Abstract
Three hybridomas, designated C2, V17, and B2, were produced from BALB/c mice after immunization with measles virus. All three were directed against the virus hemagglutinin (HA). The HA is a structural peptide of the virus and constitutes a major target for the host immune response during measles infection. The monoclonal anti-HA antibodies have biological functions such as (i) measles virus neutralization in vitro, (ii) binding to acutely and persistently infected cells, and (iii) inhibition of HA-mediated Rhesus monkey erythrocyte agglutination. Different idiotypes, designated HAMM-1, HAMM-2, and HAMM-3, were defined on C2, V17, and B2, respectively, by syngeneic anti-idiotype sera against those three monoclonal antibodies. A limited cross-reactivity with the HAMM-1 idiotype was detected in sera from some BALB/c mice immunized with measles virus. The anti-idiotype sera could significantly inhibit the biological functions of the HAMM-1 and HAMM-3 idiotypes bearing monoclonal anti-HA-antibodies. This suggests a possible role for auto-anti-idiotypes in the immune response after infection with measles virus.
Full text
PDF



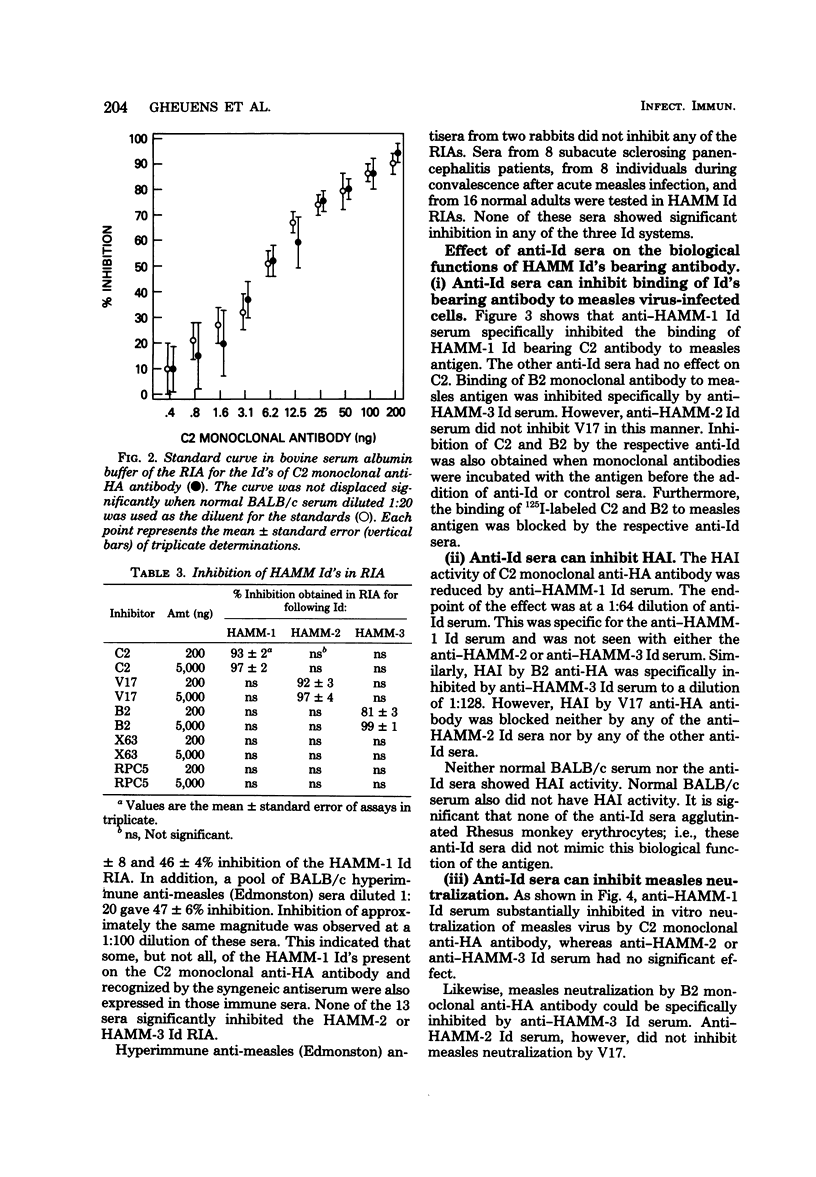
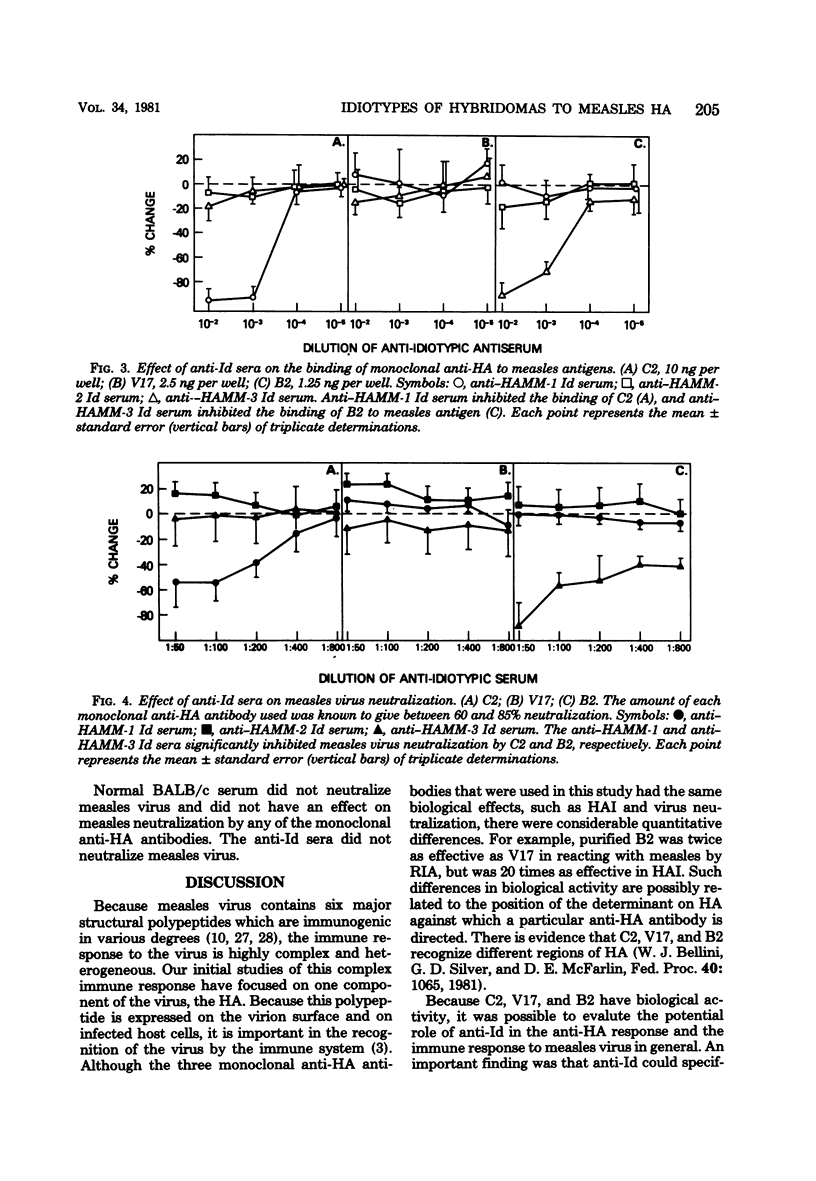


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Williamson A. R., Askonas B. A. One cell-one immunoglobulin. Origin of limited heterogeneity of myeloma proteins. Biochem J. 1970 Jan;116(2):241–248. doi: 10.1042/bj1160241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bellini W. J., McFarlin D. E., Silver G. D., Mingioli E. S., McFarland H. F. Immune reactivity of the purified hemagglutinin of measles virus. Infect Immun. 1981 Jun;32(3):1051–1057. doi: 10.1128/iai.32.3.1051-1057.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Trudgett A., McFarlin D. E. Purification of measles virus with preservation of infectivity and antigenicity. J Gen Virol. 1979 Jun;43(3):633–639. doi: 10.1099/0022-1317-43-3-633. [DOI] [PubMed] [Google Scholar]
