Abstract
Lesch–Nyhan disease (LND) is caused by deficiency of the purine salvage enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT). Affected individuals exhibit over-production of uric acid, along with a characteristic neurobehavioural syndrome that includes mental retardation, recurrent self-injurious behaviour and motor disability. Prior studies involving relatively small numbers of patients have provided different conclusions on the nature of the motor disorder. The current study includes the results of a multi-centre international prospective study of the motor disorder in the largest cohort of patients studied to date. A total of 44 patients ranging from 2 to 38 years presented a characteristic motor syndrome that involved severe action dystonia superimposed on baseline hypotonia. Although some patients also displayed other extrapyramidal or pyramidal signs, these were always less prominent than dystonia. These results are compared with a comprehensive review of 122 prior reports that included a total of 254 patients. Explanations for the differing observations available in the literature are provided, along with a summary of how the motor disorder of LND relates to current understanding of its pathophysiology involving the basal ganglia.
Keywords: cerebral palsy, choreoathetosis, dystonia, neurogenetics
Introduction
Lesch–Nyhan disease (LND) is an X-linked recessive disorder caused by deficiency of the purine salvage enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT). Affected individuals have hyperuricaemia and a characteristic neurobehavioural phenotype that includes mental retardation, self-injurious behaviour and motor disability (Lesch and Nyhan, 1964; Jinnah and Friedmann, 2000). A number of detailed studies have addressed the genetic (Jinnah et al., 2000; Jinnah et al., 2004), metabolic (Seegmiller, 1989; Jinnah and Friedmann, 2000; Puig et al., 2001), cognitive (Anderson et al., 1992; Matthews et al., 1995; Schretlen et al., 2001) and behavioural (Nyhan, 1976; Anderson and Ernst, 1994; Robey et al., 2003; Schretlen et al., 2005) features of this disease. In comparison, fewer studies have specifically addressed the motor disorder.
In their original report of two affected brothers in 1964, Lesch and Nyhan described choreoathetosis and increased muscle tone as the salient motor abnormalities (Lesch and Nyhan, 1964). Subsequently, several retrospective studies described larger numbers of patients. The largest of these included 19 patients, all of whom were described as having choreoathetosis, with a minority also having dystonia (Christie et al., 1982). All of these patients were described as also showingsigns of corticospinal dysfunction, such as scissoring of the legs or extensor plantar reflexes. The second largest series included 14 patients, most of whom were described as having choreoathetosis and spasticity (Mizuno, 1986). In keeping with these reports, most modern textbooks of paediatric neurology emphasize choreoathetosis and pyramidal signs in their descriptions of LND. However, one of the most detailed studies that included eight patients described dystonia rather than choreoathetosis as the major motor abnormality (Watts et al., 1982). All of these patients also were described as having hypotonia rather than hypertonia, and pyramidal signs were uncommon. Another five patients were described as showing a combination of choreoathetosis, dystonia, ballismus and ataxia (Jankovic et al., 1988). Pyramidal signs were not noted in these patients. Finally, many reports of smaller numbers of cases have described various combinations of choreoathetosis, dystonia, ballismus, spasticity, hypotonia or ataxia. The reasons for these incongruent descriptions of the motor disorder of LND remain unclear.
To further delineate the motor disorder of LND, 44 patients from 40 different families were evaluated systematically by the same group of investigators using defined criteria for the diagnosis of motor disorders. The evaluations revealed a relatively uniform motor phenotype that consists of severe generalized dystonia superimposed on hypotonia, sometimes with less prominent choreoathetosis or spasticity. These results are compared with a comprehensive review of the published literature, and the potential reasons for the differing descriptions are considered. The motor phenotype is also considered in the light of current knowledge of pathophysiology in LND.
Methods
Prospective evaluation
This study included 44 LND patients. They were recruited after referral for clinical evaluation and management, after referral to centres devoted to the long-term care of patients with developmental disabilities or metabolic diseases, or after participation in other studies. A detailed assessment of the motor syndrome has not been reported for the current patients, excepting no. 29 (De Gregorio et al., 2005). Some of the results have been summarized in abstract form (Jinnah et al., 1998; Visser et al., 2004).
The focus of this study was on the motor features of classic LND due to complete HPRT deficiency. A definitive molecular (hprt gene mutation predicting null enzyme function) or biochemical (<1% residual HPRT enzyme activity in blood or fibroblast samples) diagnosis was established for 40 of 44 patients. Of the remaining four patients, three declined laboratory confirmation and one died before testing could be arranged. Among these four patients were three who met clinical criteria for LND with expression of the full syndrome including mental retardation, self-injurious behaviours, motor disability and over-production of uric acid. The remaining patient (no. 3) exhibited the full syndrome except for self-injurious behaviours. This patient was included because he had a cousin who was diagnosed with LND on the basis of the full clinical syndrome that included self-injury. Because the focus of this study was on the classic form of the disease due to complete HPRT deficiency, attenuated or partial forms of the disease known to be due to partial HPRT deficiency were excluded. Only one patient with classic LND who was evaluated for these studies was excluded, because of an incomplete record.
The first author directly evaluated 33 of the 44 patients. The remaining nine patients were evaluated by clinicians who had experience in movement disorders or had worked with the first author during evaluation of other LND cases. Detailed videotapes available for three of these patients were reviewed by the first author to confirm diagnostic impressions. The evaluation began with a complete history. Before their evaluation for this study, many patients had been evaluated only once by a general neurologist and subsequently followed in institutions or clinics devoted to paediatrics or metabolic diseases. Therefore, historical records on the evolution of neurological symptoms and signs were not always available.
The examination consisted of a standardized written protocol with specific attention to the motor features. Video recordings of the examination were made for 33 patients. All movement disorders and assessments of muscle tone were defined according to standard published criteria (Barbeau et al., 1981; Fahn, 1988; Jankovic and Fahn, 1998; Sanger et al., 2003). Ballism was defined as involuntary rapid flinging movements, typically of the limbs. Choreoathetosis was defined as involuntary non-rhythmic and unpatterned movements with variable timing and distribution. Dystonia was defined as involuntary, sustained, patterned and sometimes repetitive contractions of opposing muscles often leading to twisting movements or abnormal posturing. Spasticity was defined as an abnormality of muscle tone with a velocity-dependent increase in resistance to externally imposed movement, often worse in extension than flex-ion. The determination of spasticity was made independent from other pyramidal signs. Dystonia was discriminated from spasticity by the lack of velocity-dependence, exaggeration by attempted action and frequently marked variation in tone with emotional state (Sanger et al., 2003).
This study is primarily descriptive, but to obtain an estimate of the severity of individual motor defects for comparative purposes, we applied a semi-quantitative rating scale that was modelled after one used in a prior study of LND (Jankovic et al., 1988). The scale was defined as follows: 0 = motor abnormality absent; 1 = motor abnormality mild and insufficiently severe to be significantly disabling; 2 = motor abnormality moderate with significant disability but most functions still possible with added effort; 3 = motor abnormality severe enough to preclude meaningful function.
Literature review
The Medline database through December 2005 was reviewed for reports that included the keywords Lesch–Nyhan or hypoxanthine-guanine phosphoribosyltransferase. Additional patients were identified by surveying the reference lists of these and other articles. A list of the 122 reports reviewed is available from the authors by request. The clinical data were recorded in a database, and information for patients reported more than once was combined into a single entry wherever possible. Because the focus of this study is on the classic form of the disease, reports of attenuated variants due to partial enzyme deficiency were again excluded. Because the disorder is X-linked and recessive, virtually all cases were male, and the rarely reported female cases were excluded.
Prospective evaluation
Demographics
The 44 patients ranged from 2 to 38 years (Table 1). They were followed for an average duration of 3.3 years (range 0–18 years). All but one of the patients were male. The female patient had a clinical phenotype indistinguishable from that of the males.
Table 1.
