Abstract
Neuropeptide Y (NPY) is abundant in the extended amygdala, a conceptual macrostructure in the basal forebrain important for regulation of negative affective states. NPY has been attributed a central role in anxiety-like behavior, fear, nociception, and reward in rodents. Deletion of the NPY gene in mice produces a high-anxiety high-alcohol-drinking phenotype. NPY infused into the brains of rats selectively bred to consume high quantities of alcohol suppresses alcohol drinking by those animals, an effect that is mediated by central amygdala (CeA). Likewise, alcohol-preferring rats exhibit basal NPY deficits in CeA. NPY infused into the brains of alcohol-dependent rats blocks excessive alcohol drinking by those animals, an effect that also has been localized to the CeA. NPY in CeA may rescue dependence-induced increases in anxiety and alcohol drinking via inhibition of downstream effector regions that receive GABAergic inputs from CeA. It is hypothesized here that NPY modulates anxiety-like behavior via Y2R regulation of NPY release, whereas NPY modulation of alcohol-drinking behavior in alcohol-dependent animals occurs via Y2R regulation of GABA release.
Keywords: Amygdala, BNST, Stress, Anxiety, Alcohol Withdrawal, Alcohol Dependence, Alcohol Self-Administration, Neuropeptide Y, Y2 receptor
1. Neuropeptide Y Distribution in Extended Amygdala
NPY is a 36-amino acid polypeptide that results from cleavage of its 97-amino acid precursor, preproNPY, and exhibits a high degree of phylogenetic conservation across species (Allen et al., 1987; Larhammar et al., 1987). NPY is widely distributed in the mammalian central nervous system, particularly in the cortex, striatum, amygdala, and hypothalamus (Gray & Morley, 1986; de Quidt & Emson, 1986; Miyazaki & Funakoshi, 1988; Heilig & Widerlöv, 1995). At least five NPY receptor subtypes have been identified in mammals (Larhammar & Salaneck, 2004), of which the best characterized are Y1, Y2, and Y5 receptors.
The extended amygdala is a conceptual macrostructure in the basal forebrain that includes the central nucleus of the amygdala (CeA), lateral division of the bed nucleus of the stria terminalis (BNST), and shell of the nucleus accumbens (NAc; Alheid & Heimer, 1988). The extended amygdala has been attributed a prominent role in regulation of negative affective (e.g., anxiety) states (Koob, 2008). The constituent regions of the extended amygdala are highly interconnected and each of these regions is densely populated by neuropeptides with pro-stress and anti-stress profiles (Koob, 2008; Gilpin & Roberto, 2012).
An emerging story in the stress/fear and alcohol fields has been the further subdivision of the CeA into medial and lateral aspects based on the connectivity and functionality of these subregions. Both the lateral and medial CeA are composed of GABAergic projection neurons and interneurons (Sun & Cassell, 1993; Veinante & Freund-Mercier, 1998). The lateral CeA projects heavily to the BNST (Krettek & Price, 1978; Weller & Smith, 1982), and reciprocal connections between CeA and BNST frequently co-transmit neuropeptides (e.g., Sakanaka et al., 1986). Within the amygdala as a whole and also within the CeA, there is a lateromedial flow of information, although there is not yet complete understanding of how emotional processing maps onto these complex intra-amygdala connections (Ehrlich et al., 2009; Pape & Pare, 2010). The medial division of the CeA is the major output region of the amygdala and sends inhibitory projections to various effector regions (Pitkänen, 2000) that initiate appropriate behavioral and physiological responses to emotionally relevant stimuli (e.g., stressors, alcohol, and drugs).
In the context of alcohol dependence, perhaps the extended amygdala neuropeptides most frequently examined are (1) corticotropin-releasing factor (CRF), which is important for initiating not only the neuroendocrine stress response but also the extra-hypothalamic (i.e., extended amygdala) stress response (Koob, 1999), and (2) neuropeptide Y (NPY), which is a peptide with robust anxiolytic effects mediated by the amygdala (Heilig et al., 1993; Sajdyk et al., 1999). CRF and NPY exhibit largely opposite behavioral profiles as well as a high degree of neuroanatomical overlap (Valdez & Koob, 2004), particularly in the extended amygdala where they drive inhibitory neurotransmission in opposite directions and have opposite effects on modulation of neurotransmission by alcohol (Gilpin & Roberto, 2012).
The amygdala is densely populated by NPY-containing fibers and NPY receptors (Allen et al., 1984; de Quidt & Emson, 1986; Dumont et al. 1990; Gustafson et al. 1997; Migita et al., 2001). NPY acts post-synaptically at Y1 receptors (Y1Rs) and pre-synaptically at Y2 receptors (Y2Rs), both of which are abundantly expressed in amygdala (Dumont et al., 1993; Gustafson et al., 1997; Kopp et al., 2002; Parker & Herzog, 1999). It has long been recognized that Y2Rs function both as autoreceptors that regulate pre-synaptic NPY release (Chen et al., 1997), and also as heteroceptors that regulate excitatory and inhibitory transmission via effects on pre-synaptic release (Gilpin et al., 2011; Greber et al., 1994).
