Abstract
Background
Human pancreatic cancer is currently one of the fifth-leading causes of cancer-related mortality with a 5-year survival rate of less than 5%. Since pancreatic carcinoma is largely refractory to conventional therapies, there is a strong medical need for the development of novel and innovative cancer preventive strategies. The forkhead transcription factors of the O class (FOXO) play a major role in cell proliferation, angiogenesis, metastasis and tumorigenesis. The objectives of this study were to examine whether FKHRL1/FOXO3a modulates antitumor activity of (-)-epigallocatechin-3-gallate (EGCG), an active ingredient in green tea, in pancreatic cancer model in vivo.
Methods
PANC-1 cells were orthotopically implanted into Balb c nude mice and gavaged with EGCG after tumor formation. Cell proliferation and apoptosis were measured by Ki67 and TUNEL staining, respectively. The expression of PI3K, AKT, ERK, and FOXO3a / FKHRL1 and its target genes were measured by the Western blot analysis and/or q-RT-PCR. FOXO-DNA binding were measured by gelshift assay.
Results
EGCG-treated mice showed significant inhibition in tumor growth which was associated with reduced phosphorylation of ERK, PI3K, AKT, and FKHRL1/FOXO3a, and modulation of FOXO target genes. EGCG induced apoptosis by up-regulating Bim and activating caspase-3. EGCG modulated markers of cell cycle (p27/KIP1), angiogenesis (CD31, VEGF, IL-6, IL-8, SEMA3F and HIF1α), and metastasis (MMP2 and MMP7). The inhibition of VEGF by EGCG was associated with suppression of neuropilin. EGCG inhibited epithelial mesenchymal transition by upregulating the expression of E-cadherin and inhibiting the expression of N-cadherin, and Zeb1. These data suggest that EGCG inhibits pancreatic cancer orthotopic tumor growth, angiogenesis and metastasis which are associated with inhibition of PI3K/AKT and ERK pathways and activation of FKHRL1/FOXO3a.
Conclusions
EGCG can be used for the prevention and/or treatment of pancreatic cancer.
Keywords: Pancreatic Cancer, EGCG, cancer prevention, FOXO
BACKGROUND
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the United States with an overall 5-year survival rate of <5% [1]. Its biology is characterized by the propensity of early and aggressive invasion and metastasis, such that <10% of patients have surgically respectable disease at the time of diagnosis. The poor prognosis of pancreatic cancer is related with late presentation, aggressive local invasion, early metastasis, and poor response to conventional chemotherapy and radiotherapy [1, 2]. Several factors are associated with increased risk for pancreatic cancer and these include chronic pancreatitis, smoking, diabetes, prior gastric surgery, exposure to certain classes of organic solvents, radiation, and specific gene polymorphisms [3, 4]. Heritable as well as several acquired gene mutations have been identified in pancreatic tumors [5]. The K-Ras oncogene is primarily mutated in codon 12 in > 90% of pancreatic tumors and the mutation results in a constitutively active form of ras that can lead to increased cell proliferation. Mutations in the tumor suppressor gene p53, the cyclin-dependent kinase inhibitor p16, and SMAD4, a downstream target of TGFβ also exhibit high mutation frequencies in pancreatic tumors [6, 7]. Therefore, understanding the pathogenesis of the preinvasive stage, and developing effective strategies to prevent pancreatic neoplasms are of paramount importance.
Limited options for the management of pancreatic cancer and its increasing incidence necessitate the search for novel approaches. The most accepted compounds for chemoprevention in humans are naturally occurring dietary substances. Green tea, which is widely consumed in China, Japan and India, contains polyphenolic compounds, which account for 30% of the dry weight of the leaves [8, 9]. Various studies have demonstrated that green tea catechins inhibit carcinogenesis and growth of established cancers at various organ sites [10–12]. Epidemiological studies revealed that the incidences of stomach and prostate cancers are the lowest in the world among a population that consumes green tea on a regular basis [10, 13–16]. The chemopreventive effects of green tea are mediated by its catechins with (–)-epigallocatechin-3-gallate (EGCG) being the most abundant and powerful catechin in cancer prevention and treatment [12]. The chemopreventive activity of EGCG was studied extensively, but the underlying mechanisms are not yet completely understood. Pleiotropic effects of EGCG include anti-oxidant activities, cell signaling modulation, apoptosis induction, cell cycle arrest as well as inhibition of matrix metalloproteinases (MMPs), urokinase-plasminogen activator, telomerase, DNA methyltransferase, and proteasome [17, 18]. We and others have demonstrated that EGCG inhibits growth and induces apoptosis in human pancreatic cancer cells in vitro through regulation of Bcl-2 family members and MAP kinase pathway, generation of reactive oxygen species and activation of caspases [19–24], thus it holds great promise for development as a chemopreventive agent in pancreatic cancer. Furthermore, the efficacy of EGCG for the prevention of pancreatic cancer in an orthotopic animal model system has not yet been examined. The orthotopic model system allows us to study the effects of anticancer agents in a natural environment under the influence of stroma.
Transcription factors of the Forkhead box O (FOXO) class are predominantly regulated through phosphoinositide 3-kinase/AKT (also known as PKB) pathway [25]. FOXO1a / FKHR, FOXO3a / FKHRL1, and FOXO4 / AFX are members of FOXO subfamily [26]. The PI3K/AKT pathway phosphorylates all of these FOXO proteins, resulting in impairment of DNA binding ability and inhibition of FOXO-dependent transcription [27]. Inhibition of the PI3K/AKT and ERK pathways lead to dephosphorylation and nuclear translocation of active FOXOs, which causes cell cycle arrest and apoptosis [28]. Conversely, loss of PTEN activity results in increased AKT activity leading to inhibition of FOXO activity through phosphorylation and cytoplasmic sequestration [28]. FOXO transcriptional activity controls cellular proliferation and apoptosis downstream of PTEN, PI3K, AKT and ERK [29]. Since inactivation and loss of PTEN, and overexpression of AKT are frequently observed in pancreatic cancer [30], targeting a downstream target such as FOXO may be an attractive strategy for pancreatic cancer prevention and/or treatment.