Case demographics
| Case | Family | Age first seen (year : month) | Age at last visit (year : month) | HPRT mutation | HPRT enzyme | Medications | Presenting problem | Evolution | Onset of self-injury |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 2 | C610G | <1% | Sodium bicarbonate, diazepam | Motor delay, opisthotonus, posturing at 3 months | Posturing of limbs at 6 months, chorea at 2 years | 2 years |
| 2 | 2 | 0 : 2 | 2 | IVS6-1G>A | <1% | Baclofen | Lactic acidosis with stones in diapers at 2 months | Hypotonia with posturing at 5 months, opthisthotonus at 9 months | None |
| 3 | 3 | 2 | 2 | Declined | Declined | None | Motor delay at 4 months | Dystonic posturing at 1 year | None |
| 4 | 4 | 2 | 2 | A131G | <2% | Domperidone, omeprazole, sodium bicarbonate, clonazepam, melatonin | Motor delay during first year | Posturing at 2 years | 1 year |
| 5 | 5 | 2 | 3 | NA | <1% | Risperidone | Motor delay at 4 months | Jerky arm movements at 8 months | 2 years |
| 6 | 6 | 0 : 9 | 3 | 611delA | <1% | Sodium bicarbonate | Motor delay at 3 months | Hypotonia with posturing at 14 months | 2 years |
| 7 | 7 | 2 | 3 | 10del1 | NA | Gabapentin | Motor delay at 6 months | Limb posturing at 6–12 months | 2 years |
| 8 | 8 | 1 | 3 | E9 deletion | <1% | Diazepam | Motor delay at 3 months | Dystonic posturing at 12 months | 2 years |
| 9 | 9 | 1 : 7 | 4 | IVS4+1G>A | <1% | Sodium bicarbonate | Motor delay at 4 months | Hypotonia with posturing at 9 months | None |
| 10 | 10 | 4 | 4 | IVS8+1G>A | <1% | None | Motor delay at 6 months | Dystonic posturing at 18 months | 2 years |
| 11 | 11 | 0 : 8 | 8 | NA | <1% | Diazepam | Motor delay at 7 months | Dystonia at 1 year, deceased at 9 years | 2 years |
| 12 | 12 | 6 | 9 | IVS7+5G>A | NA | Sodium bicarbonate, iron sulphate | Motor delay at 7 months | Posturing ~12 months | None |
| 13 | 13 | 8 | 8 | T203C | <1% | Risperidone, folate | Motor delay at 6 months | Retrocollis and posturing at 6 months | 5 years |
| 14 | 14 | 0 : 5 | 10 | G118A | <1% | Sodium bicarbonate, diazepam | Motor delay at 4 months | Hypotonia with limb posturing at 2 years | None |
| 15 | 15 | 10 | 10 | IVS7+5G > A | <1% | None | Motor delay at 3 months | Limb posturing at 18 months | 4 years |
| 16 | 16 | 10 | 10 | NA | <1% | Baclofen, diazepam, risperidone, metaclopramide, lansoprazole, sodium citrate | Orange crystals in diaper at 3 months, motor delay at 5 months | Backward arching at 12 months, limb dystonia at 4 years | 2 years |
| 17 | 17 | 1 | 11 | E4 del | <1% | Sodium bicarbonate | Motor delay at 4 months | Limb posturing, opisthotonus at 11 months | None |
| 18 | 18 | 3 | 11 | 100 ins GG | <1% | Sodium bicarbonate, diazepam, risperidone | Motor delay at 8 months | Limb posturing at 12 months | 8 years |
| 19 | 19 | 0 : 4 | 12 | C115G | <1% | None | Motor delay at 4 months | Opisthotonus at 6 months | Present, onset unknown |
| 20 | 20 | 12 | 12 | E1 deleted | <1% | Lansoprazole, cisapride, melatonin | Motor delay at 6 months | NA | 1 year |
| 21 | 21 | 12 | 12 | G209A | <1% | Carbamazepine, clonazepam, folate | Motor delay at 8 months | Posturing and flinging limbs by 1 year | 6 years |
| 22 | 22 | 8 | 13 | A611G | NA | Metaclopramide, cimetidine | Motor delay at 7 months | Twisting and flinging at 3 years | 4 years |
| 23 | 15 | 13 | 13 | IVS7+5G>A | <1% | Diazepam, omeprazole, cetirizine, fluticazone | Delivery by emergent Caesarean section for fetal distress, motor delay at 6 months | Limb posturing at 18 months | 3 years |
| 24 | 16 | 13 | 13 | NA | <1% | Intrathecal baclofen, clonazepam, dantrolene, sertraline | Motor delay and backward arching at 6 months | NA | 3 years |
| 25 | 20 | 14 | 14 | E1 deleted | <1.5% | Prevacid, propulsid, melatonin | Motor delay at 6 months | NA | 1 year |
| 26 | 23 | 13 | 15 | C508T | NA | Gabapentin, alprazolam | Motor delay at 9 months | NA | 15 months |
| 27 | 24 | 1 | 19 | Declined | Declined | Dantrolene, diazepam, sucralfate, paracetamol, ibuprofen | Motor delay during first year | Flinging limbs at 2 years followed by posturing | 2 years |
| 28 | 25 | 17 | 19 | Declined | Declined | Diazepam, thioridazine | Motor delay at 5 months | Deceased at 19 years | Severe, onset unknown |
| 29 | 26 | 20 | 20 | IVS8+4A>G plus mosaic X-inactivation | Tissue-dependent | Paroxetine, omeprazole | Motor delay at 5 months | Opisthotonic spasms at 1 year | 3 year |
| 30 | 27 | 21 | 21 | C508T | NA | NA | Worsening in late teenage years | Present, onset unknown | |
| 31 | 28 | 22 | 22 | G580C | <1% | Alprazolam, paroxetine, valproic acid, acetazolamide | NA | NA | Present, onset unknown |
| 32 | 29 | 14 | 23 | A140G | <1% | Chronulac, iron | Motor delay during first year | NA | 2 years |
| 33 | 29 | 14 | 23 | A140G | <1% | Chronulac, iron | Motor delay during first year | NA | 2 years |
| 34 | 30 | 17 | 23 | IVS1+1G>T | NA | Baclofen, lactulose, magnesium | Motor delay at 6 months | NA | 2 years |
| 35 | 31 | 19 | 23 | Declined | Declined | Diazepam paroxetine prevacid docusate | Delivery by emergency cesarean section for foetal distress, motor delay at 3 months | Posturing at 8 months, worsening in late teenage years | 3 years |
| 36 | 32 | 23 | 23 | NA | <1% | Intrathecal baclofen, clonazepam, risperidone, clomipramine | Motor delay at 4 months | Dystonic limb movements at 12 months | 3 years |
| 37 | 33 | 18 | 27 | 428-432del TGCAG, insAGCAAA | <1% | Sodium citrate | Motor delay and backward arching at 4 months | Posturing at 18 months | 1 year |
| 38 | 34 | 22 | 30 | A140T | NA | Polycitra, omeprazole | NA | NA | Present, onset unknown |
| 39 | 35 | 28 | 31 | C151T | NA | Albuterol, theophylline, prednisone, montelukast, gabapentin, alprazolam | Motor delay in early infancy | NA | 5 years |
| 40 | 36 | 32 | 32 | G580T | NA | Paroxetine, carbamazepine, olanzepine | Motor delay at 6 months | Choreoathetosis at 2 years, worsening in second decade | 2 years |
| 41 | 37 | 32 | 32 | E2-3 deleted | <2% | Baclofen, primidone, phenobarbital | Motor delay at 6–12 months | Posturing at 1 year | Present, onset unknown |
| 42 | 38 | 25 | 33 | del E4 | <1% | Diazepam, folic acid | Hypotonia and fisted hands at 3 months | Abnormal arm postures at 6 months | 2 years |
| 43 | 39 | 34 | 34 | 371insTT | NA | None | Motor delay at 4 months | NA | 6 years |
| 44 | 40 | 38 | 38 | G580A | <1.5% | Baclofen, diazepam, paroxetine, gaviscon, omeprazole, dulcolax | NA | NA | Present, onset unknown |
Some information was not available for all patients because knowledgeable informants or early medical records were not available (NA). All cases except two were taking allopurinol in addition to the medications listed. The molecular genetic basis for the majority of cases has been presented in prior studies (Jinnah et al., 2000; Torres et al., 2000; De Gregorio et al., 2005).
Medication use at evaluation
All but one patient were receiving allopurinol for control of uric acid. Twelve were receiving medications to alkalinize the urine to promote uric acid solubility. Eleven were taking medications for gastro-oesophageal reflux.
Seven were taking medications to reduce muscle tone (baclofen, dantrolene, paracetamol). Nineteen were taking benzodiazepines to alleviate both increased muscle tone and mood lability (diazepam, clonazepam, alprazolam). Seven were taking dopamine receptor antagonists (risperidone, thioridazine, olanzepine) for control of self-injury and two were taking metaclopramide to promote gastrointestinal motility. Eleven were taking medications for mood stabilization or depression (paroxetine, gabapentin, carbamazepine, clomipramine, sertraline). Though this study was not designed to assess treatment responses, none of these medications was clearly associated with a dramatic improvement of the motor disorder. Motor abnormalities among those taking dopamine receptor antagonists were similar to those who had never taken them.
Presentation and developmental progression
Information concerning presenting signs was available from the histories in 39 patients. All but two first came to medical attention for motor delay (Table 1). Motor delay became apparent most often at 3–6 months of age as hypotonia, with failure to sit upright unsupported. Overt involuntary movements most often were noted between 6 and 12 months of age, although abnormal movements sometimes were appreciated by parents or caretakers earlier, or delayed for up to 4 years.
After some evolution during the first few years, the severity of the motor disorder appeared relatively static rather than progressive in most cases. The majority of patients were reviewed more than once over a period of several years, and progression of the motor disorder was not significant beyond 5–6 years of age. The oldest patients did not appear to be more seriously affected than the youngest ones. However, several patients evidenced signs of increasing disability from long-standing abnormal movements. For example, three patients had histories noting objective signs of worsening motor function, such as loss of grasp or ability to stand with assistance, often in a stepwise fashion. Six developed severe scoliosis, and one required a surgical procedure to stabilize the spine.
Only two patients presented first with difficulties attributable to excessive production of uric acid. One presented with lactic acidosis, presumably due to nephrolithiasis with obstruction of urine flow and renal failure. The other presented with orange crystals in the diapers, most probably due to crystalluria combined with microhaematuria. No patient presented with self-injurious behaviours, which consistently emerged later than the other problems, usually between 1 and 8 years of age.
Extrapyramidal signs
Abnormal movements were relatively minor at rest in all patients, and became most obvious with stress, excitement, anticipation or voluntary movement. The most prominent feature of the motor syndrome in all patients was dystonia (Table 2, Fig. 1). Essentially, all parts of the body were affected. Multidirectional cervical dystonia was universal. Truncal twisting and arching was present in all, particularly with efforts to stand. Dystonia of the upper limbs prevented their use for most tasks such as feeding or grasping in all patients. All regularly used wheelchairs because lower limb dysfunction prevented them from walking or standing unassisted. Oromandibular and lingual dystonia were evident during speaking or eating in most. Several also exhibited blepharospasm, most prominently during ocular testing. Several developed fixed abnormal postures of the hands or feet, and fixed contractures of the hamstring muscles with incomplete extension at the knee were common. Muscle hypertrophy resulting from long-standing dystonia was evident in several patients in the neck and arms.
Table 2.
