Abstract
Attention-deficit/hyperactivity disorder (ADHD) is the most common behavioral disorder of childhood. Preliminary studies with proton magnetic resonance spectroscopy (1HMRS) of the brain have reported differences in brain metabolite concentration-to-Cr ratios between individuals with ADHD and unaffected controls in several frontal brain regions including anterior cingulate cortex. Using multivoxel 1H-MRS, we compared 14 individuals affected with ADHD to 20 individuals without ADHD from the same genetic isolate. After controlling by sex, age and multiple testing, we found significant differences at the right posterior cingulate of the Glx/Cr ratio density distribution function between ADHD cases and controls (P<0.05). Furthermore, we found several interactions of metabolite concentration-to-Cr ratio, age, and ADHD status: Ins/Cr and Glx/Cr ratios at the left posterior cingulate, and NAA/Cr at the splenius, right posterior cingulate, and at the left posterior cingulate. We also found a differential metabolite ratio interaction between ADHD cases and controls for Ins/Cr and NAA/Cr at the right striatum. These results show that: 1) NAA/Cr, Glx/Cr and Ins/Cr ratios, as reported in other studies, exhibit significant differences between ADHD cases and controls; 2) differences of these metabolite ratios between ADHD cases and controls evolve in specific and recognizable patterns throughout age, a finding that replicates previous results obtained by structural MRI, where is demonstrated that brain ontogeny follows a different program in ADHD cases and controls; 3) Ins/Cr and NAA/Cr ratios, at the right striatum, interact in a differential way between ADHD cases and controls. As a whole, these results replicate previous 1H-MRS findings and add new intriguing differential metabolic and ontogeny patterns between ADHD cases and controls that warrant further pursue.
Keywords: 1H-MRS, ADHD, ontogeny, MRI, Genetics
INTRODUCTION
ADHD, defined as a persistent syndrome characterized by inattention, excessive motor activity, and impulsivity, is the most common behavioral disorder of childhood and affects 8–12% of children worldwide (Biederman and Faraone 2005). ADHD affected individuals are at increased risk for poor educational achievement, low income, underemployment, legal difficulties, and impaired social relationships (Faraone et al. 1996). A conservative estimate, based on an ADHD prevalence of 5%, estimated the costs attributable to ADHD in the United States at $42.5 billion per year (range $36 to $52.4 billion)(Pelham et al. 2007). Although ADHD occurs as a single disorder in a minority of diagnosed individuals, it is generally comorbid with other behavioral and emotional disorders such as oppositional defiant disorder (ODD), conduct disorder (CD), and substance abuse (Palacio et al. 2004). Genetic and environmental factors play a key role in conferring susceptibility to ADHD (Pineda et al. 2007; Lopera et al. 1999).
Extensive literature derived from brain imaging studies strongly supports the presence of anatomical and developmental differences between ADHD cases and control individuals. These differences can be summarized as follows. First, the caudate nucleus and globus/pallidus are smaller in the ADHD vs. control individuals (Swanson et al. 2007). Second, ADHD individuals have relatively larger posterior brain regions and smaller anterior brain regions (Swanson et al. 2007). Third, areas coordinating multiple brain regions, such as rostrum and splenium of the corpus callosum and the cerebellum vermis lobules VIII-X are smaller in ADHD individuals than in control groups (Swanson et al. 2007). Fourth, throughout of the cerebrum, there is a delay in the age of attaining peak cortical thickness in children with ADHD compared to controls (Shaw et al. 2007). Fifth, a meta-analysis of seven studies including a total of 114 patients with ADHD (or related disorders) and 143 comparison subjects showed a reduction in the gray matter in the right putamen/globus pallidus region (Ellison-Wright et al. 2008). Sixth, recent studies in adults, as are the majority of patients in this study (see below), report that the posterior cortex is particularly compromised in adults with ADHD (Proal et al. 2011). These established structural variations suggest the presence of both variable brain neuronal regulation and/or function, as well as different structural patterns of brain ontogeny between ADHD cases and controls.