Hypoxia is one of the main activators of VEGF expression in tumor cells through direct transcriptional activation by HIFs [31]. However, hypoxia also upregulates vascular endothelial growth factor (VEGF) at the nontranscriptional level. For example, it has been shown previously that under hypoxic conditions, VEGF mRNA was stabilized and VEGF secretion was more efficient [32]. Neuropilin-2 (NRP-2) is a coreceptor for VEGF on endothelial cells. NRP-2 is overexpressed in pancreatic ductal adenocarcinoma (PDAC) cells relative to nonmalignant ductal epithelium [33, 34]. Interleukin-8 (IL-8) is associated with tumorigenesis by promoting angiogenesis and metastasis. In pancreatic cancer exogenous IL-8 up-regulated the expression of VEGF, neuropilin (NRP)-2, and extracellular signal-regulated kinase (ERK)1/2 in BxPC-3 cells [35]. IL-8 might be a malignant factor in human pancreatic cancer by induction of VEGF and NRP-2 expression and ERK activation. Reduction of NRP-2 expression in PDAC cells decreased survival signaling, migration, invasion, and ability to grow under anchorage-independent conditions [35]. In vivo, reduction of NRP-2 led to decreased growth of xenograft tumors and decreased vascular area, which was associated with decreased Jagged-1 levels. Thus, NRP-2 is a potential therapeutic target on PDAC cells.
The purpose of this study was to determine whether EGCG inhibited tumor growth, angiogenesis, and metastasis of pancreatic cancer cells orthotopically implanted in Balb C nude mice. Our data showed that EGCG inhibited tumor growth, angiogenesis and metastasis in PANC-1 tumors in nude mice through regulation of multiple signaling pathways, and has a great potential for pancreatic cancer prevention and/or treatment.
METHODS
Reagents
Antibodies against PTEN, phospho-AKT, AKT, phospho-ERK, ERK, p27/KIP1, cyclin D1, pFOXO3a, FOXO3a, Bim, PCNA, caspase-3, and β-actin were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Enhanced chemiluminescence (ECL) Western blot detection reagents were purchased from Amersham Life Sciences Inc. (Arlington Heights, IL). Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay kit was purchased from EMD Biosciences / Calbiochem (San Diego, CA). EGCG was purchased from LKT Laboratories, Inc. (St. Paul, MN). Kits for Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) and caspase-3 assays were purchased from EMD Biosciences / Calbiochem (San Diego, CA).
Antitumor activity of EGCG
PANC-1 cells (0.1 × 106 cells mixed with Matrigel, Becton Dickinson, Bedford, MA, 50:50 ratio, in a final volume of 75 μl) were injected into the pancreas of Balb/c nu/nu mice (4–6 weeks old) as per approved protocol (The University of Texas Health Science Center at Tyler, Tyler, Texas). The mice were purchased from the National Cancer Institute, Frederick, MD. After one week, mice (7 mice per group) were treated with EGCG (0, 60, 80 and 100 mg/kg body weight) through gavage (Monday to Friday, 5 days a week for 28 days, once daily). At the end of the experiment, mice were euthanized and tumors were isolated and weighed.
Western Blot Analysis
Western blots were performed as we described earlier [36]. In brief, cells were lysed in a buffer containing 10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.5 mM EDTA, 1 mM EGTA, 1% SDS, 1 mM sodium orthovanadate, and a mixture of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 2 μg/ml aprotinin). Lysates were sonicated for 10 s, centrifuged for 20 min at 10,000 × g and stored at −70 °C. Equal amounts of lysate proteins were run on 10% SDS-PAGE and electrophoretically transferred to nitrocellulose. Nitrocellulose blots were blocked with 6% nonfat dry milk in TBS buffer (20 mM Tris-HCl (pH 7.4), and 500 mM NaCl), and incubated with primary antibody in TBS containing 1% bovine serum albumin overnight at 4 °C. Immunoblots were washed three times (15, 5 and 5 min each) with TBST (TBS and 0.01% Tween 20). Immunoreactivity was detected by sequential incubation with horseradish peroxidase-conjugated secondary antibody and ECL reagents.
Electrophorectic mobility shift assay (EMSA)
FOXO probes were end-labeled with [γ-32P] dATP by incubating oligodeoxyribonucleotide strands with 5 × reaction buffer and 10 U T4 polynucleotide kinase for 1 h at 37°C. Then labeled oligonucleotides were allowed to anneal at room temperature for 10 min and 20 μg protein from each sample was used in 25 μl binding reactions, which consisted of 1 μg poly dI-dC, in 5× binding buffer (50 mM Tris HCl; pH 8.0, 750 mM KCl, 2.5 mM EDTA, 0.5% Triton-X 100, 62.5% glycerol (v/v) and 1 mM DTT). To determine specificity of DNA binding, samples were incubated with or without 20 ng of unlabeled competitor DNA for 10 min at room temperature. Then 0.1 ng of labeled probe was added and samples were further incubated for 20 min at room temperature. Samples were separated on a 5% non-denaturing polyacrylamide gel in 0.5% TBE and visualized by autoradiography.
Evaluation of mRNA expression levels by quantitative Real Time-PCR
For the quantification of gene amplification, Real-time PCR was performed using an ABI 7300 Sequence Detection System in the presence of SYBR- Green. Briefly, RNA was isolated with TRIzol (Life Technologies) and reverse transcribed. cDNA reactions were amplified with QPCR SYBR Green Mix (Applied Biosystems). Gene specific primers were purchased from Applied Biosystems.
Target sequences were amplified using incubated at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. HK-GAPD is used as endogenous normalization control. All assays were performed in triplicate and were calculated on the basis of ΔΔCt method.
Immunohistochemistry
Imunohistochemistry of tumor tissues collected was performed as we described elsewhere [20]. TUNEL assays were performed as per manufacturer's instructions (Roche Applied Sciences).
Statistical Analysis
The mean and SD were calculated for each experimental group. Differences between groups were analyzed by one or two way ANOVA, followed by Bonferoni's multiple comparison tests using PRISM statistical analysis software (GrafPad Software, Inc., San Diego, CA). Significant differences among groups were calculated at P < 0.05.
