Abstract

As stem cells are a cornerstone of regenerative medicine, research efforts have been extensively focused on controlling their self-renewal and differentiation. It is well known that stem cells are tightly regulated by a combination of physical and chemical factors from their complex extracellular surroundings; thus, conventional cell culture approaches based purely on using soluble factors to direct stem cell fate have resulted in limited success. To account for the complexities of native stem-cell niches, biomaterials are actively investigated as artificial extracellular matrices in order to mimic the natural microenvironment. This Perspective highlights important areas related to the design of biomaterials to control stem cell behavior, such as cell-responsive ligands, mechanical signals, and delivery of soluble factors.
Although regenerative medicine is a relatively new field of biomedical research, its application to organ reconstruction provides a promising therapeutic option. Regenerative therapeutics may provide cures to patients with various ailments by replacing or regenerating human tissues or organs to restore normal physiological functions.1 A wide range of biomedical engineering approaches have been pursued for regenerative therapies, and some have led to clinical applications. Regardless of the approach, acquiring an adequate source of cells is an important prerequisite. Stem cells, defined by their inherent ability to self-renew continuously, or to differentiate into a multitude of different cell types, are thus a highly promising choice for regenerative medicine.1
Although the extensive research on utilizing stem cells for regenerative medicine has resulted in remarkable discoveries, there are still major hurdles that need to be overcome before stem cells can become a viable source of cells for regenerative medicine. Aside from the ethical concerns associated with embryonic stem cells, the precise control of stem cell fate, self-renewal or differentiation into specific cell types, has been a glaring challenge for scientists and engineers.
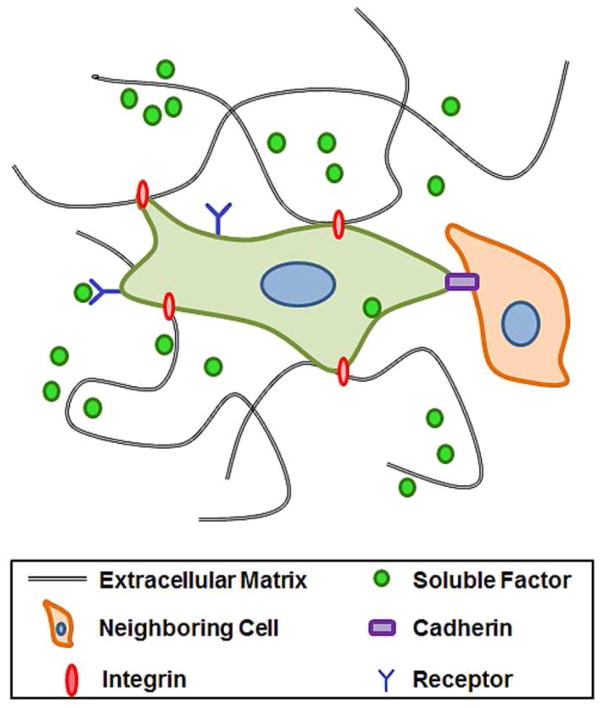
To control stem cell differentiation, researchers have traditionally relied on two-dimensional (2D) surfaces for culture. However, research over the last few decades has shown that stem cells reside in a complex microenvironment, and their fate is determined by several factors, such as soluble and physical signals from extracellular matrix (ECM) and neighboring cells (Figure 1).2 Moreover, these factors are spatially and temporally controlled. To mimic the native microenvironment of stem cells with the ultimate goal of directing their self-renewal and differentiation outcomes, more researchers are now utilizing biomaterials-based approaches.
Figure 1.
Schematic representation of the stem cell niche. Stem cells form complex interactions within their microenvironment between extracellular matrix, neighboring cells, and soluble factors.
Biomaterials for controlling stem cell fate
Biomaterials for stem cells have become an important part of regenerative medicine because they can serve as a biomimetic platform for relevant biological studies.3 Furthermore, these materials can ultimately be used as scaffolds for constructing artificial tissues or organs for clinical applications.
