Abstract
Polygalacturonase (PG) is the major enzyme responsible for pectin disassembly in ripening fruit. Despite extensive research on the factors regulating PG gene expression in fruit, there is conflicting evidence regarding the role of ethylene in mediating its expression. Transgenic tomato (Lycopersicon esculentum) fruits in which endogenous ethylene production was suppressed by the expression of an antisense 1-aminocyclopropane-1-carboxylic acid (ACC) synthase gene were used to re-examine the role of ethylene in regulating the accumulation of PG mRNA, enzyme activity, and protein during fruit ripening. Treatment of transgenic antisense ACC synthase mature green fruit with ethylene at concentrations as low as 0.1 to 1 μL/L for 24 h induced PG mRNA accumulation, and this accumulation was higher at concentrations of ethylene up to 100 μL/L. Neither PG enzyme activity nor PG protein accumulated during this 24-h period of ethylene treatment, indicating that translation lags at least 24 h behind the accumulation of PG mRNA, even at high ethylene concentrations. When examined at concentrations of 10 μL/L, PG mRNA accumulated within 6 h of ethylene treatment, indicating that the PG gene responds rapidly to ethylene. Treatment of transgenic tomato fruit with a low level of ethylene (0.1 μL/L) for up to 6 d induced levels of PG mRNA, enzyme activity, and protein after 6 d, which were comparable to levels observed in ripening wild-type fruit. A similar level of internal ethylene (0.15 μL/L) was measured in transgenic antisense ACC synthase fruit that were held for 28 d after harvest. In these fruit PG mRNA, enzyme activity, and protein were detected. Collectively, these results suggest that PG mRNA accumulation is ethylene regulated, and that the low threshold levels of ethylene required to promote PG mRNA accumulation may be exceeded, even in transgenic antisense ACC synthase tomato fruit.
PG (EC 3.2.1.15) catalyzes the hydrolytic cleavage of α-(1–4) galacturonan linkages and is a key enzyme involved in the large changes in pectin structure that accompany the ripening of many fruit (Fischer and Bennett, 1991). The tomato (Lycopersicon esculentum L.) fruit PG gene is one member of a family of PG genes present in tomato, but its expression is confined to fruit and it is transcriptionally activated during ripening (Bird et al., 1988; DellaPenna et al., 1989; Montgomery et al., 1993). In spite of extensive research on the regulation of the tomato fruit PG gene, the role of ethylene in regulating PG gene expression remains controversial. Some observations suggest that PG expression is ethylene regulated. First, treatment of MG tomato fruit with ethylene induced PG mRNA accumulation (Grierson et al., 1986; Maunders et al., 1987; Bird et al., 1988). Second, PG mRNA and protein levels were greatly reduced in tomato fruit of the ripening-impaired mutant Nr, which contains a mutation that blocks ethylene perception (Tucker et al., 1980; DellaPenna et al., 1987; Knapp et al., 1989; Lanahan et al., 1995). Third, analysis of the PG promoter revealed sequences with similarity to ethylene-responsive elements identified in the ethylene-regulated E8 and E4 gene promoters (Nicholass et al., 1995). Fourth, inhibitors of ethylene action, such as silver thiosulfate and norbornadiene, reduced PG mRNA accumulation during tomato fruit ripening, suggesting that continuous ethylene perception is required for PG expression (Lincoln et al., 1987; Davies et al., 1988). These observations imply that expression of the PG gene is ethylene regulated.
In contrast, however, tomato fruits expressing an antisense ACC synthase gene that strongly blocked ethylene production were shown to accumulate PG mRNA (Oeller et al., 1991; Theologis et al., 1993). In these experiments PG mRNA accumulated at the appropriate developmental time, suggesting that transcription of the gene was regulated by factors other than ethylene. However, in the same fruits PG protein did not accumulate, and treating the fruits with propylene induced its accumulation. These results suggested that PG gene transcription is ethylene independent but that translation of PG mRNA or the stability of the PG protein is ethylene dependent. Based on these results and others, it has been proposed that ripening-associated genes are regulated by two independent pathways: one that is ethylene independent and developmentally regulated and another that is ethylene dependent (Theologis et al., 1993). In the context of this model, PG gene expression has thus been used as a marker for the ethylene-independent and developmentally regulated pathway (Montgomery et al., 1993; Giovannoni, 1997).