Proton magnetic resonance spectroscopy (1H-MRS), is a non-invasive technique for evaluating brain chemistry in vivo (Sun et al. 2005). 1H-MRS can obtain the spectra of metabolites linked directly as well as indirectly to neurotransmission pathways; these metabolites include N-acetylaspartate (NAA), Inositol (Ins), Choline (Cho) and Glutamate-Glutamine complex (Glx) to creatine (Cr) ratios (in the brain, creatine is a relatively constant element indicating that neuronal energetic metabolism is taking place and therefore used as an internal standard). For instance, NAA/Cr intensity is thought to be a marker of neuronal integrity and is the most important proton signal in studying central nervous system (CNS) pathology. Decreases in the NAA/Cr signal are associated with neuronal loss. Besides, changes in Cho/Cr levels are associated to acute demyelinating disease. Glx is a neurotransmitter of the glutaminergic system, which is the major stimulatory system in the CNS. Because it plays a fundamental role in neuron maturation (it regulates the processes of proliferation and migration of neural precursors and immature neurons during brain development) as well as in learning and memory processes, changes in Glx/Cr ratios have been associated to process affecting both brain’s ontogeny and physiology of the CNS (Smigielska-Kuzia and Sobaniec 2007).
Thus far, such neurometabolic markers reflect the specific stage of brain development as well as providing an index of neuronal function, both of which are ultimately related to neuronal activity and/or viability number (Sun et al. 2005). These features make 1H-MRS potentially useful to study potential differences in brain function metabolism in individuals with ADHD. Preliminary 1H-MRS studies showed the presence of significant differences between ADHD cases and controls. For instance, at the frontal-striatal region and at the right dorsolateral frontal region, ADHD individuals exhibit higher Glx-to-Cr (Glx/Cr) ratios than controls at the frontal–striatal region and at the right dorsolateral frontal region (MacMaster et al. 2003). In addition, patients with hyperactive-impulsive subtype ADHD were found to have significantly lower NAA levels in the dorsolateral prefrontal cortex than inattentive ADHD patients and control subjects (Hesslinger et al. 2001). Furthermore, at the anterior cingulate cortex, the glutamate plus glutamine to myo-inositol-containing ratio compounds in children with ADHD, were significantly higher than children with ADHD plus bipolar disorder and controls at the anterior cingulate cortex (Moore et al. 2006). This latter finding contrasts with results found in the largest sample of ADHD adult cases and controls analyzed by 1H-MRS, which showed a significant reduction of the Glx/Cr ratio in the right anterior cingulate cortex in patients with ADHD when compared to controls (Perlov et al. 2007). The latter result may be explained fact that the other studies investigated children and adolescents with ADHD, whereas the Perlov et al. study focused on adult patients. Finally, a meta-analysis of available data reported increased Choline-to-Cr (Cho/Cr) compounds including the prefrontal cortex, striatum, and anterior cingulated cortex (Perlov et al. 2009).
In the present study we compared the 1H-MRS profiles of individuals with ADHD (cases) with those of individuals without ADHD (controls) in brain areas showing evidence of anatomic abnormalities and previously shown to be associated with ADHD (Krain and Castellanos 2006). The areas specifically examined in this study were striatum, cingulated gyrus, splenium of the corpus callosum, medial and lateral thalamus, and cerebellar vermis.
Even though the main goal of this study was to determine the effect of the ADHD diagnosis and to explore potential effects of demographic covariates such age and sex on brain metabolite concentration, this study also provides useful information to control these variables, which are potential confounders in future 1H-MRS studies focused in detecting specific effects of biomarkers and/or genetic polymorphisms on brain function.
METHODS
Subjects
Individuals were ascertained as part of an ADHD genetic study of a genetic isolate, the Paisa community of Antioquia, in Colombia, South America (Arcos-Burgos et al. 2004; Arcos-Burgos and Muenke 2002; Palacio et al. 2004; Arcos-Burgos et al. 2002). Demographic, clinical assessment and genetic information have been provided elsewhere (Palacio et al. 2004). Imaged individuals, to the best of our knowledge, were not biologically related. They were neither sedated nor receiving medications for treatment of ADHD. Participation required informed consent of adults or parental consent for minors. The study was approved by the Institutional Review Board of the University of Antioquia, in Medellin, Colombia, and the National Human Genome Research Institute, National Institute of Health, Bethesda, MD, USA.