RESULTS
EGCG inhibits the growth of PANC-1 cells orthotopically implanted in Balb C Nude mice
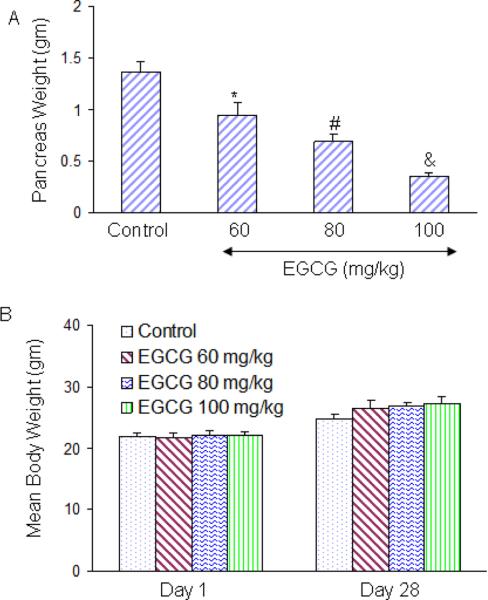
We first examined the effects of EGCG on growth of PANC-1 tumor cells orthotopically implanted in Balb C nude mice. PANC-1 cells were injected into pancreas and after one week of cancer cell implantation, mice were treated with EGCG (0–100 mg/kg body weight) through gavage (Monday to Friday, once daily) for 28 days. As shown in Fig. 1A, EGCG inhibited pancreatic tumor growth in Balb C nude mice in a dose-dependent manner. Furthermore, EGCG had no effect on the body weight of tumor bearing mice, although mice gained weight during the treatment. We did not observe any toxicity in the liver, spleen and intestine of mice treated with EGCG.
Fig. 1. EGCG inhibits the growth of PANC-1 tumors orthotopically inplanted in Balb C Nude mice.
(A) Upper Panel, PANC-1 cells (0.1 × 106 cells mixed with Matrigel, 50:50 ratio) were orthotopically implanted into the pancreas of Balb C nude mice. Tumor bearing mice were treated with EGCG (0–100 mg/kg body weight) through gavage (Monday to Friday, once daily) for 28 days. At the end of the experiment, pancreatic tumor weights were recorded. Data represent the mean ± S.D. *, #, & = significantly different from control, P < 0.05. (B), Body weight of tumor-bearing mice during the experiment. Data represent the mean ± S.D.
EGCG induces apoptosis and inhibit tumor cell proliferation
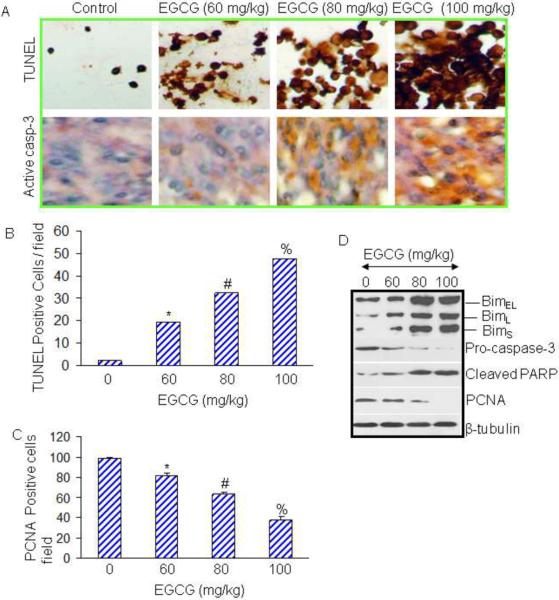
Most anticancer agents induce apoptosis through activation of caspases. We next examined whether EGCG induced tumor cell apoptosis through activation of caspase-3. Apoptosis and caspase-3 were measured by TUNEL and immunohistochemistry using active anti-caspase-3 antibody, respectively (Fig. 2A). EGCG induced apoptosis and activated caspase-3 is a dose-dependent manner. Quantification of TUNEL positive cells demonstrated a dose-dependent induction of tumor cell apoptosis (Fig. 2B). Activation of caspase-3 by EGCG correlated with induction of apoptosis in tumor tissues.
Fig. 2. Effects of EGCG on apoptosis, cell proliferation and expression of Bim, caspase-3, PARP and PCNA in tumor tissues.
(A), Immunohistochemistry was performed in tumor tissues isolated from control and EGCG treated mice. Apoptosis was measured by TUNEL assay and caspase-3 was measured by IHC using antibody which recognizes active caspase-3. (B and C), Quantification of TUNEL and PCNA positive cells. Data represent the mean ± S.D. *, #, % = significantly different from control, P < 0.05. (D), Effects of EGCG on markers of apoptosis and cell proliferation. Western blot analyses were performed to examine the expression of Bim, caspase-3, PARP, and PCNA in tumor tissues. The β-actin was used as a loading control.
We next examined the effects of EGCG on cell proliferation in tumor tissues derived from control and EGCG treated mice using anti-PCNA antibody (Fig. 2C). EGCG inhibited tumor cell proliferation in a dose-dependent manner.
Since EGCG induced apoptosis and inhibited tumor cell proliferation, we next confirmed these phenomena by measuring the expression of proteins by Western blot analysis (Fig. 2D). EGCG induced the expression of all the isoforms of Bim (BimEL, BimL and BimS). Furthermore, EGCG treatment of mice resulted in reduction of procaspase-3, induction of cleaved PARP, and inhibition of PCNA expression in a dose-dependent manner. Overall, these data suggest that EGCG inhibited cell proliferation and induced apoptosis in pancreatic tumor tissues through inhibition of PCNA, and induction of Bim and cleavage of caspase-3 and PARP.