Neurological features
| Case | Family | Resting muscle tone
|
Extrapyramidal signs
|
Pyramidal signs
|
Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hypotonia | Spasticity | Rigidity | Dystonia | Choreoathetosis | Ballism | Hyperreflexia | Clonus | |||
| 1 | 1 | Yes | No | No | 3 | 1 | 0 | No | No | Malignant hyperthermia with levomepromazine |
| 2 | 2 | Yes | No | No | 3 | 0 | 0 | No | No | Inspiratory stridor, opisthotonus |
| 3 | 3 | Yes | No | No | 3 | 1 | 1 | No | No | None |
| 4 | 4 | Yes | No | No | 3 | 0 | 2 | No | No | Non-verbal, inspiratory stridor |
| 5 | 5 | Yes | No | No | 3 | 0 | 0 | No | No | Gastrostomy tube, opisthotonus, non-verbal |
| 6 | 6 | Yes | No | No | 3 | 1 | 0 | Legs | Ankles | Hypophonia, dysphagia |
| 7 | 7 | Yes | No | No | 3 | 0 | 0 | No | No | Episodic apnoea with cyanosis, gastrostomy tube |
| 8 | 8 | Yes | No | No | 3 | 1 | 0 | No | No | None |
| 9 | 9 | Yes | No | No | 3 | 1 | 0 | No | No | None |
| 10 | 10 | Yes | No | No | 3 | 0 | 0 | No | No | Violent retrocollis |
| 11 | 11 | Yes | Legs | No | 3 | 0 | 0 | Legs | No | Opisthotonus, recurrent cardiopulmonary arrests |
| 12 | 12 | Yes | Legs | No | 3 | 0 | 0 | Legs | Ankles | Episodic apnoea with cyanosis |
| 13 | 13 | Yes | No | No | 3 | 0 | 1 | No | No | None |
| 14 | 14 | Yes | No | No | 3 | 1 | 1 | Legs | No | Hypophonia |
| 15 | 15 | Yes | No | No | 3 | 0 | 0 | No | No | Epileptic seizures |
| 16 | 16 | Yes | No | No | 3 | 0 | 0 | No | No | Blepharospasm, gastrostomy tube |
| 17 | 17 | Yes | No | No | 3 | 2 | 0 | No | No | Hypophonia |
| 18 | 18 | Yes | No | No | 3 | 1 | 0 | Legs | No | Violent retrocollis |
| 19 | 19 | Yes | No | No | 3 | 2 | 1 | No | No | Opisthotonus |
| 20 | 20 | Yes | No | No | 3 | 1 | 0 | Legs | No | None |
| 21 | 21 | Yes | Legs | Arms | 3 | 0 | 1 | Knees | Knees | Epileptic seizures |
| 22 | 22 | Yes | No | No | 3 | 0 | 1 | Left arm, legs | Ankles | Gastrostomy tube, noisy breathing |
| 23 | 15 | No | Legs, arms | Arms | 3 | 0 | 0 | Legs | Ankles | Gastrostomy tube |
| 24 | 16 | Yes | No | No | 3 | 0 | 0 | No | No | Recurrent head bobbing |
| 25 | 20 | Yes | Left arm/leg | No | 3 | 1 | 0 | Left tricep and legs | Left knee, both ankles | Hypophonia |
| 26 | 23 | Yes | No | No | 3 | 1 | 1 | Legs | Ankles | Hypophonia |
| 27 | 24 | Yes | No | No | 3 | 0 | 1 | Triceps, legs | Ankles | None |
| 28 | 25 | Yes | No | No | 3 | 2 | 2 | Legs | No | Hypophonia, opthisthotonus, violent retrocollis, severe scoliosis, sudden death at 19 years |
| 29 | 26 | Legs | No | Arms | 3 | 0 | 0 | No | No | None |
| 30 | 27 | No | Left arm | No | 3 | 1 | 1 | Left tricep, legs | Left tricep, right ankle | Severe episodic retrocollis and scoliosis |
| 31 | 28 | Yes | No | No | 3 | 1 | 0 | No | No | Hypophonia |
| 32 | 29 | Yes | No | No | 3 | 1 | 0 | Legs | Right ankle | Stuttering, hypophonia, opisthotonus |
| 33 | 29 | Yes | No | No | 3 | 1 | 0 | Legs | No | Stuttering, hypophonia, opisthotonus |
| 34 | 30 | Yes | No | No | 3 | 0 | 0 | Legs | Left ankle | Opisthotonus, periodic sustained upward gaze |
| 35 | 31 | Trunk | Legs | Arms | 3 | 1 | 0 | Legs | Ankles | Severe scoliosis |
| 36 | 32 | Yes | No | No | 3 | 0 | 0 | No | No | Severe scoliosis |
| 37 | 33 | Yes | No | No | 3 | 2 | 1 | Legs | No | Opisthotonus, severe scoliosis |
| 38 | 34 | Yes | No | No | 3 | 1 | 0 | Legs | Ankles | Anarthria |
| 39 | 35 | Yes | No | No | 3 | 0 | 0 | No | No | Opisthotonus, recurrent aspiration, gastrostomy tube, severe scoliosis |
| 40 | 36 | No | No | No | 3 | 0 | 0 | No | No | Gastrostomy tube |
| 41 | 37 | Legs | No | Arms | 3 | 0 | 0 | No | No | Gastrostomy tube, epileptic seizures |
| 42 | 38 | Yes | Legs | No | 3 | 2 | 1 | Legs | No | Gastrostomy tube, hypophonia, opisthotonus |
| 43 | 39 | No | Left arm/leg | Right arm/leg | 3 | 1 | 0 | Left tricep, legs | No | Gastrostomy tube |
| 44 | 40 | Yes | Legs | No | 3 | 0 | 0 | Left arm, legs | Left knee, ankles | Cardiopulmonary arrest, cervical myelopathy |
Other motor abnormalities (ataxia, tremor, myoclonus) were absent. The extensor plantar reflex was not included among the ‘pyramidal’ signs because it could not be unequivocally distinguished from the ‘striatal toe’ response.
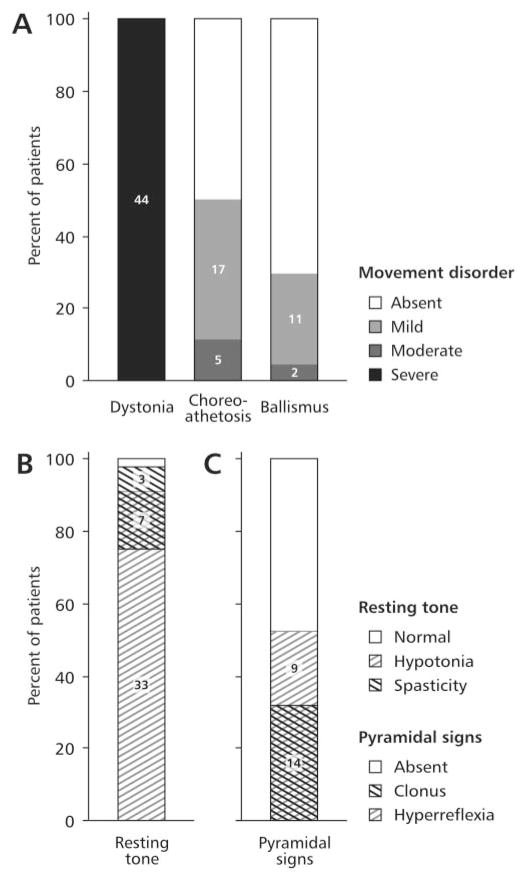
Fig. 1.
Summary of the motor signs in current series of 44 LND patients. The most common signs were extrapyramidal (A), abnormalities of resting tone (B) and pyramidal signs (C). Overlapping hatch marks show patients with overlapping signs. The extensor plantar reflex was omitted because it could not be unequivocally distinguished from the striatal toe response.
Severe opisthotonus or truncal arching was observed directly in 11 (25%), and movements compatible with opisthotonus were described frequently in the medical records as severe or prolonged ‘arching’ or ‘backward bending’ of the trunk. Sudden and rapid backward thrusting of the head without concomitant truncal involvement was observed directly in four and described in the records of several others. Such spasms were absent at rest, but emerged when patients attempted to stand or were transferred from the seated to supine position. Severe, sustained truncal arching with dystonic tremor sometimes resembled a generalized epileptic seizure, except that consciousness was preserved and there was no post-ictal depression of consciousness.
Although dystonia was universal and always the most severe extrapyramidal disorder, choreoathetosis was present in 22 (50%) and ballism was observed in 13 (30%). Ballism was most common in the arms, though it occasionally appeared in the legs. These movements were seen almost exclusively with excitement or agitation. Ballism was often triggered by the presence of an object near enough to strike, raising the question of whether this represented impulsive or voluntary acts of aggression. However, the movements otherwise appeared typical of ballism, and their speed and fluidity contrasted with the marked impairment in all other voluntary movements.
Resting muscle tone
When fully relaxed, 40 (91%) patients were considered to have hypotonia (Table 2; Fig. 1). Spasticity was noted in 10 (23%) patients. In six of these the spasticity was limited to the legs, and in three others it was markedly asymmetrical. Rigidity was noted in six (14%), but cogwheeling was not apparent.
Corticospinal tract signs
A total of 23 patients (52%) had pathologically brisk muscle stretch reflexes (Table 2). Pathological reflexes were limited to the legs in 17 of these patients, and they were asymmetrical in 5. Clonus was observed in 14 (32%) cases, and in 13 of these cases it was limited to the legs.
Several patients demonstrated an abnormal resting posture of the great toe. In some, the toe was plantar flexed and deviated laterally at the metacarpophalangeal joint in a fixed position above or below the adjacent toe. In others, the toe remained in extension for lengthy periods without plantar stimulation, but eventually returned to a normal position. Plantar stimulation often led to varied responses, with an extensor on some occasions and a flexor on others. These phenomena were considered more characteristic of a striatal toe response than a classic Babinski sign, but distinction between these signs was not attempted.
