Abstract
Bioremediation of polychlorinated biphenyls (PCBs) has been precluded in part by the lack of a cost-effective method to stimulate microbial degradation in situ. A common limitation is the lack of an effective method of providing electron donors and acceptors to promote in situ PCB biodegradation. Application of an electric potential to soil/sediment could be an effective means of providing electron-donors/-acceptors to PCB dechlorinating and degrading microorganisms. In this study, electrical stimulation of microbial PCB dechlorination/ degradation was examined in sediment maintained under simulated in situ conditions. Voltage was applied to open microcosms filled with PCB-impacted (Aroclor 1242) freshwater sediment from a Superfund site (Fox River, WI). The effect of applied low voltages (1.5 to 3.0V) on the microbial transformation of PCBs was determined with: 1) spiked PCBs, and 2) indigenous weathered PCBs. The results indicate that both oxidative and reductive microbial transformation of the spiked PCBs was stimulated but oxidation was dominant and most effective with higher voltage. Chlorobenzoates were produced as oxidation metabolites of the spiked PCBs, but increasing voltage enhanced chlorobenzoate consumption, indicating that overall degradation was enhanced. In the case of weathered PCBs, the total concentration decreased 40–60% in microcosms exposed to electric current while no significant decrease of PCB concentration was observed in control reactors (0 V or sterilized). Single congener analysis of the weathered PCBs showed significant loss of di- to penta-chlorinated congeners, indicating that microbial activity was not limited to anaerobic dechlorination of only higher chlorinated congeners. Degradation was most apparent with the application of only 1.5 V where anodic O2 was not generated, indicating a mechanism of degradation independent of electrolytic O2. Low voltage stimulation of the microbial degradation of weathered PCBs observed in this study suggests that this approach could be a cost-effective, environmentally sustainable strategy to remediate PCBs in situ.
Keywords: In situ bioremediation, polychlorinated biphenyls, electrical stimulation, biotransformation
1. Introduction
Polychlorinated biphenyls (PCBs) are a well-known class of persistent organic contaminants due to their inherent stability (Dobson, S. and van Esch, G.J., 1993; Fiedler, H., 1997), tendency to bioaccumulate (Serrano, R. et al., 2000; Wong, C.S. et al., 2003), and potential toxicity to endocrine systems and neurodevelopment in humans and wildlife (Danse, I.R. et al., 1997; Safe, S.H., 1994). Bioremediation has the potential to effectively remove PCBs from aquatic sediments in situ without negative environmental impact associated with dredging. The optimal delivery of electron donors and acceptors to PCB-dechlorinating and -degrading microorganisms is vital to stimulate biodegradation and to prevent the generation and accumulation of potentially toxic side products. Several methods to supply electron donors and acceptors into subsurface environments have been proposed including direct gas addition techniques (e.g. sparging of gases and membrane-supplied gas; Gibson, T.L. et al., 1998; Ma, X. et al., 2003; Newell, C.J. et al., 2001) and the addition of liquid and solid chemicals (e.g. fermentable substrates, Fe species, oxygen- and hydrogen-releasing compounds; Adamson, D.T. et al., 2003; Nebe, J. et al., 2009). However, these approaches require periodic reapplication to sustain the amendment's effect because chemical compounds and gases added to the subsurface easily diffuse away and are consumed via abiotic reactions, and the delivery system can be costly. Thus, a new approach, one that can provide a continuous source at a controlled rate in situ, is needed.
A promising alternative would be to use electrodes as cathodic electron sources or anodic electron sinks for microbial communities responsible for PCB dechlorination and degradation. Electrodes can offer a continuous and finely controlled supply of electron donors and acceptors to microorganisms in the subsurface (Aulenta, F. et al., 2009; Strycharz, S.M. et al., 2008; Thrash, J.C. and Coates, J.D., 2008; Zhang, T. et al., 2010). The use of electric potential can create desired electron flows through indirect and direct stimulation of microbial metabolism. Indirectly, electrolytic hydrogen and oxygen have also been used to induce microbial denitrification/nitrification in wastewater (Sakakibara, Y. and Nakayama, T., 2001; Watanabe, T. et al., 2002), reductive dehalogenation of organohalides (Skadberg, B. et al., 1999; Weathers, L.J. et al., 1997), and aerobic mineralization of aromatic hydrocarbons (Goel, R.K. et al., 2003). Biostimulation by water electrolysis commonly requires higher voltage and expensive catalysts, but the use of microbial catalysts with an electrode would be more cost-effective and require less voltage. For example, Geobacter spp. have been reported to be capable of using a graphite electrode as a direct electron donor for the reductive dechlorination of tetrachlorethene at −300 mV versus a standard hydrogen electrode (SHE) (Strycharz, S.M. et al., 2008) and as an electron sink for anaerobic oxidation of aromatic hydrocarbons at +500 mV vs. SHE (Zhang, T. et al., 2010). Moreover, Aulenta and colleagues stimulated microbial dechlorination of trichloroethene with and without the assistance of methyl viologen, a redox mediator in bioelectrochemical reactors with mixed cultures (Aulenta, F. et al., 2009; Aulenta, F. et al., 2007). Electrolytic hydrogen and oxygen have also been used to induce microbial denitrification/nitrification in wastewater (Sakakibara, Y. and Nakayama, T., 2001; Watanabe, T. et al., 2002), reductive dehalogenation of organohalides,(Skadberg, B. et al., 1999; Weathers, L.J. et al., 1997), and aerobic mineralization of aromatic hydrocarbons (Goel, R.K. et al., 2003).
To date, most studies on electrode-based biostimulation have investigated either cathodic stimulation for microbial reduction or anodic promotion for microbial oxidation using dual-chamber bioelectrochemical systems and potentiostats. These approaches are useful to ascertain the effect of an electric current on desired microbially driven reactions and to maintain a precise redox potential in a bioelectrochemical chamber. For example, Sun et al. (Sun, M. et al., 2010) reported that the application of an electric current creates a redox gradient and provides electron donor for the potential degradation of contaminants in a sediment cap system. The size and location of such zones are controllable by the voltage applied, time, and location of electrodes, suggesting that electrode-based biostimulation has the potential to engineer redox conditions for selective stimulation of microbial activities that remediate contaminants of concern. Furthermore, using electric potential to integrate concurrent reductive and oxidative conditions, potentially optimal for complete mineralization of PCBs, may stimulate in situ biotreatment of PCBs most efficiently. Thus, in situ electrode-based treatment may offer a controllable process that results in extensive bioremediation of PCBs in aquatic sediment.
In this study, we examined the effect of electrical biostimulation on microbial PCB dechlorination and degradation in bioelectrochemical reactors prepared with sediment from Fox River, WI, a PCB impacted Superfund site. These cells were tested under in situ-like conditions in order to determine the practicality and efficacy of the approach. Specifically, we investigated the effect of different applied voltages on the anaerobic dechlorination and aerobic degradation of PCBs and observed that a relatively low voltage effectively stimulated the biodegradation of both low concentrations of indigenous weathered PCBs and high concentration PCBs spiked to the sediment.
2. Experimental Methods
2.1 Chemical, suppliers, and purities
PCBs supplied to the cultures and used as GC standards were of the highest purity available (99%) and were purchased from Accustandard (New Haven, CT). Other chemicals, suppliers, and purities are listed in the Supporting Information (SI).
