Abstract
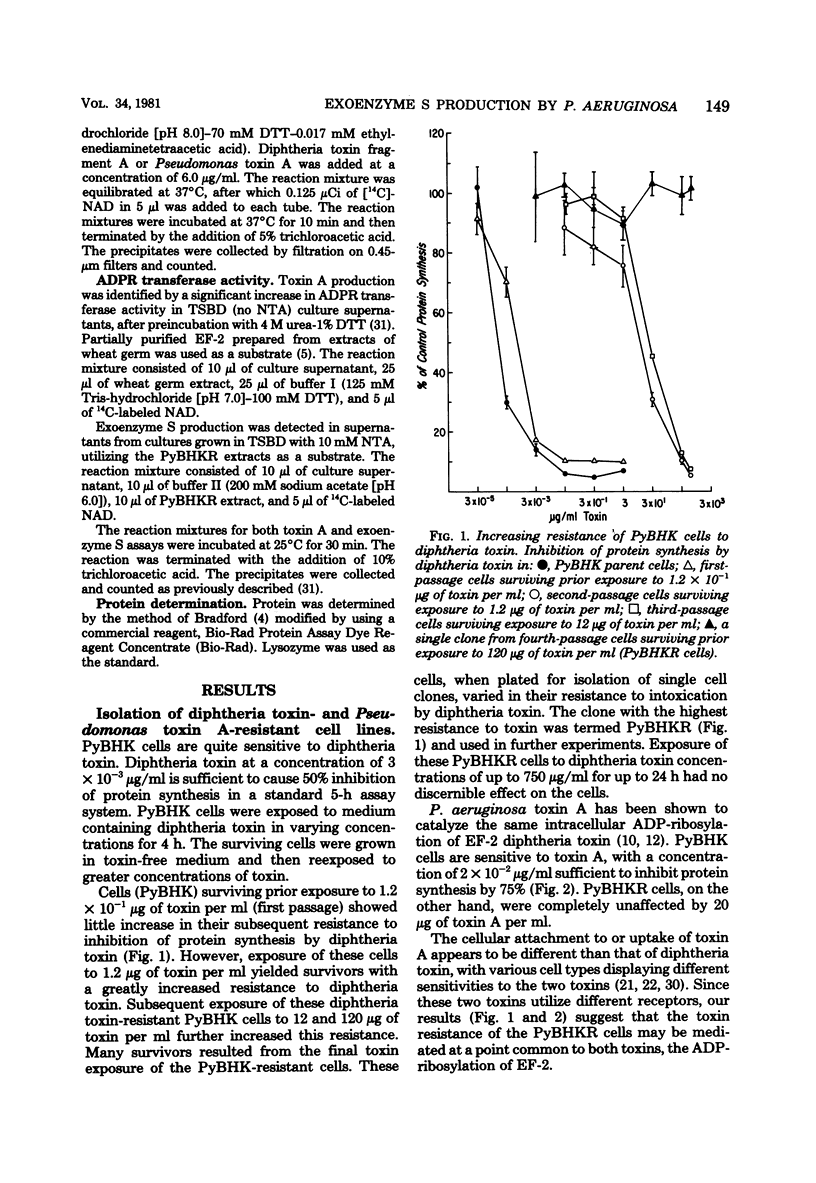
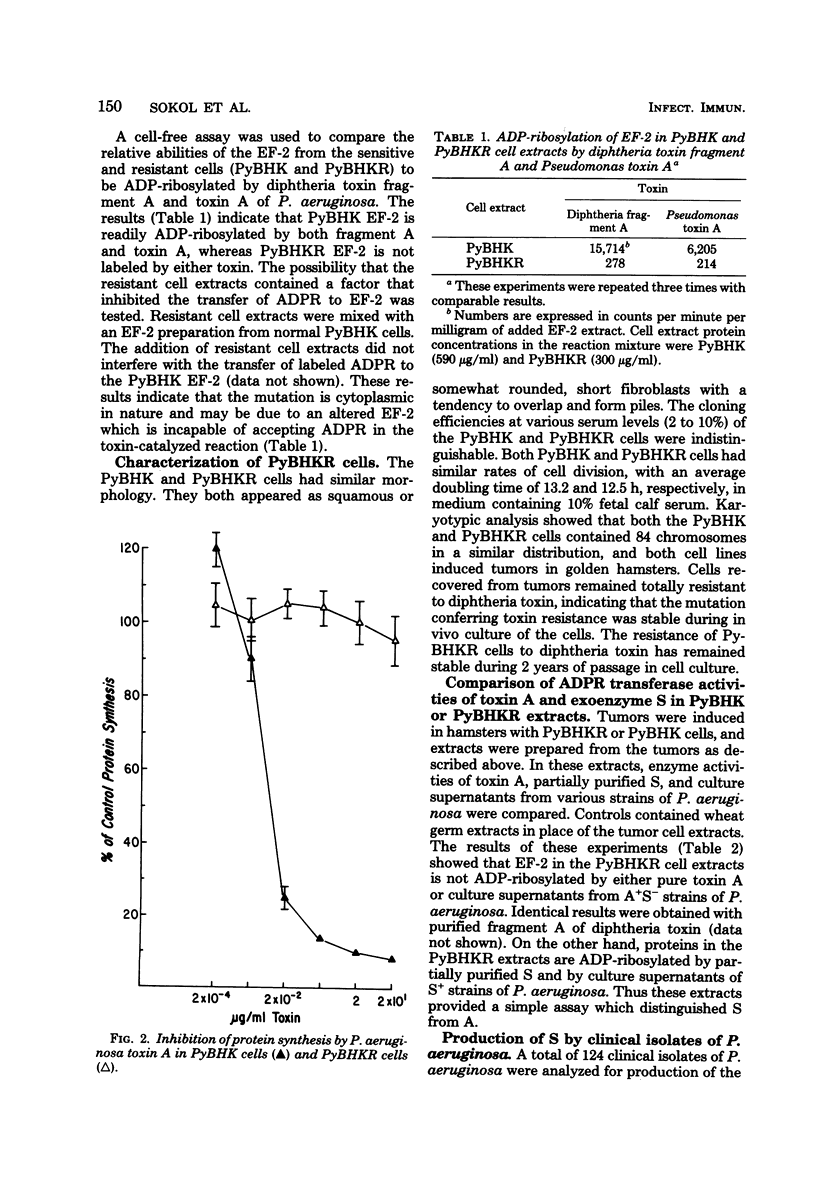
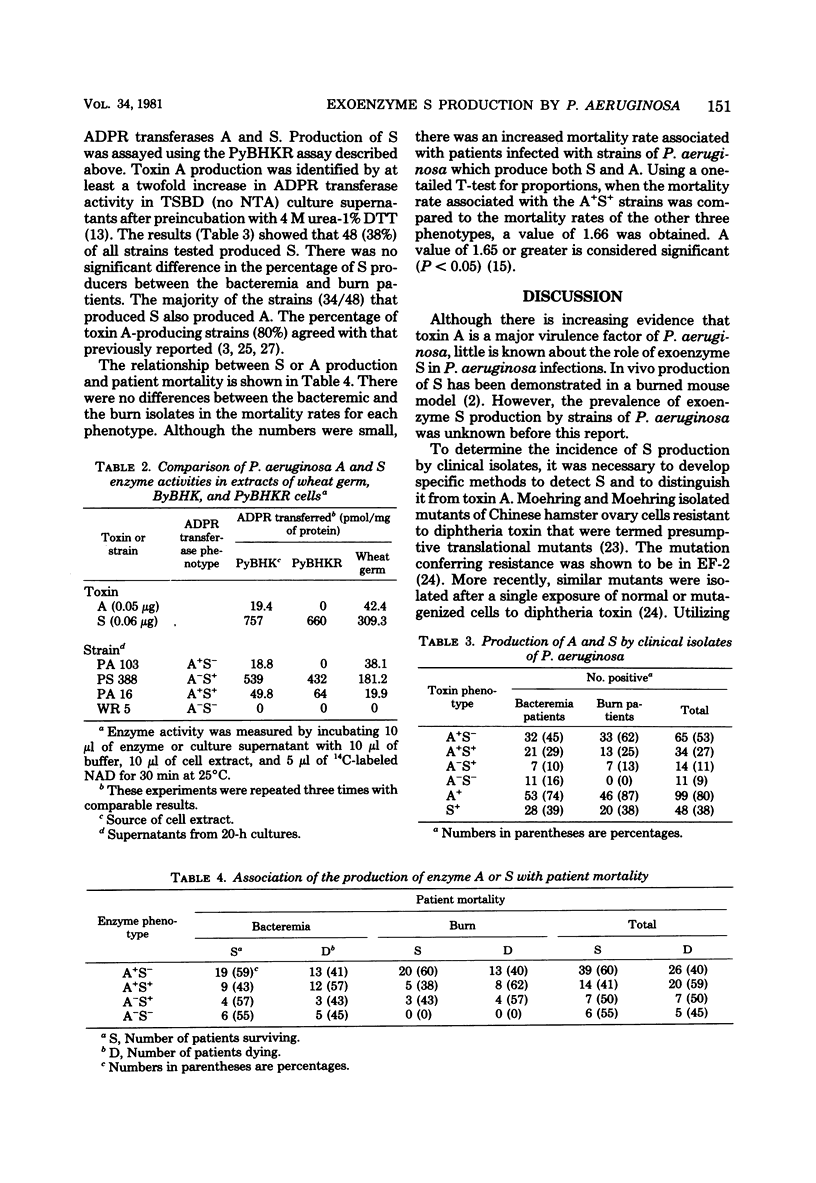
Exoenzyme S differs from toxin A and diphtheria toxin in that it does not adenosine diphosphate (ADP)-ribosylate elongation factor-2, but rather catalyzes the transfer of the ADP-ribose moiety of nicotinamide adenine dinucleotide to a number of different proteins in extracts of eucaryotic cells. Polyoma-transformed BHK-21 cells were isolated which were resistant to diphtheria toxin and toxin A. Extracts from these cells are ADP-ribosylated by exoenzyme S but not toxin A or diphtheria toxin, providing an assay which distinguishes between S and A activities. A total of 124 clinical isolates of P. aeruginosa were analyzed for production of toxin A and exoenzyme S. Exoenzyme S production was detected in 38% of the strains, whereas 80% of the strains produced toxin A.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltch A. L., Griffin P. E., Hammer M. Pseudomonas aeruginosa bacteremia: relationship of bacterial enzyme production and pyocine types with clinical prognosis in 100 patients. J Lab Clin Med. 1979 Apr;93(4):600–606. [PubMed] [Google Scholar]
- Bjorn M. J., Pavlovskis O. R., Thompson M. R., Iglewski B. H. Production of exoenzyme S during Pseudomonas aeruginosa infections of burned mice. Infect Immun. 1979 Jun;24(3):837–842. doi: 10.1128/iai.24.3.837-842.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Vasil M. L., Sadoff J. C., Iglewski B. H. Incidence of exotoxin production by Pseudomonas species. Infect Immun. 1977 Apr;16(1):362–366. doi: 10.1128/iai.16.1.362-366.