To complement recent clinical successes using harvested and decellularized scaffolds,4 biomaterials scientists continue to develop new synthetic materials to recapitulate the instructive cellular niches of natural tissue. By engineering biological activity into synthetic materials, instructive biomaterials can be produced with more control and reproducibility than their natural counterparts. Different types of natural or synthetic polymeric biomaterials have been explored to direct stem cell differentiation. Recently, Kloxin et al. pioneered the use of photolabile hydrogels to create dynamic architectures amenable to real-time modulation of material properties, formation of spatio-selective network porosity, and generation of temporal concentration gradients of specific functional groups.5 Numerous other types of polymers have been synthesized and used with controllable biochemical and biophysical properties, which are described in detail in elsewhere.6–8 Ideally, these biomaterials should be carefully designed to act as artificial ECM to present a combination of chemical and physical factors that provide necessary signals to direct stem cell fate. Critical aspects of designing biomaterials that can be used to direct stem cell responses include: i) presentation of cell-responsive ligands, ii) delivery of soluble factors, and iii) mechanical stimuli.
Cell-Responsive Ligands
It is important that biomaterials are designed to be cell-responsive, such that cells recognize and interact with the material. One approach to provide bio-responsive elements to materials is to present cell-recognition ligands. For example, Arg-Gly-Asp (RGD) is a peptide sequence found in fibronectin and collagen that is responsible for cell adhesion to ECM.9 Thus, RGD peptide has become a de facto ligand for cell adhesion, and chemical modification to present the RGD peptides has become a gold standard in biomaterials design. Other peptide sequences, such as Tyr-Ile-Gly-Ser-Arg (YIGSR) and Ile-Lys-Val-Ala-Val (IKVAV) found in laminin, have also been utilized as cell-responsive ligands.10
Previous research efforts have demonstrated the profound impact of ligands and matrix components on stem cell differentiation. For example, Benoit et al. showed that human mesenchymal stem cells (MSCs) were preferentially driven to differentiate into osteogenic, adipogenic, or chondrogenic pathways by immobilizing simple moieties reminiscent of exposed functional groups in the native extracellular space in crosslinked poly(ethylene glycol) (PEG) hydrogels.11 In addition to ECM components, stem cells are also in contact with neighboring cells through cell junction proteins such as cadherins. This interaction has also been shown to influence stem cell fate; thus, biomaterials that mimic cell–cell contact could also provide an important strategy for controlling stem cells. For example, Yue et al. demonstrated that stem cells that were cultured on hydrogels modified with N-cadherin remained undifferentiated and showed high proliferative capacity.12 In addition to engineering cell–matrix and cell–cell interactions within biomaterials, the spatial presentation of such molecules is highly important. For example, the spatial organization and density of RGD peptides have been shown to influence stem cell renewal and differentiation.13,14 Interestingly, recent advancements in micro-and nanoscale engineering have made it possible to fabricate and to pattern biomaterials with precise dimensions and ligand organization, enabling new directions in using architecture and spatial patterning to regulate cellular behavior.15,16
Soluble factors
Soluble factors secreted by cells, such as growth factors and cytokines, are important regulators of stem cell renewal and differentiation.17 For example, bone morphogenic protein-2 (BMP-2) is an important growth factor in osteogenic differentiation,18 whereas transforming growth factor beta (TGF-β) family has been implicated in various differentiation pathways.19 In addition to the growth factors that induce differentiation, others have been shown to maintain pluripotency of stem cells.20
Due to the difficulties associated with synthesizing and purifying such proteins, there have been increasing efforts to use small molecules that can replace the function of signaling proteins. Synthetic or naturally occurring chemicals have proven to be potent regulators of stem cell differentiation: several of them, either by themselves or in combination with other biological factors, have now become standard tools for differentiating stem cells. For instance, a cocktail of dexamethasone, ascorbic acid, and β-glycerophosphate is commonly used to induce osteogenic differentiation.21 Also, 5-azacytidine, a chemotherapeutic agent, has been shown to induce cardiomyogenic differentiation.22 To identify such small molecules, chemical libraries are increasingly being screened to identify molecules that can direct cell fate decisions in a reproducible and scalable manner.