2.2 Sediment bioelectrochemical reactors (SBRs)
PCB-impacted freshwater sediment from Fox River (Neenah, WI) was used as test material for SBRs. Sediment was collected from the Lower Fox River (FR) site (Operable Unit 1; Little Lake Butte des Morts; Latitude: 44.2, Longitude: −88.4) during dredging (September 2008). More information on the sites is available in US EPA report (US EPA Region 5, 2009). Characteristics of PCB contamination in FR sediment were examined. Two types of SBRs were used in this study: mesocosm-scale SBRs to observe physiochemical changes (i.e. pH, redox potential, and gas production) in FR sediment during voltage application and microcosm-scale SBRs to examine the overall effect of electrolysis on PCB dechlorination/degradation as a function of the applied voltage. A schematic diagram of the SBRs is shown in Figure S1. Live and sterilized sediment was thoroughly homogenized in an anoxic chamber with and without the addition of spiked PCBs (0.6 μmol/g sediment of PCB1 [2-chlorobiphenyl] and 0.1 μmol/g sediment of PCB61 [2,3,4,5-tetrachlorobiphenyl]). Sterilized sediment was prepared by autoclaving 4 times in 24-h interval. Homogenized sediment (70 ml) was transferred to each reactor. Before the installation of electrodes into sediment, 10 mL of homogenized sediment was sampled for PCB analysis and 16S rRNA gene analysis at T=0. Electrodes were prepared using Ti foil (2.5 cm×2.5 cm×0.25 mm, 99.7 %; Aldrich) and Ti wire (0.5 mm diam., 99.9 %; Aldrich). The dimensions of the electrode were5.0 cm×6.0 cm×0.25 mm (surface are: 50.6 cm2) and 2.5 cm×2.5 cm×0.25 mm (surface area: 12.5 cm2) for mesocosm and microcosm-scale SBRs, respectively. Electrodes were vertically positioned on the bottom of the reactor and spaced 2 cm apart. They were connected to a programmable DC power source (Model 9130, BK precision). Three constant voltages (1.5, 2.2, and 3.0 V) were applied to the reactor and the current was monitored by a digital multimeter (Model 2500, Keithley) with insertion of a Ω resistor. The potential of the cathode and the anode was measured against a 1M Ag/AgCl reference electrode. Pore-water was periodically collected from the electrode using a Rhizon soil water sampler (5-cm long, 2.5-cm diam.; Soilwater Corp.) connected with a Mininert® syringe valve and placed into a 0.5-mL headspace-analysis vial sealed with a Mininert® valve cap to monitor electrolytic O2 and H2. Dissolved O2 and H2 in pore water were measured using microelectrode DO probes (MI-730, Microelectrodes Inc.) and gas chromatography (GC)-thermal conductivity detector with Ar as carrier gas, respectively. Control reactors were maintained in parallel without power for both live and sterilized SBRs. Water lost due to evaporation was periodically replenished with sterilized deionized water to maintain the original salts composition. All SBR experiments were terminated after 88-days of incubation and the entire contents of the SBRs were homogenized for PCB analysis, and 16S rRNA and bphA gene analysis. Approximately 60 mL of the homogenized sediment was air-dried at room temperature in a desiccator containing CaCl2·2H2O prior to extraction of PCBs and 10 mL of sediment was frozen and stored at −20°C prior to DNA extraction.
2.3 Extraction and analysis of PCBs
To quantify absolute concentration of weathered PCBs, sediment samples were extracted using an Accelerated Solvent Extractor (Dionex) following EPA method 3545. The dried sediment was transferred to an eleven ml stainless steel extraction cell containing 0.6 g Cu and 2.4 g fluorosil between two cellulose filters on the bottom of the cell and the remainder of the cell was filled with anhydrous Na2SO4. To correct for extraction efficiency, 10 μl of a 400 μg/l solution of PCB 166 in hexane was pipetted on top of the Na2SO4. The sample containing the surrogate was extracted with approximately 20 ml of hexane at 100 °C and purged with 1 MPa nitrogen. The sample was evaporated to a final volume of 1 ml at 30°C under nitrogen using a N-EVAP 111 nitrogen evaporator (Organomation Associates, Inc. Berlin MA, US). Before PCB analysis, 10 μl of a solution containing 400 μg l−1 of PCB 30 and 400 μg/l of PCB 204 (as internal standards) was added to the sample.
PCB congeners were analyzed using a Hewlett-Packard 6890 series II gas chromatograph (GC) with a DB-1 capillary column (60 m×0.25 mm×0.25 mm; JW Scientific, Folsom, CA) and a 63Ni electron capture detector by a modified method of EPA 8082. PCB congeners in a mixture containing 250 μg/l Aroclor 1232, 180 μg/l Aroclor 1248 and 180 μg/l Aroclor 1262 were quantified with a 10-point calibration curve using PCB 30 and PCB 204 as internal standards. Individual congeners and respective concentrations were obtained from Mullin et al. (Mullin et al, 1984). Fifty-five additional congeners not present in the Aroclor 1232:1248:1262 mixture that were potential dechlorination products were added to the calibration table containing the Aroclor congeners. The additional congeners were quantified with 10-point calibration curves at concentrations of 2, 5, 10, 20, and 40 μg/l (in duplicate) for the low range calibration and 40, 100, 200, 400, and 800 μg l−1 (in duplicate) for the high range calibration and using PCB 30 and PCB 204 as internal standards. Using this protocol, 173 congeners were resolved in 130 individual peaks (not including the standards PCB 30 and PCB 204 and the surrogate PCB166). Co-eluting peaks were indicated as multiple congeners. The final concentration of individual congeners in samples was corrected for the recovery efficiency of the surrogate PCB 166; typically recovery efficiencies of PCB 166 were 60 % or greater.
2.4 Analysis of Metabolites
To monitor the oxidative degradation pathway, dried sediment samples from SBRs and control reactor were assayed for chlorobenzoate (CBA) metabolites. The dried sediments (0.2 g) were acidified with HCl (0.1 mL; 2 N) and extracted with 0.9 mL of acetonitrile containing 5 μM 2,4,6-chlorobenzoates (2,4,6-CBA) as an internal standard. Samples were mixed with a Vortex mixer for 30 minutes and then filtered through 0.2 μm PTFE syringe tip filters (Millipore). The organic phase was evaporated and derivatized with a diethyl ether solution (0.5 mL) of diazomethane (Hudlicky, M., 1980) for 1 hour at room temperature in order to transform the carboxylic acids into methyl esters. The samples were dried and resuspended in 0.5 mL isooctane. The detection of metabolites was performed with GC-MS (Trace GC ultra, Thermo) equipped with a RTX-5 column (0.25 mm film thickness, 0.25mm i.d., 15 m length). Helium gas was used as the carrier with a flow rate of 0.6 mL/min. Both injector and detector temperature were set at 250 °C. Sample injection was splitless (2μL) and the oven temperature was 80 °C for 1.5 min, ramped to 170 °C at a rate of 25 °C/min, then ramped to 270 °C at a rate of 15 °C/min and was held at 270 °C for 3 min. CBA detection ions were m/z 111, 139, and 171 for monochlorinated CBAs, m/z 145, 173, and 204 for dichlorinated CBAs, and 180, 207, and 240 for trichlorinated CBAs.
The quantification of CBAs in acetonitrile phase was performed using high-pressure liquid chromatography (VP model, Shimadzu) equipped with a C18 column (Supelco, 15 cm × 4.6 mm, 3 μm particle size). The injection volume was 30 μL and the detector wavelength was 233 nm. The mobile phase was 1% acetic acid/acetonitrile (1/1 v/v) at a flow rate of 0.7 mL/min. A calibration standard of 2-; 3-; 4-; 2,6-; 2,5-; 2,4-; 2,3-; 3,4-; 3,5; 2,3,5; and 2,4,6-CBA was analyzed with the samples to determine the relative retention times in the system.
