Abstract
Cadmium (Cd) is a common environmental contaminant. Adult exposure to Cd alters the immune system, however, there are limited studies on the effects of prenatal exposure to Cd. Pregnant C57Bl/6 mice were exposed to an environmentally relevant dose of CdCl2 (10 ppm) and the effects on the immune system of the offspring were assessed at 20 weeks of age. Prenatal Cd exposure caused an increase in the percent of CD4−CD8−CD44+CD25− (DN1) thymocytes in both sexes and a decrease in the percent of CD4−CD8−CD44−CD25+ (DN3) thymocytes in females. Females had an increase in the percent of splenic CD4+ T cells, CD8+ T cells, and CD45R/B220+ B cells and a decrease in the percent of NK cells and granulocytes (Gr-1+). Males had an increase in the percent of splenic CD4+ T cells and CD45R/B220+ B cells and a decrease in the percent of CD8+ T cells, NK cells, and granulocytes. The percentage of neutrophils and myeloid-derived suppressor cells were reduced in both sexes. The percent of splenic nTreg cells was decreased in all Cd-exposed offspring. Cd-exposed offspring were immunized with a streptococcal vaccine and the antibody response was determined. PC-specific serum antibody titers were decreased in Cd exposed female offspring but increased in the males. PspA-specific serum IgG titers were increased in both females and males compared to control animals. Females had a decrease in PspA-specific serum IgM antibody titers. Females and males had a decrease in the number of splenic anti-PspA antibody-secreting cells when standardized to the number of B cells. These findings demonstrate that very low levels of Cd exposure during gestation can result in long term sex-specific alterations on the immune system of the offspring.
Keywords: cadmium, prenatal exposure, thymocytes, splenocytes, serum antibody, antibody-secreting cells
Introduction
Cadmium (Cd) is a heavy metal that poses a hazard to human health due to its toxicity. There is sufficient evidence in humans to classify Cd and Cd compounds as carcinogens based on epidemiological studies demonstrating a link between Cd and lung (Park et al., 2012), and breast (Julin et al., 2012), cancers. Occupational exposure to the heavy metal and its compounds primarily occurs in mining, smelting, processing, and battery manufacturing. Environmental and non-occupational exposures come from various foods, contaminated water, contaminated dust and tobacco smoke. Smokers generally have Cd blood levels 4–5 times those of non-smokers (Elinder et al., 1976).
Cd levels in the environment vary widely due to its ability to be transported through air, water, and soil. Cd levels in soils, particularly areas in which phosphate fertilizers have been applied, can range from 10 to 200 µg/g (Cook, 1995). Former zinc smelter sites often leave significant levels in the area surrounding the plant because of the pyrometallurgical process that removed contaminating metals by progressively raising the temperature of the ore until each metal sublimated. Cd’s sublimation temperature is lower than Zn, thus, Cd fumes were released into the environment and as the Cd-fumes cooled and condensed, Cd settled around the plant.
Humans normally absorb Cd into the body either by ingestion or inhalation (Lauwerys et al., 1986). The daily intake is estimated to be approximately 10–50 µg, but can reach levels of 200–1000 µg in highly contaminated areas (Nordberg, 2006). The average Cd intake from food in one study showed values of 38–300 µg/week (Olsson et al., 2002), while absorption from cigarette smoke is 1–3 µg/pack/day (Faroon et al., 2008). The biologic half-life of Cd in the human body is estimated to be 15–20 years because humans do not have an effective Cd elimination pathway (Jin et al., 1998). Excessive Cd accumulation in the body often results in diseases such as kidney failure, respiratory disease, neurological disorders, and occasionally death (Waalkes et al., 1992). Studies on the immunomodulatory effects of Cd in adult humans and experimental animals are inconsistent probably due to varying doses, route of administration, length of Cd exposure, and differences in the sensitivity of immune systems of different animal species (Mackova et al., 1996; Liu et al., 1999; Lafuente et al., 2004).
Pharmacokinetic studies demonstrate that Cd does not readily reach the fetus, however, it accumulates in high concentrations in the placenta (Piasek et al., 2001) and the teratological effects of Cd in rodents have been extensively documented (Hovland et al., 1999; Scott et al., 2005; Minetti and Reale, 2006; Jacquillet et al., 2007). In a likely scenario for human environmental Cd exposure, the exposure to the conceptus could conceivably begin during gestation because of the mother’s residence in a Cd-contaminated area. There are limited reports on the teratological effects associated with Cd exposure for humans; however, maternal exposure to environmental Cd, higher placental concentration (Loiacono et al., 1992), and/or fetal Cd exposure (Frery et al., 1993) has been associated with lower birth weights in humans. Therefore, the present study determined the immunotoxic effects of prenatal-only exposure to Cd in mice.
Morselt (Morselt et al., 1988) reported that the thymus, the primary site of T-cell production, is a target organ of Cd-induced toxicity. Thymocytes mature through a series of stages defined by expression of the cell surface markers CD4 and CD8. The most immature thymocytes are CD4−CD8− double-negative (DN). This population gives rise to CD4+CD8+ double-positive (DP) cells, which then give rise to mature CD4+CD8− single-positive (SP) and CD4−CD8+ SP cells. The DN population can be further subdivided in mice based on the expression of surface markers CD25 and CD44: CD44+CD25−(DN1) cells differentiate into CD44+CD25+(DN2) cells, which then develop into CD44−CD25+(DN3) cells, which differentiate into the CD44−CD25−(DN4) population. Prenatal exposure to an environmentally relevant dose of CdCl2 (10 ppm w/v) affects thymocyte development of newborn (<12 h old) offspring (Hanson et al., 2010). Follow up studies assessed the effects on the immune system of the offspring at two additional time points following birth (2 and 7 woa) (Hanson et al., 2012). At these ages, thymus and spleen cell populations in Cd-exposed offspring varied very little from controls, with only the macrophage population in males showing a significant increase (Hanson et al., 2012). There were, however, changes in spleen cell cytokine production at 7 woa with significantly lower interferon-γ production by male and female offspring. Males also had significantly reduced IL-2 production (Hanson et al., 2012). There was no effect on IL-10 and IL-4 production at 7 woa (Hanson et al., 2012). The acquired immune response was determined by assessing anti-streptococcal antibody responses in animals vaccinated with heat-killed Streptococcus pneumoniae. Interestingly, serum antibody responses to both a T-independent (phosphorylcholine, PC) and a T-dependent antigen (Pneumococcal surface protein A, PspA) were dramatically higher in the Cd-treated offspring than the control animals at 7 woa (Hanson et al., 2012). The relative percentage of CD4+CD25+FoxP3+ (nTreg) and CD8+CD223+ T cells in the spleen was also markedly different between the two groups at 7 woa (Hanson et al., 2012).
A frequent question with prenatal exposure studies is whether changes noted at a relatively early age, e.g., 7 woa, are temporary or whether the changes persist to an older age denoting a permanent (possible) epigenetic effect. Thus, we matured a cohort of animals to 20 woa and measured similar immune cell populations and the animal’s ability to respond to streptococcal T-independent and T-dependent antigens. At 20 woa, thymic DN1 and DN3 cell populations were altered in Cd-exposed offspring. The number of splenocytes and splenic B cells was increased in both sexes. Several spleen cell populations were significantly changed from the controls in a gender specific manner. The percentage of nTreg cells in the Cd-treated offspring was significantly decreased, however, CD8+CD223+ T cells, a T cell prone to immune exhaustion in chronic viral infection, were not affected. The acquired immune system was assessed at 20 woa using the streptococcal vaccine. Cd-treated male offspring had increased serum antibody titers to the T-independent antigen, PC. In contrast, Cd-treated female offspring had decreased PC-specific serum antibody titers. Serum antibody titers specific for the T-dependent antigen, PspA, were significantly lower in the Cd-treated female offspring while the male offspring had no difference in PspA specific serum antibody titers compared to control animals. An assessment of the number of antibody secreting cells (ASC) demonstrated an increase in the anti-PC ASC in the spleens of males. The number of anti-PspA ASC was significantly lower in both sexes for the Cd-treated offspring when normalized to 1 × 106 B cells, and in the spleens of female offspring. Collectively, the data demonstrate that prenatal exposure to Cd causes phenotypic and functional changes to the immune system of the offspring that is persistent to at least 20 woa.