Here we have re-examined the role of ethylene in the regulation of PG mRNA and protein accumulation in transgenic tomato fruit expressing an antisense ACC synthase gene that blocks endogenous ethylene synthesis. Our data indicate that PG mRNA accumulation is ethylene regulated in a concentration- and time-dependent manner. The implications of these results on current models of ethylene-dependent and ethylene-independent regulation of ripening-associated genes are discussed.
MATERIALS AND METHODS
Plant Material and Fruit Treatment
Greenhouse-grown tomato (Lycopersicon esculentum Mill. cv VF36) fruits expressing an antisense ACC synthase gene (line A11.1; Oeller et al., 1991; Theologis et al., 1993) were harvested at the MG stage 37 d after anthesis. Ethylene production rates of harvested fruit were determined, and only fruit producing less than 0.1 nL g−1 h−1 ethylene were included in the experiments. Fruits were placed in humidified 20-L bottles and subjected to a continuous flow of air or ethylene at 20°C. The ripening stage of fruit was determined according to the internal changes associated with ripening, as described by Su et al. (1984): MG1/2, jelly-like materials in at least one but not all locules, few seeds are cut; MG3, jelly-like materials in all locules, seeds not cut; and MG4, some internal red color in locules.
Fruit internal ethylene concentration was determined by withdrawing air samples with a hypodermic syringe from the interior of fruit submerged in water. The air sample was then transferred to a new syringe to avoid injection of fruit juices into the gas chromatograph. Immediately after the air sample was taken the fruits were frozen in liquid nitrogen for further analysis of mRNA and protein levels.
RNA Analysis
Total RNA was isolated from 5 to 10 g fresh weight of frozen pericarp as previously described (Lashbrook et al., 1994). Poly(A+) mRNA was isolated using an Oligotex mRNA kit (Qiagen, Chatsworth, CA). RNA gel blotting to nylon membranes (Hybond N, Amersham) was as described by the manufacturer. The filters were probed with the full-length PG cDNA insert of pPG 1.9 radiolabeled with [32P]dATP (DellaPenna et al., 1987). Hybridization was for 16 h at 65°C in 10% (w/v) dextran sulfate, 2% (w/v) SDS, 1 m NaCl, and 100 mg/mL base-denatured salmon-sperm DNA. The final wash was at 65°C in 0.1× SSC and 0.2% (w/v) SDS for 1 h. Radioactivity in the blot was quantified by exposure to a phosphor imager plate and scanning with a phosphor imager (Fujix BAS 1000, Fuji Medical Systems, Stamford, CT).
Protein Extraction and Enzyme Assay
Protein was extracted from pericarp tissue as previously described (Moore and Bennett, 1994) with slight modifications. Following the high-salt extraction, proteins were precipitated by the addition of ammonium sulfate to 75% saturation. After the sample was centrifuged, the resulting protein pellet was resuspended in 20 mm sodium acetate, pH 4.0, and dialyzed extensively against the same buffer. The dialyzed extract was clarified by centrifugation, and aliquots containing 10 mg of proteins were used for the enzyme assay. The protein content of the extract was determined by the method of Bradford (1976) using BSA as a standard.
PG enzyme activity was assayed as described by Gross (1982) following the modifications by Moore and Bennett (1994). Enzyme samples were incubated at 37°C for 16 h with 0.01% (w/v) sodium azide to prevent microbial growth.
PAGE and Western Blots
Protein (10 mg) of the preparation described above was separated by SDS-PAGE (10%, w/v), transferred to a PVDF membrane (Millipore), and incubated with PG antiserum as previously described (DellaPenna et al., 1986). Detection of the PG protein-antibody complex was carried out using a chemiluminescence western blotting kit (Boehringer Mannheim) according to the manufacturer's instructions.
