Abstract
Introduction and hypothesis
The objective of this study was to compare puborectal muscle integrity and bulk in women with both major levator ani (LA) defects on MRI and pelvic organ prolapse (POP) to women with normal LA muscle and normal support.
Methods
This is a case-control study comparing 24 cases with known major LA defects and POP to 24 controls with normal LA and normal support. Axial T-2 weighted MRI scans of the pelvis were evaluated for integrity of the puborectal muscle and degree of muscle bulk.
Results
There were no significant group differences in age, body mass index, vaginal deliveries, or hysterectomy status. In all 48 subjects, the puborectal muscle was visible and had no disruption noted. There was no difference in muscle bulk between groups (control/case, thin 42% vs. 25%, average 42% vs. 38%, thick-17% vs. 38%; P=0.47).
Conclusions
Defects and loss of muscle bulk in the puborectal muscle are not seen on MRI in women with major LA defects and POP.
Keywords: Levator ani, Pelvic organ prolapse
Introduction
Standardized anatomical terminology identifies three major components of the levator ani muscle: pubococcygeal, iliococcygeal, and puborectal [1, 2]. Each of these elements has at least one component that decussates behind the rectum near the anorectal junction and contributes to pulling the pelvic organs toward the pubic bone creating an angulation in the anorectal region [3]. It is known that vaginal birth is associated with levator ani (LA) injury [4]. It is also known that loss of this levator muscle action is associated with pelvic floor disorders, specifically pelvic organ prolapse (POP) and fecal incontinence [4–6]. Until recently, it has not been possible to examine each of the different components of the levator ani muscle to evaluate their individual structural integrity. Recent work has described the appearance of each of the different components of the levator ani muscle on ultrasound (US) [7] and MRI [8], making it is possible to evaluate the status of the puborectal muscle specifically.
A portion of the levator ani muscle arises from the pubic bone. Some authors use the term puborectal muscle to describe all the muscle arising from the pubic bone while others, such as Terminologia Anatomica, the international standard for anatomical terms, divides it into two components: the pubococcygeal and puborectal. To avoid confusion between the two different uses of the term “puborectal,” we will use “puborectalTA” and “pubococcygealTA” to designate the Terminologia Anatomica definitions.
The pubococcygeusTA lies medial to the puborectalisTA, arises high on the pubis near the superior pubic ramus, and inserts into the vagina, perineal body, and anus. The puborectalTA arises near the inferior pubic ramus and passes dorsal to the rectum at the anorectal angle just cephalad to the anal sphincter. These distinctions are visible on MRI and US. It is clear that levator avulsion involves the pubic portion of the levator ani muscle. Prior studies have shown that the pubococcygealTA is universally involved in the levator avulsion, but it is not clear whether the puborectalTA muscle is as well.
The aim of this study is to specifically compare the status of the puborectalTA (muscle integrity and bulk) in women with both major levator defects and POP on magnetic resonance (MR) imaging to women with no levator defects and normal pelvic support. We hypothesize that there is no difference in puborectalTA integrity or bulk between groups.
Methods
We performed a secondary analysis of MR images obtained as part of two institutional-review-board-approved case control studies of POP and stress urinary incontinence [5, 9]. Inclusion criteria for case subjects were twofold. First, subjects had to have a major LA injury (defined as greater than 50% loss of the pubic portion of the LA) on MRI. Secondly, they had to have clinically significant prolapse (anterior, apical, or posterior support ≥1 cm below the hymen). Women were excluded if they had previous surgery for pelvic floor dysfunction (e.g., pelvic organ prolapse, urinary incontinence, or fecal incontinence). Women who had undergone hysterectomy were eligible if the surgery had been at least 2 years prior to enrollment and if the indication for surgery was not pelvic floor dysfunction. Inclusion criteria for control subjects were (1) no loss of the pubic portion of the LA on MRI and (2) normal pelvic organ support on exam (anterior and posterior wall support ≥1 cm above the hymen). Controls were recruited via community-based media advertising and excluded if they had pelvic floor dysfunction symptoms such as vaginal bulge or incontinence.