2. Extended Amygdala NPY in Negative Affective States
2.1. NPY in Anxiety-Like and Fear Behaviors
Both endogenous NPY and exogenously administered NPY exert powerful anxiolytic effects in rats in a multitude of behavioral assays, including the elevated plus-maze, social interaction, fear-potentiated startle, and operant conflict tests (Britton et al., 1997; Broqua et al., 1995; Heilig et al., 1989, 1992; Sajdyk et al., 1999). NPY knockout (KO) mice exhibit increased basal levels of anxiety-like behavior under some conditions (Palmiter et al., 1998). Y1R KO mice exhibit alterations in anxiety-like behavior that depend on the task (Karl et al., 2006), but it does appear that Y1Rs are necessary for the anxiolytic effects of exogenously administered NPY (Karlsson et al., 2008). Finally, Y2R KO mice exhibit decreased anxiety-like behavior and improved ability to cope with forced swim stress (Tschenett et al., 2003), perhaps via increased NPY release following the loss of negative feedback that occurs following deletion of this autoreceptor.
The ability of NPY to reduce anxiety-like behavior has been localized to the amygdala, although it is not yet clear whether this effect is mediated by the CeA, the basolateral amygdala (BLA), or if contributions are made by both nuclei (Heilig et al., 1993; Sajdyk et al., 1999). Rats that exhibit an extreme behavioral response (high anxiety-like behavior and hyperarousal) to a traumatic stressor exhibit NPY deficiencies in several brain regions, including amygdala (Cohen et al., 2012). Site-specific deletion of the Y2R gene in CeA or BLA reduces anxiety-like behavior and depressive-like behavior (CeA only) in mice, perhaps due to changes in GABAergic transmission and/or NPY release (Tasan et al., 2010). Infusion of Y2R agonists (NPY3–36, C2-NPY) into the BLA can increase or decrease anxiety-like behavior depending on the compound and dose (Sajdyk et al., 2002a,b). Pre- and post- synaptic NPY receptors in the amygdala appear to interact in a complex way to affect behavior, perhaps because inhibitory neurons in the lateral division of the CeA synapse on each other and also on inhibitory neurons in the medial division of the CeA that project to brainstem effector regions (Ciocchi et al., 2010).
In traditional rodent models of fear conditioning, infusion of NPY directly into the amygdala of mice reduces fear behavior as measured by both conditioned freezing and fear-potentiated startle (Fendt et al., 2009). Pharmacological and genetic studies confirmed that this effect is not mediated by Y1Rs (Fendt et al., 2009), which suggests that NPY modulation of fear behavior likely occurs via Y2Rs in amygdala, consistent with data on anxiety-like behavior and alcohol-related behaviors in rats.
NPY is present in high quantities in the BNST (Allen et al., 1984), where it is co-localized with CRF, most of which comes from the CeA (Sakanaka et al., 1986). The BNST is densely innervated by GABAergic projections from CeA that also co-localize Y2Rs (Tasan et al., 2010), and is well situated to modulate affective-like behavior via projections to effector regions (e.g., brainstem and hypothalamus). Indeed, chronic restraint stress in mice increases basal GABAergic transmission in BNST and reduces the ability of NPY to modulate inhibitory transmission via Y2Rs in that region (Pleil et al., 2012).
2.2. NPY in Pain Behavior
Brain NPY modulates nociception and pain-related behaviors in rat models. For example, infusion of NPY into the ventricles produces robust decreases in nociception in a rat model of neuropathic pain, and these effects are synergistic with the anti-nociceptive effects of morphine (Upadhya et al., 2009). Furthermore, a Y1R agonist ([Leu31, Pro34]-NPY) mimics the anti-nociceptive effect of NPY, whereas ventricular infusion a Y1R antagonist (BIBP3226) attenuates the anti-nociceptive effect of morphine (Upadhya et al., 2009). Interestingly, micro-infusion of NPY into the periaqueductal grey (PAG), important in top-down pain control and a terminal region for projections from extended amygdala (e.g., CeA & BNST), also produces anti-nociceptive effects in rats (Wang, 2004). Neuroadaptations in brain NPY may contribute to the lower nociceptive thresholds seen in alcohol-dependent rats, especially since CRF1Rs have been implicated in alcohol withdrawal-induced allodynia (Edwards et al., 2011).
2.3. NPY in Reward-Related Behavior
NPY also modulates reward behavior, perhaps via interactions with dopamine (DA) in NAc. Rats exhibit conditioned-place preference (CPP) for a context repeatedly paired with intra-NAc shell infusions of NPY (Brown et al., 2000), and this CPP can be blocked by pre-treatment with a DA receptor antagonist (cis-flupenthixol; Josselyn & Beninger, 1993). In agreement with those findings, infusion of NPY directly into the NAc shell produces increases in extracellular dopamine, as measured by in vivo microdialysis (Sørensen et al., 2009). NPY content in NAc is altered by chronic drug exposure; for example, chronic cocaine treatment produces long-lasting decreases in NPY in the NAc of rats (Wahlestedt et al., 1991). Furthermore, inbred strains that are prone to drink high amounts of alcohol exhibit NPY deficiencies in NAc shell relative to low-drinking strains (Misra & Pandey, 2006).