RESULTS
Concentration Dependence of PG mRNA Accumulation in Response to Ethylene
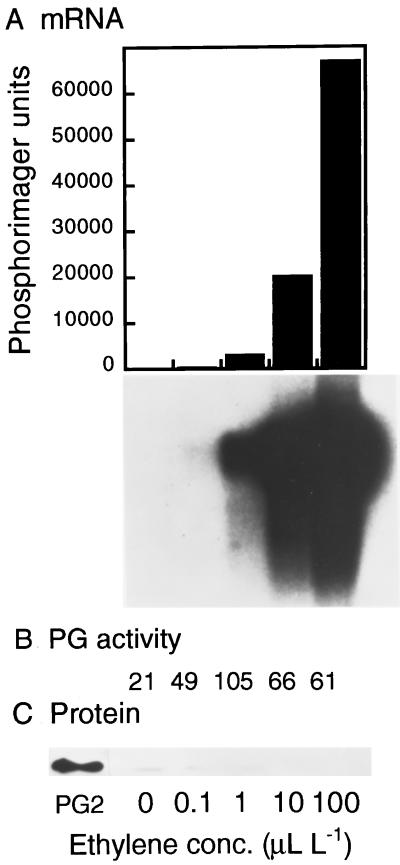
To minimize the effects of endogenous ethylene production in MG fruit, we examined the response of PG mRNA accumulation to exogenous ethylene in tomato fruit in which ethylene production was suppressed by more then 99% by the action of an ACC synthase antisense transgene (Oeller et al., 1991; Theologis et al., 1993). Fruits at the MG stage were treated with different ethylene concentrations for 24 h and then PG mRNA accumulation was determined. The results shown in Figure 1A indicate that 0.1 to 1 μL/L ethylene was sufficient to induce accumulation of PG mRNA, whereas none was detected in untreated fruit. Increasing the ethylene concentration to 10 and 100 μL/L induced higher levels of PG mRNA accumulation. Although the time used in this study was substantially longer than that typically used (24 versus 8 h) to characterize ethylene-responsive genes, the ethylene concentration dependence of PG mRNA accumulation was similar to that found in other ethylene-inducible genes (Lincoln et al., 1987; Giovannoni, 1997). After 24 h of exposure to 100 μL/L ethylene, PG mRNA levels were comparable to those found during ripening of normal fruits. In contrast to the effects of ethylene on PG mRNA accumulation, PG enzyme activity was very low and did not change appreciably during 24-h ethylene treatments (Fig. 1B). This observation is consistent with the inability to detect immunologically PG protein in the same protein samples extracted from ethylene-treated fruit (Fig. 1C).
Figure 1.
Regulation of PG mRNA, enzyme activity, and protein accumulation by different ethylene concentrations. MG transgenic ACC synthase antisense tomato fruit were treated with different ethylene concentrations for 24 h, and PG mRNA, enzyme activity, and protein levels were determined. A, mRNA gel-blot hybridization analysis showing the accumulation of PG mRNA. Each lane contained 4.6 μg of poly(A+) mRNA. Blots were hybridized with a radiolabeled full-length PG cDNA probe. Washes were carried out at high stringency (0.1× SSC and 0.2% [w/v] SDS at 65°C for 1 h). Radioactivity in the blot was estimated with a phosphor imager and plotted as a histogram above the gel blot. B, PG enzyme assays were performed by incubating 10 mg of crude cell wall protein extracts with polygalacturonic acid, and PG activity was determined by a reducing sugar assay. C, Immunoblot of cell wall protein extracts. Each lane contained 10 mg of protein, and PG protein was detected with antibody by using a chemiluminescence kit. PG2 indicates the mobility of purified PG.