Materials and methods
Breeding and Cd Exposure
C57Bl/6 mice at 8–10 woa were obtained from Harlan Laboratories (Indianapolis, IN). The C57Bl/6 strain of mouse was used for these experiments due to its reported teratogenic susceptibility to Cd exposure (Hovland et al., 1999). The mice were bred as part of a cohort of mice that were assayed for shorter term effects of prenatal Cd exposure at 7 woa (Hanson et al., 2012). Briefly, after a one week (minimum) acclimation in our vivarium, 2 females were placed in a cage with one male for 72 hours to maximize pregnancy rate. Females were inspected for a vaginal plug and if present, this day was declared as gestational day 0. Dams used as controls had free access to water and Cd-treated dams had free access to 10 ppm of CdCl2 (Sigma-Aldrich) dissolved in water. To reduce the number of animals used for the study only one dose was used at a level that would reproduce human dose environmental exposure, as described in the Introduction. The dose of 10 ppm was chosen because it is the greatest concentration that will elicit immunomodulatory effects in adult rodents without causing systemic effects (Lafuente et al., 2003). Cd administration was stopped at birth. All offspring were weaned at 3 woa and the dams were euthanized. Animals tested for the results reported herein are from the same cohort tested at birth and Cd levels were assessed in the dams and representative offspring using inductively coupled plasma optical emission spectrometry (ICPOES) as reported in Hanson et al. (2010). The kidney Cd levels in the dams at the time of weaning the litter was 4.37±0.76 µg/g tissue and Cd levels slightly above the level of detection by the ICP-OES (2.5ppb) were only detectable in the liver of offspring if tissues from ≥3 animals were pooled (Hanson et al., 2010). At approximately 20 woa, 3 offspring (at least 1 male and 1 female) selected from each of the litters were euthanized and thymi and spleens were removed. The 20 woa time point would approximate a 25 year-old human with a fully developed immune system. All animal procedures were approved by the WVU Institutional Animal Care and Use Committee.
Tissue Isolation and Cell Preparation
Thymi and spleens were harvested from euthanized mice and single cell suspensions prepared as previously described (Hanson et al., 2012). The cell preparations from each mouse were analyzed individually.
Cell staining and flow cytometry
Single cell suspensions of thymocytes and splenocytes were stained as described in Hanson et al. (Hanson et al., 2012). SP and DP cell subpopulations were identified using anti-CD4 and anti-CD8. To identify the different DN subpopulations, CD44 and CD25 expression were detected on the CD4−CD8− population. Splenocytes were stained using combinations of the directly conjugated antibodies. A detailed list of these antibodies, conjugate, clone, dilution and source is provided in Supplemental Table 1. Data were acquired using a FACSAria and analyzed employing FACSDiva software (BD Biosciences Pharmingen, San Diego, CA). A total of 10,000 events were collected for each sample.
To detect the CD8+CD223+T cells in spleen and thymus, a combination of fluorochrome-conjugated antibodies against CD8, CD28 and CD223 (CD223 is also called LAG-3) was used as above.
CD4+ nTreg cells expressing the transcription factor forkhead box P3 (FoxP3) were detected using antibodies (eBioscience) against membrane-associated CD4 and CD25, in addition to intracellular FoxP3. Splenocyte and thymocyte surface staining with anti-CD4 and anti-CD25 was performed as above followed by fixation/permeabilization for 30 min, washed and stained for 30 min with anti-FoxP3 antibody or the rat IgG2a isotype control. Cells were washed twice, resuspended in PBSAz and analyzed by flow cytometry.
Preparation of S. pneumonia vaccine and immunization
Functional analysis of the immune system was performed by immunizing the offspring with a heat-killed vaccine of Streptococcus pneumoniae strain R36A. S. pneumoniae strain R36A is an avirulent, non-encapsulated strain. The kinetics of the serum antibody response and the predominant types of antibody isotypes to phosphorylcholine (PC) and pneumococcal surface protein A (PspA) have been well characterized (Wu et al., 2000; Wu et al., 2001).
The vaccine was prepared as described by Salazar et al. (Salazar et al., 2005) and Hanson et al. (Hanson et al., 2012). Briefly, S. pneumoniae strain R36A, was grown to mid-log phase in supplemented Todd-Hewitt broth (Becton Dickinson Company, Sparks, MD) at 37°C. The strain was heat killed at 60°C for 2 hr, washed twice and resuspended in saline at 2 × 109 CFU/ml. Sterility and CFU were confirmed by culture on blood agar plates and the heat-killed stock was stored at −80°C. Mice were immunized intraperitoneally with 2 × 108 CFU in 100 µl, Spleens and blood were collected 10 days following immunization.
Preparation of PspA
PspA for use in the ELISA and ELISpot assays was prepared as described in (Hanson et al., 2012). Briefly, plasmid UAB055, which contains the truncated PspA gene attached to a 6-His tag (gift of Dr. Susan Hollingshead, Department of Microbiology, UAB, Birmingham, AL), was transformed into BL21 (DE3) pLysS (EMD Biosciences, San Diego, CA). A selected transformant colony was grown in ampicillin (100 µg/ml; Sigma-Aldrich) supplemented Luria broth (Becton Dickinson Company) to an OD600 of 0.5, induced with 1 mM IPTG and harvested. His-tagged PspA protein was purified from periplasmic extract using BugBuster HisBind purification kit (EMD Biosciences), dialyzed extensively against PBS, adjusted to 0.25 mg/ml and stored at 4°C.
Serum antibody ELISA
Blood samples were collected from immunized mice and sera obtained by standardized methods and stored at −20°C until assayed as described in Hanson et al. (Hanson et al., 2012). For ELISA assays, Immulon 2 plates (Thermo LabSystems, Franklin, MA) were coated overnight at 4°C with 2 µg/ml PC-BSA (Biosearch Technologies, Inc, Novato, CA) or 5 µg/ml PspA. Plates were washed (PBS + 0.05% Tween-20) and blocked with 1% BSA in PBS at 37°C for 1 hr. Plates were washed and 100 µl/well serum diluted in PBS was added starting at 1/400 for PC and 1/50 for PspA with twofold dilutions thereafter, and allowed to bind overnight at 4°C. Plates were then washed and incubated with alkaline phosphatase (AP) conjugated antibodies (Southern Biotechnology Associates, Birmingham, AL) for 3 h at 37°C. After washing, 100 µl/well phosphatase substrate (Sigma-Aldrich) dissolved in diethanolamine buffer, pH 9.8 was added. Absorbance values were read at 405nm (A405) at timed intervals using a µQuant spectrophotometer (Bio-Tek Instruments, Winooski, VT) using KCJunior software (Bio-Tek Instruments, Winooski, VT). To determine the titer, a standard pooled sera was diluted and plated on each ELISA plate. The titer for each sample was determined by comparison to the standard sera when the A405 for the standard was 0.4 at a 1:3200 dilution to measure all anti-PC antibody isotypes and 0.2 for anti-PspA IgM and IgG isotypes. These dilutions were chosen because they were in the linear part of the curve for the respective isotypes. In some instances, the anti-PspA IgG1, IgG2a, and IgG2b titers were determined at an A405 of 0.2 after 2 h of incubation.
Quantification of the number of splenic antibody secreting cells (ASC)
ELISpot assays were performed as described in Salazar et al. (Salazar et al., 2005). Briefly, 96-well filter plates (Pall Life Sciences, Ann Arbor, MI) were coated with 50 µl PC-BSA (10 µg/ml) or 50 µl PspA (10 µg/ml) overnight at 4°C. Plates were washed with PBS and blocked with 200 µl/well RPMI medium + 25% fetal bovine serum (Hyclone, Logan, UT) for 2 h at 37°C. Plates were washed, and cells (100 µl/well) were then added at a concentration of 5 × 106 cells/ml or 1 × 106 cells/ml. All samples were plated in triplicate. Plates were incubated for 4–6 h at 37°C in a 5% CO2 incubator. Plates were washed and goat anti-mouse alkaline phosphatase (AP) conjugated IgG or IgM antibodies (Southern Biotechnology Associates), diluted 1/2000 in PBS + 1% BSA +.05% Tween-20, were added to the appropriate wells (100 µl/well). Plates were incubated overnight at 4°C and washed. SIGMA-ALDRICH FAST 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium tablets (Sigma-Aldrich) were dissolved in distilled water and added at 100 µl per well. Color development was stopped by washing with distilled water. The number of spots per well was counted using a dissection microscope (Olympus Optical Co., Melville, NY). The number of ASC was calculated by using the mean number of spots from triplicate wells. Data are expressed as the number of ASC per 1 × 106 B cells or as the number of ASC per spleen.