2.5 Quantitative PCR
Genomic DNA was extracted from both frozen and dried sediment (0.1 g) using an UltraClean soil DNA kit (MoBio, Carlsbad, CA) according to manufacturers' protocols. The concentration of nucleic acids was determined at 260 nm using a nanodrop spectrophotometer (NanoDrop® ND-1000). Extracted DNA samples had an A260/280 ratio of ≥ 1.7 and an A260/230 ratio of 2.0–2.2. The gene copies of total putative dehalogenating members of the phylum Chloroflexus and bphA gene partially encoding the ISPa subunit of the Rieske nonheme oxygenases of the toluene/biphenyl subfamily in each sample were estimated using qPCR. Dehalogenating Chloroflexus 16S rRNA and bphA genes were quantified using primer 348F/884R(Fagervold, S.K. et al., 2005) and bphAf668-3/bphAr1153-2 (Witzig, R. et al., 2006), respectively. PCR conditions for enumerating Chloroflexi16S rRNA genes were as follows: 15 min at 95°C, followed by 35 cycles of 30s at 95°C, 30s at 61°C, and 30s at 72°C. The bphA genes were enumerated in the PCR condition: 5 min at 95°C, followed by 35 cycles of 45s at 95°C, 30s at 52°C, and 45s at 72°C. The qPCR reaction (25 μL) contained 1× SYBR Green PCR master mix (BioRad), forward and reverse primer (400 nM each), and DNA template (1 μL). All DNA templates were diluted to 2 ng/μl in water. qPCR was performed in a MyiQ thermal cycler (BioRad). Standard curves for qPCR were prepared with a dilution series of gel-purified348F-884R and bphAf668-3-bphAr1153-2 PCR product amplified from PCB dechlorinating bacteria DF1(May, H.D. et al., 2008) and Burkholderia xenovorans strain LB400, respectively. The standard curve consisted of triplicate dilutions over 6 orders of magnitude and was run for every plate with the samples. The specificity of qPCR amplification was verified by melting curve analysis. Amplification efficiencies of dilutions of DF1 16S rRNA gene PCR product used as standards were 93~98 % (r2 = 0.99, Y-intercept=39.2~41.1), and amplification efficiencies of the LB 400 bphA gene PCR product were 92~97% (r2 = 0.99, Y-intercept=40.4~ 43.6). Amplification efficiencies of samples were determined using three dilutions (1:1, 1:5, and 1:10) for three representative samples. Amplification efficiencies of samples using 348F-884R and amplification efficiencies of samples using bphAf668-3-bphAr1153-2 fell within the range of the standard curve, indicating that there were no detectable PCR inhibitors present. The lower detection limit was defined as the lowest template concentration, which resulted in a threshold cycle (Ct) that was significantly less than the total number of cycles performed. Mostly, the gene copies of 16S rRNA and bphA in the samples were greater than the detection limits of 80 (Ct=32–34) and 120 (Ct= 34–36) copies for respective 16S rRNA and bphA gene. Water was used as a control template and its Ct values ranged from 38 to 42.
2.6 Aerobic flask culture experiments
Aerobic flask culture experiments were conducted with PCB1 (100 μM) in 25 mL shake flasks with loose-fit metal cap. Wet sediment (0.5 ml drawn from homogenized SBRs after 88-days of incubation) was added to 10 mL of liquid mineral medium (Dercova, K. et al., 2008) and incubated in an orbital shaker (100 rpm) at 25°C. At the desired time intervals samples (0.25 mL) were removed from the culture and filtered through 0.2 μm PTFE syringe tip filters (Millipore) in preparation for CBA analysis by HPLC.
3. Results and Discussion
3.1 Characteristics of PCB contamination in FR sediment
The total PCB concentration (weathered) in FR sediment was 20.2±4.0 mg/kg dry sediment (0.077±0.008 μmol/g dry sediment, n=12). Homolog analysis showed that di-, tri-, and tetrachlorinated PCBs comprised about 90% of the total congeners (Figure S2A). PCBs with more than five chlorines accounted for about 10% of the total and mono-PCBs were detected at less than 0.5%. The average number of chlorine atoms per molecule PCB (Cl/BP) in the FR sediment was 3.11±0.04, which is similar to the Cl/BP of Aroclor 1016 (3.1) and generally lower than that of Aroclors 1242 (3.4) or 1248 (3.9), (Frame, G.M. et al., 1996). Individual congener analysis (Figure S2B) showed that PCB5+8 (24'+23) and PCB28 (244') dominated (~40 % of total concentration) and that the majority of congeners (greater than 80%) had unflanked chlorine atoms and was ortho-chlorinated. The observation that most of the congeners had 2 or 3 chlorine atoms combined with the fact that the main component discharged into the river was Aroclor 1242 from manufacturing industries (e.g. paper mills)(US EPA Region 5, 2009; Imamoglu, I. and Christensen, E.R., 2002)suggests that natural attenuation (presumably dechlorination) of PCBs occurred in the lower FR. Moreover, the congener pattern observed is similar to the pattern that resulted from microbial dechlorination of Aroclor 1242 in a microcosm study with Silver Lake sediment (Massachusetts, MA; Quensen, J.F. et al., 1990).
3.2 Effect of applied voltage on physiochemical changes across SBRs
Figure S3 shows physiochemical changes across the sediment in SBRs as a function of current density. Overall, the redox potential decreased (−0.2~−0.4 V vs. Ag/AgCl) near the cathode where H2 was generated and increased (> 0 V) near the anode where O2 was generated with time. The pH gradient trend was opposite. The time required to establish such gradients across SBRs depends on the current density, which is affected by voltage. At high current density (0.067 mA/cm2, 3.8∑4.4 V), the potential at the electrode was great enough to generate both O2 and H2, which created distinct gradients rapidly, while such gradient were less apparent across the sediment except for the area near the cathode at the low current density (0.003 mA/cm2, 2.0−2.5 V). Several visual changes were observed as well. Figure S4 shows a digital photograph of the SBR with high current density at T=19 and 41 days. First, a horizontal yellowish brown layer (A) was formed at 2~3 cm below the top of sediment and near the anode. Likely, this would be iron precipitation as a result of the oxidation of ferrous ions released from anode corrosion. Black vertical lines (B) were observed 3 cm from the cathode. We speculate that this is iron sulfide was produced by ferrous iron reacting with sulfide, indicating the presence and activity of sulfate-reducing bacteria. Since the redox potential at the anode is above 0 V, the color of sediment near the anode is lighter (C) and such area expands with time. This is likely related to the oxygen level. Also, gas pockets developed in the middle of the reactor. In summary, the application of an electric current to an aquatic sediment created distinct anaerobic, microaerophilic, and aerobic zones within the redox gradients that form across the systems. As a consequence, microbial metabolisms may be influenced differently in a particular zone, which suggests that the voltage application potentially stimulates both reductive dechlorination and aerobic oxidation of PCBs in the sediment. Physiochemical results from the mesocosm-scale SBR provided information for the optimal setup (i.e. the range of voltage, gas production, electrode spacing) of the microcosm-scale SBRs used to observe the effect of the applied voltage in microbial PCB transformation.