Time Dependence of PG mRNA Accumulation in Response to Ethylene
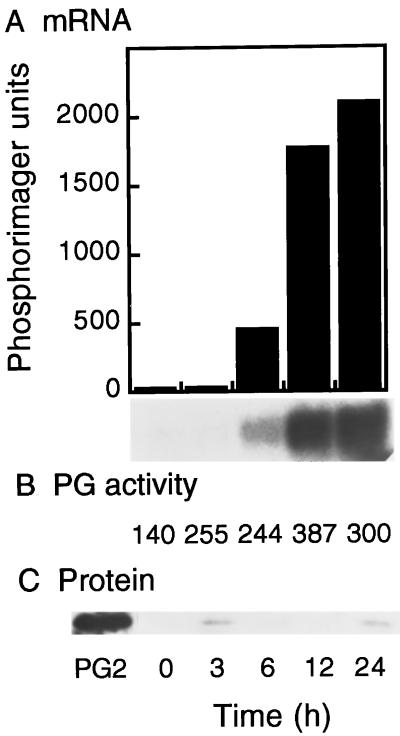
The time dependence of PG mRNA accumulation in response to ethylene was also examined. MG transgenic antisense ACC synthase fruits were treated with 10 μL/L ethylene for periods up to 24 h, and the levels of PG mRNA and protein were determined. Accumulation of PG mRNA was first detected clearly after 6 h of exposure to ethylene, and at this time PG mRNA levels were approximately 12-fold higher than the basal level of PG mRNA detected at 0 and 3 h (Fig. 2A). At 6 to 12 h of exposure to ethylene, the PG mRNA level increased by an additional 3-fold and remained approximately constant at 12 to 24 h (Fig. 2A). PG enzyme activity increased slightly during the first 3 h of ethylene treatment and stayed at a similar level during the 24 h of exposure to ethylene (Fig. 2B). It should be noted that the levels of PG enzyme activity detected in this experiment are very low, amounting to approximately 15% of enzyme activity levels detected in wild-type ripe fruit (compare with Fig. 4B). Immunoblot analysis indicated the presence of extremely low levels of PG protein after 3 h of exposure to ethylene, which did not increase during the 24 h of ethylene treatment, despite the large increase in PG mRNA during this same period (Fig. 2C). The results shown in Figures 1 and 2 indicate that PG mRNA accumulation is not tightly coupled to PG translation, and that there is a time lag of at least 24 h between PG mRNA accumulation and the accumulation of significant levels of PG protein or PG enzyme activity.
Figure 2.
Regulation of PG mRNA, enzyme activity, and protein accumulation by time of ethylene treatment. MG transgenic ACC synthase antisense tomato fruits were treated with 10 μL/L ethylene for the indicated times, and PG mRNA, enzyme activity, and protein levels were determined. A, mRNA-hybridization analysis. Each lane contained 3 μg of poly(A+) mRNA. B, PG enzyme assays were performed by incubating 10 mg of crude cell wall protein extracts with polygalacturonic acid, and PG activity was determined by a reducing sugar assay. C, Immunoblot of cell wall protein extracts. Each lane contained 10 mg of protein, and PG protein was detected with antibody by using a chemiluminescence kit. PG2 indicates the mobility of purified PG.
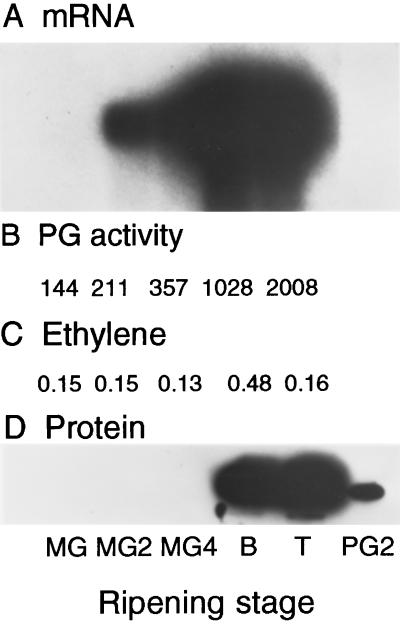
Figure 4.