Magnetic resonance imaging was conducted in the axial, sagittal, and coronal planes in the supine position using two-dimensional fast spin proton density MR scans (echo time, 15 ms; repetition time, 4,000 ms). The scans early in the study period were performed on a 1.5 Tesla superconducting magnet (Signa; General Electric Medical Systems, Milwaukee, WI) and later, on a 3 Tesla Philips Achieva System (Amsterdam, The Netherlands). The slice thickness was 4 mm with a slice gap of 1 mm, yielding an image spacing of 5 mm. A 160×160 mm field of view and an imaging matrix of 256×256 were used.
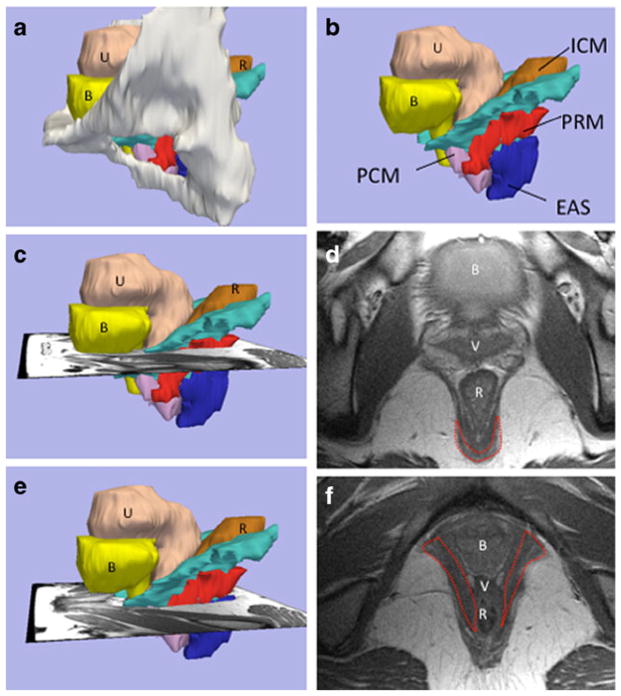
The puborectalTA was identified on MR scans in its characteristic location as previously described by Kearney et al. 2004 and Margulies et al. 2006 [2, 8]. This later technique imports MR images into the 3-D Slicer 2.1b1 program, thus allowing the appearance of a muscle in one plane to be compared with the same muscle in other planes in the same 3-D space. This creates a 3-D model, which allows a better understanding of such pelvic anatomy as the subdivisions of the levator ani muscle complex. Specifically, the puborectalTA decussates behind the anorectal junction cranial to the external anal sphincter, lies lateral to the iliococcygeal and pubococcygeal muscle lateral to the rectum and vagina, and immediately adjacent to the ischiorectal fat, and projects towards the pubic bone just cephalad to the perineal membrane (Fig. 1). The puborectalTA was evaluated in its entirety from its origin at the pubic bone to its decussation behind the rectum, as we viewed the contiguous cross-sectional MR images.
Fig. 1.
Structural orientation to levator ani subdivisions and visibility of the puborectal muscle in MR images at two different levels (chosen from multiple contiguous sections as we followed the puborectalTA muscle from its origin at the pubic bone to its decussation behind the rectum); a lateral view of 3-D model with pubic bones, (B bladder, U uterus, R rectum), b levator subdivisions: without bones (PCM pubococcygeus muscle, ICM iliococcygeus muscle, PRM puborectal muscleTA, EAS external anal sphincter). c Level of the PRM decussation showing the scan plane for d, revealing the MRI at level of the PRM decussation (outlined), e level where the PRM “arms” project towards the pubic bone; f MRI at the level of the PRM “arms” (outlined in red)
Determination of levator ani muscle defect status was performed by two examiners, blinded to subject demographic status. Each examiner independently graded LA defect status on MR images using a system previously described by our group [10, 11]. Both the first author and an additional author evaluated a separate group of 80 MRIs to gain familiarity with the morphological appearance of the levator ani muscle subdivisions as previously described by Kearney et al. [2]. The left and right levator ani muscles were scored separately. When scores of the two reviewers differed, scans were reviewed jointly by both individuals to assign a final score. Major injury was determined to exist if more than 50% of the muscle was visibly missing [5].