3. NPY & Alcohol in Genetic Animal Models
3.1. NPY & Alcohol in Non-Rodent Species
Neuropeptide Y-like proteins and their receptors have been implicated in the response to ethanol even in C. elegans and Drosophila. For example, NPR-1 is an NPY receptor-like protein in C. elegans that negatively regulates the development of acute alcohol tolerance (Davies et al., 2004). In Drosophila, NPY-like neuropeptide F (NPF) and its receptor NPFR1 regulate initial sensitivity to acute alcohol via interactions with the protein kinase C (PKC) pathway (Chen et al., 2008). More recently, it was shown that mating and sexual deprivation respectively increase and decrease NPF levels in male Drosophila, which lead in turn to respective reduction and enhancement of alcohol preference, consistent with the rodent literature (Shohat-Ophir et al., 2012).
3.2. NPY Knockout & Transgenic Rodents
Deletion of the NPY gene in mice produces a phenotype marked by excessive alcohol consumption and reduced sensitivity to the sedative effects of high alcohol doses (Thiele et al., 1998). Conversely, transgenic mice that overexpress NPY consume less alcohol than controls and exhibit increased sensitivity to the sedative effects of high alcohol doses (Thiele et al., 1998). NPY KO mice also exhibit increased sensitivity to the locomotor activating effects of low alcohol doses, but this effect is dependent on the genetic background of the mouse (Thiele et al., 2000). Mice lacking the Y1R are very similar to NPY KO mice in that they consume high quantities of alcohol and exhibit reduced sensitivity to the sedative effects of alcohol (Thiele et al., 2002). Mice lacking the Y2R consume normal or lower quantities of alcohol relative to controls (Thiele et al., 2004), although Y2R KO mice in this study were not from the same high-alcohol-drinking background as the NPY KO and Y1R KO mice described above.
3.3. NPY in the Extended Amygdala of Alcohol-Preferring Rats
Multiple lines of rats selectively bred to drink high quantities of alcohol (>5.0 g/kg/day) exhibit NPY deficiencies in CeA relative to their counterparts selectively bred for low alcohol preference. More specifically, alcohol-preferring (P) rats and high-alcohol-drinking (HAD1) rats exhibit less NPY immunoreactivity in CeA relative to non-preferring (NP) and low-alcohol-drinking (LAD1) rats, respectively (Ehlers et al., 1998; Hwang et al., 1999; Pandey et al., 2005). Similarly, P rats exhibit basal deficits in NPY mRNA in CeA relative to NP rats (Suzuki et al., 2004). Another line of alcohol-preferring (AA) rats exhibits basal deficits in Y2R mRNA in the medial amygdala relative to non-preferring (ANA) counterparts and genetically heterogeneous controls, although Y2R transcript in CeA was not measured in that study (Caberlotto et al., 2001). Collectively, these results are consistent with the notion that NPY deficits in amygdala, and particularly in the CeA, promote a high-anxiety high-alcohol-drinking phenotype.
3.4. Amygdalar NPY Effects on Alcohol Drinking in Alcohol-Preferring Rats
NPY infused into the ventricles suppresses alcohol consumption in P rats and HAD1 rats, but not in their low-preferring counterparts (Badia-Elder et al., 2001, 2003). The magnitude and duration of this effect are both augmented in P rats that have endured a period of abstinence prior to NPY administration and availability of alcohol (Gilpin et al., 2003, 2005). The ability of NPY to suppress alcohol drinking in P rats has been localized to the CeA (Pandey et al., 2005), and this effect is not secondary to effects on feeding (Gilpin et al., 2008a). Furthermore, the ability of intra-CeA NPY infusion to suppress alcohol consumption in P rats is enhanced following a period of abstinence (i.e., reversal of alcohol deprivation effect; Gilpin et al., 2008a).
4. NPY in Animal Models of Alcohol Dependence
4.1. Alcohol Effects on Inhibitory Transmission in Extended Amygdala
Antagonism of GABAARs in CeA reduces alcohol self-administration in rats (Hyytiä & Koob, 1995). Acute alcohol increases GABAergic synaptic transmission in CeA (Roberto et al., 2003), and this effect is rapid, reversible, and has a significant pre-synaptic component. Chronic alcohol exposure facilitates GABA release in the CeA, mainly via actions at pre-synaptic GABAergic terminals (Roberto et al., 2004, 2010). Acute alcohol enhances GABAergic transmission similarly in the CeA of alcohol-naïve and alcohol-dependent rats (see Figure 1), suggesting a lack of tolerance for the acute effects of alcohol in this brain region (Roberto et al., 2004). Microdialysis studies have confirmed that alcohol dependence produces large increases in extracellular GABA concentrations in CeA, and also that alcohol-dependent rats exhibit a lack of tolerance for acute alcohol-induced increases in CeA GABA dialysate levels (Roberto et al., 2004). Because antagonism of GABAARs in BNST also reduces alcohol self-administration in rats (Hyytiä & Koob, 1995), it is reasonable to hypothesize that the effects of alcohol on inhibitory transmission in BNST may be similar to effects in CeA, although this has not yet been shown.