- Bona C., Hooghe R., Cazenave P. A., Leguérn C., Paul W. E. Cellular basis of regulation of expression of idiotype. II. Immunity to anti-MOPC-460 idiotype antibodies increases the level of anti-trinitrophenyl antibodies bearing 460 idiotypes. J Exp Med. 1979 Apr 1;149(4):815–823. doi: 10.1084/jem.149.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brient B. W., Nisonoff A. Quantitative investigations of idiotypic antibodies. IV. Inhibition by specific haptens of the reaction of anti-hapten antibody with its anti-idiotypic antibody. J Exp Med. 1970 Nov;132(5):951–962. doi: 10.1084/jem.132.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebers G. C., Zabriskie J. B., Kunkel H. G. Oligoclonal immunoglobulins in subacute sclerosing panencephalitis and multiple sclerosis: a study of idiotypic determinants. Clin Exp Immunol. 1979 Jan;35(1):67–75. [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. The chromic chloride method of coupling antigens to erythrocytes: definition of some important parameters. J Immunol Methods. 1976;10(1):61–66. doi: 10.1016/0022-1759(76)90007-7. [DOI] [PubMed] [Google Scholar]
- Golub E. S. Idiotypes and the network hypothesis. Cell. 1980 Dec;22(3):641–642. doi: 10.1016/0092-8674(80)90536-x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Black S. J., Herzenberg L. A. Regulatory circuits and antibody responses. Eur J Immunol. 1980 Jan;10(1):1–11. doi: 10.1002/eji.1830100102. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Köhler G., Shulman M. J. Cellular and molecular restrictions of the lymphocyte fusion. Curr Top Microbiol Immunol. 1978;81:143–148. doi: 10.1007/978-3-642-67448-8_22. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamoyi E., Estess P., Capra J. D., Nisonoff A. Heterogeneity of an intrastrain cross-reactive idiotype associated with anti-p-azophenylarsonate antibodies of A/J mice. J Immunol. 1980 Jun;124(6):2834–2840. [PubMed] [Google Scholar]
- McFarland H. F., McFarlin D. E. Cellular immune response to measles, mumps, and vaccinia viruses in multiple sclerosis. Ann Neurol. 1979 Aug;6(2):101–106. doi: 10.1002/ana.410060204. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., Bellini W. J., Mingioli E. S., Behar T. N., Trudgett A. Monospecific antibody to the haemagglutinin of measles virus. J Gen Virol. 1980 Jun;48(Pt 2):425–429. doi: 10.1099/0022-1317-48-2-425. [DOI] [PubMed] [Google Scholar]