Statistical Analyses
Results are expressed as mean ± SEM of 9–13 offspring per sex per treatment group. Data expressed in percent of total or percent of control for cell populations were analyzed using the nonparametric Mann-Whitney (Wilcoxon) test. T-test was used to analyze cell numbers, PspA and PC titers, and ASC, between Cd-exposed and control offspring. All experiments were repeated at least one time. An alpha value of p≤0.05 was considered significant.
Results
The total number of thymocytes and splenocytes were changed in 20 week-old offspring exposed to Cd prenatally
The total number of thymocytes and splenocytes were enumerated for each animal at 20 woa. Total thymocyte number was significantly higher in Cd-exposed versus control offspring. Cd-exposed male offspring had 7.1±0.77 × 107 versus 4.6±0.46 × 107 thymocytes in the control offspring (Supplemental Figure 1). Female Cd-exposed offspring had 9.6±0.11 × 107 versus the control female offspring that had 4.7±0.12 × 107 thymocytes (Supplemental Figure 1).
At 20 woa, Cd-exposed males had significantly more splenocytes (13.5±3 × 107) than control males (6.7±1.1 × 107) (Supplemental Figure 1). Female Cd-exposed offspring and control females had comparable numbers of splenocytes (10.3±1.2 × 107 and 10.2±1.6 × 107, respectively) (Supplemental Figure 1).
Prenatal Cd exposure changed the relative percentages of thymocyte subpopulations in 20 week-old offspring
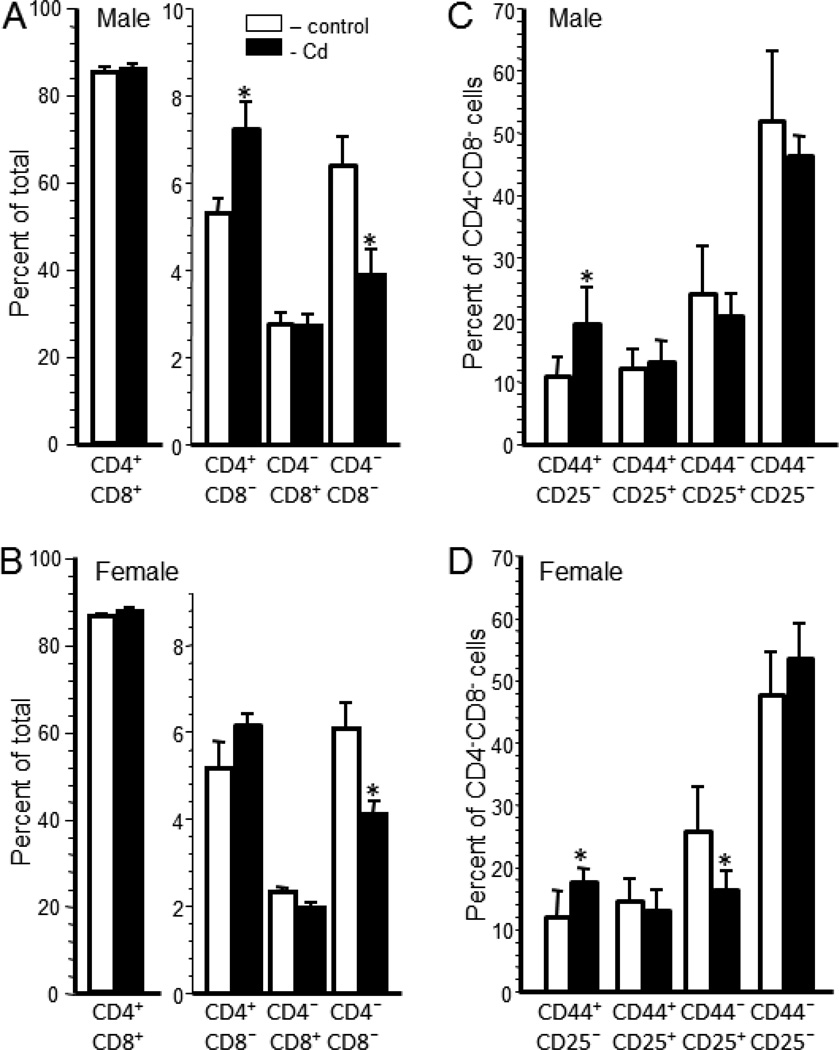
Thymocyte populations of representative offspring from each litter was determined by the expression of cell surface markers. The percentage of double positive cells (DP; CD4+CD8+) was similar for Cd-exposed and control male (Figure 1A) and female offspring (Figure 1B). The percentage of single positive (SP) CD4+CD8− thymocytes was significantly higher in the Cd-exposed male offspring (7.25±0.61%) than in the control offspring (5.35±0.30%)(Figure 1A). Cd-exposed female offspring also had a higher percentage of SP CD4+CD8− thymocytes (6.17±0.25%) than the control female offspring (5.19±0.6%), however, the difference was not significant (Figure 1B). The percentage of SP CD4−CD8+ thymocytes was essentially identical between treatment groups in both sexes (Figure 1A and B). The percentage of CD4−CD8− (double negative; DN) thymocytes was significantly lower in the Cd-exposed offspring for both sexes. Male Cd-exposed offspring had 3.91±0.57% DN thymocytes versus 6.42±0.65% in the control offspring (Figure 1A). Female Cd-exposed offspring had 4.1±0.3% DN thymocytes versus 6.1±0.6% in the control offspring (Figure 1B).
Figure 1. Prenatal Cd exposure induced lasting changes in thymocyte populations of 20 week-old offspring.
Thymocytes were isolated from mice that were prenatally exposed to 10 ppm Cd (solid bars) or water (open bars) and analyzed by flow cytometry as described in the Materials and Methods. Each bar represents the mean ± SEM. Data shown are representative of 2 experiments where the n equals at least 5 in each group. Thymocyte populations were determined based on CD4 and CD8 cell surface expression for male (A) and female (B) offspring. DN subpopulation phenotype was determined based on CD44 and CD25 cell surface expression for male (C) and female (D) offspring. *p<0.05
The percentage of each subtype of DN thymocyte was also determined using the membrane markers CD44 and CD25. The percentage of DN1 (CD44+CD25−) thymocytes was significantly greater in males (19.56±2.63%) and females (17.65±0.75%) in the Cd-exposed offspring versus the control males (11.14±1.32%) and females (11.04±1.75%) (Figure 1C and D). Although the percentage of DN3 (CD44−CD25+) cells was lower for the Cd-treated offspring of both sexes, only the females showed a significant difference. Cd-treated females had 16.22±1.14% versus the control females with 25.8±7.2% DN3 cells. There was no significant difference between treatment groups in the percentage of DN2 (CD44+CD25+) or DN4 (CD44−CD25−) thymocytes for either sex (Figure 1C and D).