EGCG activates FOXO3a through inhibition of ERK and PI3K/AKT pathways
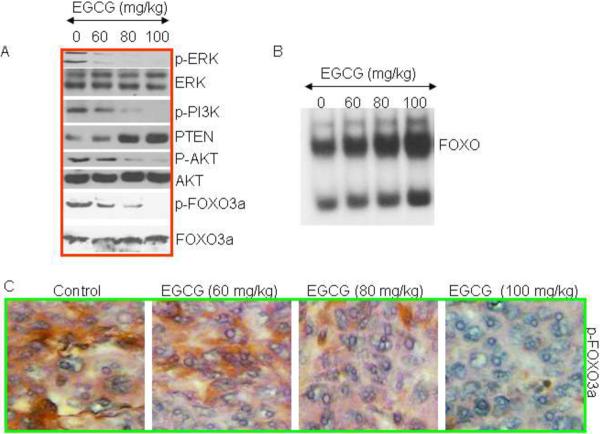
We have previously demonstrated that inhibition of ERK and PI3K/AKT pathway synergistically activate FOXO transcription factors in prostate and pancreatic cancer cells [36, 37]. We therefore measured the effects of EGCG on the expression of ERK, PI3K, PTEN, AKT and FOXO3a /FKHRL1 in tumor tissues derived from control and EGCG treated mice (Fig. 3). Western blot analyses were performed to examine the expression of p-ERK, ERK, p-PI3K (Tyr458), p-AKT (Ser473), AKT, p-FOXO3a (Ser256), and FOXO3a. As shown in Fig. 3A, EGCG inhibited the phosphorylation of ERK, PI3K (Tyr458), p-AKT (Ser473), and p-FOXO3a (Ser256) in pancreatic tumor tissues. It also induced the expression of PTEN, a negative regulator of AKT, in tumor tissues. EGCG has no significant effect on the expression of total ERK, AKT and FOXO3a proteins. These data confirm our hypothesis that EGCG activates FOXO3a transcription factor (dephosphorylation of FOXO3a means its activation) by inhibiting ERK and PI3K/AKT pathways in pancreatic tumor tissues.
Fig. 3. Effects of EGCG on ERK, PI3K/AKT and FOXO proteins isolated from tumor tissues.
(A), Western blot analyses were performed to examine the expression of p-ERK, ERK, p-PI3K (Tyr458), PTEN, p-AKT (Ser473), AKT, p-FOXO3a (Ser256), and FOXO3a. (B), Effects of EGCG on FOXO DNA binding. Nuclear extracts were prepared and incubated with labeled FOXO probe. Gelshift assay was performed as we described in Materials and Methods. (C), Expression of phospho-FOXO3a. Immunohistochemistry was performed to examine the expression of p-FOXO3a in tumor tissues isolated from control and EGCG treated mice.
Since EGCG inhibits the phosphorylation of FOXO3a, which may result in enhanced nuclear translocation and DNA binding, we measured the FOXO-DNA interaction by gelshift assay (Fig. 3B). EGCG enhanced FOXO-DNA binding in a dose-dependent manner. We next confirmed the phosphorylation of FOXO3a in tumor tissues by immunohistochemistry (Fig. 3C). Treatment of mice with EGCG resulted in inhibition of phosphorylation of FOXO3a, as measured by phospho-specific FOXO3a antibody. Overall these data suggest that EGCG inhibits ERK and PI3K/AKT pathways, induces PTEN, enhances FOXO-DNA binding activity and thus activates FOXO3a in pancreatic tumor tissues.
EGCG inhibits angiogenesis and the expression of IL-6 and IL-8
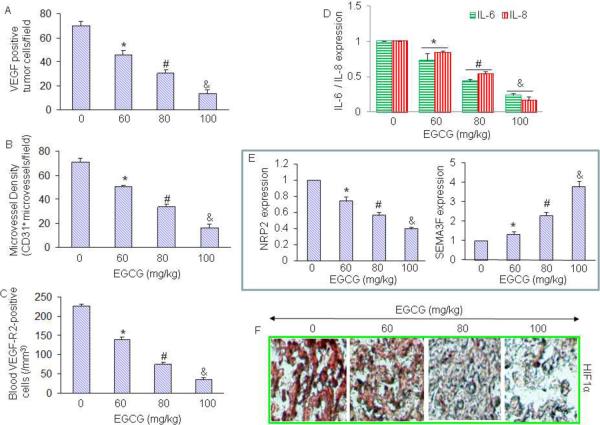
Since angiogenesis plays a major role in tumor growth [38], we sought to measure the effects of EGCG on angiogenesis by measuring VEGF positive tumor cells, microvessel density (staining tumor tissues with anti-CD31 antibody), and blood VEGFR2-positive cells (marker of angiogenesis) (Fig. 4A–C). Treatment of mice with EGCG resulted in significant inhibition in VEGF positive tumor cells, reduction in microvessel density (as measured by CD31+ microvessels / field) and blood VEGFR2-positive cells in a dose-dependent manner.
Fig. 4. Effects of EGCG on angiogenesis, and production of IL-6, IL-8, NRP-2 and and HIF1α.
(A), Quantification of VEGF-positive cells in tumor tissues on day 28. Sections from tumor tissue were stained with anti-VEGF antibody, and the number of VEGF-positive tumor cells was counted. The results are shown as the mean SD. *, # and & = significantly different from control, P < 0.05. (B), Sections from tumor tissue were stained with anti-CD31 antibody, and the number of CD31+ blood vessels was counted. The results are shown as the mean SD. *, # and & = significantly different from control, P < 0.05. (C), VEGF receptor 2 (VEGF-R2)-positive circulating endothelial cells in mice on day 28. The blood cells from peripheral blood attached to the slide were stained with anti-VEGF-R2 antibody, and the number of VEGF-R2-positive cells was counted under a microscope. The results are shown as the mean SD. *, # and & = significantly different from control, P < 0.05. (D), The expression of IL-6 and IL-8 in tumor tissues was measured by q-RT-PCR. The results are shown as the mean SD. *, # and & = significantly different from control, P < 0.05. (E), The expression of NRP2 and SEMA3F in tumor tissues was measured by q-RT-PCR. The results are shown as the mean SD. *, # and & = significantly different from control, P < 0.05. (F), Immunohistochemistry of HIF1α. Sections from tumor tissue were stained with anti-HIF1α antibody, and the photographs were taken.
It has been shown that proliferation of cancer cells is under the control of a complex network of cytokines, like interleukin IL-6 and IL-8. These cytokines have been shown to enhance angiogenesis in tumor tissues. Recent studies have demonstrated that the IL-8/IL-8 receptor axis plays an important role on the induction and/or maintenance of tumor EMT and its ability to remodel the tumor microenvironment [39, 40]. We therefore measure the expression of IL-6 and IL-8 in tumor tissues by q-RT-PCR. Treatment of tumor bearing mice with EGCG resulted in suppression of IL-6 and IL-8 in a dose-dependent manner (Fig. 4D). These data suggest that IL-6 and IL-8 may mediate antitumor activity of EGCG.