1H-MRS methodology
Potential differences between ADHD cases and controls with respect to brain metabolism, as assessed by the ratio of N-acetylaspartate (NAA), Inositol (Ins), Choline (Cho) and Glutamate-Glutamine complex (Glx) to creatine (Cr), were explored in 14 cerebral regions namely: right striatum (RS), left striatum (LS), splenium of the corpus callosum, right anterior cingulate (RAC), left anterior cingulate (LAC), right medial cingulate (RMC), left medial cingulate (LMC), right posterior cingulate (RPC), left posterior cingulate (LPC), right lateral thalamus (RLT), left lateral thalamus (LLT), right medial thalamus (RMT), left medial thalamus (LMT), and cerebellar vermis.
To obtain measures of metabolic brain activity using 1H-MRS, T2-weighted high resolution anatomic images were obtained in the axial, coronal and sagittal planes (TE=103 ms, TR=5910 ms, 3 mm slice thickness, and 5:27 (min:sec) imaging time). Axial images were oriented parallel to the orbitomeatal anatomical reference plane. The T2-weighted images were used to guide multi-voxel MR spectroscopy volume selection. The 2D chemical shift imaging (CSI) point-resolved spectroscopic sequence (PRESS) technique (TE = 30 ms, TR = 1500 ms, NEX = 3, resolution 10mm × 10mm × 10mm, acquisition time = 6:05) was localized in the inferior vermis using the anatomical reference images. Three-dimensional CSI PRESS sequence (TE = 30 ms, TR = 1500 ms, NEX = 3, resolution 13.3 mm × 13.3 mm × 13.8mm, acquisition time = 10:23) explored the center of the brain including the striatum, thalamus, and the cingulate gyrus relative to anatomic images. Saturation bands around the 2D and 3D Volumes of Interest (VOI) were used to prevent contamination of the spectra from subcutaneous fat signal. All MR data were obtained on a 1.5 T Symphony Master Class Siemens Clinical Imaging System using an 8-channel head array coil. The spectra were transferred off-line to be processed automatically using LCModel (Castillo et al. 2000; Provencher 2001). Average data from voxels covering the left and right striatum (3–4 voxels), lateral (2 voxels) and medial (2 voxels) aspects of the thalamus, anterior (1 voxel), medial (1 voxel) and posterior (1 voxel) cingulate gyrus, and inferior vermis (2 voxels) were analyzed. Voxels containing cerebrospinal fluid were excluded from analyses.
Criteria for acceptable reliability were those recommended by the LCModel provider (Provencher 2001). This multivoxel approach allowed us to explore most of the structures linked to dysfunction of frontal-striatal-cerebellar circuits in ADHD. Total scanning duration was 45 minutes. Absolute metabolite quantification was not attempted because of the requirement for markedly increased data acquisition time, which is particularly problematic for patients with ADHD. Statistical differences of brain metabolite ratios between the two groups were ascertained with the general linear model at an uncorrected type I error probability of 5%, as this was an exploratory analysis.
We chose certain target regions (striatum, cingulate gyrus, cerebellar vermis and splenium of the corpus callosum) based on prior evidence of anatomic abnormalities and added the medial and lateral thalamus, a region that has been difficult to quantify volumetrically (Krain and Castellanos 2006).
Statistical Analyses
Exploratory Analysis
For each metabolite-region combination, measures by subject are reported as arithmetic averages of multi-voxel MR spectroscopy registered data. Because of the presence of zero ratios and missing observations clustered at vermis and splenius, we used two approaches to solve these common problems when acquiring data by 1H-MRS. First, we replaced zero ratios by the minimum registered value as estimated by the density distribution function for each metabolite; second, we deleted rows presenting with at least one missing cell at the vermis and splenius. Results obtained for each one of these two scenarios were compared in order to define any significant effect of null cells.
Potential differences between individuals with ADHD and unaffected individuals regarding age and sex were explored by a two-tailed t-test and a χ2 test. The effect of age on metabolite concentration was explored using ratios dispersion and correlation matrices by region while controlling for the variable sex as a modifier (Kadota et al. 2001).
Differences in correlation coefficients (ρ) between individuals with ADHD and unaffected individuals were tested using a two-tailed Z test to contrast the hypothesis H0: ρAffected = ρunaffected. The false discovery rate (FDR) procedure (Benjamini and Hochberg 1995; Benjamini et al. 2001), in which every P-value is compared against a specific sequentially weighted threshold, was used to correct for multiple testing.