Prenatal Cd exposure changed the relative percentages of splenic cell populations in 20 week-old offspring
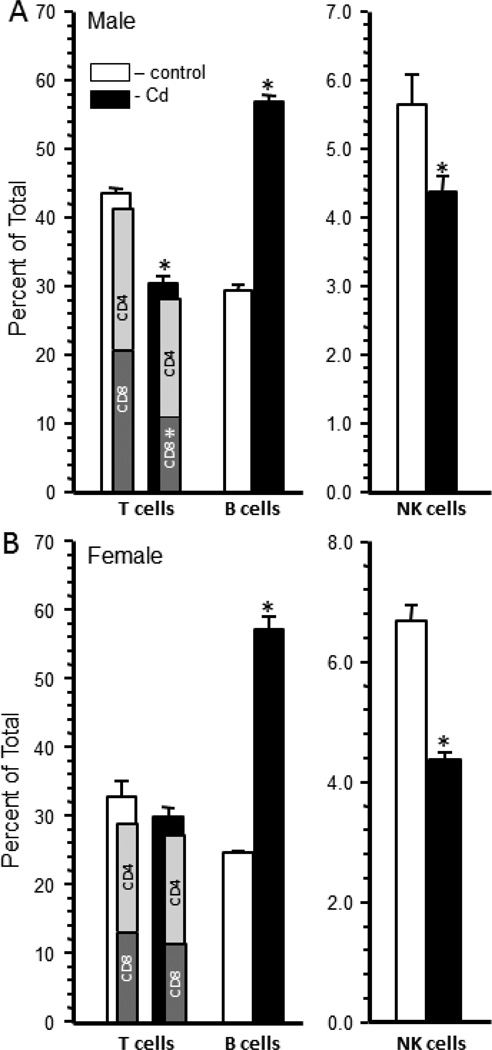
Splenocyte populations of representative offspring from each litter were determined for CD4+ T cells, CD8+ T cells, B cells, NK cells, Gr-1+ granulocytes, and CD11b+ monocytes/macrophages. The total percentage of T cells was also determined using the T cell receptor-beta (TCRβ) marker. The percentage of TCRβ positive splenocytes was significantly reduced in the Cd-exposed males (30.44±1.01%) versus the control male offspring (43.58±0.7%) (Figure 2A). Male offspring from Cd-treated dams had a decrease in CD8+ T cells compared to the offspring of control dams (Figure 2A). The CD4:CD8 ratio for the control offspring was 0.92 or approximately 1:1 whereas this ratio was 1.48 or approximately 1.5:1 in the Cd-treated male offspring (Figure 2A). There was no significant difference in the total percentage of TCRβ positive splenocytes or the relative percentage of CD4+ and CD8+ T cells within the TCRβ-positive splenocytes between the Cd-exposed and control female offspring (Figure 2B). The CD4:CD8 ratio was, therefore, approximately 1:1 in the female offspring.
Figure 2. Prenatal Cd exposure changed the splenocyte phenotype of 20 week-old male offspring.
Spleen cells were isolated from male and female mice that were exposed to 10 ppm Cd (solid bars) or water (open bars) and analyzed by flow cytometry after staining with anti-TCR-β, anti-CD4, anti-CD8, anti-CD45R (B cells) and anti-NK1.1 (NK-cells) as described in the Materials and Methods. Each bar represents the mean ± SEM. Data are representative of 2 experiments where the n equals at least 5 in each group. *p<0.05
The percentage of CD45R+ B cells was significantly higher in the Cd-treated offspring than the control offspring (Figure 2A and B). Male Cd-exposed offspring had 56.8±1.0 B cells versus 29.2±1 in the control offspring (Figure 2A). Female Cd-exposed offspring had 57.3±1.9 B cells versus 24.8±.2% in the control female offspring (Figure 2B). The actual number of B cells in the spleens of Cd-exposed offspring was also significantly increased over control animals. Male Cdexposed offspring had 8.21±2.1 × 107 B cells versus the control offspring that had 2.85±0.65 × 107 B cells (Supplemental Figure 1). Cd-exposed female offspring had 6.4±0.8 × 107 B cells versus control that had 4.1±1.1 × 107 B cells (Supplemental Figure 1).
The percentage of NK cells was significantly lower in the Cd-exposed offspring (Figure 2A and B). Male Cd-exposed offspring had 4.6±0.25% NK cells versus the control offspring that had 5.3±0.26% NK cells (Figure 2A). Females had a slightly higher difference with female Cd-exposed offspring having 4.3±0.3 NK cells versus 6.7±.6% NK cells in the control offspring (Figure 2B).
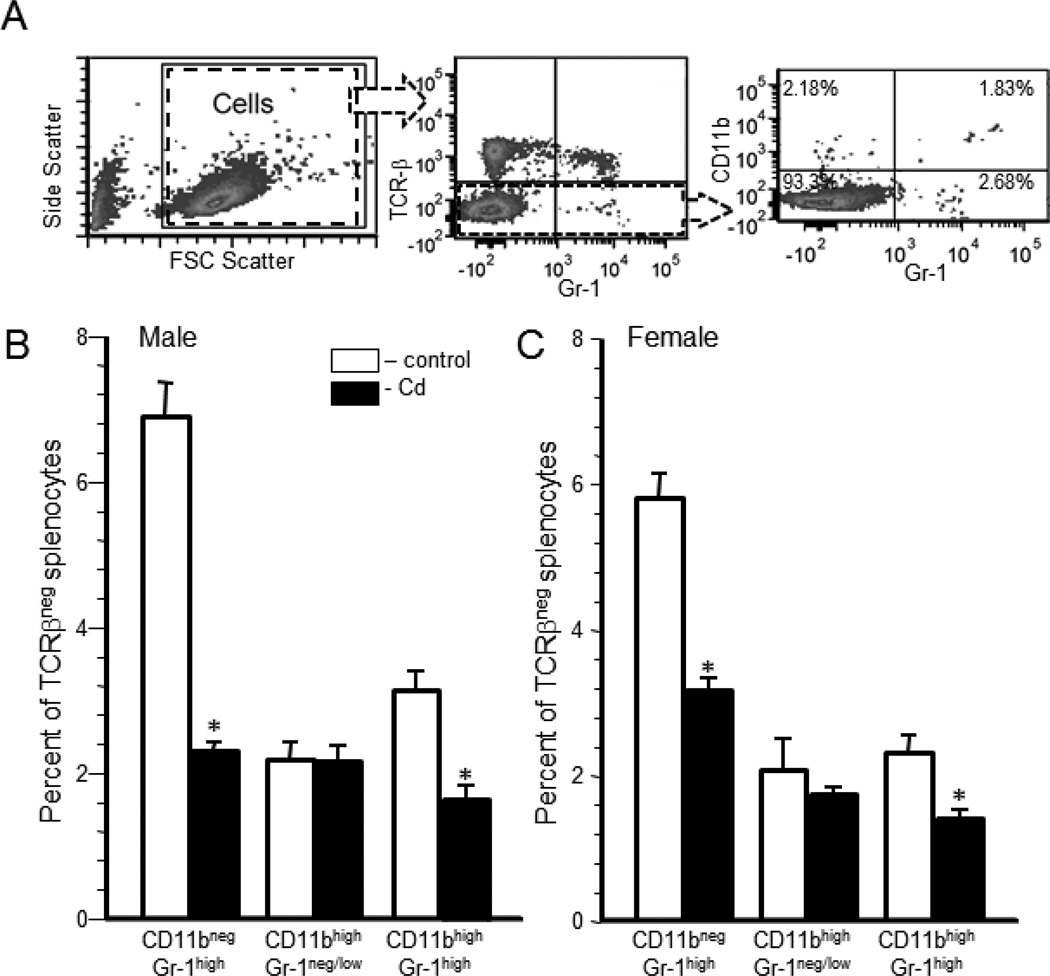
Within the TCRβ-negative cells, a further analysis was performed using CD11b+ and Gr-1 markers. Figure 3A represents a typical scatter plot from a Cd-exposed male that demonstrates how the populations were determined. Live cells (Figure 3A, left panel) were analyzed for TCR-β (Figure 3A, middle panel). The TCR-β negative population was analyzed for CD11b and Gr-1 expression (Figure 3A, right panel). Four populations were determined: CD11bnegGr-1neg/low, CD11bnegGr-1high, CD11bhighGr-1neg/low, and CD11bhighGr-1high (Figure 3B and C). There were no significant differences between the Cd-exposed offspring and control offspring in the CD11bnegGr-1neg/low population (data not shown). Cd-exposed male offspring had a significantly lower percentage of the CD11bnegGr-1high population (2.3±0.1%) versus the control offspring (6.9±0.5%) (Figure 3B). Female Cd-exposed offspring also had a significantly lower percentage of CD11bnegGr-1high cells (3.18±0.1%) compared to the control offspring (5.79±0.3%) (Figure 3C). There was no difference in the percentage of the CD11bhighGr-1neg/low population for either sex (Figure 3B and 3C). A significant difference in the CD11bhighGr-1high population between the Cd-exposed offspring and control offspring was noted in both sexes. Male Cd-exposed offspring had 1.67±0.15% CD11bhighGr-1high cells versus the control offspring that had 3.15±0.2% (Figure 3B). Female Cd-exposed offspring had 1.4±0.1% CD11bhighGr-1high cells compared to the control offspring that had 2.31±0.2% (Figure 3C).