Stimulating stem cells with soluble factors has been the conventional method for regulating stem cells phenotypes. It is, however, becoming increasingly appreciated that providing these soluble factors in the context of biomaterials could further enhance our ability to direct stem cells. This is due to a number of factors, such as slower release of soluble factors encapsulated within biomaterials, which prolongs their bioavailability and efficacy.23 In addition, the release of these factors from microscale reservoirs may generate localized gradients, which may be more biologically relevant. For example, TGF-β1 encapsulated in microparticles has been shown to direct chondrogenic differentiation of MSCs in hydrogel matrices.24
Mechanical stimuli
Cells are able to recognize mechanical signals from their surroundings by forming focal adhesions, complex sensory machinery involving a group of cell surface receptors and intracellular proteins that mediate mechanical signals from ECM to regulate a variety of gene expressions.25 This mechanotransduction has been shown to be an important regulator of stem cells. Seminal work by Engler et al. elegantly demonstrated the importance of mechanotransduction in stem cell fate, in which the substrate elasticity alone could direct MSC differentiation into specific lineages (Figure 2).26 This principle has been applied to create biomimetic stem-cell niches with suitable mechanical environments. For example, Chatterjee et al. varied the stiffness of PEG hydrogel across a wide range (10–300 kPa), and determined the optimal stiffness (~225 kPa) for inducing osteogenic differentiation of encapsulated cells.27 Furthermore, Connelly et al. determined the conditions for chondrogenic differentiation in RGD-coupled polysaccharide hydrogel.28
Figure 2.
Substrate elasticity can direct stem cell differentiation. (A) Native tissues exhibit a range of stiffness. (B) Stem cells cultured on hydrogel with varying elasticity modified with cell-adhesive type I collagen directed differentiation into different lineages (neurogenic, 0.1–1 kPa; myogenic, 8–17 kPa; osteogenic, 25–40 kPa). (C) Microarray profiles of transcription factors show neurogenic markers (left) are highest on 0.1–1 kPa gels, myogenic markers (center) are highest on 11 kPa gels, and osteogenic markers (right) are highest on 34 kPa gels. Reprinted with permission from ref. 26. Copyright 2006 Elsevier.
Controlling the substrate elasticity is one major advantage of using biomaterials. Conventional plastic-based 2D tissue–culture platforms do not allow the control of surface elasticity, and its rigidity (~3 GPa) is not physiologically relevant. On the other hand, the elasticities of soft biomaterials such as hydrogels and polymeric scaffolds are within the range of biological tissues, and can be readily controlled by varying the crosslinking density via concentrations of polymers or crosslinking molecules.
A new paradigm in biomaterial design—targeting glycosaminoglycans (GAGs) to regulate stem cell fate
In line with previously stated methods of using engineered biomaterials to direct stem cell differentiation, it is important to utilize novel signaling mechanisms and biological tools. An emerging class of molecules for directing cellular behavior are glycosaminoglycans (GAGs), which are linear polysaccharides that are present on the cell surface and in the ECM.29,30 It is known that cell-surface proteoglycans (heavily glycosylated proteins), which project their GAG components outward, can mediate physical cues from ECM to influence cell phenotypes. Thus, it may be possible to use cell-surface GAGs to regulate various cellular activities. Recently, Kiessling and co-workers developed an array of self-assembled monolayers that present several different types of GAG-binding peptides and their combinations derived from ECM proteins, and demonstrated that proliferation and differentiation of stem cells could be mediated by GAG-binding peptides.31
In this issue of ACS Nano, Kiessling and co-workers present a novel biomaterial design strategy that incorporates GAG-binding peptides as cell-responsive ligands into a biomaterial to mediate physical cues imparted by the material elasticity (Figure 3).32 In this research, they utilized a chemical modification strategy to conjugate a GAG-binding peptide derived from vitronectin, GKKQRFRHRNRKG, onto a hydrogel surface. Stem cells were able to adhere and to proliferate on the hydrogel surface while maintaining their pluripotency without other integrin-binding ligands such as RGD peptides, confirming that GAG-binding peptides act as a potent bioactive ligand for stem cells.