Kolmogorov-Smirnov and Mann-Whitney Tests
We performed the Kolmogorov-Smirnov (KS) and Mann-Whitney (MW) non-parametric tests in order to determinate, by region, (i) if the distribution of the ratios is the same for affected and unaffected subjects and (ii) if significant differences were present in the metabolite ratios when comparing affected and unaffected individuals, respectively. To eliminate potential confounder effects of sex and age over the ratio in each region, both tests were applied on the residuals of the linear model calculated as follows. First, define yij as the i-th metabolite ratio (Ins/Cr: i = 1; NAA/Cr: i=2; Cho/Cr: i=3; Glx/Cr: i=4) in the j-th region (see above), and fit the linear model yij= β0,j+β1,jSex+β2,jAge; second, calculate the predicted metabolite ratio as ŷij = β̂0 j+β̂1 jSex+β̂2 jAge and third, calculate the residuals r̂ij = yij − ŷij.
Generalized Linear Models
To study correlations between metabolites and the presence of ADHD diagnosis while controlling by confounding factors such as age and sex by region, we modeled the metabolites’ measurements using the following linear models: (1) y=β0+β1Age+β2Diagnosis+β3Sex, (2) y=β0+β1log(Age)+β2Diagnosis+β3Sex, (3) y*=β0+β1Age+β2Diagnosis+β3Sex and (4) y*=β0+β1log(Age)+β2Diagnosis+β3Sex, with y*=log(y). In addition, because metabolite ratios are positive values, a Gamma regression model with identity link function was also fitted to the data using the same structure as in model (1). Finally, we explored potential differences among cases and controls of correlations among metabolites (independent of demographic and phenotype covariates). For purposes of selection, some goodness of fit statistical measures including the Akaike Information Criteria (AIC), log-Likelihood, R2, and the adjusted R2 were estimated for all models (data not reported). Data processing and statistical analysis were performed in R (R Development Core Team 2011).
RESULTS
A total of 34 individuals, 21 (61.7%) females and 13 males (38.3%), ages 8 to 54 y/o, 14 (41.2%) with ADHD and 20 controls without ADHD underwent 1H-MRS. Two individuals, one ADHD affected and one unaffected were excluded because of the substantial presence of missing data. Table 1 (Online Supplementary Material) summarizes demographic data (age and sex) of the remaining 32 individuals subject to analysis. Further information to the ADHD phenotype status, regarding either the presence or not of Oppositional Defiant Disorder, Conduct Disorder, Nicotine Abuse, Alcohol Abuse, Other Substances Abuse, Anxiety and Depression is included. Detailed information regarding DSM-IV clinical subtypes and latent classes endorsement have been published elsewhere (Palacio et al. 2004; Acosta et al. 2008). Similarly, detailed information regarding neuropsychological characterization including intelligence coefficient (IQ) as well as other additional potential neuropsychological endophenotypes has been published elsewhere (Pineda et al. 2011). No significant difference between individuals with and without ADHD regarding age and sex was detected (t=−0.12, df = 26.93, P=0.9028; χ2=1.03, df = 1, P= 0.3104, respectively).