- Mingioli E. S., Strober W., Tourtellotte W. W., Whitaker J. N., McFarlin D. E. Quantitation of IgG, IgA and IgM in the CSF by radioimmunoassay. Neurology. 1978 Oct;28(10):991–995. doi: 10.1212/wnl.28.10.991. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Choppin P. W. A comparison of the polypeptides of four measles virus strains. Virology. 1977 May 15;78(2):463–474. doi: 10.1016/0042-6822(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Noppe M., Lowenthal A., Karcher D., Gheuens J. A two-site immunoradiometric assay for the determination of alpha-albumin. J Immunol Methods. 1979 May 10;27(1):75–81. doi: 10.1016/0022-1759(79)90240-0. [DOI] [PubMed] [Google Scholar]
- Norrby E., Gollmar Y. Identification of measles virus-specific hemolysis-inihibiting antibodies separate from hemagglutination-inhibiting antibodies. Infect Immun. 1975 Feb;11(2):231–239. doi: 10.1128/iai.11.2.231-239.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Hammarskjöld B. Structural components of measles virus. Microbios. 1972 Jan;5(17):17–29. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Sher A., Cohn M. Effect of haptens on the reaction of anti-idiotype antibody with a mouse anti-phosphorylcholine plasmacytoma protein. J Immunol. 1972 Jul;109(1):176–178. [PubMed] [Google Scholar]
- Trudgett A., Bellini W. J., Mingioli E. S., McFarlin D. E. Antibodies to the structural polypeptides of measles virus following acute infection and in SSPE. Clin Exp Immunol. 1980 Mar;39(3):652–656. [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Weiner H. L., Fields B. N. Immune response in subacute sclerosing panencephalitis: reduced antibody response to the matrix protein of measles virus. J Immunol. 1979 Aug;123(2):884–889. [PubMed] [Google Scholar]
- Weigert M., Raschke W. C., Carson D., Cohn M. Immunochemical analysis of the idiotypes of mouse myeloma proteins with specificity for levan or dextran. J Exp Med. 1974 Jan 1;139(1):137–147. doi: 10.1084/jem.139.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]