Dysarthria and dysphagia
Speech was delayed in all patients for whom developmental histories were available. Most began using words by 2–4 years of age. A few were not speaking at much older ages, such as one who was still not using words by age 14 years.
When speech occurred, it was always dysarthric. In all patients, dysarthria was severe enough that speech was difficult to understand without frequent repetition. One was anarthric because of extreme cervical and oromandibular dystonia but communicated effectively with a keyboard. Most communicated using only single words or very short phrases. Speech had the typical characteristics of striatal dysarthria, with slow and irregular enunciation associated with excessive activation of pharyngeal, lingual, and perioral muscles. During casual conversation, a trailing volume was common and sufficiently soft to be considered pathologically hypophonic in 10 (23%) patients, and 2 (4%) of these also exhibited stuttering without obvious jaw dystonia. Spasmodic dysphonia with intermittent breathiness, intermittent strained-strangled quality or phonic breaks was not apparent. None had a high-pitched, nasal or strained-strangled quality suggestive of spastic dysarthria. None displayed poor modulation of tempo or volume suggestive of cerebellar dysarthria.
Chewing and swallowing was laboured in most patients. Many also had gastro-oesophageal reflux with frequent emesis (Table 1). Together, these problems were sufficiently severe to require gastrostomy tube in nine (20%) patients.
Other features
Respiratory abnormalities were evident in several patients. Two patients had prominent inspiratory stridor without cyanosis or apnoea during examination and another had a history of recurrent ‘noisy breathing’ (Table 2). In all three patients, the respiratory problems were noted during wakefulness and not during sleep. Two other patients had frequent episodes of apnoea and cyanosis without stridor, also during wakefulness but not during sleep. Extensive pulmonary evaluations were normal for both, and EEG studies provided no evidence for epilepsy. Two other patients each had at least one episode of cardiopulmonary arrest leading to multiple hospitalizations, and extensive evaluations failed to disclose a cause. One of these patients expired without obvious cause, presumably due to an unwitnessed respiratory event. Another case with no history of cardiopulmonary events expired during sleep with no apparent cause.
Three patients had histories compatible with generalized tonic–clonic epileptic seizures, though none was witnessed by the evaluators, and the possibility of prolonged opisthotonic posturing with dystonic tremor could not be excluded. One case had episodic and briefly sustained upwards or lateral conjugate eye deviation resembling ocular tics. Two cases with a history of neuroleptic use exhibited repetitive orobuccolingual stereotypies typical of tardive dyskinesia. Other tics or stereotypies were absent. None of the patients had evidence for cerebellar dysfunction such as appendicular or axial ataxia or ocular dysmetria. Tremor and myoclonus were absent.
Literature review
Reported cases
A total of 122 reports describing 254 LND patients reported from 1959 to 2005 were examined. Overall, the average age at presentation was 6.1 months, with a range of 1 day to 7 years. The average age at the time of case description was 9.5 years, with a range of 1 day to 33 years.
Presentation
At least one presenting problem was recorded for 158 patients, and 130 of these included neurological problems (Table 3). The most common presenting problems were signs of motor delay, abnormalities of muscle tone or ‘cerebral palsy’. These descriptions are not mutually exclusive, as all indicate abnormal motor development. Less common neurological presentations included opisthotonus, apparent epileptic seizures, ‘spastic torticollis’, fisting of the hands, scissoring of the legs, nystagmus and microcephaly.
Table 3.
Presenting features in reported cases
| Presenting feature | Number (n = 158) | Per cent of total |
|---|---|---|
| Neurological | 130 | 82.3 |
| Developmental delay | 92 | 58.2 |
| Cerebral palsy | 19 | 12.0 |
| Hypertonia | 12 | 7.6 |
| Hypotonia | 8 | 5.1 |
| Opisthotonus | 4 | 2.5 |
| Seizures | 2 | 1.3 |
| Other* | 8 | 5.1 |
| Urate over-production | 18 | 11.4 |
| Renal failure | 8 | 5.1 |
| Crystalluria | 5 | 3.2 |
| Gout | 3 | 1.9 |
| Other† | 3 | 1.9 |
| Other | 21 | 13.3 |
| Vomiting | 6 | 3.8 |
| Affected family | 6 | 3.8 |
| Colic or irritability | 6 | 3.8 |
| Other‡ | 2 | 1.3 |
This table summarizes the presenting features in 158 of the 254 cases for whom information was available. A list of the reports reviewed is available by request. The number of cases may sum to more than the total because more than one presenting feature was recorded for several cases.
Includes fisting of the hands, scissoring of the legs, nystagmus, microcephaly, ‘spastic torticollis’ and ‘abnormal tone’;
includes hyperuricaemia, kidney stone without renal failure, and acidosis;
includes unexplained fevers and non-specific terminology such as ‘poor feeding’ or ‘lethargy’.
Presenting problems among the remaining patients were due mostly to excessive production of uric acid (Table 3). Most commonly, these included renal failure or uric acid crystals in the urine. Gout was uncommon, being noted as the presenting feature for only three patients. Only two patients presented with self-injury. A few patients were noted to present with such non-specific problems as ‘colic’, ‘irritability’ or difficulty feeding. A few pre-symptomatic patients also were identified because of a known affected relative.
Developmental progression
No prospective studies of the evolution and natural history of LND have been reported. However, in one retrospective study of eight patients followed for up to 8 years, most presented with hypotonia and/or motor delay between 3 and 9 months of age, and then developed dystonia between 6 and 24 months of age (Watts et al., 1982). Another study of six patients followed over several years provided a similar picture of early motor delay followed by development of athetosis and spasticity (Michener, 1967). Limited information concerning the natural history of neurological features was reported for a total of 69 patients. The most frequently noted initial neurological problems included hypotonia and delayed motor development. Involuntary movements or spasticity usually were not present at birth, but emerged at an average of 16 months (range 1 month to 6 years). Involuntary movements or spasticity then appeared to remain static, as few were described as showing progressive motor dysfunction beyond 2 years.
Neurological features
A summary of all neurological features noted at the time each case was reported is provided in Table 4. The neurological features were described with varying degrees of detail. No information concerning the neurological features was provided for 34 patients. A detailed description of the neurological features was available for only 42 patients. Among the 220 patients for whom any neurological information was provided, various extrapyramidal signs were described. These included chorea, choreoathetosis, athetosis, dystonia, opisthotonus and ballismus. Most patients were reported to have more than one extrapyramidal sign. Other features commonly reported as pyramidal signs included spasticity, hyperreflexia, an extensor plantar reflex, scissoring of the legs and clonus.
Table 4.
Neurological features in reported cases
| Feature | Number (n = 220) | Per cent of total |
|---|---|---|
| Extrapyramidal | 178 | 80.9 |
| Choreoathetosis | 106 | 48.2 |
| Opisthotonus | 58 | 26.4 |
| Dystonia | 51 | 23.2 |
| Athetosis | 51 | 23.2 |
| Ballism | 12 | 5.5 |
| Chorea | 7 | 3.2 |
| Pyramidal | 145 | 65.9 |
| Spasticity | 118 | 53.6 |
| Hyperreflexia | 61 | 27.7 |
| Extensor plantar | 31 | 14.1 |
| Scissoring legs | 28 | 12.7 |
| Clonus | 11 | 5.0 |
| Abnormal muscle tone | 96 | 43.6 |
| Hypotonia | 44 | 20.0 |
| Hypertonia | 66 | 30.0 |
| Hypotonia and hypertonia | 14 | 6.4 |
| Other | 84 | 48.1 |
| Dysarthria | 67 | 30.5 |
| Dysphagia | 8 | 3.6 |
| Seizures | 18 | 8.2 |
| Ataxia | 8 | 3.6 |
| Rigidity | 4 | 1.8 |
This table summarizes the neurological features in 220 of the 254 cases for whom information was available. The number of cases or percentages may sum to more than the total because more than one feature was recorded for several cases. The table employs the terminology in the original published reports with no attempt to combine similar or overlapping terms such as chorea and choreoathetosis. The extensor plantar reflex and scissoring of the legs are listed as pyramidal signs in accordance with the published reports, though they may also be considered extrapyramidal as noted in the text.
Descriptions of muscle tone varied. Among the 96 patients for whom muscle tone was described, hypertonia was noted in 69%, hypotonia in 46% and both hypotonia and hypertonia in 14 of these patients. Increased muscle tone was most often described as spasticity or dystonia, although such non-specific terms as ‘hypertonic’ or ‘stiffness’ were used for several cases. Only three patients were described as having rigidity.
Uncommonly reported neurological features included apparent epileptic seizures, ataxia, unspecified dyskinetic movements and myoclonic-like limb jerks. Varying degrees of self-injurious behaviours were documented for nearly all patients but other motor stereotypies and motor or vocal tics were not apparent. None was reported to have tremor.
Neuroimaging
Brain CT scans have been reported for 22 patients, including 12 that were part of a retrospective study (Table 5). Atrophy was reported for four, with the remainder being interpreted as normal. Results of brain MRI have been reported for 25 patients. Only three were noted to have visible atrophy. However, a 17% reduction of total cerebral volume and 34% reduction in basal ganglia volumes were found in a quantitative study when seven patients were compared with normal controls (Harris et al., 1998).
Table 5.