3.3 Effect of applied voltage on the microbial dechlorination/degradation of the spiked PCBs added to FR sediment
Tests were conducted in microcosm-scale SBRs to determine the effect that an applied voltage would have on the microbial transformation of PCBs (~100 ppm) added to FR sediment. Selection of an optimal voltage to stimulate anaerobic PCB-dechlorinating and aerobic PCB-degrading activities may be critical, since the voltage applied will affect redox potential in sediment (Bard, A.J. and Faulkner, L.R., 2001). Based on the potentials required for the oxidation of water (anode) and reduction of protons(cathode) (Bard, A.J. and Faulkner, L.R., 2001), we tested three different voltages; 1.5, 2.2, and 3.0 V. Ti electrodes inserted in freshwater sediment (low conductivity and circumneutral pH condition) produced both anodic oxygen and cathodic hydrogen when the potential difference between the anode and cathode was set beyond 2.2~2.5 V (data not shown). At <2.2 V, hydrogen was solely produced at the cathode (maximally 0.01~0.02 mmol/day based on the passed current), presumably coupled with the oxidation of organic and inorganic materials in the sediment at the anode since the generation of anodic oxygen is thermodynamically and kinetically limited at this potential (e.g. <0.2 mg O2/day is calculated to be produced at ~+1.1 V of anodic potential (measured) vs. SHE based on Faraday's law). Based on the passed current monitored by a digital multimeter (Figure 6), the production rate of O2 was 0.53~2.63 mg O2/day and 1.31~6.57 mg O2/day for the SBR with 2.2 and 3.0 V, respectively.
Figure 6.
Current production in SBRs with and without the spiked PCBs (1 and 61) combined at 0.7 μmol/g sediment with the applied voltage of 1.5 (A), 2.2 (B), and 3.0 V (C). Red and black line represents SBR with the added PCB and without the added PCB, respectively.
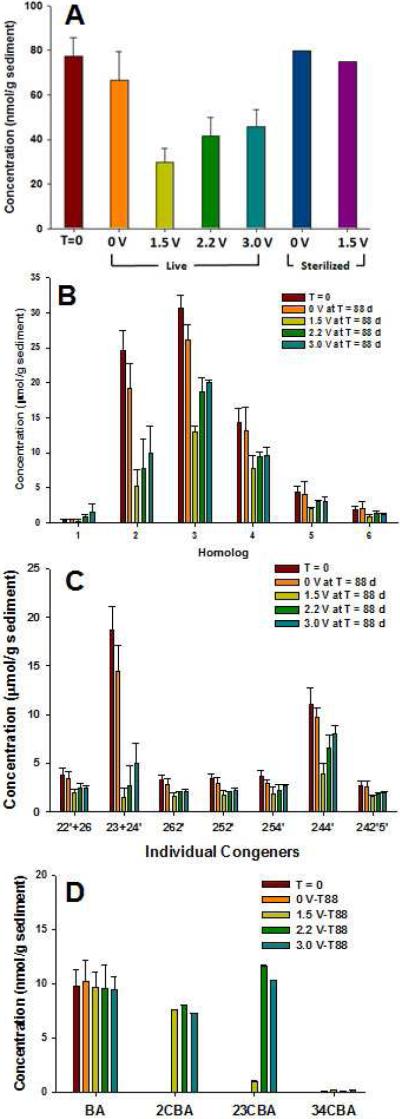
PCB1 and PCB61 were added at high enough concentrations to be readily available and discernable from weathered PCBs (the concentration of the combined spiked PCBs was ~10 fold higher than total weathered Aroclor concentration in the sediment). PCB61 is often reductively dechlorinated without difficulty in aquatic sediment (Wu, Q. et al., 2000)and PCB1 is readily degraded by aerobic microbes (Harkeness M.R. et al., 1993); neither congener is typically observed in Aroclors. Overall, the concentration of the spiked PCBs decreased in SBRs with electric current after 88-days of incubation, however, there was no significant change for spiked PCBs in sterilized SBRs (Figure 1), indicating that abiotic loss of spiked PCBs in an open reactor via vaporization of the spiked PCBs or direct anodic/cathodic reaction of PCBs with electrodes was negligible. PCB61 was transformed to PCB23 [2,3,5-trichlorobiphenyl], which was further reduced to PCB9 [2,5-dichlorobiphenyl], at all tested voltages, even when 3.0 V was applied and O2 was generated, indicating a robust native population of anaerobic PCB dechlorinating microorganisms that was not adversely affected by the electrolytic generation of O2. The total concentration of PCB61 and its dechlorination products PCBs 23 and 9 decreased when the voltage was raised above 1.5 V (Figure 1Aand S3), suggesting enhancement of activity by aerobic PCB degrading microorganisms in addition to the PCB dechlorinators. In the case of PCB1, the concentration decreased by more than 60% regardless of the voltage applied or the lack of electrolytic O2. The concentration of biphenyl did not change appreciably with voltage or in any of the controls (1.09±0.47 nmol/g sediment at T=0 and 0.58±0.48 nmol/g sediment at T=88 days).
Figure 1.
(A) Concentration of PCB61 and its dechlorination products (PCB23 and PCB9) and (B) concentration of PCB1 in live and sterilized SBRs and control reactors at 88 days as a function of the applied voltage. Data represent the average from triplicate samples from the homogenized contents of each reactor, standard deviations are included for PCB1 in B. Another version of Figure 1A with standard deviations is in SI (Figure S5).
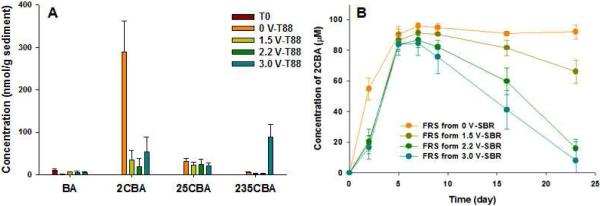
GC/MS analysis revealed that mono-, di-, and tri-chlorobenzoates (CBAs), degradation metabolites of PCBs, were formed in all SBRs spiked with PCBs and receiving voltage. Figure S6 presents an example of CBA detection and identification by GC/MS in a dried sediment sample from the control reactor (0 V). Due to the addition of the spiked PCBs at nearly 10-fold greater than the concentration of indigenous weathered PCBs, it is likely that most of the CBAs detected (Figure 2A) originated from the spiked PCBs. This was particularly so in the control reactor with no added voltage where the amount of 2CBA detected (Figure 2A) was nearly equal to that of the PCB1 consumed (Figure 1B). In contrast, the concentration of 2CBA detected was much lower in SBRs maintained with voltage and it and other CBAs could come from PCB1 or PCB61. However, the decrease in concentrations of PCB1 with voltage and PCB61 with >2.2 V would argue for the accumulation of far more 2CBA than was detected with voltage applied. It is possible that more 2CBA was produced but the added voltage stimulated further degradation of the 2CBA. In addition, 25CBA and 235CBA were generated when voltage was applied, especially 235CBA with 3.0 V. These products were likely generated from the oxidation of PCB9 and PCB23, which were detected as dechlorination products of PCB61, confirming the stimulation of O2-dependent PCB degradation at ≥2.2V.Only benzoate (less than 10 nmol/g sediment) was detected in SBRs with sterilized sediment.
Figure 2.
(A) The formation of benzoate (BA) and chlorobenzoates (CBA) in live control and SBRs with the spiked PCBs 1 and 61 as a function of applied voltage and (B) aerobic degradation of PCB 1 to 2-CBA in shake culture flask with 88-day homogenized sediment collected from SBRs as inoculants (B). The number in CBAs represents the location of chlorine atoms on the ring. Error bars represent the standard deviation of triplicate samples for each reactor.