Figure 3.
Stem cell control with glycosaminoglycan (GAG)-binding ligands. (A) Pluripotency of stem cells, as identified by pluripotency markers Oct4 and SSEA-3, was better maintained cultured on GAG-binding peptides than on Arg-Gly-Asp (RGD) peptides. (B) Stem cell spreading and proliferation. Reprinted from ref. 32. Copyright 2012 American Chemical Society.
More significantly, the proliferative capacity of stem cells could be controlled by the elasticity of hydrogel substrate, in which increasing hydrogel stiffness resulted in increased proliferation rate. Further investigation into intracellular pathways revealed the activation of YAP/TAZ proteins, transcription factors involved in mechanosensing and stem cell pluripotency. The results of this work validated previous research that cell-surface proteoglycans can act as ECM receptors to regulate stem cell behavior, as well as provide a new approach to designing biomaterials by utilizing GAG-binding peptides.
Future Outlook
Many recent contributions have shed light on the complex systems of mechanical and chemical spatiotemporal cues that regulate stem cell differentiation. However, much work remains before we can rationally design synthetic materials capable of providing autonomous direction to pluripotent stem cell populations. Insightful contributions, like those from Kiessling and colleagues, that connect the extracellular environment to changes in stem cell gene expression represent key advances in our ability to establish design parameters for biomaterials in regenerative medicine. To realize the goals of regenerative medicine—to replace or to regenerate aged, injured, or diseased organs or tissues—concerted efforts are required from biomaterials scientists and stem-cell biologists. This work is an exciting and vibrant area of research at the interface of stem-cell biology and materials chemistry. Using synthetic materials to recapitulate reliable and instructive stem-cell niches will require distensible command of fluid transport, bioactive molecule incorporation, surface chemistries that facilitate cell–matrix and cell–cell interactions, and degradation/elimination mechanisms. From stem-cell biologists, future advances in this area will require further identification of rapidly accessible biomarkers to discriminate between stem cells and their differentiated progeny as well as more mechanistic understanding of the biological cues that regulate stem cell fate decisions.
Acknowledgments
The authors would like to acknowledge funding from the National Science Foundation (CBET 10-33746 (N.P.)) and from the National Institutes of Health (EB-000246-18 (N.P.), EB-012726-02 (N.P), and HL-092836-05 (to A.K)).
Footnotes
Conflict of Interest
The authors claim no conflicts of interest.
References
- 1.Atala A, Lanza R, Thomson JA, Nerem RM. Principles of Regenerative Medicine. Academic Press; San Diego: 2008. [Google Scholar]
- 2.Moore KA, Lemischka IR. Stem Cells and Their Niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 3.Stabenfeldt SE, Brown AC, Barker TH. Engineering ECM Complexity into Biomaterials for Directing Cell Fate. In: Roy K, editor. Biomaterials as Stem Cell Niche. Springer; New York: 2010. [Google Scholar]
- 4.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. Clinical Transplantation of a Tissue-Engineered Airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 5.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marklein RA, Burdick JA. Controlling Stem Cell Fate with Material Design. Adv Mater. 2010;22:175–189. doi: 10.1002/adma.200901055. [DOI] [PubMed] [Google Scholar]
- 7.Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for Stem Cell Differentiation. Adv Drug Deliver Rev. 2008;60:215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Hubbell JA. Biomaterials in Tissue Engineering. Nat Biotech. 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 9.Ruoslahti E. RGD and Other Recognition Sequences for Integrins. Ann Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 10.Weber LM, Hayda KN, Haskins K, Anseth KS. The Effects of Cell–Matrix Interactions on Encapsulated β-Cell Function within Hydrogels Functionalized with Matrix-Derived Adhesive Peptides. Biomaterials. 2007;28:3004–3011. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small Functional Groups for Controlled Differentiation of Hydrogel-Encapsulated Human Mesenchymal Stem Cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue XS, Murakami Y, Tamai T, Nagaoka M, Cho CS, Ito Y, Akaike T. A Fusion Protein N-Cadherin-Fc as an Artificial Extracellular Matrix Surface for Maintenance of Stem Cell Features. Biomaterials. 2010;31:5287–5296. doi: 10.1016/j.biomaterials.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Hsiong SX, Huebsch N, Fischbach C, Kong HJ, Mooney DJ. Integrin-Adhesion Ligand Bond Formation of Preosteoblasts and Stem Cells in Three-Dimensional RGD Presenting Matrices. Biomacromolecules. 2008;9:1843–1851. doi: 10.1021/bm8000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frith JE, Mills RJ, Cooper-White JJ. Lateral Spacing of Adhesion Peptides Influences Human Mesenchymal Stem Cell Behaviour. J Cell Sci. 2012;125:317–327. doi: 10.1242/jcs.087916. [DOI] [PubMed] [Google Scholar]
- 15.Zorlutuna P, Annabi N, Camci-Unal G, Nikkhah M, Cha JM, Nichol JW, Manbachi A, Bae H, Chen S, Khademhosseini A. Microfabricated Biomaterials for Engineering 3D tissues. Adv Mater. 2012;24:1782–1804. doi: 10.1002/adma.201104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tekin H, Sanchez JG, Landeros C, Dubbin K, Langer R, Khademhosseini A. Controlling Spatial Organization of Multiple Cell Types in Defined 3D Geometries. Adv Mater. 2012 doi: 10.1002/adma.201201805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannidou E. Therapeutic Modulation of Growth Factors and Cytokines in Regenerative Medicine. Curr Pharm Design. 2006;12:2397–2408. doi: 10.2174/138161206777699007. [DOI] [PubMed] [Google Scholar]
- 18.Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, Hedrick MH, Benhaim P. Bone Induction by BMP-2 Transduced Stem Cells Derived from Human Fat. J Orthopaed Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 19.Watabe T, Miyazono K. Roles of TGF-β Family Signaling in Stem Cell Renewal and Differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 20.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid Leukaemia Inhibitory Factor Maintains the Developmental Potential of Embryonic Stem Cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic Differentiation of Purified, Culture-Expanded Human Mesenchymal Stem Cells in Vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 22.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, et al. Cardiomyocytes can be Generated from Marrow Stromal Cells in Vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabata Y. Tissue Regeneration Based on Growth Factor Release. Tissue Eng. 2003;9(supplement 1):5–15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 24.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable Biodegradable Hydrogel Composites for Rabbit Marrow Mesenchymal Stem Cell and Growth Factor Delivery for Cartilage Tissue Engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger B, Spatz JP, Bershadsky AD. Environmental Sensing through Focal Adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 26.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, Young MF, Simon CG., Jr The Effect of 3D Hydrogel Scaffold Modulus on Osteoblast Differentiation and Mineralization Revealed by Combinatorial Screening. Biomaterials. 2010;31:5051–5062. doi: 10.1016/j.biomaterials.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connelly JT, Garcia AJ, Levenston ME. Interactions between Integrin Ligand Density and Cytoskeletal Integrity Regulate BMSC Chondrogenesis. J Cell Physiol. 2008;217:145–154. doi: 10.1002/jcp.21484. [DOI] [PubMed] [Google Scholar]
- 29.Koda JE, Bernfield M. Heparan Sulfate Proteoglycans from Mouse Mammary Epithelial Cells. Basal Extracellular Proteoglycan Binds Specifically to Native Type I Collagen Fibrils. J Biol Chem. 1984;259:11763–11770. [PubMed] [Google Scholar]
- 30.Rapraeger A, Jalkanen M, Bernfield M. Cell Surface Proteoglycan Associates with the Cytoskeleton at the Basolateral Cell Surface of Mouse Mammary Epithelial Cells. J Cell Biol. 1986;103:2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A Defined Glycosaminoglycan-Binding Substratum for Human Pluripotent Stem Cells. Nat Methods. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musah S, Morin SA, Wrighton PJ, Zwick DB, Jin S, Kiessling LL. Glycosaminoglycan-Binding Hydrogels Enable Mechanical Control of Human Pluripotent Stem Cell Self-Renewal. ACS Nano. 2012 doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]