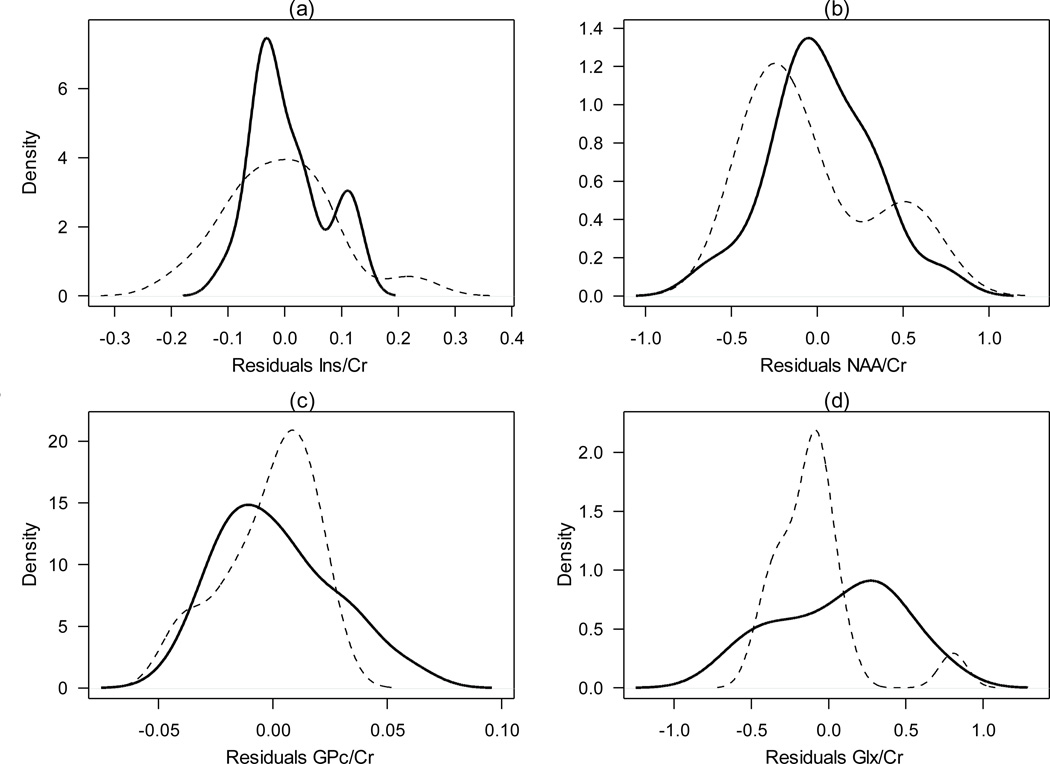
Metabolite concentration raw data i.e., mean, standard deviation and range, for ADHD affected and unaffected groups, throughout analyzed regions, are presented in Table 2 (Online Supplementary Material). After controlling by sex and age, and correcting by multiple testing using the FDR, the KS test revealed significant differences at the RPC for the Glx/Cr ratio when comparing individuals with ADHD to unaffected individuals (P<0.05, Table 1). Figure 1 depicts the density distribution functions for the Ins/Cr, NAA/Cr, GPc/Cr and Glx/Cr metabolite ratio concentrations by ADHD status, which highlights the statistically significant difference found for the Glx/Cr ratio. Furthermore, although not statistically significant, individuals with ADHD demonstrated slightly higher Glx/Cr metabolite levels than unaffected individuals (Table 2, Online Supplementary Material).
Table 1.
Kolmogorov-Smirnov and Mann-Whitney FDR-corrected P-values comparing, by region, the ratios between ADHD-affected and controls individuals.
| Region | Ins/Cr | NAA/Cr | Cho/Cr | Glx /Cr | ||||
|---|---|---|---|---|---|---|---|---|
| KS | MW | KS | MW | KS | MW | KS | MW | |
| Vermis | 0.090 | 0.221 | 0.134 | 0.094 | 0.910 | 0.758 | 0.882 | 0.573 |
| Splenium | 0.636 | 0.461 | 0.972 | 0.912 | 0.690 | 0.456 | 0.986 | 0.927 |
| RAC | 0.910 | 0.743 | 0.991 | 0.849 | 0.616 | 0.717 | 0.814 | 0.753 |
| LAC | 0.722 | 0.691 | 0.961 | 1.000 | 0.922 | 0.769 | 0.487 | 0.478 |
| RMC | 0.975 | 0.717 | 0.308 | 0.274 | 0.993 | 0.834 | 0.648 | 0.319 |
| LMC | 0.616 | 0.616 | 0.991 | 0.877 | 0.850 | 0.877 | 0.648 | 0.834 |
| RPC | 0.341 | 0.823 | 0.394 | 0.416 | 0.797 | 0.641 | 0.044 | 0.158 |
| LPC | 0.341 | 0.641 | 0.465 | 0.641 | 0.910 | 0.691 | 0.145 | 0.259 |
| RLT | 0.553 | 0.849 | 0.308 | 0.359 | 0.616 | 0.849 | 0.419 | 0.156 |
| LLT | 0.832 | 0.769 | 0.373 | 0.500 | 0.341 | 0.769 | 0.648 | 0.495 |
| RMT | 0.659 | 0.545 | 0.509 | 0.377 | 0.553 | 0.341 | 0.783 | 0.421 |
| LMT | 0.593 | 0.436 | 0.832 | 0.769 | 0.682 | 0.637 | 0.157 | 0.129 |
| RS | 0.553 | 0.416 | 0.882 | 0.691 | 0.648 | 0.916 | 0.553 | 0.904 |
| LS | 0.593 | 0.436 | 0.833 | 0.592 | 0.658 | 0.743 | 0.783 | 0.552 |
KS: Kolmogorov-Smirnov test
MW: Mann-Whitney test
Ins: Inositol; NAA; N-acetylaspartate; Cho: Choline; Glx: Glutamate-Glutamine complex; Cr: Creatine.