Figure 3. Prenatal Cd exposure changed the relative percentages of granulocyte subpopulations of 20 week-old offspring.
Spleen cells were isolated from male and female mice that were exposed to 10 ppm Cd (solid bars) or water (open bars) and analyzed by flow cytometry as described in the Materials and Methods. Live cells (Figure 3A, left panel) were analyzed for TCR-β expression (Figure 3A, middle panel). The TCR-β negative population was analyzed for CD11b and Gr-1 expression (Figure 3A, right panel). Four populations were determined: CD11bnegGr-1neg/low, CD11bnegGr-1high, CD11bhighGr-1neg/low, and CD11bhighGr-1high. Figure 3A shows a typical set of plots for a Cd-exposed male offspring. The results are shown for males (B) and females (C). The results for the CD11bnegGr-1neg/low (lower left quadrant) are not shown. Each bar represents the mean ± SEM. Data are representative of 2 experiments where the n equals at least 5 in each group. *p<0.05
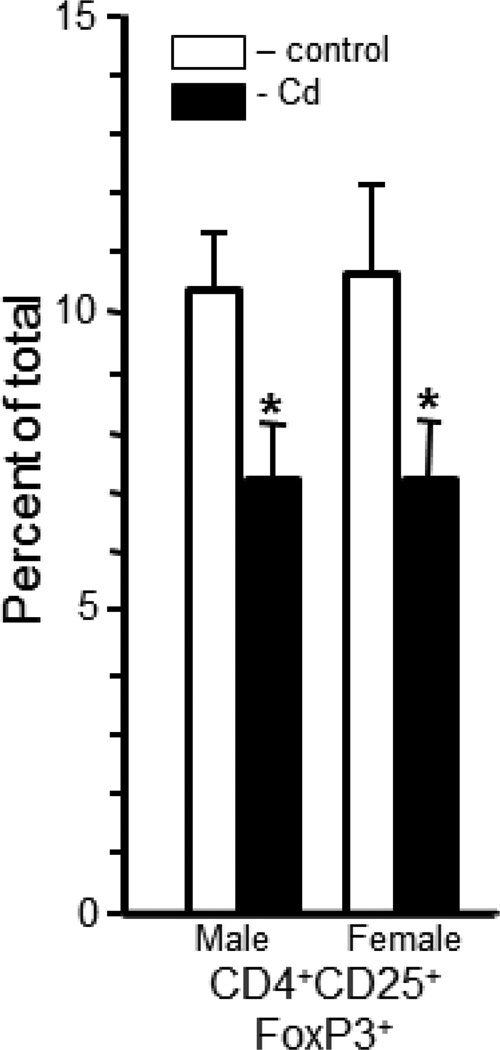
The splenic nTreg cells (CD4+CD25+FoxP3+) in the Cd-treated offspring were significantly lower than in controls (Figure 4). Cd-treated male offspring had 7.4±0.7% whereas the control male offspring had 10.5±1.0% (Figure 4). Female offspring had similar differences in the percentage of nTreg cells. Female Cd-treated offspring had 7.4±0.8% and the control offspring had 10.7±1.5% nTreg cells (Figure 4).
Figure 4. Prenatal Cd exposure altered the relative percentage of a population of CD4+ natural T regulatory cells (nTreg) in the spleens of 20 week-old offspring.
Single cell suspensions were prepared for flow cytometry and stained with anti-CD4, anti-CD25 and anti-FoxP3 to quantify nTreg cells as described in the Materials and Methods. Each bar represents the mean ± SEM. Data are representative of 2 experiments where the n equals at least 5 in each group. *p<0.05
The percentage of CD8+CD223+ T cells is increased after antigen-stimulation (Blackburn et al., 2009), after immune ‘exhaustion’ as a result of chronic viral infection (Blackburn et al., 2009) and after loss of self-tolerance (Grosso et al., 2007). The Cd-offspring had fewer CD8+CD223+ cells, however, these differences were not statistically significant (Supplemental Figure 2).
Prenatal Cd exposure changed the specific anti-PC and anti-PspA serum antibody response at 20 woa
The T-independent and T-dependent serum antibody responses were determined after vaccination with a heat-killed S. pneumoniae vaccine. The T-independent response to the PC antigen provides a functional assessment of the ability of the B cell to be stimulated by antigen and differentiate into IgM producing plasma cells. In addition, type II T-independent antigens, such as PC, can induce switching via dendritic cells to produce IgG antibodies (Colino et al., 2002).
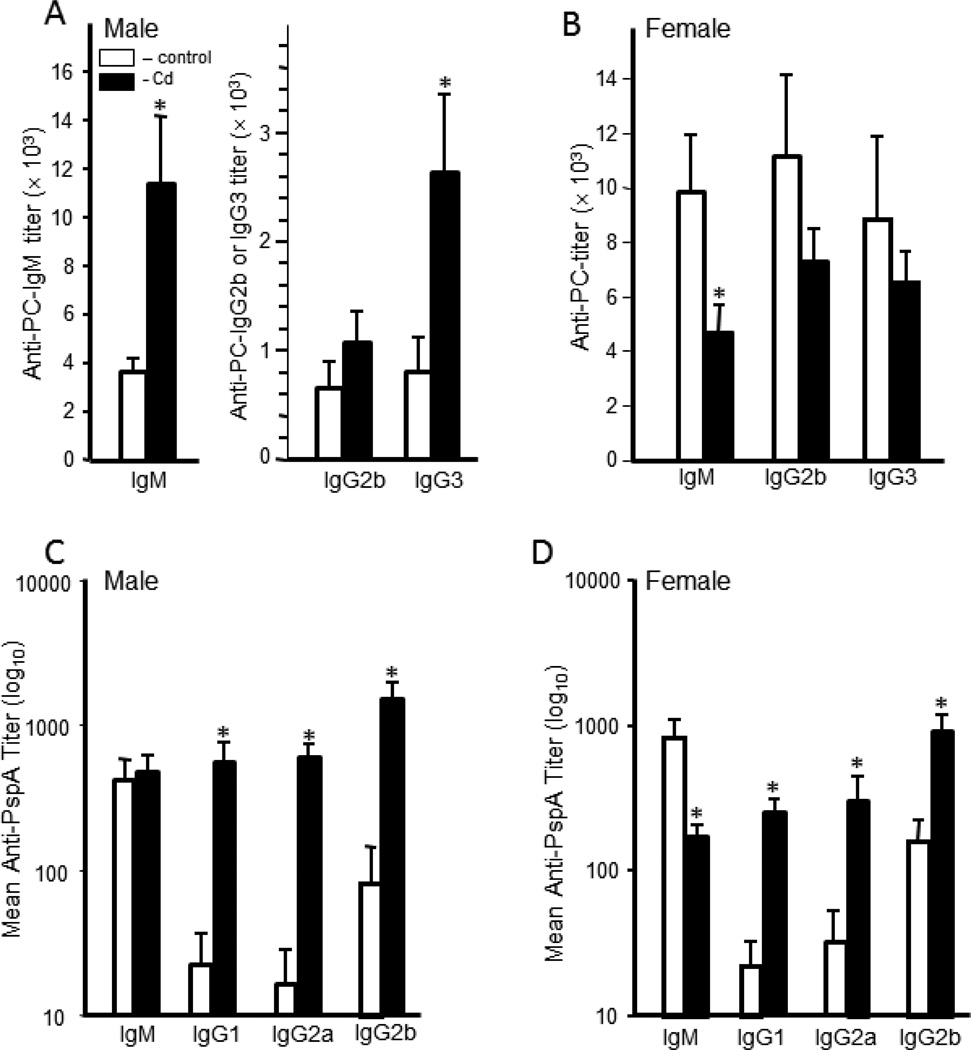
The anti-PC serum IgM and IgG3 antibody titers were significantly higher in male offspring prenatally exposed to Cd (Figure 5A). For the males, the IgM titers were approximately threefold higher in the Cd-exposed animals over the control male offspring (Figure 5A). IgG3 titers were also significantly higher in the Cd-exposed males, although the titer was much lower than the IgM titers (Figure 5A). IgG2b titers for the Cd-exposed offspring were also higher than controls; however, this difference was not statistically significant. Female offspring had a reverse pattern. For the females, the control offspring had higher anti-PC titers than the Cd-treated offspring (Figure 5B).
