Abstract
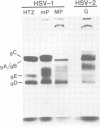
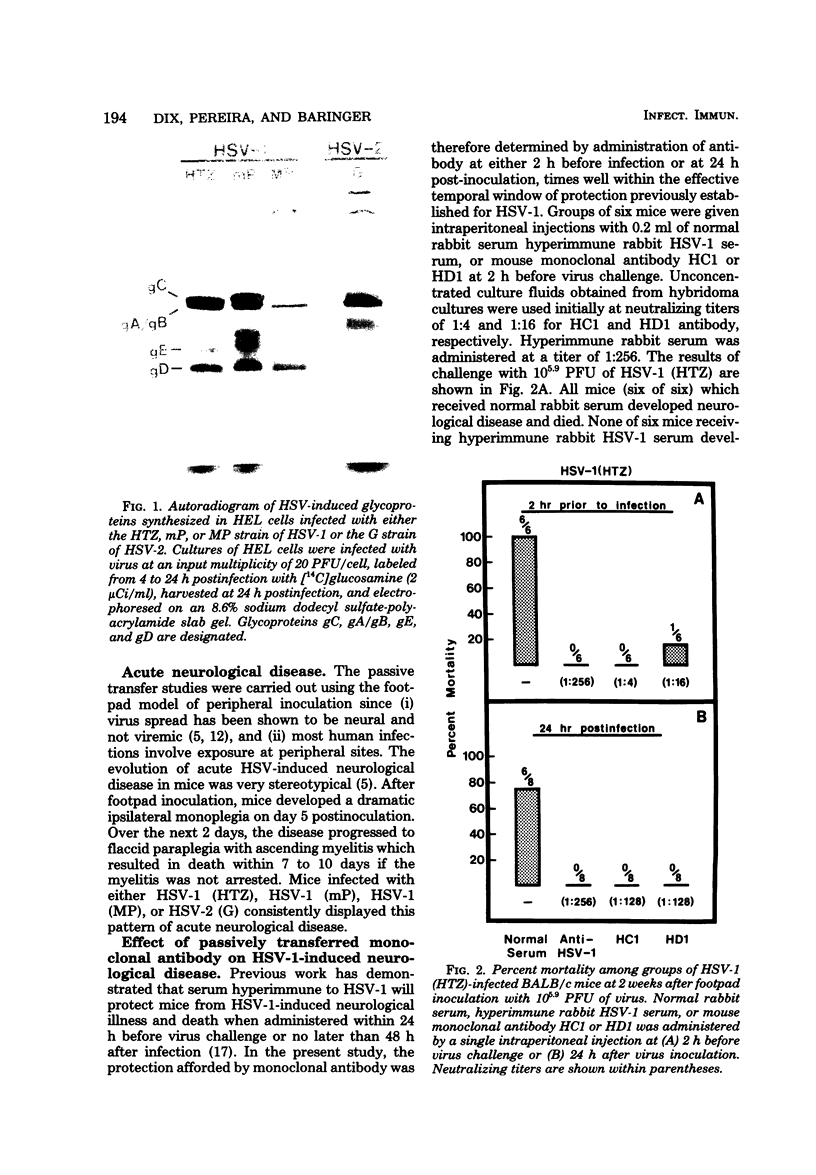
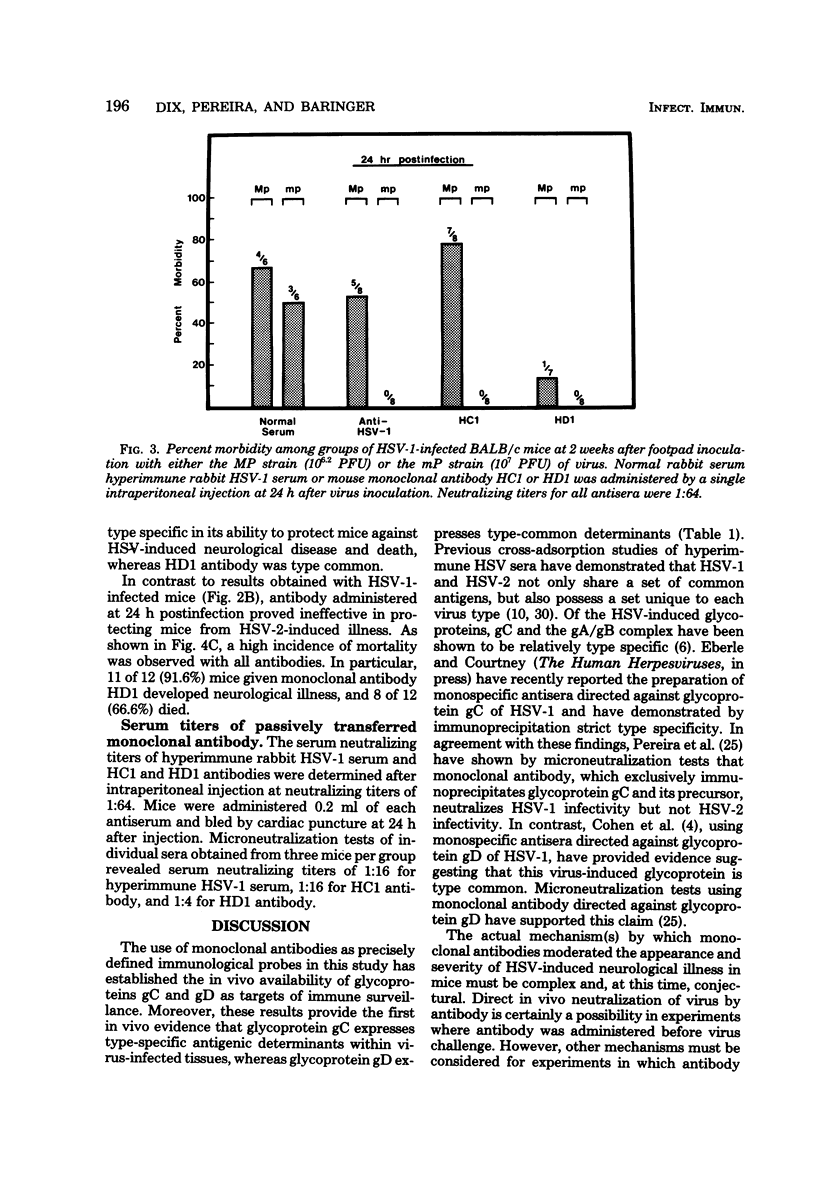
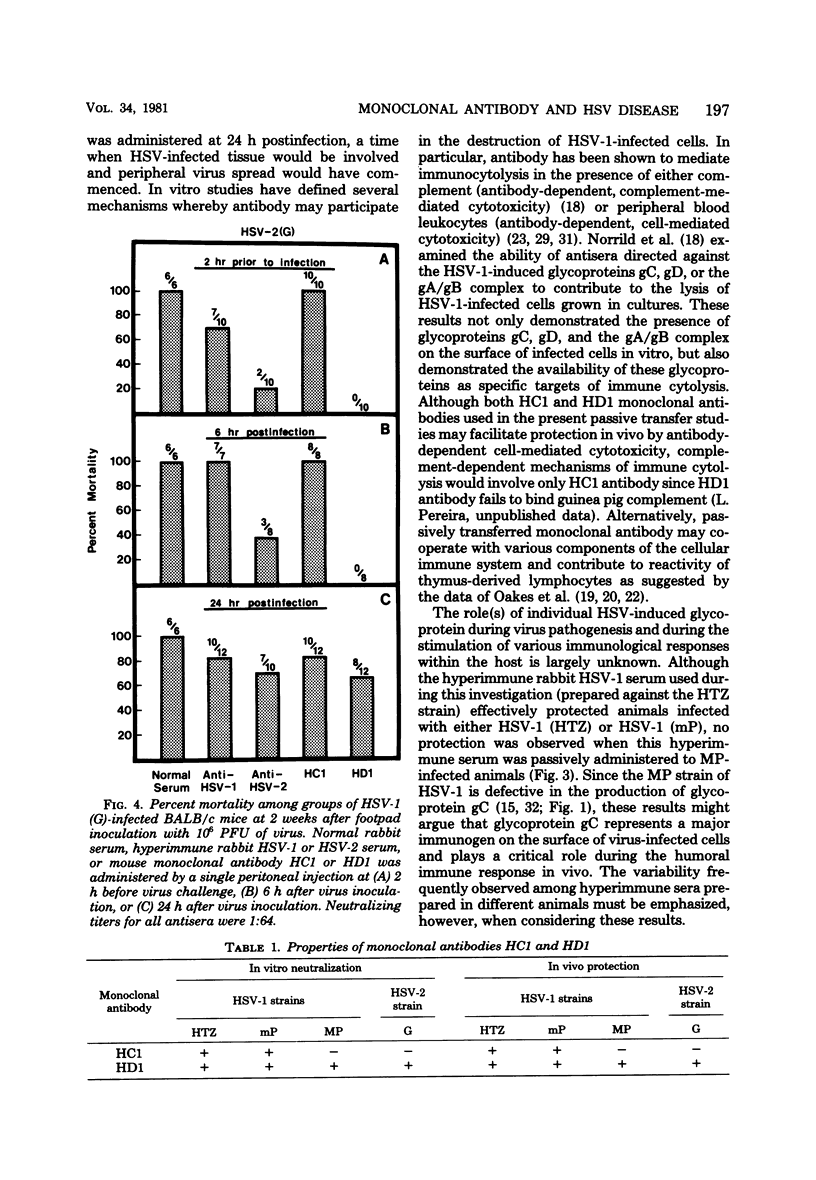
Monoclonal antibodies HCl and HD1, directed against herpes simplex virus type 1 (HSV-1) glycoproteins gC and gD, respectively, were evaluated for their ability to passively immunize mice against acute virus-induced neurological disease after footpad inoculation with HSV-1 or herpes simplex virus type 2 (HSV-2). Control virus-infected mice receiving a single intraperitoneal injection of normal serum died within 7 to 10 days after the spread of virus from footpad to spinal cord and brain. However, a single intraperitoneal injection of either HCl or HD1 antibody protected mice from neurological illness and death when administered to HSV-1 (strain HTZ)-infected mice at either 2 h before virus challenge or at 24 h after virus inoculation. To determine the in vivo specificity of the antibodies, passive transfer studies were performed with mice infected with the MP strain of HSV-1, a mutant of HSV-1 (mP) which is defective in the production of glycoprotein gC. Whereas HD1 antibody decreased the incidence of neurological illness in MP- and mP-infected mice, HCl antibody, which protected mP-infected animals, failed to protect mice infected with the MP strain. When HD1 antibody was administered to HSV-2 (strain G)-infected mice at either 2 h before virus challenge or at 6 h (but not 24 h) after virus inoculation, 100% of the infected animals receiving HD1 antibody survived. In contrast, 100% of HSV-2 (strain G)-infected animals passively immunized with HCl antibody developed neurological illness and died. These results provide in vivo evidence that the HSV-induced glycoprotein gC expresses type-specific antigenic determinants, whereas glycoprotein gD expresses type-common determinants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron S., Worthington M. G., Williams J., Gaines J. W. Postexposure serum prophylaxis of neonatal herpes simplex virus infection of mice. Nature. 