RAC: Right Anterior Cingulate, LAC: Left Anterior Cingulate; RMC: Right Medial Cingulate, LMC: Left Medial Cingulate; RPC: Right Posterior Cingulate, LPC: Left Posterior Cingulate; RLT: Right Lateral Thalamus, LLT: Left Lateral Thalamus; RMT: Right Medial Thalamus, LMT: Left Medial Thalamus; RS: Right Striatum, LS: Left Striatum.
Figure 1.
Density distribution functions by ADHD status at the RPC for (a) Ins/Cr, (b) NAA/Cr, (c) GPc/Cr and (d) Glx/Cr ratios after controlling by sex and age. P-values were corrected by multiple comparisons using the FDR. A significant difference for Glx/Cr ratio was found (P <0.05). Abbreviations as in Table 1.
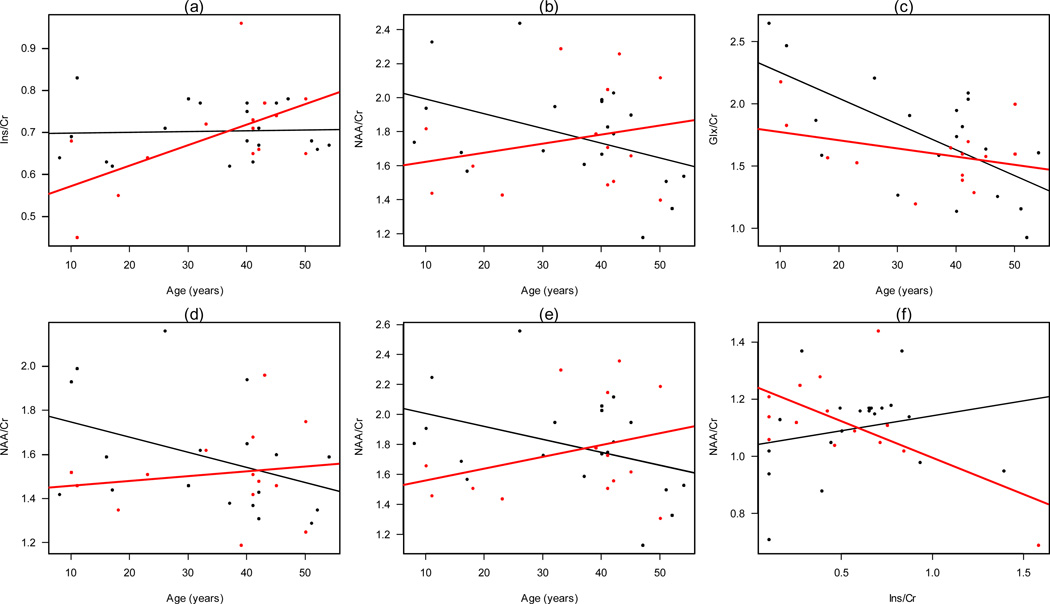
There were also statistical differences between the linear correlation coefficients (P<0.001) of ADHD individuals vs. controls. Figure 2 shows scatterplots as well as linear models depicting these differences in the LPC, RPC, RS and Splenius. Significant differences were found between cases and controls for Ins/Cr vs. age (Figure 2a), NAA/Cr vs. age (Figure 2b) and Glx/Cr vs. age (Figures 2c) in the LPC; NAA/Cr vs. age in the splenius (Figure 2d) and RPC (Figure 2e), and Ins/Cr vs. NAA in RS (Figure 2f) that was only present in ADHD patients.
Figure 2.
Scatter plots for (a) Ins vs. age, (b) NAA vs. Age and (c) Glx vs. age in LPC; (d) NAA vs. age in splenius of the corpus callosum; (e) NAA vs. age in RPC, and (f) Ins vs. NAA in RS (this significant correlation was only present in ADHD patients). Plots show differences in trend between this ratios and age between ADHD affected (red) and unaffected (black).