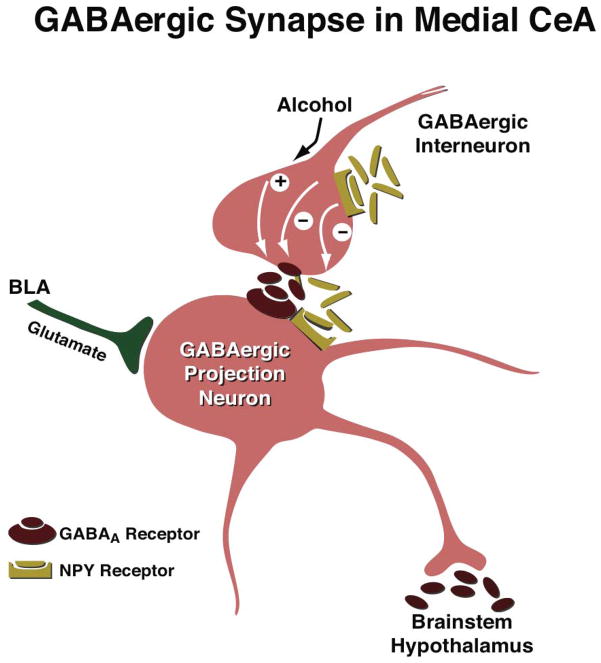
Figure 1.
Schematic diagram of the effects of acute alcohol and NPY on GABAergic synaptic transmission in the medial aspect of the CeA. GABAergic afferents in the medial CeA arise largely from the lateral CeA and intercalated GABA cells, and commonly co-express NPY and/or other neuropeptides. Glutamatergic inputs to medial CeA arise largely from BLA. In this simplified scheme, GABAARs are located post-synaptically, and G-protein coupled receptors (GPCRs) that bind NPY are located both pre- (Y2) and post-synaptically (Y1). Acute alcohol promotes pre-synaptic GABA release in the medial CeA of both alcohol-naïve and alcohol-dependent rodents, perhaps via actions at CRF1Rs (not shown here). Increased GABA release produced by alcohol inhibits GABAergic output from the medial CeA, thereby leading to excitation (i.e., disinhibition) of downstream effector regions. Conversely, NPY decreases pre-synaptic GABA release in the medial CeA via actions at Y2Rs, and this effect is enhanced following a history of either alcohol dependence or alcohol binge exposure (Gilpin et al., 2011; Sparrow et al., 2012). Reduced GABA release produced by NPY disinhibits GABAergic output from the medial CeA, thereby leading to inhibition of downstream effector regions. Chronic alcohol exposure enhances many aspects of synaptic transmission in CeA (e.g., stronger effects of acute alcohol, NPY, and CRF on GABAergic transmission), perhaps due to changes in transmitter expression and/or receptor levels. Here, it is hypothresized that what may (also) be recruited during the transition to alcohol dependence is the pre-synaptic interaction of NPY & GABA and the heteroceptor function of Y2Rs. It is also conceivable that individual differences in the balance between basal autoreceptor and heteroceptor functions of Y2Rs may predict alcohol effects in CeA as well as the propensity to abuse alcohol. Based on this model, it is hypothesized that NPY regulates anxiety-like behavior (regardless of alcohol history) via Y2R regulation of NPY release, whereas NPY modulation of alcohol-drinking behavior in alcohol-dependent animals occurs via Y2R regulation of GABA release.
4.2. NPY Effects on Inhibitory Transmission in Extended Amygdala
NPY prevents and reverses acute alcohol-induced increases in evoked GABAergic transmission in CeA via pre-synaptic effects on GABA release (Gilpin et al., 2011). As illustrated in Figure 1, NPY blocks alcohol effects on GABA release via activation of pre-synaptic Y2Rs in slice electrophysiology recordings from the CeA of rats. NPY alone does not decrease GABAergic transmission in CeA unless post-synaptic Y1Rs are blocked, suggesting that functional Y1Rs in CeA “buffer” the effects of NPY at pre-synaptic Y2Rs. Importantly, NPY normalizes alcohol dependence-induced increases in GABA release in CeA, suggesting that chronic alcohol produces neuroadaptations in NPY systems that affect inhibitory transmission in CeA (Gilpin et al., 2011). In agreement with these findings, NPY reduces evoked inhibitory post-synaptic currents (eIPSCs) in the CeA of mice, and this effect is enhanced in slices taken from mice with a history of binge alcohol drinking (Sparrow et al., 2012).