To determine whether the microbial communities in the SBRs exposed to the applied voltage possessed the capability to further biodegrade CBAs aerobically, each community was incubated in aerobic shake flasks with PCB1 and CBA formation and degradation was monitored. Figure 2B indicates that the communities enriched under all 4 conditions in the SBRs (0.0, 1.5, 2.2 and 3.0 V) would generate 2CBA from PCB1, but only the communities enriched with applied voltage consumed the accumulated 2CBA, and the rate of 2CBA degradation was greatest with microorganisms from the SBRs incubated at the highest voltage. Note that we did not observe the production of2CBA in un-inoculated and sterile control flasks. Taken together, the analysis of the microbial dechlorination/degradation of the spiked congeners added to FR sediment indicate that 1) reductive and oxidative microbial processes occurred under all conditions, regardless of the voltage applied and the generation of electrolytic O2, 2) the oxidation of PCB61 and its dechlorination products PCB 23 and 9 to their corresponding CBAs increased when the voltage applied was greater than 1.5, i.e. when O2 was generated, and 3) the application of electric current stimulated more complete mineralization of PCBs, at least PCB1, by supporting CBA degradation.
3.4 Effect of applied voltage on the microbial dechlorination/degradation of weathered PCBs in FR sediment
In addition to the spiked PCBs, changes in the total concentration and congener distribution profile of the weathered PCBs in FR sediment were examined by subtracting PCBs 1, 61 and the dechlorination products of 61 (PCBs 9 and 23) from the complete single congener analysis data sets. Figure 3A presents the effect of applied voltage on the microbial dechlorination and degradation of the weathered PCBs in FR sediment after 88 days. No transformation of PCBs was observed in sterilized controls. PCB removal increased with more voltage applied, reaching ~50% with 3.0 V. This trend is reflected in the distribution of the homologs and the most prevalent congeners (Figures 3B and 3C). The addition of the spiked PCBs could potentially stimulate (“prime”) microbial dechlorination and degradation (DeWeerd, K.A. and Bedard, D.L., 1999; Van Dort, H.M. et al., 1997) regardless of the application of voltage, which may explain the ~20% removal of weathered PCBs in the live no voltage control (Figure 3A). Despite this observed loss, greater degradation of weathered PCBs was observed in SBRs exposed to electric current than in the live no-current control, which suggests that the application of electric current stimulates broader and more extensive degradation of weathered Aroclor. However, CBA formation from the weathered PCBs could not be discerned due to the high concentrations of model PCBs added.
Figure 3.
Total concentration (A) of weathered Aroclors in sterilized and live SBRs filled with FR sediment with the addition of PCBs 1 and 61. Homolog concentration of weathered PCBs (B), and concentration of major PCB congeners which account for ~ 60 % of initial total weathered Aroclor concentration (C) in live SBRs with PCBs 1 and 61 added. The concentrations of PCBs 1, 61 and the dechlorination products of 61, have been subtracted for these figures (, note that weathered FR sediment originally contains no or negligible amount of PCB 1, 9, 23, and 61 and we assume that PCB9 and 23 only comes from the spiked PCB61). As for individual congeners, numbers represent the positions of chlorine atoms on the carbon atoms of each benzene ring. The symbol of plus indicates co-eluted congeners in one chromatographic peak. The concentration of PCB1 and PCB61 added to SBRs is 0.6 and 0.1 mmol/g sediment, respectively. The sample size of T=0 and 0V at 88 days is 18 (triplicate samples from 6 reactors) and 5 (triplicate samples from one reactor and one sample from two reactors), respectively. For SBRs with the applied voltage at 88 days, triplicate samples were analyzed from the homogenized contents of each. Error bars represent the standard deviation of triplicate samples.
To clearly observe electrical stimulation of biodegradation of weathered PCBs, the FR sediments were tested in SBRs as described above but without the addition of model PCBs. Removal of weathered PCBs was 40–62% when voltage was supplied to the 3 SBRs, with the greatest decrease in PCBs occurring with only 1.5 V (Figure 4A). The removal of PCBs in the SBRs with 1.5, 2.2, and 3.0 V was statistically significant in comparison to the removal (~15%) of 0 V control reactors (based on the t-test, two-sample assuming unequal variances, α=0.05, see SI). Such removal (~ 62 %) of weathered Aroclor from sediments in only 88 days via electrolytic biostimulation is promising when compared with natural microbial attenuation of weathered Aroclor from sediment, which took significantly longer in field tests and/or microcosm experiments (Bedard, D.L., 2008; Harkness, M.R. et al., 1993). Single congener/homolog analysis (Figure 4B and 4C) showed that the concentration of di-, tri-, and tetrachlorinated PCB congeners decreased, especially PCBs 5 and 8 (PCB-23 and PCB-24') and PCB28 (PCB-244'), without an accumulation of lesser-chlorinated congeners in the SBRs. Again, this appears to be an oxidation-dominated biodegradation pattern since there is no apparent accumulation of lesser-chlorinated PCBs, as was observed when model PCBs were added.
Figure 4.
Total concentration (A) of weathered Aroclors in sterilized and live SBRs filled with FR sediment without the addition of PCBs 1 and 61. Homolog concentration of weathered PCBs (B), concentration of major PCB congeners which account for ~ 60 % of initial total weathered Aroclor concentration (C) and BA/CBA concentration (D) in live SBRs. As for individual congeners, numbers represent the positions of chlorine atoms on the carbon atoms of each benzene ring. The symbol of plus indicates co-eluted congeners in one chromatographic peak. The sample size of T=0 and 0V at 88 days is 18 (triplicate samples from 6 reactors) and 5 (triplicate samples from one reactor and one sample from two reactors), respectively. For SBRs with the applied voltage at 88 days, triplicate samples were analyzed from the homogenized contents of each. Error bars represent the standard deviation of triplicate samples.
CBA analysis (Figure 4D) showed that small amounts of 2CBA, 23CBA, and 34CBA were detected when voltage was applied (most with >2.2 V) but none with 0V. The low amount of CBAs detected was expected due to the amount of weathered PCBs in the SBRs and the likelihood of the CBAs being transient. This is consistent with the enrichment of chlorobenzoate producing (PCB degrading) and consuming reactions with voltage in the SBRs (Fig. 2B). 23CBA likely originated from PCB5, which is one of the major congeners among the weathered Aroclor residues, but the concentration of 2CBA is too high to have come solely from PCB1 (~ 0.2 nmol/g sediment)present in weathered Aroclors and would require reductive dechlorination of the di- and tri-chlorinated PCBs. Benzoate concentration remained constant under all conditions. Thus, it is likely that both reductive and oxidative microbial transformations of the PCBs occurred in the SBRs, regardless of voltage and electrolytic O2 generation.
3.5Effect on population size of PCB-dechlorinating and -degrading microorganisms
To assess the effect of the voltage on population size of PCB transforming microorganisms, PCB-dechlorinating and -degrading microorganisms were enumerated based on the number of 16S rRNA of anaerobic dechlorinating, non-photosynthetic Chloroflexi and the bphA gene, which is associated with the aerobic degradation of PCBs (Leigh, M.B. et al., 2006; Pieper, D.H., 2005). Due to the fact that functional genes for anaerobic PCB dechlorination have not yet been conclusively identified, we used 16S rRNA sequences of closely related anaerobic dechlorinating Chloroflexi, including Dehalococcoides strains and the o-17/DF-1 clade, to develop a presumptive enumeration assay for of PCB-dechlorinating microorganism in the reactors. The melt curve analysis showed only one melting peak (~86°C and ~88 °C for the respective amplicon generated by primer348F/884R and bphAf668-3/bphAr1153-2, confirming the absence of any nonspecific amplification. QPCR analyses of the bphA gene indicated that the copy number of this gene was high (~109 per g sediment) in all of the sediments and remained steady or modestly increased under all conditions (Figures 5B and 5D). This result indicates that bphA was readily available but is not sufficient to discern the role of this or other oxidative pathways in the degradation of PCBs stimulated by the applied voltage. However this result is consistent with the presence of a native and robust community of microorganisms responsible for oxidation-dominated biodegradation pattern in SBRs. QPCR analysis of the 16S rRNA gene of the putative dechlorinating Chloroflexi shows that the number of total gene copies at T=0 was near the lower detection limit, and the total number of Chloroflexi 16S gene copies increased after 88 days under all conditions, with and without spiked PCBs. The increase was >10,000 fold in the SBRs without spiked PCBs with ≥2.2V applied, indicating that the anaerobic dechlorinators were present and increasing regardless of whether anodic O2was generated. This result implies that dechlorinating activity may occur antecedently or simultaneously with the oxidation of the PCBs, which leads to no or little accumulation of dechlorination intermediates in the sediment. In short, the application of voltage did not suppress microbial communities responsible for reductive dechlorination nor oxidative metabolism.