DISCUSSION
In this study we report the presence of significant differences of the Glx/Cr ratio density distribution function in the right posterior cingulate (RPC) in individuals with ADHD vs. controls after controlling by sex and age (FDR-corrected P<0.05, simulated P<0.0001). In addition, we disclosed several interactions of metabolite ratio-to-Cr, ADHD status, and age. These metabolite ratios included Ins/Cr and Glx/Cr at the LPC, and NAA at the splenius, RPC and LPC. Finally, we found differential metabolite ratios interaction in ADHD cases vs. controls for Ins and NAA at the RS.
From these results we might infer three main conclusions: 1) NAA/Cr, Glx/Cr and Ins/Cr ratios, as reported in other studies, are significantly different in individuals with and without ADHD; 2) the trend of these metabolite ratios differences in ADHD cases vs. controls evolves in a differential with age, suggesting that brain ontogeny follows a differential metabolic program in ADHD cases compared to controls, which may correlate with differences in attaining peak cortical thickness (Shaw et al. 2007); 3) Some metabolite concentration-to-Cr ratios may also interact in a differential way, as is the case of Ins and NAA at the RS.
The strengths of this study include: 1) this is the first report using a multivoxel approach to study ADHD, which allowed us to simultaneously explore most of the structures linked to dysfunction of the frontal-striatal-cerebellar circuits in ADHD; 2) ADHD cases and controls were ascertained from a genetic isolate, where environmental and genetic influences have shown to be homogeneous (Arcos-Burgos et al. 2004; Arcos-Burgos and Muenke 2002; Palacio et al. 2004); 3) imaged participants were neither sedated nor receiving medications for ADHD treatment; 4) correction for multiple comparisons was applied following the FDR procedure (Benjamini and Hochberg 1995).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to extend their deepest gratitude to all the patients and families from Antioquia, Colombia who took part in our research on ADHD. This research was supported in part by the Division of Intramural Research, National Human Genome Research Institute, National Institutes of Health, United States of America.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Acosta MT, Castellanos FX, Bolton KL, Balog JZ, Eagen P, Nee L, Jones J, Palacio L, Sarampote C, Russell HF, Berg K, Arcos-Burgos M, Muenke M. Latent class subtyping of attention-deficit/hyperactivity disorder and comorbid conditions. J Am Acad Child Adolesc Psychiatry. 2008;47(7):797–807. doi: 10.1097/CHI.0b013e318173f70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcos-Burgos M, Castellanos FX, Lopera F, Pineda D, Palacio JD, Garcia M, Henao GC, Palacio LG, Berg K, Bailey-Wilson JE, Muenke M. Attention-deficit/hyperactivity disorder (ADHD): feasibility of linkage analysis in a genetic isolate using extended and multigenerational pedigrees. Clin Genet. 2002;61(5):335–343. doi: 10.1034/j.1399-0004.2002.610503.x. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Castellanos FX, Pineda D, Lopera F, Palacio JD, Palacio LG, Rapoport JL, Berg K, Bailey-Wilson JE, Muenke M. Attention-deficit/hyperactivity disorder in a population isolate: linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am J Hum Genet. 2004;75(6):998–1014. doi: 10.1086/426154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcos-Burgos M, Muenke M. Genetics of population isolates. Clin Genet. 2002;61(4):233–247. doi: 10.1034/j.1399-0004.2002.610401.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statisyical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Castillo M, Smith JK, Kwock L. Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am J Neuroradiol. 2000;21(9):1645–1649. [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mennin D, Gershon J, Tsuang MT. A prospective four-year follow-up study of children at risk for ADHD: psychiatric, neuropsychological, and psychosocial outcome. J Am Acad Child Adolesc Psychiatry. 1996;35(11):1449–1459. doi: 10.1097/00004583-199611000-00013. [DOI] [PubMed] [Google Scholar]