Recent data from our lab show that chronic high-dose alcohol exposure produces allostatic shifts in NPY content in BNST, such that once-normal NPY levels in BNST actually represent deficits in alcohol-withdrawn rats (Baynes & Gilpin, 2012). Similar to its effects in CeA, NPY modulates GABA release in BNST (Kash & Winder, 2006) via activation of pre-synaptic Y2Rs, supporting the notion that Y2Rs function not only as autoreceptors regulating NPY release (Chen et al., 1997), but also as heteroceptors regulating the release of other neurotransmitters (Greber et al., 1994). This dual role of pre-synaptic Y2Rs (regulating NPY and GABA release) may explain apparent discrepancies in the literature regarding the effects of NPY and Y2R compounds on some behaviors (see Section 6 below).
4.3. NPY Effects on Alcohol Dependence-Related Behaviors
Repeated intra-ventricular NPY administration during prior alcohol withdrawals blocks increases in alcohol self-administration during subsequent withdrawals (Gilpin et al., 2011), a hallmark behavioral feature of the transition to alcohol dependence. This effect may be due to NPY reversal of withdrawal-induced increases in anxiety-like behavior, as has been suggested with other anti-anxiety compounds chronically administered in a similar protocol (Breese et al., 2005). Activation of NPY systems in the CeA suppresses alcohol self-administration in alcohol-dependent rats at doses that do not affect alcohol self-administration in non-dependent rats (Gilpin et al., 2008b; Thorsell et al., 2007). Rats withdrawn from chronic high-dose alcohol exhibit NPY mRNA and protein deficits in CeA that parallel increases in anxiety-like behavior (Roy & Pandey, 2002; Zhang & Pandey, 2003). Finally, NPY blocks stress-induced reinstatement of alcohol-seeking behavior (Cippitelli et al., 2010), an effect that may be attributable to effects of NPY on inhibitory neurotransmission in CeA (Gilpin et al., 2011).
Pre-synaptic Y2Rs are likely to mediate NPY effects on post-dependent alcohol-related behaviors. Intra-ventricular administration of a Y2R antagonist (BIIE0246) reduces alcohol consumption by rats (Thorsell et al., 2002) and mice (Sparrow et al., 2012), and alcohol-dependent rats exhibit increased sensitivity to the suppressive effects of BIIE0246 on alcohol drinking during protracted abstinence (Rimondini et al., 2005). Systemic administration of a Y2R antagonist (JNJ-31020028) that is able to cross the blood-brain barrier dose-dependently reverses the increased anxiety-like behavior observed in rats during hangover (i.e., withdrawal) from a single bolus injection of alcohol (Cippitelli et al., 2011). These behavioral effects of systemic and whole-brain Y2R manipulations are likely the sum of drug actions at multiple loci. In the CeA, because NPY blocks alcohol effects on GABA release via Y2Rs (Gilpin et al., 2011), it is hypothesized that infusion of a Y2R antagonist into the CeA would actually increase alcohol self-administration in situations where the heteroceptor function of Y2Rs has been recruited (e.g., alcohol dependence).
5. CeA Disinhibits Downstream Effector Regions
Traditionally, compounds that promote GABAergic transmission (e.g., barbiturates, benzodiazepines, and neuroactive steroids) reduce anxiety in animals and humans via interaction with the GABAA receptor complex (for recent review, see Rudolph & Knoflach, 2011). Therefore, it may seem counterintuitive that pro-anxiety pro-alcohol-drinking peptides (e.g., CRF) increase GABAAR-mediated inhibitory transmission in CeA, whereas anti-anxiety anti-alcohol-drinking peptides (e.g., NPY) decrease GABAergic transmission in the same region. However, this pattern of results can be explained by closer examination of the connectivity within and between amygdaloid nuclei. The amygdala is organized such that lateral nuclei (i.e., lateral and basolateral amygdala) project to medial nuclei (i.e., central amygdala). Within the CeA, information also flows from lateral aspects to the medial subdivision of the CeA, the major output region of the amygdala, which sends inhibitory projections to effector regions (e.g., hypothalamus and brainstem).
Medial CeA projection neurons receive dense inhibitory inputs from lateral CeA and intercalated GABA cells. In the slice electrophysiology experiments described above, GABAAR-mediated inhibitory transmission was pharmacologically isolated and post-synaptic potentials (PSPs) were evoked by local electrical stimulation in the medial CeA. Although it is not possible in this preparation to discern whether inhibitory PSPs reflect GABAergic transmission from local interneurons in CeA or inhibitory afferents from other nearby regions, recorded increases in evoked GABAergic transmission in CeA (e.g., following application of CRF) promote inhibition of GABAergic neurons projecting out of CeA. Conversely, recorded decreases in evoked GABAergic transmission in CeA (e.g., following application of NPY) facilitate GABA release from CeA efferents onto downstream effector regions. Therefore, recorded increases in GABAergic transmission reflect a net disinhibition of downstream regions (e.g., hypothalamus, PAG, locus coeruleus, nucleus of the solitary tract, pedunculopontine tegmental nucleus), whereas recorded decreases in GABAergic transmission reflect a net inhibition of downstream target regions.