Neuropilin-2 (NRP2) is a receptor expressed by tumor cells and endothelial cells (EC) that binds both semaphorin 3F (SEMA3F), a potent inhibitor of tumor angiogenesis and metastasis, and vascular endothelial growth factor (VEGF), a potent stimulator of tumor angiogenesis. Tumor angiogenesis is mediated by a balance of angiogenesis activators, such as VEGF, and angiogenesis inhibitors, such as SEMA3F. VEGF and SEMA3F belong to two disparate families, yet they bind the same receptor NRP2 [41, 42]. Our data demonstrate that EGCG inhibited the expression of NRP2 and up-regulated the expression of SEMA3F in PANC-1 tumor tissues (Fig. 4E), suggesting the involvement of NRP2 receptors in modulating the anti-angiogenic effects of EGCG.
Hypoxia is one of the main activators of VEGF expression in tumor cells through direct transcriptional activation by HIFs [31]. Since solid tumors grow in hypoxic environment which induces the expression of HIF1α, we measured the expression of HIF1α by immunohistochemistry. As shown in Fig. 4F, treatment of tumor-bearing mice with EGCG resulted in a dose-dependent inhibition of HIF1α in tumor tissues. The inhibition of VEGF by EGCG was positively correlated with the expression of HIF1 in tumor tissues.
EGCG inhibits the expression of p27/Kip1, a downstream target of FOXO
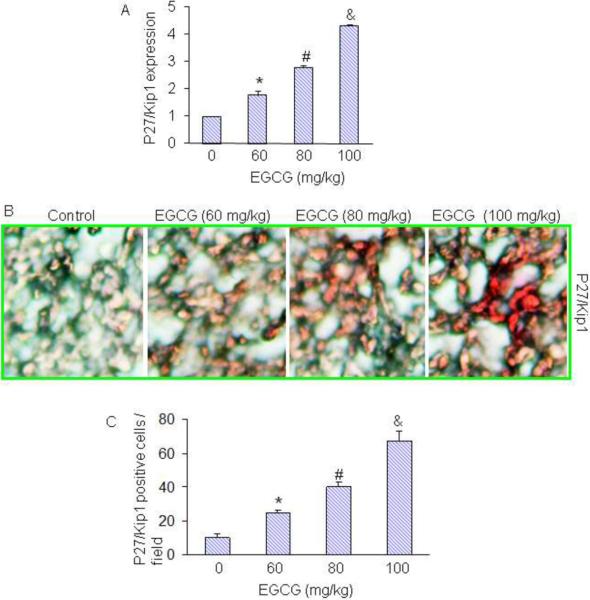
Since cell cycle inhibitor p27/Kip1 is one of the targets of the FOXO transcription factor, we next examined the effects of EGCG on the expression of p27/Kip1. EGCG induced the expression of p27/Kip1 as measured by q-RT-PCR (Fig. 5A). We also confirmed the expression of p27/Kip1 by immunohistochemistry. As shown in Fig. 5B, EGCG induced the expression of p27/Kip1 in tumor tissues in a dose-dependent manner. Quantification of p27/Kip1 positive cells confirmed the induction of p27/Kip1 in a dose-dependent manner. These data suggest that EGCG can cause growth arrest in tumor cells by inducing the expression of p27/Kip1.
Fig. 5. Effects of EGCG on the expression of cell cycle inhibitor p27/Kip1.
Tumor samples were collected on day 28 from the experiment described above. (A), The expression of p27/Kip1 was measured by q-RT-PCR. The data represent mean SD. *, # and & = significantly different from control, P < 0.05. (B), Immunohistochemical procedure for detection of cell cycle inhibitor p27/Kip1 was performed. (C), Quantification of p27/Kip1-positive cells. The data represent mean SD. *, # and & = significantly different from control, P < 0.05.
EGCG inhibits markers of metastasis
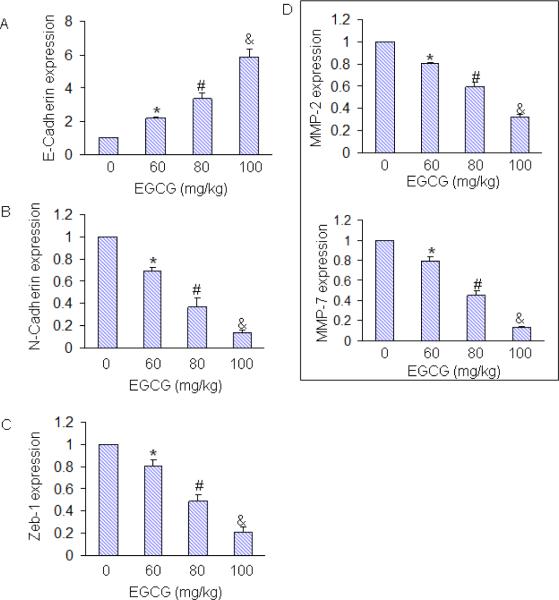
The switch of tumor cells from an epithelial to a mesenchymal-like phenotype [designated as epithelial-to-mesenchymal transition (EMT)] is known to induce tumor cell motility and invasiveness, therefore promoting metastasis of solid carcinomas [43]. During EMT, expression of Zeb-1 transcription factor is induced, as a result the expression of E-cadherin is inhibited and the expression of N-cadherin is upregulated. The expression of matrix metalloproteinases (MMPs), which digest the different components of the extracellular matrix (ECM) and basement membrane, also enhanced during EMT [44–47]. Treatment of mice with EGCG induced the expression of E-cadherin and inhibited the expression of N-cadherin, Zeb-1, MMP-2 and MMP-7 in tumor tissues (Fig. 6). Our data demonstrate that EGCG can inhibit / reverse tumor metastasis by inducing a “cadherin switch” and inhibiting the expression of Zeb1 and MMPs. Thus, EGCG not only inhibit tumor growth but also tumor metastasis.
Fig. 6. Effects of EGCG on markers of metastasis in tumor tissues.
Tumor samples were collected on day 28 from the experiment described above. The expression of E-cadherine, N-cadherin, Zeb1, MMP-2 and MMP-7 was measured by q-RT-PCR. The data represent mean SD. *, # and & = significantly different from control, P < 0.05.