- Hesslinger B, Thiel T, Tebartz van Elst L, Hennig J, Ebert D. Attention-deficit disorder in adults with or without hyperactivity: where is the difference? A study in humans using short echo (1)H-magnetic resonance spectroscopy. Neurosci Lett. 2001;304(1–2):117–119. doi: 10.1016/s0304-3940(01)01730-x. [DOI] [PubMed] [Google Scholar]
- Kadota T, Horinouchi T, Kuroda C. Development and aging of the cerebrum: assessment with proton MR spectroscopy. AJNR Am J Neuroradiol. 2001;22(1):128–135. [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26(4):433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lopera F, Palacio LG, Jimenez I, Villegas P, Puerta IC, Pineda D, Jimenez M, Arcos-Burgos M. Discrimination between genetic factors in attention deficit. Rev Neurol. 1999;28(7):660–664. [PubMed] [Google Scholar]
- MacMaster FP, Carrey N, Sparkes S, Kusumakar V. Proton spectroscopy in medication-free pediatric attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;53(2):184–187. doi: 10.1016/s0006-3223(02)01401-4. [DOI] [PubMed] [Google Scholar]
- Moore CM, Biederman J, Wozniak J, Mick E, Aleardi M, Wardrop M, Dougherty M, Harpold T, Hammerness P, Randall E, Renshaw PF. Differences in brain chemistry in children and adolescents with attention deficit hyperactivity disorder with and without comorbid bipolar disorder: a proton magnetic resonance spectroscopy study. Am J Psychiatry. 2006;163(2):316–318. doi: 10.1176/appi.ajp.163.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio JD, Castellanos FX, Pineda DA, Lopera F, Arcos-Burgos M, Quiroz YT, Henao GC, Puerta IC, Ramirez DL, Rapoport JL, Bailey-Wilson J, Berg K, Muenke M. Attention-deficit/hyperactivity disorder and comorbidities in 18 Paisa Colombian multigenerational families. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1506–1515. doi: 10.1097/01.chi.0000142279.79805.dc. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. J Pediatr Psychol. 2007;32(6):711–727. doi: 10.1093/jpepsy/jsm022. [DOI] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Hesslinger B, Buechert M, Ahrendts J, Feige B, Bubl E, Hennig J, Ebert D, Tebartz van Elst L. Reduced cingulate glutamate/glutamine-to-creatine ratios in adult patients with attention deficit/hyperactivity disorder -- a magnet resonance spectroscopy study. J Psychiatr Res. 2007;41(11):934–941. doi: 10.1016/j.jpsychires.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Matthies S, Drieling T, Maier S, Bubl E, Hesslinger B, Buechert M, Henning J, Ebert D, Tebartz Van Elst L. Spectroscopic findings in attention-deficit/hyperactivity disorder: review and meta-analysis. World J Biol Psychiatry. 2009;10(4 Pt 2):355–365. doi: 10.1080/15622970802176032. [DOI] [PubMed] [Google Scholar]
- Pineda DA, Lopera F, Puerta IC, Trujillo-Orrego N, Aguirre-Acevedo DC, Hincapie-Henao L, Arango CP, Acosta MT, Holzinger SI, Palacio JD, Pineda-Alvarez DE, Velez JI, Martinez AF, Lewis JE, Muenke M, Arcos-Burgos M. Potential cognitive endophenotypes in multigenerational families: segregating ADHD from a genetic isolate. Atten Defic Hyperact Disord. 2011;3(3):291–299. doi: 10.1007/s12402-011-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda DA, Palacio LG, Puerta IC, Merchan V, Arango CP, Galvis AY, Gomez M, Aguirre DC, Lopera F, Arcos-Burgos M. Environmental influences that affect attention deficit/hyperactivity disorder: study of a genetic isolate. Eur Child Adolesc Psychiatry. 2007;16(5):337–346. doi: 10.1007/s00787-007-0605-4. [DOI] [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, Lerch JP, He Y, Zijdenbos A, Kelly C, Milham MP, Castellanos FX. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry. 2011;68(11):1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. ISBN 3-900051-07-0, URL http://www.R-project.org/. [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigielska-Kuzia J, Sobaniec W. Brain metabolic profile obtained by proton magnetic resonance spectroscopy HMRS in children with Down syndrome. Adv Med Sci. 2007;52(Suppl 1):183–187. [PubMed] [Google Scholar]
- Sun L, Jin Z, Zang YF, Zeng YW, Liu G, Li Y, Seidman LJ, Faraone SV, Wang YF. Differences between attention-deficit disorder with and without hyperactivity: a 1H-magnetic resonance spectroscopy study. Brain Dev. 2005;27(5):340–344. doi: 10.1016/j.braindev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17(1):39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.