This model suggests that anxiogenic peptides (e.g., CRF) in CeA promote anxiety-like and alcohol-drinking behaviors associated with alcohol dependence via disinhibition of downstream regions responsible for generating behavioral and physiological responses to alcohol and alcohol withdrawal. Conversely, anxiolytic peptides (e.g., NPY) in CeA may rescue the high-anxiety excessive-drinking state associated with alcohol dependence via inhibition of downstream effector regions. The hypothesized role of this circuitry in anxiety- and alcohol-related behaviors is in general agreement with data from the fear conditioning literature (Davis et al., 2010; Paré et al., 2004; Tye et al., 2011).
6. Predicting Behavior from Inhibitory Transmission in CeA
From the model described in the previous section and illustrated in Figure 1, certain predictions can be made about the effects of NPY (and other peptides) on anxiety-like and alcohol-related behaviors both prior to and following the transition to alcohol dependence, some of which have been previously confirmed and others which have been not yet been tested. The overarching hypothesis put forth here is that NPY affects anxiety-like behavior (regardless of alcohol history) via Y2R regulation of NPY release, whereas NPY modulation of alcohol-drinking behavior in alcohol-dependent animals occurs via Y2R regulation of GABA release. The implication is that, while alcohol dependence produces changes in expression of NPY and its receptors, what may be critical for mediating the effects of NPY on excessive alcohol drinking by alcohol-dependent (and perhaps alcohol-preferring) animals is the recruitment of Y2R heteroceptor function and NPY-GABA interactions in amygdala.
Within this model, one obvious prediction is that a Y2R antagonist should mimic the effects of NPY in the CeA on anxiety-like behavior, but oppose the effects of NPY on alcohol consumption in alcohol-dependent animals and also oppose NPY effects on GABA release in CeA. This hypothesis is supported by data showing that application of Y2R antagonist (BIIE0246) to rat CeA slice increases evoked GABAergic transmission and abolishes the ability of NPY to block alcohol effects on GABA release (Gilpin et al., 2011). In this scenario, there would appear to be a delicate balance between the autoreceptor and heteroceptor functions of pre-synaptic Y2Rs in CeA, a balance that may differ in non-dependent and alcohol-dependent states, and also across individuals. One important experiment would be to determine the effects of Y2R blockade on both anxiety-like behavior and alcohol self-administration in the same set of animals, the results of which would have obvious implications for both the autoreceptor-heteroceptor hypothesis put forth here and also for the allostasis theory of alcohol addiction.
Another set of predictions that can be made from the electrophysiological data is that infusion of a GABAAR antagonist into the CeA should reduce alcohol drinking, and that manipulation of pre-synaptic NPY and CRF receptors should not further affect alcohol drinking following pre-treatment with a GABAAR antagonist. The latter of these hypotheses has not been tested, but infusion of a competitive GABAAR antagonist (SR 95531) into the amygdala, in particular the CeA, does reduce alcohol self-administration in rats (Hyytiä & Koob, 1995). Blocking GABAergic transmission with a drug that is peripherally administered is not likely to be viable as a long-term strategy for treating alcohol dependence in humans. As such, a more promising approach may be to target neuromodulators (e.g., NPY, CRF) of inhibitory transmission that are recruited (e.g., in extended amygdala) during the transition to alcohol dependence.
7. Conclusions
NPY is abundantly expressed in the extended amygdala and is critically involved in the regulation of negative affective states in rats. The transition to alcohol dependence produces neuroadaptations in extended amygdala NPY systems, as well as other pro- and anti-stress peptide systems. The effects of NPY in CeA on elevated anxiety and excessive alcohol drinking in alcohol-dependent rats may be differentially mediated by Y2R regulation of NPY and GABA release. NPY systems in extended amygdala may constitute a promising target in the search for potential pharmacotherapeutics to combat alcohol use disorders in humans.