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Iglewski B. H., Sokol P. A. Evidence for the role of toxin A in the pathogenesis of infection with Pseudomonas aeruginosa in humans. J Infect Dis. 1980 Oct;142(4):538–546. doi: 10.1093/infdis/142.4.538. [DOI] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. The elongation factor 2 content of mammalian cells. Assay method and relation to ribosome number. J Biol Chem. 1973 Jan 25;248(2):654–658. [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J., Bjorn M. J., Maxwell E. S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot H. N., Iglewski B. H. Synthesis of diphtheria toxin in E. coli cell-free lysate. Biochem Biophys Res Commun. 1974 Jan 23;56(2):351–357. doi: 10.1016/0006-291x(74)90849-3. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Lui P. V. Biology of Pseudomonas aeruginosa. Hosp Pract. 1976 Jan;11(1):139–147. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Differential chemical protection of mammalian cells from the exotoxins of Corynebacterium diphtheriae and Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):232–239. doi: 10.1128/iai.16.1.232-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977 Feb;23(2):183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Characterization of the diphtheria toxin-resistance system in Chinese hamster ovary cells. Somatic Cell Genet. 1979 Jul;5(4):453–468. doi: 10.1007/BF01538880. [DOI] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Selection and characterization of cells resistant to diphtheria toxin and pseudomonas exotoxin A: presumptive translational mutants. Cell. 1977 Jun;11(2):447–454. doi: 10.1016/0092-8674(77)90063-0. [DOI] [PubMed] [Google Scholar]