Accumulation of PG mRNA, enzyme activity, and protein during ripening of transgenic ACC synthase antisense tomato fruits. MG fruits were harvested and maintained under a constant flow of humidified air at 25°C for 28 d. During this period some fruit began to slowly ripen. The ripening stage of each fruit was determined (see Methods), and accumulation of PG mRNA, activity, and protein was analyzed in fruit that had attained a ripening stage defined by the physical state of the locule and seed maturity. A, mRNA-hybridization analysis. Each lane contained 3.2 μg of poly(A+) mRNA. B, PG enzyme assays were performed by incubating 10 mg of crude cell wall protein extracts with polygalacturonic acid, and PG activity was determined by a reducing sugar assay. C, Internal ethylene concentration (microliters/liter). D, Immunoblot of cell wall protein extracts. Each lane contained 10 mg of protein, and PG protein was detected with antibody by using a chemiluminescence kit. PG2 indicates the mobility of purified PG.
Induction of PG mRNA Accumulation by Low Ethylene Concentration
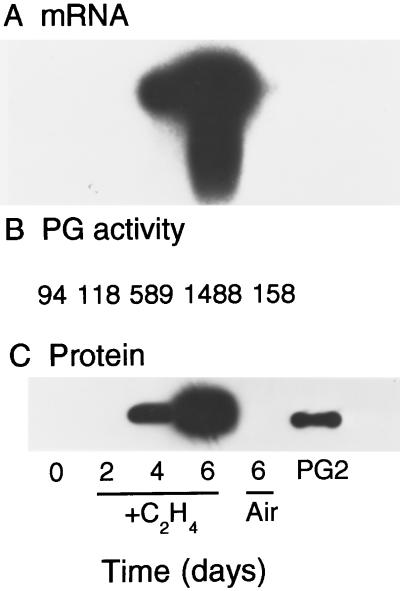
Tomato fruits expressing an antisense ACC synthase antisense gene have been shown to be suppressed in ethylene production by approximately 99%, but ethylene is still produced at a level of < 0.1 nL g−1 h−1 (Oeller et al., 1991; Theologis et al., 1993). To determine whether long-term exposure to low levels of endogenous ethylene may be sufficient to induce PG mRNA and protein accumulation, MG transgenic antisense ACC synthase fruits were treated with 0.1 μL/L ethylene. PG mRNA was readily detected after 4 d and PG enzyme activity increased approximately 5-fold compared with air-treated fruits (Fig. 3, A and B). PG protein was also immunologically detected after 4 d of ethylene treatment (Fig. 3C). At this stage the fruit locules had jellied and fruit color had advanced to faint yellow. After 6 d of exposure to ethylene, the levels of PG mRNA, enzyme activity, and immunologically detected protein increased further and were similar to those found in wild-type fruits at the beginning of the breaker stage (Fig. 3). At this stage the inner pericarp and locules had turned to light pink. Fruits treated with air for 6 d did not contain any PG mRNA, and PG activity and protein levels were similar to the level detected at the beginning of the experiment (Fig. 3). These results indicated that PG mRNA and protein accumulation are induced by ethylene, and that this process is responsive to low levels of ethylene when present for several days. The results also indicate that during the time frame of several days, both PG mRNA and protein are induced to accumulate by the same low ethylene concentration.
Figure 3.
Regulation of PG mRNA, enzyme activity, and protein accumulation by prolonged treatment with a low ethylene concentration. MG transgenic ACC synthase antisense tomato fruits were treated with 0.1 μL/L ethylene, and PG mRNA, activity, and protein accumulation were determined. A, mRNA-hybridization analysis. Each lane contained 3 μg of poly(A+) mRNA. B, PG enzyme assays were performed by incubating 10 mg of crude cell wall protein extracts with polygalacturonic acid and PG activity was determined by a reducing sugar assay. C, Immunoblot of cell wall protein extracts. Each lane contained 10 mg of protein, and PG protein was detected with antibody by using a chemiluminescence kit. PG2 indicates the mobility of purified PG.
PG mRNA Enzyme Activity, and Protein Accumulation during Ripening of Transgenic Tomato Fruit
Although ethylene production in the ACC synthase antisense fruit is severely blocked, the fruits eventually ripen and soften. We therefore examined these fruit over a long period to assess whether they accumulated low levels of ethylene, which might be sufficient to induce PG expression. Since the color change accompanying ripening of transgenic antisense ACC synthase fruit is strongly inhibited, ripening stages were determined according to the texture and color of locules and seed maturity, as judged by their ability to be cut (Su et al., 1984).