Acknowledgments
This work was supported by National Institute of Alcoholism grant AA018400.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neurosci. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Allen J, Novotny J, Martin J, Heinrich G. Molecular structure of mammalian neuropeptide Y: analysis by molecular cloning and computer-aided comparison with crystal structure of avian homologue. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:2532–2536. doi: 10.1073/pnas.84.8.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen YS, Roberts GW, Bloom SR, Crow TJ, Polak JM. Neuropeptide Y in the stria terminalis: evidence for an amygdalofugal projection. Brain Res. 1984;321:357–62. doi: 10.1016/0006-8993(84)90193-8. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li T-K. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386–90. [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li T-K. Effects of neuropeptide Y on sucrose and ethanol intake and on the elevated plus maze test of anxiety in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894–9. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- Baynes B, Gilpin NW. Alcohol dependence produces allostatic shifts in neuropeptide Y (NPY) levels in the extended amygdala of rats. Alcohol Clin Exp Res. 2012;36 (S1):16A. [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacol. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton KT, Southerland S, Van Uden E, Kirby D, Rivier J, Koob G. Anxiolytic activity of NPY receptor agonists in the conflict test. Psychopharmacol. 1997;132:6–13. doi: 10.1007/s002130050313. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6:215–222. [PubMed] [Google Scholar]
- Brown CM, Coscina DV, Fletcher PJ. The rewarding properties of neuropeptide Y in perifornical hypothalamus vs. nucleus accumbens. Peptides. 2000;21:1279–87. doi: 10.1016/s0196-9781(00)00270-9. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Thorsell A, Rimondini R, Sommer W, Hyytiä P, Heilig M. Differential expression of NPY and its receptors in alcohol-preferring AA and alcohol-avoiding ANA rats. Neuropsychopharmacol. 2001;25:91–7. [PubMed] [Google Scholar]
- Chen X, DiMaggio DA, Han SP, Westfall TC. Autoreceptor-induced inhibition of neuropeptide Y release from PC-12 cells is mediated by Y2 receptors. J Am Physiol. 1997;273:H1737–44. doi: 10.1152/ajpheart.1997.273.4.H1737. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang Y, Shen P. A protein kinase C activity localized to neuropeptide Y-like neurons mediates ethanol intoxication in Drosophila melanogaster. Neuroscience. 2008;156:42–7. doi: 10.1016/j.neuroscience.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, et al. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacol. 2010;208:417–26. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Rezvani A, Robinson JE, Eisenberg L, Levin ED, Bonaventure P. The novel, selective, brain-penetrant neuropeptide Y Y2 receptorantagonist, JNJ-31020028, tested in animal models of alcoholconsumption, relapse, and anxiety. Alcohol. 2011;45:567–576. doi: 10.1016/j.alcohol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathé AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacol. 2012;37:350–63. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AG, Bettinger JC, Thiele TR, Judy ME, McIntire SL. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron. 2004;42:731–43. doi: 10.1016/j.neuron.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Quidt ME, Emson PC. Distribution of Neuropeptide Y-like immunoreactivity in the rat central nervous system-II. Immunohistochemcial analysis. Neurosci. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Fournier A, St-Pierre S, Quirion R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J Neurosci. 1993;13:73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y, Fournier A, St-Pierre S, Schwartz TW, Quirion R. Differential distribution of Y1 and Y2 receptors in the rat brain. Eur J Pharmacol. 1990;191:501–3. doi: 10.1016/0014-2999(90)94189-5. [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF receptor antagonism. Neuropharmacol. 2011;62:1142–51. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Li T-K, Lumeng L, Hwang BH, Somes C, Jiminez P, et al. Neuropeptide Y levels of ethanol-naïve alcohol-preferring and -nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–82. [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Fendt M, Bürki H, Imobersteg S, Lingenhöhl K, McAllister KH, Orain D, Uzunov DP, Chaperon F. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacol. 2009;206:291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y Opposes Alcohol Effects on Gamma-Aminobutyric Acid Release in Amygdala and Blocks the Transition to Alcohol Dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced decreases in alcohol drinking. Pharmacol Biochem Behav. 2008b;90:475–80. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012;36:873–88. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y administration into the amygdala suppresses ethanol drinking in alcohol-preferring (P) rats following multiple deprivations. Pharmacol Biochem Behav. 2008a;90:470–4. doi: 10.1016/j.pbb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Sensitized effects of neuropeptide Y on multiple ingestive behaviors in P rats following ethanol abstinence. Pharmacol Biochem Behav. 2005;81:740–9. doi: 10.1016/j.pbb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li T-K, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–94. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Gray TS, Morley JE. Neuropeptide Y: anatomical distribution and possible function in mammalian nervous system. Life Science. 1986;38:389–401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- Greber S, Schwarzer C, Sperk G. Neuropeptide Y inhibits potassium-stimulated glutamate release through Y2 receptors in rat hippocampal slices in vitro. Br J Pharmacol. 1994;113:737–40. doi: 10.1111/j.1476-5381.1994.tb17055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EL, Smith KE, Durkin MM, Walker MW, Gerald C, Weinshank R, Branchek TA. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Mol Brain Res. 1997;46:223–35. doi: 10.1016/s0169-328x(97)00017-x. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacol. 1993;8:357–63. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Koob GK, Britton KT. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regul Pept. 1992;41:61–9. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- Heilig M, Söderpalm B, Engel JA, Widerlöv E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacol. 1989;98:524–9. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heilig M, Widerlöv E. Neurobiology and clinical aspects of neuropeptide Y. Critical Reviews in Neurobiology. 1995;9:115–136. [PubMed] [Google Scholar]
- Hwang BH, Zhang JK, Ehlers CL, Lumeng L, Li T-K. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdale between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–30. [PubMed] [Google Scholar]