DISCUSSION
Pancreatic ductal adenocarcinoma (PDA), the fourth leading cause of cancer death in the United States, is a complex disease that arises in the setting of genetic alterations (KRAS, CDKN2A/p16 (INK4a), BRCA1, SMAD4, and TP53), epigenetic perturbations (acetylation and methylation), and epicellular events (diabetes and inflammation). In this paper we have demonstrated, for the first time that EGCG-induced suppression of PANC1 tumor growth in nude mice was associated with inhibition of ERK, PI3K, AKT and FOXO3a phosphorylation, and tumor cell proliferation, and induction of apoptosis in tumor tissues. EGCG also induced the expression of FOXO target genes such as Bim, and p27/KIP1in pancreatic tumor tissues. Similarly, others have also demonstrated the antiproliferative and proapoptotic effects of EGCG in pancreatic cancer, and here we further added the role of FOXO3a, NRP2 and SEMA3F in mediating the effects of EGCG. Furthermore, IL-8 signaling blockade may provide a means of targeting invasive tumor cells. Since EGCG is a nontoxic polyphenolic compound, it can be safely used for the treatment and/or prevention of pancreatic cancer.
FOXO transcription factors have been shown to regulate tissue homeostasis in the pancreas, diabetes and cancer. In recent years, they have emerged as critical transcriptional integrators among pathways regulating proliferation, survival, differentiation and angiogenesis. FOXO regulates cell cycle and apoptotic genes such as Bim [48, 49], Fas ligand [50], p27/KIP1 [49], and Bcl-6 [51]. In the present study, all the doses of EGCG induced the expression of FOXO target genes such as Bim, and p27/Kip1 in pancreatic tumor tissues. However, the Western blot analysis of tumor tissues with the lowest dose of EGCG (60 mg/kg) showed no significant changes in PCNA expression although procaspase 3 levels were reduced with significant increase in TUNEL positive cells when compared with the untreated controls. This is interesting and would suggest for early apoptotic events that target caspase-3 activation. EGCG induced the expression of PTEN and inhibited the phosphorylation / activation of AKT in in tumor tissues. We also demonstrate that the inhibition of AKT phosphorylation is correlated with the activation of FOXO transcription factors and induction of Bim, and p27/KIP1. In another study, we have demonstrated that the inhibition of FOXO phosphorylation by resveratrol resulted in its nuclear translocation, reduced DNA binding and transcriptional activity [49]. The inhibition of PI3K/AKT pathway induced FOXO transcriptional activity resulting in induction of Bim, TRAIL, p27/KIP1, DR4 and DR5, and inhibition of cyclin D1 [49]. Inhibition of FOXO transcription factors by shRNA blocked resveratrol-induced upregulation of Bim, TRAIL, DR4, DR5, p27/KIP1 and apoptosis, and inhibition of cyclin D1 by resveratrol [49]. Together, these studies suggest that activation of FOXO transcription factors by chemopreventive agents may regulate cell cycle and apoptosis in pancreatic cancer.
The PI3K/AKT and MAPK pathways can be activated by Kras [36, 52, 53]. AKT and ERK both have been shown to directly phosphorylate and inactivate FOXO transcription factors, resulting in cytoplasmic retention, inactivation, and inhibition of the expression of FOXO-regulated genes, which control various processes such as metabolism, cell cycle, cell death and oxidative stress [54]. In the present study, EGCG inhibited the phosphorylation of ERK and AKT resulting in dephosphorylation and activation of FOXO3a. Taken together, these studies demonstrate that the activation of FOXOs has significant implication for the treatment and/or prevention of pancreatic cancer, where Kras is activated in about 90% patients, and PI3K/AKT and MEK/ERK pathways are highly activated. In addition to phosphorylation, the regulation of FOXO transcription factors by acetylation has also been demonstrated [55]. The acetylation / deacetylation of FOXO can be regulated by p300, Cbp (CREB-binding protein) and Pcaf (p300/CBP-associated factors) in response to oxidative stress or DNA binding, followed by deacetylation by class I and II histone deacetylases [55], including Sirt1 [56]. However, further studies are needed to examine the consequences of acetylation / deacetylation of FOXO transcription factors on tumorigenesis and angiogenesis.
Most solid tumors become hypoxic as they grow. In order to survive in the hypoxic environment, tumor cells have developed a coordinated set of responses to increase their blood supply [57–59]. For example, in hypoxic conditions, tumor cell expression of VEGF, a potent stimulator of angiogenesis, is induced in order to attract new blood vessels [31, 60]. A critical mediator of the hypoxic response is the hypoxia-inducible factor (HIF), a heterodimeric basic helix-loop-helix (bHLH) transcription factor composed of a HIF-α subunit (HIF1-α or HIF2-α/EPAS) and a HIF-β subunit [57, 58, 61]. Whereas the β-subunit is constitutively expressed, the stability and transcriptional activity of the a-subunits are precisely controlled by the intracellular oxygen concentration [62]. Under normoxic conditions, cells continuously synthesize, ubiquitinate and degrade the α-subunits. However, under hypoxic conditions, the degradation of the α-subunits is inhibited, resulting in accumulation of the α-subunits, dimerization with HIF1-β, binding to hypoxia response elements (HREs) within target genes and activation of transcription. For example, under hypoxic conditions, HIF-1 has been shown to bind to and activate transcription of the gene encoding VEGF [31]. A recent study has demonstrated that hypoxia causes transcriptional repression of NRP2 in tumor cells with two consequent effects that promote tumor angiogenesis and metastasis in vivo: (1) an increase in VEGF protein levels in CM that enhances VEGF-induced angiogenesis; and (2) an inhibition of the anti-tumorigenic activity of SEMA3F [41]. In the present study, EGCG inhibited the expression of VEGF, HIF1α and NRP2 and induced the expression of SEMA3F, suggesting the involvement of NRP2 in mediating the antiangiogenic effects of EGCG.
The phenomenon of epithelial-mesenchymal transition (EMT) has gained attention in the field of cancer biology for its potential contribution to the progression of carcinomas. Tumor EMT is a phenotypic switch that promotes the acquisition of a fibroblastoid-like morphology by epithelial tumor cells, resulting in enhanced tumor cell motility and invasiveness, increased metastatic propensity and resistance to chemotherapy, radiation and certain small-molecule-targeted therapies. Tumor cells undergoing EMT are also known to increase the secretion of specific factors, including cytokines, chemokines and growth factors, which could play an important role in tumor progression. Recent studies have demonstrated that the IL-8/IL-8 receptor axis plays an important role on the induction and/or maintenance of tumor EMT and its ability to remodel the tumor microenvironment [39, 40]. In the present study, EGCG inhibited the expression of IL-6 and IL-8 in tumor tissues. Our results also indicate the essential role of IL-8 signaling for the acquisition and/or maintenance of the mesenchymal and invasive features of tumor cells and suggest that IL-8 secreted by tumor cells undergoing EMT could potentiate tumor progression by inducing adjacent epithelial tumor cells into EMT. Altogether, our results emphasize the potential role of EMT in the modulation of the tumor microenvironment via secretion of multiple soluble mediators and suggest that IL-8 signaling blockade by EGCG may provide a means of targeting mesenchymal-like, invasive tumor cells.