Figure 5. Anti-streptococcal serum antibody production was markedly altered by prenatal exposure to Cd.
Twenty week-old mice that were exposed to 10 ppm Cd (solid bars) or water (open bars) in utero were immunized with heat-killed S. pneumoniae vaccine. Sera were collected 10 days after immunization to determine anti-PC and anti-PspA antibody titers in males and females. Antibody levels were determined by ELISA as described in the Materials and Methods. A) The serum anti-PC antibody titers of the male offspring; B) the serum anti-PC antibody titers of the female offspring; C) the serum anti-PspA antibody titers of the male offspring; D) the serum anti-PspA antibody titers of the female offspring. Each bar represents the mean ± SEM. Data are representative of 2 experiments where the n equals at least 5 in each group. *p<0.05
The T-dependent response requires the cooperative participation of antigen-presenting cells, T-helper cells and B cells. Thus, this measurement provides a very comprehensive picture of the ability of the animal to respond to an antigenic challenge. The T-dependent antigen PspA induced a robust IgM response in both Cd-exposed and control offspring. There was no difference in the IgM titers between Cd-exposed and control male offspring (Figure 5C). The level of anti-PspA IgM in the female controls was significantly higher than the levels in the Cd-treated offspring (Figure 5D). For both sexes, the serum IgG (all isotypes) titers were significantly and dramatically higher in the Cd-exposed offspring versus the control offspring (Figure 5C and D).
Prenatal Cd-exposure changes the number of antibody secreting cells (ASC) in the spleen
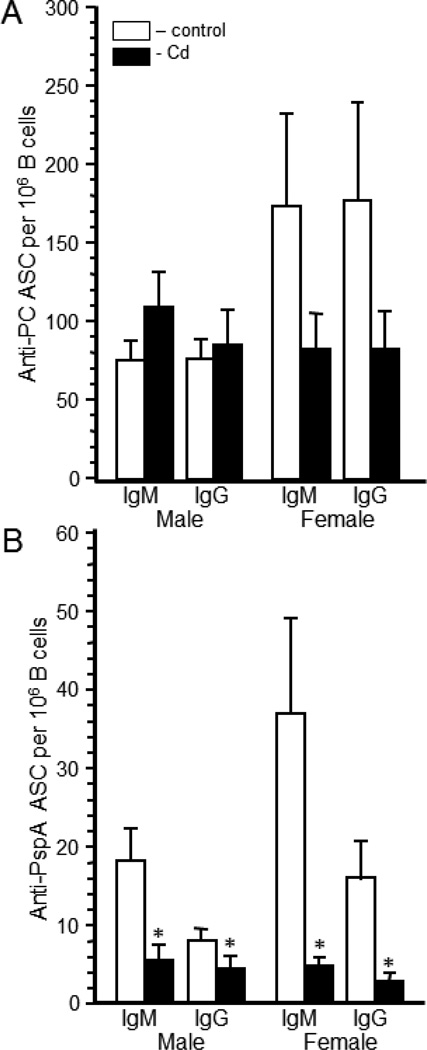
Serum titers are primarily a function of the bone marrow plasma cells (Winter et al., 2010). Given the marked differences in splenic B cell numbers between the treatment groups (Supplemental Figure 1), we assessed the number of specific antibody secreting cells in the spleen. The numbers of anti-PC ASC per 106 B cells and per spleen are shown in Figure 6 and Supplemental Figure 3, respectively. In the Cd-treated male offspring the numbers of IgM and IgG anti-PC ASC per 106 B cells were not significantly different from control offspring (Figure 6A), but the number of ASC were significantly higher when calculated on a per spleen basis (Supplemental Figure 3A). Female offspring had a reduced number of ASC per 106 B cells, which was not statistically significant (Figure 6A). When calculated on a per spleen basis (Supplemental Figure 3A), the two treatment groups had comparable numbers of ASC suggesting that there is no true difference in the overall response to PC in the females.
Figure 6. The number of anti-streptococcal antibody secreting cells (ASC) in the spleen was markedly altered by prenatal exposure to Cd.
Twenty week-old mice that were exposed to 10 ppm Cd (solid bars) or water (open bars) in utero were immunized with heat-killed S. pneumoniae vaccine. Spleen cells were collected 10 days after immunization to determine anti-PC (Figure 6A) and anti-PspA (Figure 6B) antibody forming cells (ASC) in males and females. ASC were determined by ELISpot assays as described in the Materials and Methods. The number of B cells was determined by staining an aliquot of cells with anti-CD45R as previously described. Each bar represents the mean ± SEM number of ASC per 106 splenic B cells. Data are representative of 2 experiments where the n equals at least 5 in each group. *p<0.05
The number of anti-PspA ASC was calculated on a per B cell basis (Figure 6B) as well as on a per spleen basis (Supplemental Figure 3B). Cd-exposed male and female offspring had significantly lower anti-PspA ASC on a per B cell basis (Figure 6B). However, when calculated on a per spleen basis, the number of anti-PspA ASC in the spleen was significantly lower than the control animals only in the female Cd-exposed offspring (Supplemental Figure 3B).
Discussion
This paper reports the final results of a longitudinal study on the effects of prenatal Cd exposure on the immune system from birth (Hanson et al., 2010) through two youth-stages (2 and 7 woa) (Hanson et al., 2012) and finally at 20 woa. A key finding is that prenatal exposure to Cd causes changes in the immune system that persist well beyond puberty of mice and it is speculated that this would be true for humans. The persistence of the effects would be expected if the changes in the immune system are due to a development defect, perhaps by an epigenetic change in the genome of the long-lived stem cells from which mature immune cells are derived on a daily basis throughout life.
A consistent finding at 7 and 20 woa was the dramatically increased serum antibody titers (Hanson et al., 2012). Both sexes showed very high levels of serum T-dependent anti-PspA IgG titers. Thus, this effect was persistent beyond puberty to at least the young adult stage. The T-independent anti-PC serum IgM response flip-flopped between the two ages (7 versus 20 woa) and the decrease at 20 woa in females was the only instance of a significant decrease in serum titer. Other anti-PC serum titers either were significantly increased or unchanged between Cd-treated offspring and the control offspring. Most changes in serum antibody responses were sex-independent; however, there were some instances where the changes were different between the two sexes, for example, the anti-PC IgG3 response. Serum titers have been primarily attributed to production by plasma cells in the bone marrow (Winter et al., 2010) and this is also the location of undifferentiated progenitor cell production as well as where B cell maturation occurs. Effects on serum antibody titers may suggest that prenatal Cd exposure alters the bone marrow microenvironment or cell populations in the bone marrow.
In the spleen, Cd-treated male offspring had significantly higher numbers of IgM and IgG anti-PC ASC when calculated on a per spleen basis but not when calculated on a per B cell basis. These higher total numbers of ASC (per spleen) are likely a reflection of the increase in splenic B cells (Figure S1). Female offspring showed no differences in the number of anti-PC ASC per 106 B cells or per spleen. The number of anti-PspA IgM and IgG ASC for both males and females was statistically decreased on a per B cell bases and this decrease continued to hold true on a per spleen basis for the females but not the males. However, the changes in the anti-PspA ASC, which were either decreased or showed no change, cannot be explained by changes in spleen cell numbers. We analyzed the data on the percentage of nTreg cells in the spleen for further clues for the mechanism of these results. A decrease in splenic nTreg numbers, as seen in both sexes, should seemingly result in increased antibody production, especially if the decrease in splenic nTreg cells also held true for the number or functional status of nTreg cells in the bone marrow. However, the number of nTreg would not necessarily reflect functional status of the population and the decreases in several splenic ASC values would indicate that this is not a simple case of lower nTreg numbers leading to higher antibody levels.