Figure 5.
Total putative dechlorinating Chloroflexi 16S RNA (A and C) and aerobic bphA1 gene copies (B and D) in live SBRs and control reactor with (Top) and without (Bottom) the addition of spiked PCBs before and after 88-day incubation as a function of the applied voltage. Y-axis is on logarithmic scales. Each datum point represents the mean and standard deviation of three PCR replicates of triplicate samples (n=9).
3.6Proposed mechanisms of PCB biotransformation in the SBRs
The combined results of the experiments described above indicate that anaerobic dechlorination and aerobic degradation likely occurred simultaneously in the SBRs. Concurrent anaerobic and aerobic microbial activities are possible due to the fact that the SBRs were prepared anoxically but were maintained under in situ conditions (open to air) in order to assess the efficacy of the approach. Overall, the results indicate stimulation of oxidative biodegradation of both spiked and weathered PCBs when voltage is applied. This is most apparent in the SBRs with 3 V where electrolytic O2 would promote aerobic or microaerophilic degradation of spiked and weathered PCB congeners plus transient compounds such as CBAs. However, the generation of O2 by the application of 2.2 V or 3 V, did not adversely affect the rate of dechlorination of the PCB 61 by native microorganisms, thereby indicating that the dehalorespiring anaerobes required to dechlorinate extensively chlorinated PCBs will be present and active even when O2 is present. Unexpectedly, significant amounts of added PCB1 were transformed into 2CBA with 0.0 and 1.5 V applied (Figures 1B and 2), and 2CBA was even further degraded at 1.5 V, i.e. when electrolytic O2 was not generated. Furthermore, significant degradation of the weathered PCBs occurred when 1.5 V was applied, particularly when spiked PCBs were not added (Figure 4). Regardless of the mechanism behind the decrease in the weathered PCBs when low voltage is applied, the ability to apply 1.5 V or less would be a great advantage for the development of in situ treatment systems since this low voltage could readily be provided by a solar panel or a microbial fuel cell (Shantaram, A. et al., 2005; Tender, L.M. et al., 2008).
We offer two explanations for how and why low voltage without electrolytic O2 generation may stimulate the degradation of PCBs. One possibility is that 1.5 V indirectly stimulates the PCB degradation of microaerophilic or aerobic microorganisms by promoting the movement of aquatic worms, which would actively mix O2 into the sediment. Such worms have been reported to respond to electric stimuli (Satchell, J.E., 1955) and to stimulate indigenous or bioaugmented biphenyl-degrading bacteria and improve PCB bioremediation (Luepromchai, E. et al., 2002; Singer, A.C. et al., 2001). We observed that worms moved more actively to the surface of sediment in SBRs with the added voltage (e.g. more holes with worms on the surface of sediment under overlying water in SBRs vs. control condition (0 V)), and the burrowing activity of the worms could potentially introduce O2 into the sediment even in the absence of electrolytic O2 generation. Moreover, the movement and action of the worms may disperse indigenous bacteria, nutrients and the PCBs, which may improve the overall bioavailability of the weathered Aroclor residues to microorganisms.
The other explanation requires the electrode to directly, or indirectly through an extracellular mediator, serve as an electron acceptor for the oxidation of the PCBs. Some microorganisms (e.g. Geobacter species) have been reported to anaerobically oxidize aromatic hydrocarbons using an electrode as the sole electron acceptor (Bond, D.R. et al., 2002; Zhang, T. et al., 2010). Indirectly the electrode may provide alternative electron acceptors to the degrading bacteria. The microbial oxidation of aromatic hydrocarbons including biphenyl can be coupled to the reduction of Fe(III) (Lovley, D.R. et al., 1996; Villatoro-Monzón, W. et al., 2008), nitrate (Coates, J.D. et al., 2001; Rockne, K.J. and Strand, S.E., 1998), sulfate (Selesi, D. and Meckenstock, R.U., 2009) and humus (Cervantes, F.J. et al., 2001). Within the sediment used in the SBRs there were many inorganic and organic compounds, which may have been cycled abiotically due to the delivery of electricity to the electrochemical cells. This might have resulted in a continuous supply of alternative electron acceptors with 1.5 V applied. Although anaerobic microbial PCB oxidation has not been reported, it is conceivable that such activity could occur under optimal electrochemical conditions.
Figure 6 shows current production in SBRs with and without the PCBs added to Fox River sediment. Current well above background was generated in SBRs supplied with more PCBs (spiked PCBs were added to 0.7 μmol/g sediment vs. 0.08 μmol/g sediment of weathered PCBs), indicating the current was dependent on the addition of PCBs, which suggests that the current was coupled to the degradation of PCBs. We also observed current generation dependent on the addition of PCBs in SBRs containing sediment from another site (Grasse River, NY) (Figure S7). The number of cumulative electrons for current production between days 12 and 35 in the Fox River SBR with added PCB at 1.5V was 1575 μmol, which corresponds to an oxidation of ~28 μmol PCB1 (C12H9Cl+24H2O+56e−→12CO2+Cl−+57H+). Based on the ~39 μmol PCB1 removed from the sediment, and a sediment density of 1.6 g/cm3, the current above background accounts for 70% of the degradation of PCB1. Reductive dechlorination of PCB61 (C12H6Cl4+2H2→C12H8Cl2+2H++2Cl−+4e−) may also contribute to the current production. However, the measured amount (10 μmol) of PCB61 dechlorinated would account for only 2.5% of the current production. Based on these calculations, the increased current generation when PCBs are added and consumed is possibly coupled to PCB oxidation. Whether this occurs directly with the electrode or through a mediator (alternative electron acceptor) in the sediment cannot be determined from these data. Such current production coupled to microbial PCB degradation was the greatest at 1.5 V and decreased as voltage was increased and O2 was generated. The increasing background current with 2.2 V and 3.0 V voltage applied was likely due to electrolysis of water. It is also probable that electrolytic O2 stimulated aerobic PCB-degrading microorganisms, thereby decreasing the current due to anaerobic PCB-degrading bacteria.
4. Conclusions
The application of a low current (voltage) to sediment can be an effective strategy to remediate PCBs in situ. Specifically, electrolytic biostimulation can remove approximately 60% of weathered Aroclor from sediments in only 88 days and anaerobic dechlorination and aerobic degradation may occur concurrently.
The application of electrolysis predominately resulted in oxidative biodegradation of both spiked and weathered PCBs and induced the complete degradation of some PCBs with less accumulation of CBAs in the sediment vs. in the control reactors without voltage applied.
Electrolysis-stimulated microbial oxidation of PCBs can be supported with only 1.5 V applied. The ability to use the lesser voltage would be a great advantage for the development of in situ treatment systems.
Identifying the mechanism by which PCBs are degraded without the generation of electrolytic O2 generation at low voltage requires further examination, but the results strongly indicate that an unexpected paradigm is at work, one that may be leveraged for the effective in situ treatment of weathered PCBs in aquatic sediments.