Cancer cell metastasis is a step-wise process that includes detachment of cells from the primary tumor, local proteolysis of the basement membrane, intravasation, survival of the circulation, arrest in distant organ, extravasation and invasion into the surrounding tissue and growth [63, 64]. Metastasis involves penetration of the extracellular matrix (ECM) and basement membrane, and requires the action of proteases. The MMPs are a family of zinc-dependent proteases that are capable of degrading the components of the ECM and are involved in tumor invasion [43]. The increased activities of MMP-2 and MMP-7 have been associated with increasing tumor metastases in various human cancers, suggesting an important functional role for these proteases in the metastatic process.
CONCLUSIONS
We have demonstrated that EGCG induces cell cycle arrest and apoptosis through regulation of FOXO3a. The inhibition of PI3K/AKT and MEK/ERK pathways can have synergistic effects on the activation of FOXO transcription factor, resulting in regulation of FOXO-target genes. EGCG inhibited the production of pro-inflammatory IL-6, pro-angiogenic IL-8 and VEGF as well as invasiveness-promoting MMP-2 and MMP-7 thus blocking production of tumorigenic mediators, and up-regulating negative modulator SEMA3F in the microenvironment of the tumor. Our study provides important information regarding the mechanisms by which EGCG regulates cell cycle, apoptosis and tumor growth through activation of FOXO3a. Thus, our studies suggest that inhibition of ERK and AKT pathways by EGCG act together to activate FOXO3a which can regulate pancreatic cancer growth.
Acknowledgments
We thank our lab members for critical reading of the manuscript. This work was supported in part by the grants from the National Institutes of Health (R01CA125262, RO1CA114469 and RO1CA125262-02S1) and Kansas Bioscience Authority.
List of abbreviations
- bHLH
basic helix-loop-helix
- Cbp
CREB-binding protein
- EC
endothelial cells EC
- ECM
extracellular matrix
- EGCG
(−)-epigallocatechin-3-gallate
- ERK1/2
extracellular signal-regulated kinase
- EMT
epithelial-mesenchymal transition
- FOXO
forkhead transcription factors of the O class
- HREs
hypoxia response elements
- HIF
hypoxia-inducible factor
- MMP
matrix metalloproteinases
- NRP2
Neuropilin-2
- PI3K
phosphoinositide 3-kinase
- PDA
Pancreatic ductal adenocarcinoma
- Pcaf
p300/CBP-associated factors
- SEMA 3F
semaphorin 3F
- VEGF
vascular endothelial growth factor
Footnotes
Disclosure of Potential Competing Interests The authors have declared that no competing interests exist.
Authors' contributions SS = designed the experiments, contributed reagents, wrote the manuscript, LM = performed the experiments, analyzed the data; RKS = designed the experiments, contributed reagents, wrote the manuscript
References
- 1.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 2.Magee CJ, Ghaneh P, Neoptolemos JP. Surgical and medical therapy for pancreatic carcinoma. Best Pract Res Clin Gastroenterol. 2002;16:435–55. doi: 10.1053/bega.2002.0317. [DOI] [PubMed] [Google Scholar]
- 3.Li D. Molecular epidemiology of pancreatic cancer. Cancer J. 2001;7:259–65. [PubMed] [Google Scholar]
- 4.Gold EB, Goldin SB. Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am. 1998;7:67–91. [PubMed] [Google Scholar]
- 5.Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2:25–8. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Sarkar FH. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Wientjes MG, Au JL. Pancreatic cancer: pathobiology, treatment options, and drug delivery. AAPS J. 2010;12:223–32. doi: 10.1208/s12248-010-9181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Wan SB, Yang H, Yuan J, Chan TH, Dou QP. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv Clin Chem. 2011;53:155–77. doi: 10.1016/b978-0-12-385855-9.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 11.Yang CS, Wang H. Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res. 2011;55:819–31. doi: 10.1002/mnfr.201100036. [DOI] [PubMed] [Google Scholar]
- 12.Shankar S, Ganapathy S, Srivastava RK. Green tea polyphenols: biology and therapeutic implications in cancer. Front Biosci. 2007;12:4881–99. doi: 10.2741/2435. [DOI] [PubMed] [Google Scholar]
- 13.Ahn WS, Huh SW, Bae SM, Lee IP, Lee JM, Namkoong SE, Kim CK, Sin JI. A major constituent of green tea, EGCG, inhibits the growth of a human cervical cancer cell line, CaSki cells, through apoptosis, G(1) arrest, and regulation of gene expression. DNA Cell Biol. 2003;22:217–24. doi: 10.1089/104454903321655846. [DOI] [PubMed] [Google Scholar]
- 14.Manson MM, Farmer PB, Gescher A, Steward WP. Innovative agents in cancer prevention. Recent Results Cancer Res. 2005;166:257–75. doi: 10.1007/3-540-26980-0_17. [DOI] [PubMed] [Google Scholar]
- 15.Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004;150:43–56. doi: 10.1016/j.toxlet.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Lyn-Cook BD, Rogers T, Yan Y, Blann EB, Kadlubar FF, Hammons GJ. Chemopreventive effects of tea extracts and various components on human pancreatic and prostate tumor cells in vitro. Nutr Cancer. 1999;35:80–6. doi: 10.1207/S1532791480-86. [DOI] [PubMed] [Google Scholar]
- 17.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–21. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50:170–5. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 19.Kurbitz C, Heise D, Redmer T, Goumas F, Arlt A, Lemke J, Rimbach G, Kalthoff H, Trauzold A. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells. Cancer Sci. 2011;102:728–34. doi: 10.1111/j.1349-7006.2011.01870.x. [DOI] [PubMed] [Google Scholar]
- 20.Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–52. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- 21.Shankar S, Suthakar G, Srivastava RK. Epigallocatechin-3-gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Front Biosci. 2007;12:5039–51. doi: 10.2741/2446. [DOI] [PubMed] [Google Scholar]