Transgenic MG fruit were harvested and maintained at 20°C for 28 d, the ripening stage of each individual fruit was determined by locular and seed maturity criteria, and PG mRNA levels were determined. PG mRNA was first detectable at the late MG2 stage and continued to increase through the breaker and turning stages of these slowly ripening transgenic fruit (Fig. 4A). PG enzyme activity was slightly higher in MG2 than in the MG stage and increased progressively, reaching its highest level at the turning stage (Fig. 4B). PG protein remained below the limits of detection throughout the MG4 stage but was readily detected at the breaker stage and continued to accumulate through the turning stage (Fig. 4D).
The internal ethylene concentration in these fruits was also measured and found to be similar during the first three ripening stages but increased significantly at the breaker stage (Fig. 4C). This pattern of ethylene production with a peak at the breaker stage is typical of ripening in wild-type fruit, although the levels of ethylene observed in these transgenic antisense ACC synthase fruit were vastly reduced relative to wild-type fruit. The concentration of internal ethylene (0.15 μL/L) measured in these fruits was similar to levels that induced PG mRNA and protein accumulation when applied for several days (Fig. 3).
DISCUSSION
The current model describing the regulation of tomato fruit ripening is based on the action of at least two signal transduction pathways: one that is ethylene independent and developmentally regulated and another that is ethylene dependent (Theologis et al., 1993). According to this model, it has been suggested that PG expression is developmentally regulated through the ethylene-independent signal transduction pathway but that translatability of PG mRNA or the stability of the PG protein may be ethylene dependent. However, the data presented here indicate that PG mRNA accumulation is ethylene regulated (Figs. 1–3). Two criteria have been used to establish the role of ethylene in regulating specific gene expression: (a) mRNA accumulation should be induced at physiologically active concentrations of ethylene and (b) the response to ethylene should be fast (Lincoln et al., 1987; Harpham et al., 1996). Ethylene-regulated genes in ripening tomatoes have been arbitrarily defined to show a response at the level of increased mRNA accumulation within 8 h after ethylene treatment (Lincoln et al., 1987; Giovannoni, 1997).
In the experiments presented here, PG mRNA accumulation was induced by physiologically active ethylene levels (0.1–1 μL/L). Ethylene-induced PG mRNA accumulation exhibited a typical concentration dependence, initiated at a threshold between 0.1 and 1 μL/L and a saturation greater than 100 μL/L ethylene. This type of response indicates a high sensitivity to ethylene, as well as a response that does not reach saturation under physiological conditions (Fig. 1). The accumulation of PG mRNA was also detected within 6 h following ethylene treatment at a concentration similar to that used in other studies of ethylene-regulated gene expression (10 μL/L), although the levels detected at 6 h were very low relative to the maximal levels observed after 12 to 24 h of ethylene treatment (Fig. 2). Thus, both criteria required to establish the role of ethylene in PG mRNA accumulation were met. Comparing the data shown in Figures 1 and 2 with those of other ethylene-regulated genes suggests that PG should also be classified as an ethylene-regulated gene (Lincoln et al., 1987; Montgomery et al., 1993; Harpham et al., 1996).
The observation that PG mRNA, but not PG protein, accumulated during development of transgenic ACC synthase antisense fruit suggested the hypothesis that ethylene may exert translational control over expression of this mRNA (Theologis et al., 1993). Our results indicate that, although PG mRNA translation lags behind the accumulation of PG mRNA by at least 24 h, there was no evidence that this lag was shortened by exposure to higher levels of ethylene. A significant lag in PG mRNA translation was previously reported by Biggs and Handa (1988), who observed that during ripening of normal tomato fruit the maximum accumulation of PG mRNA usually preceded the peak of PG protein by about 3 d.