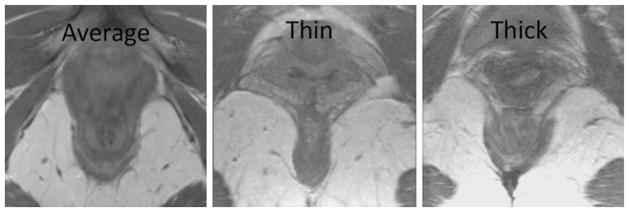
Prior to determining puborectalTA bulk in this study, representative scans were reviewed and discussed by the investigators to gain experience with the variation seen among individuals. Once parameters were set regarding muscle bulk, the puborectalTA status was assessed in two qualitative ways. First, the muscle was evaluated for avulsion by determining whether it was actually present in its normal location projecting towards the pubic bone. Second, as shown in Fig. 2, the bulk of the muscle was ultimately deemed “normal,” “thin,” or “thick” at the area of decussation behind the anus (Fig. 2). We chose a qualitative grading system for muscle bulk because it allows an overall assessment of the bulk of a muscle whose shape varies considerably among women. Both investigators were blinded to group assignment throughout the evaluation process. The top portion of the image containing the puborectalTA was masked during examination so that it was not visible while puborectalTA status was assessed. To compare group characteristics, t tests were used, and a Chi-squared test was used to compare the puborectalTA prominence percentages between groups.
Fig. 2.
Examples of PRM prominence at the point of decussation behind the anorectal junction
Results
The groups did not differ in age, parity, or hysterectomy status, but, by design, differed in pelvic organ support (Table 1). Average subject age was 52 years (+/−14.1) for controls and 53 years (+/−10.0) for cases. Average body mass index for controls was 26 kg/m2 (+/−4.1) and 25 kg/m2 (+/−4.7) for cases. Vaginal parity was 2.2 (+/−1.3) and 2.5 (+/−1.6) for controls and cases, respectively. Hysterectomy rates were 12% and 17% for controls and cases, respectively. Average point of maximal prolapse for the case group was 2.9 cm (+/−1.8). All 48 subjects were white.
Table 1.
Demographics and POP-Q measurements of groups
| Controls (n=24) | Cases (n=24) | p value | |
|---|---|---|---|
| Age (years) | 52.7 (14.1) | 53.6 (10.0) | 0.26 |
| BMI (kg/m2) | 26.3 (4.1) | 25.4 (4.7) | 0.71 |
| Vaginal parity | 2.2 (1.3) | 2.5 (1.6) | 0.71 |
| Hysterectomy | 12% | 17% | 0.08 |
| POP_Q | |||
| Aa | −1.5 (0.7) | 0.8 (1.5) | 0.005 |
| Ba | −1.5 (0.8) | 2.3 (1.8) | 0.002 |
| C | −6.6 (1.5) | −0.8 (4.0) | 0.000 |
| Bp | −1.7 (0.9) | −0.3 (2.0) | 0.002 |
| Ap | −1.8 (2.0) | −0.9 (2.7) | 0.002 |
| Genital hiatus (rest) | 2.6 (0.8) | 5.0 (1.1) | 0.154 |
Values expressed as mean (standard deviation) or percentage
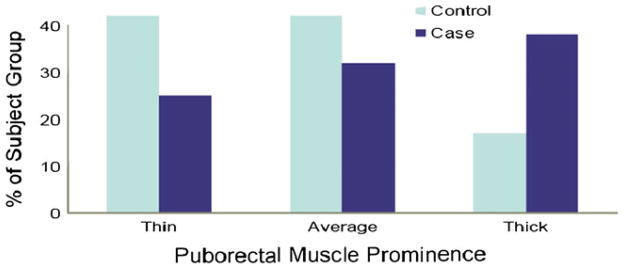
PuborectalTA findings are shown in Table 2. In all 48 women, the puborectalTA was visible on MRI in its expected location as defined above, and no subjects had an avulsion of the muscle from the pubic bone. Similarly, the muscles in the two groups did not differ statistically with respect to bulk (Table 2). Forty-two percent of the controls and 38% of the cases had normal puborectalTA prominence. Forty-two percent and 25% had thinner puborectal muscleTA (PRM) prominence in the control and case groups, respectively. Seventeen percent and 38% had thicker PRM prominence in the control and case groups, respectively (Fig. 3).