Supplementary Material
Electrolytic biostimulation can remove approximately 60% of weathered PCBs from sediments.
Anaerobic dechlorination and aerobic degradation occur concurrently.
Electrolysis-stimulated microbial degradation of PCBs can be supported with only 1.5 V applied.
The approach offers a potentially effective in situ treatment of weathered PCBs.
Acknowledgements
This work has been supported by the National Institute of Environmental Health Science (Superfund Research Program grant 5R01ES016197-02). We thank James Hahnenberg (US EPA) and William Harman (GW Partners) for providing Fox River sediment and Peter Moeller and Kevin Huncik (National Oceanic and Atmospheric Administration) for assisting us with GCMS analysis of CBAs.
Appendix. Supplementary data
Additional information about chemical purities and suppliers; a schematic diagram of the mesocosm- and microcosm-scale SBR; characteristic of weathered Aroclor in FR sediment; physiochemical changes across the SBRs; photographs of SBRs at the current density of 0.067 mA/cm3 (3.7–4.4 V); concentration of PCB61 and its dechlorination products in live SBRs and control; mass spectrum of CBAs in the control reactor; T-test for total weathered PCB concentration before and after electrical treatment; and current production in SBRs filled with Grasse River sediment (NY) with and without spiked PCB1 is in the Supporting Information.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- US EPA Region 5 . First five-year review report for fox river nrda/pcb release site. 2009. [Google Scholar]
- Adamson DT, McDade JM, Hughes JB. Inoculation of a DNAPL source zone to initiate reductive dechlorination of PCE. Environmental Science & Technology. 2003;37(11):2525–2533. doi: 10.1021/es020236y. [DOI] [PubMed] [Google Scholar]
- Aulenta F, Canosa A, Reale P, Rossetti S, Panero S, Majone M. Microbial reductive dechlorination of trichloroethene to ethene with electrodes serving as electron donors without the external addition of redox mediators. Biotechnology and Bioengineering. 2009;103(1):85–91. doi: 10.1002/bit.22234. [DOI] [PubMed] [Google Scholar]
- Aulenta F, Catervi A, Majone M, Panero S, Reale P, Rossetti S. Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of tce. Environmental Science and Technology. 2007;41(7):2554–2559. doi: 10.1021/es0624321. [DOI] [PubMed] [Google Scholar]
- Baldwin BR, Nakatsu CH, Nies L. Detection and enumeration of aromatic oxygenase by multiplex and real-time PCR. Applied and Environmental Microbiology. 2003;69(6):3350–3358. doi: 10.1128/AEM.69.6.3350-3358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard AJ, Faulkner LR. Electrochemical methods: Fundermentals and applications. John Wiley & Sons, Inc.; Hoboken, New Jersey: 2001. [Google Scholar]
- Bedard DL. A case study for microbial biodegradation: Anaerobic bacterial reductive dechlorination of polychlorinated biphenyls- from sediment to defined medium. Annual Review of Microbiology. 2008;62:253–270. doi: 10.1146/annurev.micro.62.081307.162733. [DOI] [PubMed] [Google Scholar]
- Bond DR, Holmes DE, Tender LM, Lovley DR. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295(5554):483–485. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- Cervantes FJ, Dijksma W, Duong-Dac T, Ivanova A, Lettinga G, Field JA. Anaerobic mineralization of toluene by enriched sediments with quinones and humus as terminal electron acceptors. Applied Environmental Microbiology. 2001;67(10):4471–4478. doi: 10.1128/AEM.67.10.4471-4478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates JD, Chakraborty R, Lack JG, O'Connor SM, Cole KA, Bender KS, Achenbach LA. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature. 2001;411(6841):1039–1043. doi: 10.1038/35082545. [DOI] [PubMed] [Google Scholar]
- Danse IR, Jaeger RJ, Kava R, Kroger M, London WM, Lu FC, Maickel RP, Mcketta JJ, Newell GW, Shindell S, Stare FJ, Whelan EM. Position paper of the American council on science and health: Public health concerns about environmental polychlorinated biphenyls (PCBs) Ecotoxicology and Environmental Safety. 1997;38(2):71–84. doi: 10.1006/eesa.1997.1565. [DOI] [PubMed] [Google Scholar]
- Dercova K, Cicmanova J, Lovecka P, Demnerova K, Mackova M, Hucko P, Kusnir P. Isolation and identification of PCB-degrading microorganisms from contaminated sediments. International Biodeterioration & Biodegradation. 2008;62(3):219–225. [Google Scholar]
- DeWeerd KA, Bedard DL. Use of halogenated benzoates and other halogenated aromatic compounds to stimulate the microbial dechlorination of PCBs. Environmental Science and Technololgy. 1999;33(12):2057–2063. [Google Scholar]
- Dobson S, van Esch GJ. Environmental health criteria 140: Polychlorinated biphenyls and terphenyls, World Health Organization. International Porgramme on Chemical Safety; Geneva, Switzerland: 1993. [Google Scholar]
- Fagervold SK, May HD, Sowers KR. Microbial reductive dechlorination of Aroclor 1260 in Baltimore harbor sediment microcosms is catalyzed by three phylotypes within the phylum Chloroflexi. Applied and Environmental Microbiology. 2005;73(9):3009–3018. doi: 10.1128/AEM.02958-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler H. Polychlorinated biphenyls (PCBs): Uses and environmental releases. In Subregional Awareness Raising Workshop on Persistent Organic Pollutants (POPs); Bangkok, Thailand. 1997. [Google Scholar]
- Frame GM, Wagner RE, Carnahan JC, Brown JF, Jr., May RJ, Smullen LA, Bedard DL. Comprehensive, quantitative congener-specific analyses of eight Aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996;33(4):603–623. [Google Scholar]
- Gibson TL, Abdul AS, Chalmer PD. Enhancement of in situ bioremediation of BTEX-contaminated ground water by oxygen diffusion from silicone tubing. Ground Water Monitoring & Remediation. 1998;18(1):93–104. [Google Scholar]
- Goel RK, Flora JRV, Ferry J. Mechanisms for naphthalene removal during electrolytic aeration. Water Research. 2003;37(4):891–901. doi: 10.1016/s0043-1354(02)00376-7. [DOI] [PubMed] [Google Scholar]
- Harkness MR, McDermott JB, Abramowicz DA, Salvo JJ, Flanagan WP, Stephens ML, Mondello FJ, May RJ, Lobos JH, Carroll KM, et al. In situ stimulation of aerobic PCB biodegradation in Hudson River sediments. Science. 1993;259(5094):503–507. doi: 10.1126/science.8424172. [DOI] [PubMed] [Google Scholar]
- Hudlicky M. An improved apparatus for the laboratory preparation of diazomethane. The Journal of Organic Chemistry. 1980;45(26):5377–5378. [Google Scholar]
- Imamoglu I, Christensen ER. PCB sources, transformations, and contributions in recent fox river, wisconsin sediments determined from receptor modeling. Water Research. 2002;36(14):3449–3462. doi: 10.1016/s0043-1354(02)00050-7. [DOI] [PubMed] [Google Scholar]