Lincoln et al. (1987) investigated ethylene-regulated gene expression during ripening by cloning mRNAs that accumulated in ethylene-treated unripe tomato fruit. They observed that during wild-type fruit development some of the cloned mRNAs begin to accumulate when ethylene production is at a basal level, whereas other mRNAs begin to accumulate later, after endogenous ethylene levels increase. These data suggested that gene expression during fruit development could be activated in two ways: by an increase in sensitivity to basal ethylene levels or by an increase in ethylene concentration. Theologis et al. (1993) examined the expression of the same genes (Lincoln et al., 1987) in normal and transgenic ACC synthase antisense fruits and concluded that only one of five ripening-regulated genes (E4) was ethylene regulated. Among the five ripening-related genes examined, PG has been previously shown to be the least sensitive to ethylene (Lincoln et al., 1987; Lincoln and Fischer, 1988a, 1988b). Nevertheless, our results indicate that transgenic ACC antisense fruit are not ethylene free, and suggest that the accumulation of PG mRNA in these fruits is responsive to the low level of endogenous ethylene that accumulates (Fig. 4). Thus, the group of genes (including PG) that have been proposed to be ethylene independent based on their expression in transgenic ACC synthase antisense fruit may actually represent a group of genes that are most sensitive to ethylene and are thus responsive to the very low levels of ethylene present in these transgenic fruits.
The results presented here do not question the model of dual regulatory pathways contributing to fruit ripening. It is likely that both ethylene-independent and ethylene-dependent pathways are operative in climacteric fruit and that developmental signals play an important role in the activation of ethylene production and in changing the sensitivity of climacteric fruit tissue to ethylene. However, the results presented here suggest that PG gene expression is a component of the ethylene-dependent pathway and should not be assumed to be a reliable marker for an ethylene-independent developmental pathway that may be operative in climacteric fruit ripening.
ACKNOWLEDGMENTS
We thank Dr. A. Theologis for kindly providing the transgenic antisense ACC synthase tomato seeds used in these experiments and Dr. C. Lashbrook for assistance with growing tomato plants and RNA isolation.
Abbreviations:
- MG
mature green
- PG
polygalacturonase
Footnotes
This research was supported in part by a Binational Agricultural Research and Development Fund Postdoctoral Fellowship to Y.S.
LITERATURE CITED
- Biggs MS, Handa AK. Temporal regulation of polygalacturonase gene expression in fruit of normal, mutant, and heterozygous tomato genotypes. Plant Physiol. 1988;89:117–125. doi: 10.1104/pp.89.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CR, Smith CJS, Ray JA, Moureau P, Bevan MW, Bird AS, Hughes S, Morris PC, Grierson D, Schuch W. The tomato polygalacturonase gene and ripening-specific expression in transgenic plants. Plant Mol Biol. 1988;11:651–662. doi: 10.1007/BF00017465. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davies KM, Hobson GE, Grierson D. Silver ions inhibit the ethylene-stimulated production of ripening-related mRNAs in tomato. Plant Cell Environ. 1988;11:729–738. [Google Scholar]
- DellaPenna D, Alexander DC, Bennett AB. Molecular cloning of tomato fruit polygalacturonase: analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci USA. 1986;83:6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D, Kates DS, Bennett AB. Polygalacturonase gene expression in Rutgers, rin, nor, and Nr tomato fruits. Plant Physiol. 1987;85:502–507. doi: 10.1104/pp.85.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D, Lincoln JE, Fischer RL, Bennett AB. Transcriptional analysis of polygalacturonase and other ripening associated genes in Rutgers, rin, nor, and Nr tomato fruit. Plant Physiol. 1989;90:1372–1377. doi: 10.1104/pp.90.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RL, Bennett AB. Role of cell wall hydrolases in fruit ripening. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:675–703. [Google Scholar]