1976 Jun 10;261(5560):505–506. doi: 10.1038/261505a0. [DOI] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J. Immunologic and biochemical characterization of individual polypeptides induced by herpes simplex virus types 1 and 2. Adv Pathobiol. 1976;(5):87–103. [PubMed] [Google Scholar]
- Dix R. D., Courtney R. J. Effects of cytochalasin B on herpes simplex virus type 1 replication. Virology. 1976 Mar;70(1):127–135. doi: 10.1016/0042-6822(76)90242-7. [DOI] [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970 Feb;104(2):289–295. [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Powell K. L., Robinson D. J., Sim C., Watson D. H. Type specific and type common antigens in cells infected with herpes simplex virus type 1 and on the surfaces of naked and enveloped particles of the virus. J Gen Virol. 1974 Feb;22(2):159–169. doi: 10.1099/0022-1317-22-2-159. [DOI] [PubMed] [Google Scholar]
- Klein R. J. Effect of immune serum on the establishment of herpes simplex virus infection in trigeminal ganglia of hairless mice. J Gen Virol. 1980 Aug;49(2):401–405. doi: 10.1099/0022-1317-49-2-401. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Lycke E., Sjöstrand J. Spread of herpes simplex virus in peripheral nerves. Acta Neuropathol. 1971;17(1):44–53. doi: 10.1007/BF00684740. [DOI] [PubMed] [Google Scholar]
- Luyet F., Samra D., Soneji A., Marks M. I. Passive immunization in experimental Herpesvirus hominis infection of newborn mice. Infect Immun. 1975 Dec;12(6):1258–1261. doi: 10.1128/iai.12.6.1258-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendall R. R. Comparative neurovirulence and latency of HSV1 and HSV2 following footpad inoculation in mice. J Med Virol. 1980;5(1):25–32. doi: 10.1002/jmv.1890050104. [DOI] [PubMed] [Google Scholar]