Table 2.
Puborectal muscle status among groups
| Controls (n=24) | Cases (n=24) | p value | |
|---|---|---|---|
| Puborectal muscle avulsion | 0% (0) | 0% (0) | 1.0 |
| Puborectal muscle bulk | |||
| Thin | 42% (10) | 25% (6) | |
| Average | 42% (10) | 38% (9) | |
| Thick | 17% (4) | 38% (9) | 0.47a |
Values reported as % (n)
Chi-square
Fig. 3.
PuborectalTA prominence in cases versus control groups
Discussion
Our findings show that the puborectalTA muscle remains visibly intact on MRI in women with major damage to the pubic portion of the levator ani muscle and pelvic organ prolapse below the level of the hymen. Additionally, the muscle does not appear to be thinner or more atrophic in individuals with defects and prolapse compared with women with normal muscles and support.
Levator damage is an important cause of POP and vaginal birth injury is clearly the most common etiology of levator ani injury [4, 12]. MR studies conducted on 160 primiparous women soon after delivery revealed 32 levator injuries, 29 of which involved the pubic portion of the muscle and only three involving the iliococcygeal muscle. But puborectalTA involvement could not be determined at that time because its identity on MR had not been established separately from the pubococcygeus [12]. This current study helps demonstrate that, although all of the case subjects had a >50% avulsion injury of the levator ani muscle by definition, it was not the puborectalTA specifically involved.
Learning how to reduce these injuries will depend on understanding how and why the muscle is damaged. Biomechanical modeling studies led to the stretch-induced muscle injury hypothesis that suggests specific muscle subdivisions are vulnerable to injury depending on the degree to which they are stretched during delivery [13]. Whether these hypothesized injury mechanisms are true or not depends on establishing separate clinical observations that could support or refute this hypothesis. This muscle-stretch hypothesis suggests that the medial portions of the pubococcygeus muscle are most vulnerable to stretch-induced injury, with the iliococcygeal muscle less vulnerable and the puborectalTA least vulnerable [13].
The trend towards a more prominent puborectalTA in individuals who have LA defects and prolapse is consistent with prior findings. Hsu et al. found that women with prolapse had MR imaging with a thicker levator ani muscle dorsally where the puborectalTA is found [14]. This observation is also consistent with compensatory hypertrophy hypothesis. When the ventral portion of the levator is damaged, the intact dorsal portion (specifically the puborectalTA), hypertrophies in compensation. The presence of this compensatory hypertrophy may help explain why a loss of less than 50% of the muscle is not associated with prolapse, while a loss of >50% has a significant association with prolapse [5]. Presumably, in the lesser degrees of injury, the remaining muscle can hypertrophy to take over some of the lost function while greater degrees of injury exceed the capability of remaining muscle to increase its activity.
This study did not address the question of whether or not the puborectalTA is missing or atrophic in women with fecal incontinence, which is an important area of future research. Now that this portion of the LA muscle has been identified clearly in MR [8] and 3-D ultrasound [7], it should be possible to recruit properly matched cases and controls to investigate this specific question. In addition, studying the appearance of the muscle and factors such as the anorectal angle, levator plate, and perineal descent should be feasible as well. As our ability to observe more specific detail has improved, our understanding of the relationship between levator injury and specific types of pelvic floor dysfunction will also improve.
There are several limitations that should be kept in mind in evaluating the results of this study. Given our small sample size, we deliberately chose to compare normal women to those with major muscle defects. The results therefore are not representative of what would occur in the population (with a spectrum of degrees of muscle injury), and further research would be needed in order to address that issue. The assessment used was subjective, based on the authors’ extensive experience with examining the levator ani, and specific measurements of levator ani muscle thickness were not made. This was done because of the highly variable morphology of the muscle, thus making it very difficult to quantify muscle bulk. Measuring thickness at a specific point has the disadvantage of sampling only a small portion of the overall muscle and can be influenced by simple differences between women in the shape of their muscle rather than its bulk. Also, it is not possible for the investigators to be completely blinded to subject status. We did cover the region of the MRI images where the majority of the LA defect is typically seen in order to minimize bias in the observer. It was not possible to shield the extent of prolapse in some of the scans since these portions of the image are often necessary to evaluate the puborectalisTA. Finally, future studies involving the use of MR imaging both before and after delivery are needed to investigate the etiology of LA muscle damage in a prospective fashion.