- 22.Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2012;131:30–40. doi: 10.1002/ijc.26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang SN, Fu J, Shankar S, Srivastava RK. EGCG enhances the therapeutic potential of gemcitabine and CP690550 by inhibiting STAT3 signaling pathway in human pancreatic cancer. PLoS One. 2012;7:e31067. doi: 10.1371/journal.pone.0031067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vu HA, Beppu Y, Chi HT, Sasaki K, Yamamoto H, Xinh PT, Tanii T, Hara Y, Watanabe T, Sato Y, Ohdomari I. Green tea epigallocatechin gallate exhibits anticancer effect in human pancreatic carcinoma cells via the inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor. J Biomed Biotechnol. 2010;2010:290516. doi: 10.1155/2010/290516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katoh M, Katoh M. Human FOX gene family (Review) Int J Oncol. 2004;25:1495–500. [PubMed] [Google Scholar]
- 26.Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–99. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- 27.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–82. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, Coffer PJ. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J Cell Biol. 2002;156:531–42. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–5. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozawa F, Friess H, Kleeff J, Xu ZW, Zimmermann A, Sheikh MS, Buchler MW. Effects and expression of TRAIL, and its apoptosis-promoting receptors in human pancreatic cancer. Cancer Lett. 2001;163:71–81. doi: 10.1016/s0304-3835(00)00660-1. [DOI] [PubMed] [Google Scholar]
- 33.Dallas NA, Gray MJ, Xia L, Fan F, van Buren G, 2nd, Gaur P, Samuel S, Lim SJ, Arumugam T, Ramachandran V, Wang H, Ellis LM. Neuropilin-2-mediated tumor growth and angiogenesis in pancreatic adenocarcinoma. Clin Cancer Res. 2008;14:8052–60. doi: 10.1158/1078-0432.CCR-08-1520. [DOI] [PubMed] [Google Scholar]
- 34.Li M, Yang H, Chai H, Fisher WE, Wang X, Brunicardi FC, Yao Q, Chen C. Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer. 2004;101:2341–50. doi: 10.1002/cncr.20634. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Zhang Y, Feurino LW, Wang H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci. 2008;99:733–7. doi: 10.1111/j.1349-7006.2008.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankar S, Chen Q, Srivastava RK. Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to enhance antiangiogenic effects of EGCG through activation of FOXO transcription factor. J Mol Signal. 2008;3:7. doi: 10.1186/1750-2187-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. doi: 10.1186/1750-2187-5-10. 10.1186/1750-2187-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folkman J. Angiogenesis and proteins of the hemostatic system. J Thromb Haemost. 2003;1:1681–2. doi: 10.1046/j.1538-7836.2003.00344.x. [DOI] [PubMed] [Google Scholar]
- 39.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palena C, Hamilton DH, Fernando RI. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol. 2012;8:713–22. doi: 10.2217/fon.12.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coma S, Shimizu A, Klagsbrun M. Hypoxia induces tumor and endothelial cell migration in a semaphorin 3F- and VEGF-dependent manner via transcriptional repression of their common receptor neuropilin 2. Cell Adh Migr. 2011;5:266–75. doi: 10.4161/cam.5.3.16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusy S, Potiron V, Zeng C, Franklin W, Brambilla E, Minna J, Drabkin HA, Roche J. Promoter characterization of Semaphorin SEMA3F, a tumor suppressor gene. Biochim Biophys Acta. 2005;1730:66–76. doi: 10.1016/j.bbaexp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 44.Kraljevic Pavelic S, Sedic M, Bosnjak H, Spaventi S, Pavelic K. Metastasis: new perspectives on an old problem. Mol Cancer. 2011;10:22. doi: 10.1186/1476-4598-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy N. Metastasis: Route master. Nat Rev Cancer. 2009;9:610. doi: 10.1038/nrc2721. [DOI] [PubMed] [Google Scholar]
- 46.McCarthy N. Metastasis: Influencing bad behaviour. Nat Rev Cancer. 2009;9:609. doi: 10.1038/nrc2720. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 48.Nordigarden A, Kraft M, Eliasson P, Labi V, Lam EW, Villunger A, Jonsson JI. BH3-only protein Bim more critical than Puma in tyrosine kinase inhibitor-induced apoptosis of human leukemic cells and transduced hematopoietic progenitors carrying oncogenic FLT3. Blood. 2009;113:2302–11. doi: 10.1182/blood-2008-07-167023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy SK, Chen Q, Fu J, Shankar S, Srivastava RK. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS One. 2011;6:e25166. doi: 10.1371/journal.pone.0025166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 51.Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, Lasky LA. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem. 2002;277:14255–65. doi: 10.1074/jbc.M110901200. [DOI] [PubMed] [Google Scholar]
- 52.Davis R, Singh KP, Kurzrock R, Shankar S. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep. 2009;22:1473–8. doi: 10.3892/or_00000589. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava RK, Unterman TG, Shankar S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol Cell Biochem. 2010;337:201–12. doi: 10.1007/s11010-009-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uddin S, Hussain AR, Siraj AK, Manogaran PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A, El-Solh H, Ezzat A, Bavi P, Al-Kuraya KS. Role of phosphatidylinositol 3'-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108:4178–86. doi: 10.1182/blood-2006-04-016907. [DOI] [PubMed] [Google Scholar]
- 55.Daitoku H, Fukamizu A. FOXO transcription factors in the regulatory networks of longevity. J Biochem. 2007;141:769–74. doi: 10.1093/jb/mvm104. [DOI] [PubMed] [Google Scholar]
- 56.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 57.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 58.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Fong GH. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis. 2008;11:121–40. doi: 10.1007/s10456-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 60.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–15. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang GL, Semenza GL. Purification and characterization of hypoxia inducible factor 1. J Biol Chem. 1995;270:1230–7. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 62.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 63.Barnhart BC, Simon MC. Metastasis and stem cell pathways. Cancer Metastasis Rev. 2007;26:261–71. doi: 10.1007/s10555-007-9053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pani G, Galeotti T, Chiarugi P. Metastasis: cancer cell's escape from oxidative stress. Cancer Metastasis Rev. 2010;29:351–78. doi: 10.1007/s10555-010-9225-4. [DOI] [PubMed] [Google Scholar]