- Hyytiä P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–9. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Beninger RJ. Neuropeptide Y: intraaccumbens injections produce a place preference that is blocked by cis-flupenthixol. Pharmacol Biochem Behav. 1993;46:543–52. doi: 10.1016/0091-3057(93)90542-2. [DOI] [PubMed] [Google Scholar]
- Karl T, Burne TH, Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav Brain Res. 2006;167:87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, Heilig M. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacol. 2008;195:547–57. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacol. 2006;51:1013–22. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J, Xu Z-Q, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hökfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neurosci. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- Krause J, Eva C, Seeburg PH, Sprengel R. Neuropeptide Y1 subtype pharmacology of a recombinantly expressed neuropeptide receptor. Molecular Pharmacology. 1992;41:817–821. [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978 Mar 15;178(2):225–54. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Ericsson A, Persson H. Structure and expression of the rat neuropeptide Y gene. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:2068–2072. doi: 10.1073/pnas.84.7.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D, Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38:141–151. doi: 10.1016/j.npep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Migita K, Loewy AD, Ramabhadran TV, Krause JE, Waters SM. Immunohistochemical localization of the neuropeptide Y Y1 receptor in rat central nervous system. Brain Res. 2001;889:23–37. doi: 10.1016/s0006-8993(00)03092-4. [DOI] [PubMed] [Google Scholar]
- Misra K, Pandey SC. The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: a role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacol. 2006;31:1406–19. doi: 10.1038/sj.npp.1300900. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Funakoshi A. Distribution of pancreatic polypeptide-like immunoreactivity in rat tissues. Regulatory Peptides. 1988;21:37–43. doi: 10.1016/0167-0115(88)90089-4. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog Horm Res. 1998;53:163–99. [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–73. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H-C, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parker RMC, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala. Oxford University Press; 2000. [Google Scholar]
- Pleil KE, Lopez A, McCall N, Jijon AM, Bravo JP, Kash TL. Chronic stress alters neuropeptide Y signaling in the bed nucleus of the stria terminalis in DBA/2J but not C57BL/6J mice. Neuropharmacol. 2012;62:1777–86. doi: 10.1016/j.neuropharm.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci Lett. 2005;375:129–33. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–9. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–66. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2012;10:685–97. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacol. 2002a;43:1165–72. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Smiley DL, Gehlert DR. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav. 2002b;71:419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368:143–7. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–38. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]; Life Sci. 35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- Shohat-Ophir G, Kaun KR, Azanchi R, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–5. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G, Wegener G, Hasselstrøm J, Hansen TV, Wörtwein G, Fink-Jensen A, Woldbye DP. Neuropeptide Y infusion into the shell region of the rat nucleus accumbens increases extracellular levels of dopamine. Neuroreport. 2009;20:1023–6. doi: 10.1097/wnr.0b013e32832d4848. [DOI] [PubMed] [Google Scholar]
- Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, et al. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacol. 2012;37:1409–21. doi: 10.1038/npp.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;15:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Lumeng L, McBride WJ, Li TK, Hwang BH. Reduced neuropeptide Y mRNA expression in the central nucleus of amygdala of alcohol preferring (P) rats: its potential involvement in alcohol preference and anxiety. Brain Res. 2004;1014:251–4. doi: 10.1016/j.brainres.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, Herzog H, Sperk G. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–9. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Miura GI, Marsh DJ, Bernstein IL, Palmiter RD. Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor. Pharmacol Biochem Behav. 2000;67:683–91. doi: 10.1016/s0091-3057(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Naveilhan P, Ernfors P. Assessment of ethanol consumption and water drinking by NPY Y2 receptor knockout mice. Peptides. 2004;25:975–83. doi: 10.1016/j.peptides.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O’Dell LE, Chen SA, King AR, Lekic D, et al. Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain. 2007;130:1330–7. doi: 10.1093/brain/awm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Rimondini R, Heilig M. Blockade of central neuropeptide Y (NPY) Y2 receptors reduces ethanol self-administration in rats. Neurosci Lett. 2002;332:1–4. doi: 10.1016/s0304-3940(02)00904-7. [DOI] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya MA, Dandekar MP, Kokare DM, Singru PS, Subhedar NK. Involvement of neuropeptide Y in the acute, chronic and withdrawal responses of morphine in nociception in neuropathic rats: behavioral and neuroanatomical correlates. Neuropeptides. 2009;43:303–14. doi: 10.1016/j.npep.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–89. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Intrinsic and extrinsic connections of the rat central extended amygdala: an in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res. 1998 Jun 1;794(2):188–98. doi: 10.1016/s0006-8993(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Karoum F, Jaskiw G, Wyatt RJ, Larhammar D, Ekman R, Reis DJ. Cocaine-induced reduction of brain neuropeptide Y synthesis dependent on medial prefrontal cortex. Proc Natl Acad Sci U S A. 1991;88:2078–82. doi: 10.1073/pnas.88.6.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ. Microinjection of neuropeptide Y into periaqueductal grey produces anti-nociception in rats with mononeuropathy. Sheng Li Xue Bao. 2004;56:79–82. [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982 Jan 28;232(2):255–70. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pandey SC. Effects of PKA modulation on the expression of neuropeptide Y in ratamygdaloid structures during ethanol withdrawal. Peptides. 2003;24:1397–1402. doi: 10.1016/j.peptides.2003.08.008. [DOI] [PubMed] [Google Scholar]