Neuroimaging in LND
| No of cases | Ages (years) | Imaging method | Reported findings | Reference |
|---|---|---|---|---|
| 1 | 2 | CT | Normal | Salman et al. (1987) |
| 4 | 2–10 | MRI | Two normal, one with atrophy, one with decreased T2 signal in basal ganglia | Jankovic et al. (1988) |
| 2 | 4–6 | Pneumoencephalography | Both normal | Michener (1967) |
| 1 | 5 | CT | Mild atrophy | Hara et al. (1982) |
| 1 | 7 | CT | Normal | van Bogaert et al. (1992) |
| 1 | 8 | CT | Normal | Hatanaka et al. (1990) |
| 1 | 8 | Pneumoencephalography | Cerebral atrophy | Lesch and Nyhan (1964) |
| 1 | 9 | Pneumoencephalography | Atrophy | Sass et al. (1965) |
| 12 | 10–20 | MRI | All normal | Ernst et al. (1996) |
| 1 | 11 | CT | Normal | Marmattom (2005) |
| 1 | 15 | CT and MRI | Normal | Lynch and Noetzel (1991) |
| 1 | 19 | MRI | Normal | Taira et al. (2003) |
| 1 | 21, 29 | CT twice | Mild atrophy on second scan only | Saito and Takashima (2000) |
| 7 | 22–35 | Quantitative MRI | Only two showed visible atrophy but volumetric studies revealed cerebral volumes reduced 17% | Harris et al. (1998); Wong et al. (1996) |
| 3 | NR | CT | Normal | Watts et al. (1982) |
| 12 | NR | CT | Two with mild atrophy | Wong (1988) |
One patient had two CT scans separated by 8 years, and atrophy was apparent only on the second one (Saitoh and Takashima, 2000).
NR = not reported.
Although most reports use the term ‘atrophy’ to describe cerebral volume loss, concurrent enlargement of the cranial sinuses in one imaging study suggested that the volume loss reflects poor brain development rather than a degenerative process (Harris et al., 1998). Therefore, the term ‘dystrophy’ might be more appropriate.
Autopsy reports
Autopsies including brain have been reported for a total of 23 LND patients (Table 6). These studies have not revealed any consistent neuropathological changes. Notably, complete neuropathological studies were entirely unremarkable for six patients, including electron microscopic studies for at least two. Though multiple different abnormalities have been reported, the lack of consistency suggests that many of the findings may be incidental or related to other events occurring at the time of death.
Table 6.
Neuropathology in LND
| Case | Age (year : month) | Cause of death | Brain mass (g) | Reported findings | Reference |
|---|---|---|---|---|---|
| 1 | 0 : 11 | NR | NR | Cortical neuron loss and superficial gliosis | Crome and Stern (1967) |
| 2 | 1 : 9 | NR | NR | Severe hydrocephalus with degeneration and gliosis of the cerebrum and medulla | Wada et al. (1968) |
| 3 | 2 : 10 | Seizures and opisthotonic spasms | 850 | Two small softenings in parietal lobe and brainstem | Hoefnagel et al. (1965) |
| 4 | 3 : 4 | Respiratory infection | NR | Thin cortex with dilation of the left lateral ventricle | Storey (1969) |
| 5 | 3 : 6 | NR | 1120 | Normal | Crussi et al. (1969) |
| 6 | 3 : 8 | NR | 960 | Focal degenerative changes in unspecified areas | Mahnovski et al. (1975) |
| 7 | 4 : 0 | NR | NR | Cortical neuron loss, superficial gliosis, multiple foci of cerebellar necrosis | Crome and Stern (1967) |
| 8 | 5 : 0 | Pneumonia | 1120 | Tiny birefringent crystals in perivascular spaces | Partington and Hennen (1967) |
| 9 | 6 : 0 | NR | NR | Normal | Watts et al. (1987) |
| 10 | 6 : 2 | NR | 1038 | Normal | Crussi et al. (1969) |
| 11 | 7 : 0 | Febrile illness | 1180 | Discrete cerebral oedema | Bassermann et al. (1979) |
| 12 | 10 : 0 | Pneumonia | NR | Focal areas of gliosis in cerebellum | Mitchell and McInnes (1984) |
| 13 | 11 : 0 | Uraemia with septicaemia | 950 | Diffuse vascular and demyelinative lesions of the white matter, degeneration of cerebellar granule cells, multiple foci of gliosis | Sass et al. (1965) |
| 14 | 13 : 0 | NR | 880 | PAS-positive and Sudan-negative inclusions in the olives | Warzok et al. (1982) |
| 15 | 14 : 0 | NR | NR | Normal | Watts et al. (1987) |
| 16 | 15 : 0 | Cardiopulmonary arrest | NR | Mild gliosis with increased medium spiny neurons in caudate and putamen, normal pigmented neurons in midbrain, low-normal midbrain neurons positive for TH | Saito et al. (1999) |
| 17 | 15 : 4 | Broncho-pneumonia | 1100 | Small softenings in cerebellum, chromatolysis of Purkinje cells, degeneration in dentate, diffuse foci of necrosis and gliosis in white matter | Mizuno (1986); Mizuno et al. (1976) |
| 18 | 32 : 0 | Pneumonia | NR | Mild gliosis in tegmentum and cerebellar white matter, reduced pigmented neurons in midbrain, low-normal midbrain neurons positive for TH | Saito et al. (1999) |
| 19 | NR | NR | NR | Normal | Hoefnagel (1968) |
| 20 | NR | NR | NR | Thinning of the cerebellar cortex | Hoefnagel (1968) |
| 21 | NR | NR | NR | Reduced spine density on occipital neurons | Saito and Takashima (2000) |
| 22 | NR | NR | NR | Non-specific changes compatible with an anoxic mode of death | Watts et al. (1982) |
One case was reported as LND without confirmatory laboratory findings and had atypical features of congenital blindness and marked ventricular dilation (Wada et al., 1968), raising some uncertainty regarding the diagnosis.
NR = not reported.
Discussion
Although a literature review suggests considerable variation in the motor disorder of LND, our evaluation of the largest series of patients currently available demonstrates a characteristic motor syndrome with only minor phenotypic variability. The motor disorder characteristically evolves in a manner similar to that of dyskinetic cerebral palsy, with hypotonia and/or delayed acquisition of motor skills within the first 3–6 months of age. Involuntary movements usually develop between 6 and 24 months of age. Once fully developed, the clinical course remains relatively static with severe action dystonia superimposed on a baseline of hypotonia. Many also display choreoathetosis or ballismus, but these features are always less severe than dystonia. Although many have pathological reflexes or spasticity, few have clonus. When these pyramidal signs are evident, they typically are mild, limited to the legs or asymmetrical.
Comparison with prior studies
The relatively consistent motor syndrome observed in our prospectively evaluated patients contrasts with the varied descriptions of motor abnormalities reported previously and reviewed in this study. One possible explanation for this discrepancy is that most cases were reported in non-neurological journals, often with only brief neurological evaluations made by examiners with little or no formal training in the diagnosis of motor disorders. It is widely recognized that the terminology of movement disorders has been applied using different criteria by different medical subspecialties for many years, and many of the reports on LND patients were published before the establishment of standard definitions and criteria for many motor disorders (Barbeau et al., 1981). Some uncertainties concerning nomenclature, particularly those relating to muscle tone in children, have persisted even after these definitions were established. These uncertainties have prompted recent efforts to clarify them further (Delgado and Albright, 2003; Sanger et al., 2003). To minimize difficulties associated with nomenclature, each case in the current series was examined by at least one neurologist with special training in movement disorders, using currently accepted criteria for diagnosis of abnormal motor syndromes.
Another reason for the inconsistent descriptions of the motor syndrome in LND is that some manifestations of dystonia closely resemble other neurological signs often associated with dysfunction of the corticospinal motor systems. Several major signs have been cited as evidence for corticospinal dysfunction in LND, including spasticity, the extensor plantar reflex, scissoring of the legs, hyperreflexia and clonus. The increased muscle tone of dystonia is readily mistaken for spasticity without special attention to their discriminating features. The extensor plantar reflex, or Babinski sign, is not a reliable indicator of corticospinal dysfunction among patients with extrapyramidal disease, because it cannot be reliably discriminated from the striatal toe, which is a form of dystonia (Nausieda et al., 1980; Jan, 2004; Ashour et al., 2005). Scissoring of the legs is typical in corticospinal dysfunction but also occurs with severe dystonia (Jankovic and Fahn, 1998; Furukawa et al., 2001). The many different manifestations of dystonia are not as well recognized as the more common manifestations of spasticity among evaluators without training in movement disorders, and they therefore are often mistaken as evidence for corticospinal dysfunction (Watts et al., 1982; Jan, 2004).
The third factor contributing to the varied descriptions of the motor syndrome in the published literature on LND is related to mood lability and anxiety. Patients with LND are more anxious than other children with developmental disabilities. In some, emotional lability is pronounced, including some with overt panic attacks. Anxiety and mood lability are particularly prominent when the patient encounters a stranger, such as a new care-giver or examiner. The heightened anxiety makes choreoathetosis and ballismus more apparent. Anxiety and mood lability lessen as the patients become accustomed to the evaluators, so the examination features may change during a long visit or over several visits. Consequently, even a single examiner can form different impressions on the basis of serial evaluations of the same patient. This problem was minimized in the current series because evaluations were conducted over a lengthy visit or the patients encountered the same evaluator during multiple visits over several years.
A last reason for the inconsistent descriptions of motor abnormalities in LND is that some characteristics of the disease may vary with development and ageing. There are no studies that have specifically addressed the natural history with longitudinal studies, but the available evidence suggests a stereotypical pattern of progression with hypotonia and developmental delay early in the first year of life. Other involuntary movements emerge later, between 6 and 24 months of age. Once established, significant progression of the severity of the motor syndrome with further ageing is uncommon, though progression of disability from long-standing dystonia became more apparent, for example as contractures or scoliosis.