- Pollack M., Taylor N. S., Callahan L. T., 3rd Exotoxin production by clinical isolates of pseudomonas aeruginosa. Infect Immun. 1977 Mar;15(3):776–780. doi: 10.1128/iai.15.3.776-780.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt B. A., Jr Infections caused by Pseudomonas species in patients with burns and in other surgical patients. J Infect Dis. 1974 Nov;130 (Suppl)(0):S8–13. doi: 10.1093/infdis/130.supplement.s8. [DOI] [PubMed] [Google Scholar]
- Sanai Y., Takeshi K., Homma J. Y., Kamata H. Production of exotoxin, protease and elastase of Pseudomonas aeruginosa strains isolated from patients' and environmental specimens. Jpn J Exp Med. 1978 Dec;48(6):553–556. [PubMed] [Google Scholar]
- Tapper M. L., Armstrong D. Bacteremia due to Pseudomonas aeruginosa complicating neoplastic disease: a progress report. J Infect Dis. 1974 Nov;130 (Suppl)(0):S14–S23. doi: 10.1093/infdis/130.supplement.s14. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Iglewski B. H. Comparative toxicities of diphtherial toxin and Pseudomonas aeruginosa exotoxin A: evidence for different cell receptors. J Gen Microbiol. 1978 Oct;108(2):333–337. doi: 10.1099/00221287-108-2-333. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Kabat D., Iglewski B. H. Structure-activity relationships of an exotoxin of Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):353–361. doi: 10.1128/iai.16.1.353-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S. The role of exotoxins in the pathogenesis of Pseudomonas aeruginosa infections. J Infect Dis. 1980 Oct;142(4):626–630. doi: 10.1093/infdis/142.4.626. [DOI] [PubMed] [Google Scholar]