- Giovannoni JJ. Molecular genetic analysis of ethylene-regulated and developmental components of tomato fruit ripening. In: Kanellis A, Chang C, Kende H, Grierson D, editors. Biology and Biotechnology of the Plant Hormone Ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 133–140. [Google Scholar]
- Grierson D, Maunders MJ, Slater A, Ray J, Bird CR, Schuch W, Holdsworth GA, Knapp JE. Gene expression during tomato ripening. Philos Trans R Soc Lond-Biol Sci. 1986;314:399–410. [Google Scholar]
- Gross KC. A rapid and sensitive spectrophotometric method for assaying polygalacturonase using 2-cyanoacetamide. Hortscience. 1982;17:933–934. [Google Scholar]
- Harpham NVJ, Berry AW, Holland MG, Moshkov IE, Smith AR, Hall MA. Ethylene binding in higher plants. Plant Growth Regul. 1996;18:71–77. [Google Scholar]
- Knapp J, Moureau P, Schuch W, Grierson D. Organization and expression of polygalacturonase and other ripening related genes in Ailsa Craig “Neverripe” and “Ripening inhibitor” tomato mutants. Plant Mol Biol. 1989;12:105–116. doi: 10.1007/BF00017453. [DOI] [PubMed] [Google Scholar]
- Lanahan M, Yen H-C, Giovannoni J, Klee H. The Never Ripe mutation blocks ethylene perception in tomato. Plant Cell. 1995;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrook CC, Gonzalez-Bosch C, Bennett AB. Two divergent endo-b-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell. 1994;6:1485–1493. doi: 10.1105/tpc.6.10.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln JE, Cordes S, Read E, Fischer RL. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci USA. 1987;84:2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln JE, Fischer RL. Diverse mechanisms for the regulation of ethylene-inducible gene expression. Mol Gen Genet. 1988a;212:71–75. doi: 10.1007/BF00322446. [DOI] [PubMed] [Google Scholar]
- Lincoln JL, Fischer RL. Regulation of gene expression by ethylene in wild-type and rin tomato (Lycopersicon esculentum) fruit. Plant Physiol. 1988b;88:370–374. doi: 10.1104/pp.88.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunders MJ, Holdsworth MJ, Slater A, Knapp JE, Bird CR, Schuch W, Grierson D. Ethylene stimulates the accumulation of ripening-related mRNAs in tomatoes. Plant Cell Environ. 1987;10:177–184. [Google Scholar]
- Montgomery J, Pollard V, Deikman J, Fischer RL. Positive and negative regulatory regions control the spatial distribution of polygalacturonase transcription in tomato fruit pericarp. Plant Cell. 1993;5:1049–1062. doi: 10.1105/tpc.5.9.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Bennett AB. Tomato fruit polygalacturonase isozyme 1. Plant Physiol. 1994;106:1461–1469. doi: 10.1104/pp.106.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholass FJ, Smith CJS, Schuch W, Bird CR, Grierson D. High levels of ripening-specific reporter gene expression directed by tomato fruit polygalacturonase gene-flanking regions. Plant Mol Biol. 1995;28:423–435. doi: 10.1007/BF00020391. [DOI] [PubMed] [Google Scholar]
- Oeller PW, Min-Wong L, Taylor L, Pike D, Theologis A. Reversible inhibition of tomato fruit senescence by antisense RNA. Science. 1991;254:437–439. doi: 10.1126/science.1925603. [DOI] [PubMed] [Google Scholar]
- Su L, McKeon T, Grierson D, Cantwell M, Yang SF. Development of 1-aminocyclopropane-1-carboxylic acid synthase and polygalacturonase activities during the maturation and ripening of tomato fruit. Hortscience. 1984;19:576–578. [Google Scholar]
- Theologis A, Oeller PW, Wong L, Rottmann WH, Gantz DM. Use of a tomato mutant constructed with reverse genetics to study fruit ripening, a complex developmental process. Dev Genet. 1993;14:282–295. doi: 10.1002/dvg.1020140406. [DOI] [PubMed] [Google Scholar]
- Tucker GA, Robertson NG, Grierson D. Changes in poly-galacturonase isoenzymes during the 'ripening' of normal and mutant tomato fruit. Eur J Biochem. 1980;112:119–124. doi: 10.1111/j.1432-1033.1980.tb04993.x. [DOI] [PubMed] [Google Scholar]