The evolution of the motor syndrome parallels the evolution of the cognitive disability in LND. Patients with LND exhibit mild or moderate mental retardation with slow learning in school, but there is no evidence for progressive dementia with ageing (Anderson et al., 1992; Matthews et al., 1995; Solan et al., 1997; Schretlen et al., 2001). The evolution of motor and cognitive impairments in LND resembles that seen in cerebral palsy. The early changes of the motor features may reflect the response of the developing brain to a static insult rather than an ongoing pathological process (Almli and Finger, 1984; Finger and Almli, 1984). Although LND is often classified in texts and reviews of movement disorders among the ‘heredodegenerative’ dystonias, it is more appropriately classified among the ‘dystonia-plus’ syndromes, in view of the absence of obvious progression and the lack of evidence for a degenerative process.
Several clinical phenomena observed in LND may be considered in the context of other movement disorders where dystonia is well recognized. Severe opisthotonus with coarse whole-body shaking was frequent in the current series and in the literature on LND. This phenomenon is sometimes mistaken for epileptic seizures, but it is also seen in many movement disorders, where it is best characterized as a truncal dystonia with dystonic tremor (Fricka et al., 2001; Jan, 2004). The oromandibular and lingual dystonia in LND is similar to that seen in neuro-acanthocytosis (Hardie, 1989) or tardive syndromes due to chronic exposure to dopamine receptor antagonists (Tan and Jankovic, 2000). It is interesting that self-injurious tongue biting, a nearly universal phenomenon in LND, is seen occasionally in neuro-acanthocytosis or tardive syndromes too (Jankovic, 1988). The inspiratory stridor that occurs during wakefulness but not sleep in several LND patients is similar to that reported as laryngeal breathing dystonia (Grillone et al., 1994) and in some cases of multiple system atrophy (Merlo et al., 2002). This observation may provide a clue for understanding LND patients who exhibit recurrent apnoea or even sudden death. Finally, patients with LND exhibit ocular motor apraxia characterized by delayed initiation of voluntary saccades with facilitation of reflexive saccades similar to that observed in several other extrapyramidal disorders (Jinnah et al., 2001). These additional manifestations further emphasize the extra-pyramidal nature of the motor disorder of LND.
Pathophysiology of the motor disorder
The motor syndrome of LND described in the present series is consistent with current knowledge regarding the pathophysiology of the disorder. Overall, the most frequent abnormality among the imaging (Table 5) and autopsy studies (Table 6) has been a reduction in brain volume. This reduction in volume, however, seems often small enough to escape notice in routine studies. Routine imaging studies are therefore not particularly helpful in the evaluation of these patients.
Although consistent anatomical defects in LND brains have not been apparent, biochemical and PET studies have demonstrated abnormalities of basal ganglia dopamine systems. Biochemical studies of five LND brains have documented a profound loss of dopamine and its metabolites in the basal ganglia (Lloyd et al., 1981; Saito et al., 1999). Dopamine was not significantly reduced in the midbrain, fuelling speculation that midbrain dopamine neurons were preserved but that their axonal projections to the basal ganglia failed to develop or degenerated. In support of this proposal, midbrain dopamine neurons identified by Nissl stains or by immunostaining for tyrosine hydroxylase did not appear to be reduced in numbers in two cases (Saito et al., 1999). Furthermore, two PET studies have confirmed severe abnormalities of dopaminergic fibres in the basal ganglia. In one study, the accumulation of [18F]fluorodopa into monoaminergic axons in the striatum was reduced by 60–70% in 11 LND patients (Ernst et al., 1996). In the other study, the binding of [11C]WIN 35 428 to dopamine uptake sites on dopamine axons was reduced by a similar amount in seven other patients (Wong et al., 1996). These studies suggest a severe loss of dopaminergic axons in LND, though histological evidence is currently lacking.
These biochemical and PET data are consistent with the prominent dystonia observed in LND (Visser et al., 2000). A profound loss of striatal dopamine most often causes parkinsonism in adults, but more frequently causes dystonia in children. For example, early dopamine loss associated with inherited deficiency of GTP cyclohydrolase or tyrosine hydroxylase is more often associated with dystonia rather than parkinsonism (Perlmutter and Mink, 2004). Experimental studies have also shown that the age at which striatal dopamine depletion occurs has a dramatic influence on motor function in rodents. In adult rats, destruction of 95% of nigrostriatal dopamine neurons results in a motor syndrome resembling parkinsonism (Hirsch et al., 2003). In contrast, the same lesion in neonatal rats results in spontaneous hyperactivity and aggressiveness without signs of parkinsonism (Moy et al., 1997).
Isolated dysfunction of striatal dopamine systems does not account for the pyramidal signs that appear in some cases. Even recognizing the possibility of misclassification of neurological signs, another possibility is that HPRT deficiency causes dysfunction in corticospinal motor systems, although evidence currently is lacking. Another possibility is that corticospinal tract signs reflect an indirect, acquired process. The observation that the corticospinal tract signs frequently are limited to the legs suggests the possibility of cervical myelopathy, perhaps secondary to degenerative changes of the cervical spine caused by cervical dystonia with retrocollis. In fact, cervical instability leading to myelopathy has been documented for two LND patients (Watts et al., 1982). Violent retrocollis caused atlantoaxial dislocation in another case (Hoefnagel et al., 1965). A non-traumatic high cervical fracture in one case, and an os odontoideum in another, could both have resulted from chronic and forceful involuntary movements of the neck (Shewell and Thompson, 1996).
The possibility that cervical myelopathy results from involuntary neck movements is not unique to LND, but has been described in several other conditions, including cervical dystonia, generalized dystonia, paroxysmal dystonia, dyskinetic cerebral palsy and Tourette syndrome (Anderson et al., 1962; Levine et al., 1970; Hirose and Kadoya, 1984; Ebara et al., 1989; El-Mallakh et al., 1989; Adler et al., 1996; Harada et al., 1996; Krauss and Jankovic, 1996; Rosenfeld and Friedman, 1999). Since baseline motor disability is often quite severe in LND, the potential development of a secondary myelopathy can be difficult to identify. Further studies are needed to clarify the source of the pyramidal signs in LND. Until this information is available, it is prudent to perform imaging studies of the cervical or thoracic spine in LND patients who develop true corticospinal signs.
Treatment
Although several trials have addressed the treatment of self-injury, there have been no controlled trials devoted to treatment of the motor disorder in LND. Anecdotal reports of dopamine replacement with levodopa in 11 patients described mixed results (Mizuno and Yugari, 1974; Mizuno and Yugary, 1975; Watts et al., 1982; Manzke et al., 1986; Jankovic et al., 1988; Hunter et al., 1996). Mild improvement was noted for six, mild worsening was noted for three and no effect was reported for two. There is a single report of two patients involving the use of a direct-acting dopamine agonist, bromocriptine, again with mixed results (Jankovic et al., 1988). The interpretation of these results is complicated by two issues. First, the maximal doses employed and the duration of therapy were recorded only rarely. Second, several investigators have suggested that some of the dyskinetic movements and even self-injury in LND might reflect a form of ‘endogenous’ dopamine-related dyskinesia (Proctor and McGinness, 1970; Taira et al., 2003). It is therefore possible that dopaminergic drugs reduce the severity of dystonia while simultaneously increasing other involuntary movements or distressing behaviours, rendering a negative overall impression of the treatment response. Further trials with more detailed observations of the influence of specific doses of these agents on different aspects of the condition are warranted.
Others have tried the dopamine-depleting agent tetrabenazine (Watts et al., 1974; Jankovic et al., 1988). Efforts to control self-injurious behaviours often lead to the use of dopamine receptor antagonists including pimozide, haloperidol, fluphenazine and Risperdal (Watts et al., 1982; Goldstein et al., 1985; Jankovic et al., 1988; Allen and Rice, 1996). Their benefits on self-injury are unreliable, and they do not appear to have a significant influence on dystonia. We prefer to avoid these drugs as they carry the risk of causing tardive syndromes that make assessment and management of the pre-existing motor disorder unnecessarily complicated.
Oral baclofen and benzodiazepines are often prescribed for patients with LND. While they may be useful for controlling spasticity and clonus in the minority of patients who have these problems, their value in the management of the extrapyramidal features is unclear. Two of our patients received intrathecal baclofen pumps. Both reported good results, though objective assessments were not possible because the procedures were performed before our evaluations. There was a notable paucity of reports describing results of treatment with trihexyphenidyl or other anticholinergics. In our experience, it is not useful in non-sedating doses. However, botulinum toxins can be used to treat some of the most discomforting manifestations, such as cervical or limb dystonia.
Finally, there are two case reports of thalamotomy producing little or no improvement (Michener, 1967; Bunn et al., 1975) and another case report describing 30% improvement in dystonia following chronic deep brain stimulation of the globus pallidus in LND (Taira et al., 2003). In the last case, an unexpected benefit was complete elimination of self-injurious behaviours for at least one year. Further studies of these procedures are required before they can be recommended to all patients.
Summary
The motor disorder of LND characteristically presents with hypotonia and/or delayed acquisition of motor skills within the first 3–6 months of age. Additional involuntary movements, most prominently dystonia, usually develop between 6 and 24 months of age. Other extrapyramidal and pyramidal signs may occur, but these are usually minor compared with dystonia. The more varied descriptions of the motor syndrome in the literature probably reflect differences in the use of movement disorder terminology, poor recognition of the many different manifestations of dystonia and influences related to age and anxiety at the time of evaluation. The processes whereby a defect in HPRT-mediated purine recycling leads to the neurobehavioural manifestations remain largely unknown, but a growing body of evidence has pointed to dysfunction of basal ganglia dopamine systems as a potential underlying contributor to dystonia. Current treatments are not satisfactory. Increasing our understanding of the pathophysiology of LND may allow for the rational design of new approaches for treatment.