Changes in the relative percentages of several cell populations were often dramatic and deserve highlighting. The percentage of splenic B cells in the Cd-exposed offspring was significantly increased in both sexes. The total percentage of CD8+ T cells in the spleens from male offspring was significantly lower in the Cd offspring than the controls. NK cell percentages were decreased in both sexes. Because of the important role of both of these cell populations in tumor immune surveillance and eradication, this would indicate possible reduced tumor resistance. However, further studies specifically testing tumor resistance will be required to validate this hypothesis.
The changes in the myeloid cell populations are also of probable biological significance. Two of three populations measured were reduced, often by greater than 50%. Based on previously published analyses, these cells would include neutrophils (CD11bnegGr-1high) (Lagasse and Weissman, 1996), monocytes (CD11bhighGr-1neg/low) (Lagasse and Weissman, 1996) and likely PMN-myeloid derived suppressor cells (MDSC) (CD11bhighGr-1high) (Peranzoni et al., 2010). Thus, at least two important myeloid cell populations are reduced in the Cd-exposed offspring. More definitive identification of these cells requires functional analysis that was not possible for these animals. Although speculative, the consequence of the noted reduction in neutrophils would likely be an increased susceptibility to infections that require these important innate immune cells for resolution. The other myeloid population reduced was PMN-MDSC, and although MDSC have been identified as having a role in tumor promotion, the role of the PMN-MSDC subpopulation in tumor promotion is the least defined (Dolcetti et al., 2010). Superficially, the lower number of PMN-MDSC may indicate lower tumor susceptibility; however, the full spectrum of PMN-MDSC functions and what influences these functions is just beginning to be delineated. Nonetheless, a reduction of two myeloid cell populations of the magnitude described herein suggests that significant health problems could occur later in life.
In attempting to develop a unifying hypothesis to explain these results it is important to emphasize that Cd only crosses the placenta minimally, if at all, but does accumulate in the placenta (Hazelhoff et al., 1988; Loiacono et al., 1992). Therefore, knowing and understanding how Cd affects any immune cell directly may not be as applicable as its mode of action on the placenta, as an example. The persistence of the immunotoxicity caused by prenatal Cd exposure (Hanson et al., 2010; Hanson et al., 2012) suggests a possible genetic or epigenetic mechanism. Known effects of Cd that might influence the fetal metabolism sufficiently to cause this defect include the ability of Cd to displace zinc (Zn) (Martelli et al., 2006). Prenatal Cd administration results in a Zn deficiency in the dam and the fetal liver (Hazelhoff et al., 1988) and this could affect the function of Zn-finger containing transcription factors or other signaling molecules associated with fetal development. An important Zn-dependent developmental molecule is the morphogen sonic hedgehog (Shh) (Day et al., 1999). We have shown that the activity, but not protein level, of Shh is reduced in newborn mice treated prenatally with Cd (Hanson et al., 2010) which is consistent with a Zn deficiency. Reduced Shh activity has also been implicated in Cd-induced teratology (Scott et al., 2005). Gestational Zn deprivation has also been shown to result in a persistent reduction in serum immunoglobulin levels that was still apparent three generations after exposure (Beach et al., 1982; Beach et al., 1983). Speculatively, therefore, Cd may be acting as a de facto Zn sink in the dam resulting in a fetal liver Zn deficiency which interferes with the development of immune cells and the activity of key proteins, e.g., Shh, leading to a persistent immunodeficiency. Based on the literature and our limited mechanistic studies (Hanson et al., 2010), linking the mechanism of prenatal Cd toxicity to Zn deficiency is a good candidate as the root of numerous effects downstream. Zn also plays an essential role in the functional activity of DNA methyl transferase I, methyl-CpG binding protein and histone deacetylase 1 (Ke et al., 2006). Uteroplacental insufficiency has been associated with reduced activity of these enzymes in the offspring (Ke et al., 2006) and thus, downstream epigenetic effects are possible.
In summary, this study was designed to determine if prenatal Cd-exposure produced long-term immunotoxicity in the offspring. We have shown that prenatal Cd caused numerous effects on the immune system of the offspring which persisted well beyond the age of puberty which would strongly suggest a permanent defect. The effects were diverse, not consistently sex-specific and defied a simple explanation, such as a specific effect on nTreg cells even though we saw such a reduction. We show lower percentages of CD8+ T cells and NK cells which might signal increased tumor susceptibility but lower percentages of PMN-MDSC, which have been associated with reduced anti-tumor CTL activity and lower percentages of nTreg cells which would also indicate reduced tumor susceptibility. However, this is contrary to data in humans that show higher incidences of certain types of childhood cancers born to smoking mothers. Smoking represents a major source of human exposure to Cd and on average, 1–3 µg of Cd per pack of cigarettes is absorbed by the smoker (Faroon et al., 2008). As described in Hanson et al. (Hanson et al., 2012), we estimate that the body burden of the treated dams would be ≤1.5 µg per day, and thus, our model is a good approximation of human exposure levels. It is also worrisome that we saw very high levels of specific antibodies which might be indicative of a potential increase in susceptibility to antibody-mediated autoimmune diseases. We speculate that one possible mechanism of these effects could be Zn insufficiency during gestation caused by the accumulation of Cd, a known Zn sink, in the placenta. Future mechanistic experiments will focus on the function of cell populations altered by prenatal Cd exposure.
Supplementary Material
Highlights.
Prenatal exposure to cadmium alters the immune system of 20 week old offspring
The percentage of DN1 and DN3 thymocytes was changed.
Males and females had changed percentages of numerous splenic cell populations
The antibody response of a streptococcal vaccine showed numerous changes.
Acknowledgments
Funding
This work was supported by National Institutes of Health Grants [ES015539 to J.B.B]. The flow cytometry core facility is supported by RR016440 and RR020866.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no financial conflict of interest.