- McKendall R. R., Klassen T., Baringer J. R. Host defenses in herpes simplex infections of the nervous system: effect of antibody on disease and viral spread. Infect Immun. 1979 Feb;23(2):305–311. doi: 10.1128/iai.23.2.305-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Davis W. B., Taylor J. A., Weppner W. A. Lymphocyte reactivity contributes to protection conferred by specific antibody passively transferred to herpes simplex virus-infected mice. Infect Immun. 1980 Aug;29(2):642–649. doi: 10.1128/iai.29.2.642-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E. Invasion of the central nervous system by herpes simplex virus type 1 after subcutaneous inoculation of immunosuppressed mice. J Infect Dis. 1975 Jan;131(1):51–57. doi: 10.1093/infdis/131.1.51. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Rosemond-Hornbeak H. Antibody-mediated recovery from subcutaneous herpes simplex virus type 2 infection. Infect Immun. 1978 Aug;21(2):489–495. doi: 10.1128/iai.21.2.489-495.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleske J. M., Ashman R. B., Kohl S., Shore S. L., Starr S. E., Wood P., Nahmias A. J. Human polymorphonuclear leucocytes as mediators of antibody-dependent cellular cytotoxicity to herpes simplex virus-infected cells. Clin Exp Immunol. 1977 Mar;27(3):446–453. [PMC free article] [PubMed] [Google Scholar]
- Openshaw H., Asher L. V., Wohlenberg C., Sekizawa T., Notkins A. L. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol. 1979 Jul;44(1):205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Price R. W., Walz M. A., Wohlenberg C., Notkins A. L. Latent infection of sensory ganglia with herpes simplex virus: efficacy of immunization. Science. 1975 May 30;188(4191):938–940. doi: 10.1126/science.166432. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. The role of antibody dependent cytotoxicity in recovery from herpesvirus infections. Cell Immunol. 1976 Mar 1;22(1):182–186. doi: 10.1016/0008-8749(76)90019-8. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Sim C., Watson D. H. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973 May;19(2):217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]