- Leigh MB, Prouzova P, Mackova M, Macek T, Nagle DP, Fletcher JS. Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Applied and Environmental Microbiology. 2006;72(4):2331–2342. doi: 10.1128/AEM.72.4.2331-2342.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR, Woodward JC, Chapelle FH. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Applied and Environmental Microbiology. 1996;62(1):288–291. doi: 10.1128/aem.62.1.288-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luepromchai E, Singer AC, Yang C-H, Crowley DE. Interactions of earthworms with indigenous and bioaugmented PCB-degrading bacteria. FEMS Microbiology Ecology. 2002;41(3):191–197. doi: 10.1111/j.1574-6941.2002.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Ma X, Novak PJ, Clapp LW, Semmens MJ, Hozalski RM. Evaluation of polyethylene hollow-fiber membranes for hydrogen delivery to support reductive dechlorination in a soil column. Water Research. 2003;37(12):2905–2918. doi: 10.1016/S0043-1354(03)00111-8. [DOI] [PubMed] [Google Scholar]
- May HD, Miller GS, Kjellerup BV, Sowers KR. Dehalorespiration with polychlorinated biphenyls by an anaerobic ultramicrobacterium. Applied and Environmental Microbiology. 2008;74(7):2089–2094. doi: 10.1128/AEM.01450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PH, Gurtler H, Wellington EMH. Selective recovery of Streptosporangium fragile from soil by indirect immunomagnetic capture. Microbiology. 1984;141(9):2149–2156. doi: 10.1099/13500872-141-9-2149. [DOI] [PubMed] [Google Scholar]
- Nebe J, Baldwin BR, Kassab RL, Nies L, Nakatsu CH. Quantification of aromatic oxygenase genes to evaluate enhanced bioremediation by oxygen releasing materials at a gasoline-contaminated site. Environmental Science and Technology. 2009;43(6):2029–2034. doi: 10.1021/es900146f. [DOI] [PubMed] [Google Scholar]
- Newell CJ, Aziz CE, Patrick EH, Hughes JB, Khan TA. Two novel methods for enhancing source zone bioremediation: Direct hydrogen addition and electron accepter diversion. San Diego, CA: 2001. [Google Scholar]
- Pieper DH. Aerobic degradation of polychlorinated biphenyls. Applied Microbiology and Biotechnology. 2005;67(2):170–191. doi: 10.1007/s00253-004-1810-4. [DOI] [PubMed] [Google Scholar]
- Quensen JF, Boyd SA, Tiedje JM. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganism from sediments. Applied Microbiology and Biotechnology. 1990;56(8):2360–2369. doi: 10.1128/aem.56.8.2360-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockne KJ, Strand SE. Biodegradation of bicyclic and polycyclic aromatic hydrocarbons in anaerobic enrichments. Environmental Science and Technology. 1998;32(24):3962–3967. [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. Critical Reviews in Toxicology. 1994;24(2):87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y, Nakayama T. A novel multi-electrode system for electrolytic and biological water treatment: Electric charge transfer and application to denitrification. Water Research. 2001;35(3):768–778. doi: 10.1016/s0043-1354(00)00327-4. [DOI] [PubMed] [Google Scholar]
- Satchell JE. Soil zoology. Butterworths; London: 1955. pp. 180–201. [Google Scholar]
- Selesi D, Meckenstock RU. Anaerobic degradation of the aromatic hydrocarbon biphenyl by a sulfate-reducing enrichment culture. FEMS Microbiology Ecology. 2009;68(1):86–93. doi: 10.1111/j.1574-6941.2009.00652.x. [DOI] [PubMed] [Google Scholar]
- Serrano R, Fernández M, Rabanal R, Hernández M, Gonzalez MJ. Congener-specific determination of polychlorinated biphenyls in shark and grouper livers from the northwest African Atlantic ocean. Archives of Environmental Contamination and Toxicology. 2000;38(2):217–224. doi: 10.1007/s002449910029. [DOI] [PubMed] [Google Scholar]
- Shantaram A, Beyenal H, Veluchamy RRA, Lewandowski Z. Wireless sensors powered by microbial fuel cells. Environmental Science & Technology. 2005;39(13):5037–5042. doi: 10.1021/es0480668. [DOI] [PubMed] [Google Scholar]
- Singer AC, Jury W, Luepromchai E, Yahng CS, Crowley DE. Contribution of earthworms to PCB bioremediation. Soil Biology and Biochemistry. 2001;33(6):765–776. [Google Scholar]
- Skadberg B, Geoly-Horn SL, Sangamalli V, Flora JRV. Influence of pH, current and copper on the biological dechlorination of 2,6-dichlorophenol in an electrochemical cell. Water Research. 1999;33(9):1997–2010. [Google Scholar]
- Strycharz SM, Woodard TL, Johnson JP, Nevin KP, Sanford RA, Loffler FE, Lovley DR. Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by Geobacter lovleyi. Appl. Environ. Microbiol. 2008;74(19):5943–5947. doi: 10.1128/AEM.00961-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Yan F, Zhang R, Reible DD, Lowry GV, Gregory KB. Redox control and hydrogen production in sediment caps using carbon cloth electrodes. Environmental Science and Technology. 2010;44(21):8209–8215. doi: 10.1021/es101003j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tender LM, Gray SA, Groveman E, Lowy DA, Kauffman P, Melhado J, Tyce RC, Flynn D, Petrecca R, Dobarro J. The first demonstration of a microbial fuel cell as a viable power supply: Powering a meteorological buoy. Journal of Power Sources. 2008;179(2):571–575. [Google Scholar]
- Thrash JC, Coates JD. Review: Direct and indirect electrical stimulation of microbial metabolism. Environmental Science and Technolology. 2008;42(11):3921–3931. doi: 10.1021/es702668w. [DOI] [PubMed] [Google Scholar]
- Van Dort HM, Smullen LA, May RJ, Bedard DL. Priming microbial meta-dechlorination of polychlorinated biphenyls that have persisted in housatonic river sediments for decades. Environ. Sci. Technol. 1997;31(11):3300–3307. [Google Scholar]
- Villatoro-Monzón W, Morales-Ibarria M, Velázquez E, Ramírez-Saad H, Razo-Flores E. Benzene biodegradation under anaerobic conditions coupled with metal oxides reduction. Water, Air, & Soil Pollution. 2008;192(1):165–172. [Google Scholar]
- Watanabe T, Hashimoto S, Kuroda M. Simultaneous nitrification and denitrification in a single reactor using bio-electrochemcial process. Water Science and Technology. 2002;46(4–5):163–169. [PubMed] [Google Scholar]
- Weathers LJ, Parkin GF, Alvarez PJ. Utilization of cathodic hydrogen as electron donor for chloroform cometabolism by a mixed, methanogenic culture. Environmental Science and Technology. 1997;31(3):880–885. [Google Scholar]
- Witzig R, Junca H, Hecht H-J, Pieper DH. Assessment of toluene/biphenyl dioxygenase gene diversity in benzene-polluted soils: Links between benzene biodegradation and genes similar to those encoding isopropylbenzene dioxygenases. Applied and Environmental Microbiology. 2006;72(5):3504–3514. doi: 10.1128/AEM.72.5.3504-3514.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CS, Mabury SA, Whittle DM, Backus SM, Teixeira C, DeVault DS, Bronte CR, Muir DCG. Organochlorine compounds in lake superior: Chiral polychlorinated biphenyls and biotransformation in the aquatic bood web. Environmental Science and Technology. 2003;38(1):84–92. doi: 10.1021/es0346983. [DOI] [PubMed] [Google Scholar]
- Wu Q, Sowers KR, May HD. Establishment of a polychlorinated biphenyl-dechlorinating microbial consortium, specific for doubly flanked chlorines, in a defined, sediment-free mediun. Applied and Environmental Microbiology. 2000;66(1):49–53. doi: 10.1128/aem.66.1.49-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Gannon SM, Nevin KP, Franks AE, Lovley DR. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environmental Microbiology. 2010;12(4):1011–1020. doi: 10.1111/j.1462-2920.2009.02145.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.