In conclusion, defects in the puborectalTA are not seen in women with levator ani muscle defects on MR images and pelvic organ prolapse or in women with normal support. This suggests that another portion of the LA muscle is more affected, possibly the pubococcygeus muscle. The muscles seem to be of similar bulk in cases and controls, suggesting that atrophy is not more common in women with prolapse and defects. With a better understanding of the at-risk anatomy in pelvic floor disorders, methods of prevention may be instituted.
Acknowledgments
This project was funded by National Institute of Childhood Diseases and National Institute 38865 and Office for Research on Women’s Health’s SCOR on Sex and Gender Factors Affecting Women’s Health P50 HD044406.
Footnotes
Conflict of interest None.
Contributor Information
John O. L. DeLancey, Pelvic Floor Research Group, Department of Obstetrics and Gynecology, University of Michigan, 1500 E. Medical Center Drive, SPC 5276, Ann Arbor, MI 48109, USA
Helle Christina Sørensen, Department of Obstetrics and Gynaecology, Herlev County Hospital, University of Copenhagen, Herlev Ringvej 75, DK-2730 Herlev, Denmark.
Christina Lewicky-Gaupp, Feinberg School of Medicine, Northwestern University, 680 N Lake Shore Drive, Suite 1015, Chicago, IL 60611, USA.
Tovia M. Smith, Email: toviasmith@gmail.com, Pelvic Floor Research Group, Department of Obstetrics and Gynecology, University of Michigan, 1500 E. Medical Center Drive, SPC 5276, Ann Arbor, MI 48109, USA. Department of Obstetrics and Gynecology, University of Michigan, L4000 Women’s Hospital, 1500 E. Medical Center Drive, Ann Arbor, MI 48109, USA
References
- 1.Terminologia anatomica=International anatomical terminology. x. Stuttgart: Thieme; 1998. p. 292. [Google Scholar]
- 2.Kearney R, Sawhney R, DeLancey JO. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet Gynecol. 2004;104(1):168–173. doi: 10.1097/01.AOG.0000128906.61529.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawson JO. Pelvic anatomy. I. Pelvic floor muscles. Ann R Coll Surg Engl. 1974;54(5):244–252. [PMC free article] [PubMed] [Google Scholar]
- 4.Dietz HP, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol. 2005;106(4):707–712. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]
- 5.DeLancey JO, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 6.Piloni V, et al. Measurement of the anorectal angle by defecography for the diagnosis of fecal incontinence. Int J Colorectal Dis. 1999;14(2):131–135. doi: 10.1007/s003840050198. [DOI] [PubMed] [Google Scholar]
- 7.Shobeiri SA, et al. Appearance of the levator ani muscle subdivisions in endovaginal three-dimensional ultrasonography. Obstet Gynecol. 2009;114(1):66–72. doi: 10.1097/AOG.0b013e3181aa2c89. [DOI] [PubMed] [Google Scholar]
- 8.Margulies RU, et al. Appearance of the levator ani muscle subdivisions in magnetic resonance images. Obstet Gynecol. 2006;107(5):1064–1069. doi: 10.1097/01.AOG.0000214952.28605.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLancey JO, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179(6):2286–2290. doi: 10.1016/j.juro.2008.01.098. discussion 2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kearney R, et al. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107(1):144–149. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan DM, et al. Interrater reliability of assessing levator ani muscle defects with magnetic resonance images. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):773–778. doi: 10.1007/s00192-006-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLancey JO, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101(1):46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lien KC, et al. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103(1):31–40. doi: 10.1097/01.AOG.0000109207.22354.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu Y, et al. Quantification of levator ani cross-sectional area differences between women with and those without prolapse. Obstet Gynecol. 2006;108(4):879–883. doi: 10.1097/01.AOG.0000233153.75175.34. [DOI] [PubMed] [Google Scholar]