References
- Beach RS, Gershwin ME, Hurley LS. Gestational Zinc Deprivation in Mice: Persistence of Immunodeficiency for Three Generations. Science. 1982;218:469–471. doi: 10.1126/science.7123244. [DOI] [PubMed] [Google Scholar]
- Beach RS, Gershwin ME, Hurley LS. Persistent immunological consequences of gestation zinc deprivation. Am J Clin Nutr. 1983;38:579–590. doi: 10.1093/ajcn/38.4.579. [DOI] [PubMed] [Google Scholar]
- Colino J, Shen Y, Snapper CM. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein-and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J Exp Med. 2002;195:1–13. doi: 10.1084/jem.20011432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook ME. National Workshop on Cadmium Transport into Plants. Ottawa, Ontario: Canadian Network of Toxicology Centres; 1995. Anthropogenic Sources of Cadmium in Canada. [Google Scholar]
- Day ES, Wen D, Garber EA, Hong J, Avedissian LS, Rayhorn P, Shen W, Zeng C, Bailey VR, Reilly JO, Roden JA, Moore CB, Williams KP, Galdes A, Whitty A, Baker DP. Zinc-dependent structural stability of human Sonic hedgehog. Biochemistry. 1999;38:14868–14880. doi: 10.1021/bi9910068. [DOI] [PubMed] [Google Scholar]
- Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- Elinder CG, Lind B, Kjellstrom T, Linnman L, Friberg L. Cadmium in kidney cortex, liver, and pancreas from Swedish autopsies. Estimation of biological half time in kidney cortex, considering calorie intake and smoking habits. Arch Environ Health. 1976;31:292–302. doi: 10.1080/00039896.1976.10667239. [DOI] [PubMed] [Google Scholar]
- Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, Rudisill C. In: Toxicological Profile for Cadmium. Registry AfTSaD., editor. Atlanta: Centers for Disease Control; 2008. [PubMed] [Google Scholar]
- Frery N, Nessmann C, Girard F, Lafond J, Moreau T, Blot P, Lellouch J, Huel G. Environmental exposure to cadmium and human birthweight. Toxicology. 1993;79:109–118. doi: 10.1016/0300-483x(93)90124-b. [DOI] [PubMed] [Google Scholar]
- Hanson ML, Brundage KM, Schafer R, Tou JC, Barnett JB. Prenatal cadmium exposure dysregulates sonic hedgehog and Wnt/[beta]-catenin signaling in the thymus resulting in altered thymocyte development. Toxicology and Applied Pharmacology. 2010;242:136–145. doi: 10.1016/j.taap.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ML, Holaskova I, Elliott M, Brundage KM, Schafer R, Barnett JB. Prenatal cadmium exposure alters postnatal immune cell development and function. Toxicol Appl Pharmacol. 2012;261:196–203. doi: 10.1016/j.taap.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelhoff RW, Roelofsen AM, Herber RF, Peereboom-Steg JH. Cadmium and zinc concentrations in fetal and maternal rat tissue after parenteral administration of cadmium during pregnancy. Archives of Toxicology. 1988;62:285–290. doi: 10.1007/BF00332488. [DOI] [PubMed] [Google Scholar]
- Hovland DN, Jr, Machado AF, Scott WJ, Jr, Collins MD. Differential sensitivity of the SWV and C57BL/6 mouse strains to the teratogenic action of single administrations of cadmium given throughout the period of anterior neuropore closure. Teratology. 1999;60:13–21. doi: 10.1002/(SICI)1096-9926(199907)60:1<13::AID-TERA6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jacquillet G, Barbier O, Rubera I, Tauc M, Borderie A, Namorado MC, Martin D, Sierra G, Reyes JL, Poujeol P, Cougnon M. Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. American Journal of Physiology -Renal Physiology. 2007;293:F1450–F1460. doi: 10.1152/ajprenal.00223.2007. [DOI] [PubMed] [Google Scholar]
- Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19:529–535. [PubMed] [Google Scholar]
- Julin B, Wolk A, Bergkvist L, Bottai M, Åkesson A. Dietary Cadmium Exposure and Risk of Postmenopausal Breast Cancer: A Population-Based Prospective Cohort Study. Cancer Research. 2012;72:1459–1466. doi: 10.1158/0008-5472.CAN-11-0735. [DOI] [PubMed] [Google Scholar]
- Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, Yu X, Wang L, Callaway CW, Gill G, Chan GM, Albertine KH, McKnight RA, Lane RH. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiological Genomics. 2006;25:16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- Lafuente A, González-Carracedo A, Esquifino AI. Differential effects of cadmium on blood lymphocyte subsets. Biometals. 2004;17:451–456. doi: 10.1023/b:biom.0000029441.20037.72. [DOI] [PubMed] [Google Scholar]
- Lafuente A, Gonzalez-Carracedo A, Romero A, Esquifino AI. Effect of cadmium on lymphocyte subsets distribution in thymus and spleen. J Physiol Biochem. 2003;59:43–48. doi: 10.1007/BF03179867. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Lauwerys R, Buchet JP, Roels H, Bernard A, Gennart JP. [Biological aspects of occupational exposure to cadmium and several other metals] Revue d'epidemiologie et de sante publique. 1986;34:280–285. [PubMed] [Google Scholar]
- Liu J, Liu Y, Habeebu SS, Klaassen CD. Metallothionein-null mice are highly susceptible to the hematotoxic and immunotoxic effects of chronic CdCl2 exposure. Toxicol.Appl.Pharmacol. 1999;159:98–108. doi: 10.1006/taap.1999.8718. [DOI] [PubMed] [Google Scholar]
- Loiacono NJ, Graziano JH, Kline JK, Popovac D, Ahmedi X, Gashi E, Mehmeti A, Rajovic B. Placental cadmium and birthweight in women living near a lead smelter. Arch Environ Health. 1992;47:250–255. doi: 10.1080/00039896.1992.9938357. [DOI] [PubMed] [Google Scholar]
- Mackova NO, Lenikova S, Fedorocko P, Brezani P, Fedorockova A. Effects of cadmium on haemopoiesis in irradiated and non-irradiated mice: 2. Relationship to the number of circulating blood cells and haemopoiesis. Physiol Res. 1996;45:101–106. [PubMed] [Google Scholar]
- Martelli A, Rousselet E, Dycke C, Bouron A, Moulis JM. Cadmium toxicity in animal cells by interference with essential metals. Biochimie. 2006;88:1807–1814. doi: 10.1016/j.biochi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Minetti A, Reale CA. Sensorimotor developmental delays and lower anxiety in rats prenatally exposed to cadmium. J Appl Toxicol. 2006;26:35–41. doi: 10.1002/jat.1102. [DOI] [PubMed] [Google Scholar]
- Morselt AFW, Leene W, DeGroot C, Kipp JBA, Evers M, Roelofsen AM, Bosch KS. Differences in immunological susceptibility to cadmium toxicity between two rat strains as demonstrated with cell biological methods. Effect of cadmium on DNA synthesis of thymus lymphocytes. Toxicology. 1988;48:127–139. doi: 10.1016/0300-483x(88)90095-9. [DOI] [PubMed] [Google Scholar]
- Nordberg GF. Lung cancer and exposure to environmental cadmium. Lancet Oncol. 2006;7:99–101. doi: 10.1016/S1470-2045(06)70548-4. [DOI] [PubMed] [Google Scholar]
- Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A. Cadmium in blood and urine--impact of sex, age, dietary intake, iron status, and former smoking--association of renal effects. Environ Health Perspect. 2002;110:1185–1190. doi: 10.1289/ehp.021101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park RM, Stayner LT, Petersen MR, Finley-Couch M, Hornung R, Rice C. Cadmium and lung cancer mortality accounting for simultaneous arsenic exposure. Occup Environ Med. 2012 doi: 10.1136/oemed-2011-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Piasek M, Blanusa M, Kostial K, Laskey JW. Placental cadmium and progesterone concentrations in cigarette smokers. Reprod.Toxicol. 2001;15:673–681. doi: 10.1016/s0890-6238(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Salazar KD, de la Rosa P, Barnett JB, Schafer R. The Polysaccharide Antibody Response after Streptococcus pneumoniae Vaccination Is Differentially Enhanced or Suppressed by 3,4-Dichloropropionanilide and 2,4-Dichlorophenoxyacetic Acid. Toxicological Sciences. 2005;87:123–133. doi: 10.1093/toxsci/kfi244. [DOI] [PubMed] [Google Scholar]
- Scott WJ, Jr, Schreiner CM, Goetz JA, Robbins D, Bell SM. Cadmium-induced postaxial forelimb ectrodactyly: association with altered sonic hedgehog signaling. Reprod.Toxicol. 2005;19:479–485. doi: 10.1016/j.reprotox.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Coogan TP, Barter RA. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit Rev Toxicol. 1992;22:175–201. doi: 10.3109/10408449209145323. [DOI] [PubMed] [Google Scholar]
- Winter O, Moser K, Mohr E, Zotos D, Kaminski H, Szyska M, Roth K, Wong DM, Dame C, Tarlinton DM, Schulze H, MacLennan ICM, Manz RA. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood. 2010;116:1867–1875. doi: 10.1182/blood-2009-12-259457. [DOI] [PubMed] [Google Scholar]
- Wu ZQ, Khan AQ, Shen Y, Schartman J, Peach R, Lees A, Mond JJ, Gause WC, Snapper CM. B7 requirements for primary and secondary protein-and polysaccharide-specific Ig isotype responses to Streptococcus pneumoniae. J Immunol. 2000;165:6840–6848. doi: 10.4049/jimmunol.165.12.6840. [DOI] [PubMed] [Google Scholar]
- Wu ZQ, Vos Q, Shen Y, Lees A, Wilson SR, Briles DE, Gause WC, Mond JJ, Snapper CM. In Vivo Polysaccharide-Specific IgG Isotype Responses to Intact Streptococcus pneumoniae are T Cell Dependent and Require CD40- and B7-Ligand Interactions. Journal of Immunology. 2001;163:659–667. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.