamamoto Y, Tsujimoto S, et al. The cyclooxygenase-2 selective inhibitor, etodolac, but not aspirin reduces neovascularization in a murine ischemic hind limb model. Eur J Pharmacol. 627(1–3):223–228. doi: 10.1016/j.ejphar.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 34.Tokudome T, Kishimoto I, Yamahara K, et al. Impaired recovery of blood flow after hind-limb ischemia in mice lacking guanylyl cyclase-A, a receptor for atrial and brain natriuretic peptides. Arterioscler Thromb Vasc Biol. 2009;29(10):1516–1521. doi: 10.1161/ATVBAHA.109.187526. [DOI] [PubMed] [Google Scholar]
- 35.Yan J, Tie G, Park B, et al. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg. 2009;50(6):1412–1422. doi: 10.1016/j.jvs.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park B, Hoffman A, Yang Y, et al. Endothelial nitric oxide synthase affects both early and late collateral arterial adaptation and blood flow recovery after induction of hind limb ischemia in mice. J Vasc Surg. 51(1):165–173. doi: 10.1016/j.jvs.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biscetti F, Straface G, De Cristofaro R, et al. High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes. 59(6):1496–1505. doi: 10.2337/db09-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki H, Shibata R, Kito T, et al. Therapeutic angiogenesis by transplantation of induced pluripotent stem cell-derived Flk-1 positive cells. BMC Cell Biol. 11:72. doi: 10.1186/1471-2121-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bir SC, Esaki J, Marui A, et al. Angiogenic properties of sustained release platelet-rich plasma: characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg. 2009;50(4):870 e2–879 e2. doi: 10.1016/j.jvs.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Yeo C, Saunders N, Locca D, et al. Ficoll-Paque versus Lymphoprep: a comparative study of two density gradient media for therapeutic bone marrow mononuclear cell preparations. Regen Med. 2009;4(5):689–696. doi: 10.2217/rme.09.44. [DOI] [PubMed] [Google Scholar]
- 41.Grigoriadis AE, Kennedy M, Bozec A, et al. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood. 115(14):2769–2776. doi: 10.1182/blood-2009-07-234690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amann B, Luedemann C, Ratei R, et al. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009;18(3):371–380. doi: 10.3727/096368909788534942. [DOI] [PubMed] [Google Scholar]
- 43.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 44.Gyongyosi M, Lang I, Dettke M, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized study. Nat Clin Pract Cardiovasc Med. 2009;6(1):70–81. doi: 10.1038/ncpcardio1388. [DOI] [PubMed] [Google Scholar]
- 45.Walter DH, Krankenberg H, Balzer JO, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 4(1):26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 46.Lasala GP, Minguell JJ. Vascular disease and stem cell therapies. Br Med Bull. 98:187–197. doi: 10.1093/bmb/ldr017. [DOI] [PubMed] [Google Scholar]