Acknowledgments
We gratefully acknowledge the patients and their families for participating in these studies. We appreciate the expertise of Patrick O’Neill in verifying the mutations for some patients. We thank Harvey Singer for helpful comments on the manuscript. Support was provided by the Lesch–Nyhan Syndrome Children’s Research Foundation, the Fondo de Investigaciones Sanitarias REDEMETH G03/054 and National Institutes of Health grants NS01985 and HD33095.
Abbreviations
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- LND
Lesch–Nyhan disease
Footnotes
For Permissions, please email: journals.permissions@oxfordjournals.org
References
- Adler CH, Zimmerman RS, Lyons MK, Simeone F, Brin MF. Perioperative use of botulinum toxin for movement disorder-induced cervical spine disease. Mov Disord. 1996;11:79–81. doi: 10.1002/mds.870110114. [DOI] [PubMed] [Google Scholar]
- Allen SM, Rice SN. Risperidone antagonism of self-mutilation in a Lesch-Nyhan patient. Prog Neuro-Psychopharmacol Biol Psychiat. 1996;20:793–800. doi: 10.1016/0278-5846(96)00059-0. [DOI] [PubMed] [Google Scholar]
- Almli CR, Finger S. Early brain damage: research orientations and clinical observation. Vol. 1. New York: Academic Press; 1984. [Google Scholar]
- Anderson LT, Ernst M. Self-injury in Lesch-Nyhan disease. J Autism Dev Disord. 1994;24:67–81. doi: 10.1007/BF02172213. [DOI] [PubMed] [Google Scholar]
- Anderson LT, Ernst M, Davis SV. Cognitive abilities of patients with Lesch-Nyhan disease. J Autism Dev Disord. 1992;22:189–203. doi: 10.1007/BF01058150. [DOI] [PubMed] [Google Scholar]
- Anderson WW, Wise BL, Itabashi HH, Jones M. Cervical spondylosis in patients with athetosis. Neurology. 1962;12:410–12. doi: 10.1212/wnl.12.6.410. [DOI] [PubMed] [Google Scholar]
- Ashour R, Tintner R, Jankovic J. Striatal deformities of the hand and foot in Parkinson’s disease. Lancet Neurol. 2005;4:423–31. doi: 10.1016/S1474-4422(05)70119-8. [DOI] [PubMed] [Google Scholar]
- Barbeau A, Duvoisin RC, Gerstenbrand F, Lakke JPWF, Marsden CD. Classification of extrapyramidal disorders. J Neurol Sci. 1981;51:311–27. doi: 10.1016/0022-510x(81)90109-x. [DOI] [PubMed] [Google Scholar]
- Bassermann R, Gutensohn W, Jahn H, Springmann JS. Pathological and immunological observations in a case of Lesch-Nyhan syndrome. Eur J Pediatr. 1979;132:93–8. doi: 10.1007/BF00447375. [DOI] [PubMed] [Google Scholar]
- Bunn DN, Moss IK, Nicholls A, Scott JT, Snaith ML, Watson MR. Clinical and biochemical observations on three cases of hypoxanthine-guanine phosphoribosyltransferase deficiency. Ann Rheum Dis. 1975;34:249–55. doi: 10.1136/ard.34.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie R, Bay C, Kaufman IA, Bakay B, Borden M, Nyhan WL. Lesch-Nyhan disease: clinical experience with nineteen patients. Dev Med Child Neurol. 1982;24:293–306. doi: 10.1111/j.1469-8749.1982.tb13621.x. [DOI] [PubMed] [Google Scholar]
- Crome L, Stern J. The pathology of mental retardation. London: J. & A. Churchill; 1967. [Google Scholar]
- Crussi FG, Robertson DM, Hiscox JL. The pathological condition of the Lesch-Nyhan syndrome. Am J Dis Child. 1969;118:501–6. doi: 10.1001/archpedi.1969.02100040503016. [DOI] [PubMed] [Google Scholar]
- De Gregorio L, Jinnah HA, Nyhan WL, Trombley L, O’Neill JP. Lesch-Nyhan disease in one member of a female monozygotic twin pair heterozygous for a mutation in HPRT. Mol Genet Metab. 2005;85:70–7. doi: 10.1016/j.ymgme.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Albright AL. Movement disorders in children: definitions, classifications, and grading systems. J Child Neurol. 2003;18:S1–8. doi: 10.1177/0883073803018001S0301. [DOI] [PubMed] [Google Scholar]
- Ebara S, Harada T, Yamazaki Y. Unstable cervical spine in athetoid cerebral palsy. Spine. 1989;14:1154–9. doi: 10.1097/00007632-198911000-00005. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, Rao K, Barwick M. Cervical myelopathy secondary to movement disorders: case report. Neurosurg. 1989;24:902–5. doi: 10.1227/00006123-198906000-00018. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Hardy K, et al. Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N Engl J Med. 1996;334:1568–72. doi: 10.1056/NEJM199606133342403. [DOI] [PubMed] [Google Scholar]
- Fahn S. Concept and classification of dystonia. Adv Neurol. 1988;50:8. [PubMed] [Google Scholar]
- Finger S, Almli CR. Early brain damage: neurobiology and behavior. Vol. 2. New York: Academic Press; 1984. [Google Scholar]
- Fricka KB, Kim C, Newton PO. Spinal lordosis with marked opisthotonus secondary to dystonia musculorum deformans: case report with surgical management. Spine. 2001;26:2283–8. doi: 10.1097/00007632-200110150-00026. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Graf WD, Wong H, Shimadzu M, Kish SJ. Dopa-responsive dystonia simulating spastic paraplegia due to tyrosine hydroxylase (TH) gene mutations. Neurology. 2001;56:260–63. doi: 10.1212/wnl.56.2.260. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Anderson LT, Reuben R, Dancis J. Self-mutilation in Lesch-Nyhan disease is caused by dopaminergic denervation. Lancet. 1985;1:338–9. doi: 10.1016/s0140-6736(85)91107-9. [DOI] [PubMed] [Google Scholar]
- Grillone GA, Blitzer A, Brin MF, Annino DJ, Saint-Hilaire MH. Treatment of adductor laryngeal breathing dystonia with botulinum toxin type A. Laryngoscope. 1994;104:30–2. doi: 10.1288/00005537-199401000-00007. [DOI] [PubMed] [Google Scholar]
- Hara K, Kashiwamata S, Ogasawara N, Ohishi H, Natsume R, Yamanaka T, et al. A female case of the Lesch-Nyhan syndrome. Tohoku J Exp Med. 1982;137:275–82. doi: 10.1620/tjem.137.275. [DOI] [PubMed] [Google Scholar]
- Harada T, Ebara S, Anwar MM. The cervical spine in athetoid cerebral palsy: a radiological study of 180 patients. J Bone Joint Surg. 1996;78:613–19. [PubMed] [Google Scholar]
- Hardie RJ. Acanthocytosis and neurological impairment—a review. Q J Med. 1989;71:291–306. [PubMed] [Google Scholar]
- Harris JC, Lee RR, Jinnah HA, Wong DF, Yaster M, Bryan N. Craniocerebral magnetic resonance imaging measurement and findings in Lesch-Nyhan syndrome. Arch Neurol. 1998;55:547–53. doi: 10.1001/archneur.55.4.547. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Higashino H, Woo M, Yasuhara A, Sugimoto T, Kobayashi Y. Lesch-Nyhan syndrome with delayed onset of self-mutilation: hyperactivity of interneurons at the brainstem and blink reflex. Acta Neurol Scand. 1990;81:184–7. doi: 10.1111/j.1600-0404.1990.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Hirose G, Kadoya S. Cervical spondylotic radiculo-myelopathy in patients with athetoid-dystonic cerebral palsy: clinical evaluation and surgical treatment. J Neurol Neurosurg Psychiatry. 1984;47:775–80. doi: 10.1136/jnnp.47.8.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hoglinger G, Rousselet E, Breidert T, Parin K, Feger J, et al. Animal models of Parkinson’s disease in rodents induced by toxins: an update. J Neural Transm Suppl. 2003;65:89–100. doi: 10.1007/978-3-7091-0643-3_6. [DOI] [PubMed] [Google Scholar]
- Hoefnagel D. Summary: pathology and pathologic physiology. Fed Proc. 1968;27:1042–6. [PubMed] [Google Scholar]
- Hoefnagel D, Andrew ED, Mireault NG, Berndt WO. Hereditary choreoathetosis, self-mutilation, and hyperuricemia in young males. N Engl J Med. 1965;273:130–5. doi: 10.1056/NEJM196507152730303. [DOI] [PubMed] [Google Scholar]
- Hunter TC, Melancon SB, Dallaire L, Taft S, Skopek TR, Albertini RJ, et al. Germinal HPRT splice donor site mutation results in multiple RNA splicing products in T-lymphocyte cultures. Somat Cell Mol Genet. 1996;22:145–50. doi: 10.1007/BF02369904. [DOI] [PubMed] [Google Scholar]
- Jan MMS. Misdiagnoses in children with dopa-responsive dystonia. Pediatr Neurol. 2004;31:298–303. doi: 10.1016/j.pediatrneurol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Orofacial and other self-mutilations. Adv Neurol. 1988;49:365–81. [PubMed] [Google Scholar]
- Jankovic J, Caskey CT, Stout JT, Butler IJ. Lesch-Nyhan syndrome: a study of motor behavior and cerebrospinal fluid neurotransmitters. Ann Neurol. 1988;23:466–9. doi: 10.1002/ana.410230507. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Fahn S. Dystonic disorders. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Baltimore, MD: Williams & Wilkins; 1998. pp. 513–51. [Google Scholar]
- Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2000. pp. 2537–70. [Google Scholar]
- Jinnah HA, Harris JC, Reich SG, Visser JE, Barabas G, Eddey GE. The motor disorder of Lesch-Nyhan disease. Mov Disord. 1998;13(Suppl 2):98. [Google Scholar]
- Jinnah HA, DeGregorio L, Harris JC, Nyhan WL, O’Neill JP. The spectrum of inherited mutations causing HPRT deficiency: 75 new cases and a review of 196 previously reported cases. Mutat Res. 2000;463:309–26. doi: 10.1016/s1383-5742(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Lewis RF, Visser JE, Eddey GE, Barabas G, Harris JC. Ocular motor dysfunction in Lesch-Nyhan disease. Pediatr Neurol. 2001;24:200–4. doi: 10.1016/s0887-8994(00)00265-4. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Harris JC, Nyhan WL, O’Neill JP. The spectrum of mutations causing HPRT deficiency: an update. Nucleosides Nucleotides Nucleic Acids. 2004;23:1153–60. doi: 10.1081/NCN-200027400. [DOI] [PubMed] [Google Scholar]
- Krauss JK, Jankovic J. Severe motor tics causing cervical myelopathy in Tourette’s syndrome. Mov Disord. 1996;11:563–6. doi: 10.1002/mds.870110512. [DOI] [PubMed] [Google Scholar]
- Lesch M, Nyhan WL. A familial disorder of uric acid metabolism and central nervous system function. Am J Med. 1964;36:561–70. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- Levine RA, Rosenbaum AE, Waltz JM, Scheinberg LC. Cervical spondylosis and dyskinesias. Neurology. 1970;20:1194–9. doi: 10.1212/wnl.20.12.1194. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, Goldstein M, et al. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med. 1981;305:1106–11. doi: 10.1056/NEJM198111053051902. [DOI] [PubMed] [Google Scholar]
- Lynch BJ, Noetzel MJ. Recurrent coma and Lesch-Nyhan syndrome. Pediatr Neurol. 1991;7:389–91. doi: 10.1016/0887-8994(91)90073-t. [DOI] [PubMed] [Google Scholar]
- Mahnovski V, Dozic S, Vulovic D, Marjanovic B, Tasic G. Necropsy findings in a case of Lesch-Nyhan syndrome. Arch Dis Child. 1975;50:666. doi: 10.1136/adc.50.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke H, Gustmann H, Koke HB, Nyhan WL. Hypoxanthine and tetra-hydrobiopterin treatment of a patient with features of the Lesch-Nyhan syndrome. Adv Exp Med Biol. 1986;195A:197–204. doi: 10.1007/978-1-4684-5104-7_31. [DOI] [PubMed] [Google Scholar]
- Maramattom BV. Self-mutilation in the Lesch-Nyhan syndrome. Neurology. 2005;65:E25. doi: 10.1212/01.wnl.0000184611.55051.f3. [DOI] [PubMed] [Google Scholar]
- Matthews WS, Solan A, Barabas G. Cognitive functioning in Lesch-Nyhan syndrome. Dev Med Child Neurol. 1995;37:715–22. doi: 10.1111/j.1469-8749.1995.tb15017.x. [DOI] [PubMed] [Google Scholar]
- Merlo IM, Occhini A, Pacchetti C, Alfonsi E. Not paralysis, but dystonia causes stridor in multiple system atrophy. Neurol. 2002;58:649–52. doi: 10.1212/wnl.58.4.649. [DOI] [PubMed] [Google Scholar]
- Michener WM. Hyperuricemia and mental retardation. Am J Dis Child. 1967;113:195–206. doi: 10.1001/archpedi.1967.02090170059003. [DOI] [PubMed] [Google Scholar]
- Mitchell G, McInnes RR. Differential diagnosis of cerebral palsy: Lesch-Nyhan syndrome without self-mutilation. Can Med Assoc J. 1984;130:1323–4. [PMC free article] [PubMed] [Google Scholar]
- Mizuno T. Long-term follow-up of ten patients with Lesch-Nyhan syndrome. Neuropediatrics. 1986;17:158–61. doi: 10.1055/s-2008-1052518. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Endoh H, Konishi Y, Miyachi Y, Akoaka I. An autopsy case of the Lesch-Nyhan syndrome: normal HGPRT activity in liver and xanthine calculi in various tissues. Neuropaediatrie. 1976;7:351–5. doi: 10.1055/s-0028-1091635. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Yugari Y. Self-mutilation in Lesch-Nyhan syndrome. Lancet. 1974;1:761. doi: 10.1016/s0140-6736(74)92990-0. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Yugary Y. Prophylactic effect of L-5-hydroxytryptophan on self-mutilation in the Lesch-Nyhan syndrome. Neuropediatrics. 1975;6:13–23. [Google Scholar]
- Moy SS, Criswell HE, Breese GR. Differential effects of bilateral dopamine depletion in neonatal and adult rats. Neurosci Biobehav Rev. 1997;21:425–35. doi: 10.1016/s0149-7634(96)00040-1. [DOI] [PubMed] [Google Scholar]
- Nausieda PA, Weiner WJ, Klawans HL. Dystonic foot response of Parkinsonism. Arch Neurol. 1980;37:132–6. doi: 10.1001/archneur.1980.00500520030003. [DOI] [PubMed] [Google Scholar]
- Nyhan WL. Behavior in the Lesch-Nyhan syndrome. J Autism Child Schizophren. 1976;6:235–52. doi: 10.1007/BF01543464. [DOI] [PubMed] [Google Scholar]
- Partington MW, Hennen BKE. The Lesch-Nyhan syndrome: self-destructive biting, mental retardation, neurological disorder and hyperuricemia. Dev Med Child Neurol. 1967;9:563–72. doi: 10.1111/j.1469-8749.1967.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Dysfunction of dopaminergic pathways in dystonia. Adv Neurol. 2004;94:163–70. [PubMed] [Google Scholar]
- Proctor P, McGinness JE. Levodopa side-effects and the Lesch-Nyhan syndrome. Lancet. 1970;687:1367. doi: 10.1016/s0140-6736(70)92399-8. [DOI] [PubMed] [Google Scholar]
- Puig JG, Torres RJ, Mateos FA, Ramos TH, Arcas JM, Buno AS, et al. The spectrum of hypoxanthine-guanine phosphoribosyltransferase deficiency: clinical experience based on 22 patients from 18 Spanish families. Medicine. 2001;80:102–12. doi: 10.1097/00005792-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Robey KL, Reck JF, Giacomini KD, Barabas G, Eddey GE. Modes and patterns of self-mutilation in persons with Lesch-Nyhan disease. Dev Med Child Neurol. 2003;45:167–71. doi: 10.1017/s001216220300032x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M, Friedman JH. Cervical stenosis and dystonic cerebral palsy. Mov Disord. 1999;14:194–5. doi: 10.1002/1531-8257(199901)14:1<194::aid-mds1045>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ito M, Hanaoka S, Ohama E, Akaboshi S, Takashima S. Dopamine receptor upregulation in Lesch-Nyhan syndrome: a postmortem study. Neuropediatrics. 1999;30:66–71. doi: 10.1055/s-2007-973462. [DOI] [PubMed] [Google Scholar]
- Saito Y, Takashima S. Neurotransmitter changes in the pathophysiology of Lesch-Nyhan syndrome. Brain Dev. 2000;22(Suppl 1):S122–31. doi: 10.1016/s0387-7604(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Salman RA, Glickman RS, Super S. Lesch-Nyhan syndrome: report of two cases. J Oral Med. 1987;42:13. [PubMed] [Google Scholar]
- Sanger TD, Delgado MR, Gaebler-Spira D, Hallet M, Mink JW, Task F. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:89–97. doi: 10.1542/peds.111.1.e89. [DOI] [PubMed] [Google Scholar]
- Sass JK, Itabashi HH, Dexter RA. Juvenile gout with brain involvement. Arch Neurol. 1965;13:639–55. doi: 10.1001/archneur.1965.00470060075008. [DOI] [PubMed] [Google Scholar]
- Schretlen DS, Harris JC, Park KS, Jinnah HA, Ojeda del Pozo N. Neurocognitive functioning in Lesch-Nyhan disease and partial hypoxanthine-guanine phosphoribosyltransferase deficiency. J Int Neuropsychol Soc. 2001;7:805–12. doi: 10.1017/s135561770177703x. [DOI] [PubMed] [Google Scholar]
- Schretlen DS, Ward J, Meyer SM, Yun J, Puig JG, Nyhan WL, et al. Behavioral aspects of Lesch-Nyhan disease and its variants. Dev Med Child Neurol. 2005;47:673–7. doi: 10.1017/S0012162205001374. [DOI] [PubMed] [Google Scholar]
- Seegmiller JE. Contributions of Lesch-Nyhan syndrome to the understanding of purine metabolism. J Inherit Metab Dis. 1989;12:184–96. doi: 10.1007/BF01800725. [DOI] [PubMed] [Google Scholar]
- Shewell PC, Thompson AG. Atlantoaxial instability in Lesch-Nyhan syndrome. Spine. 1996;21:757–62. doi: 10.1097/00007632-199603150-00020. [DOI] [PubMed] [Google Scholar]
- Solan A, Matthews W, Barabas G, Robey K. Cognition in LND: a two-year follow-up study. Dev Med Child Neurol. 1997;39:492–3. [PubMed] [Google Scholar]
- Storey B. The Lesch-Nyhan syndrome. Med J Aust. 1969;2:696–9. doi: 10.5694/j.1326-5377.1969.tb107349.x. [DOI] [PubMed] [Google Scholar]
- Taira T, Kobayashi T, Hori T. Disappearance of self-mutilating behavior in a patient with Lesch-Nyhan syndrome after bilateral chronic stimulation of the globus pallidus interna. J Neurosurg. 2003;98:414–6. doi: 10.3171/jns.2003.98.2.0414. [DOI] [PubMed] [Google Scholar]
- Tan EK, Jankovic J. Tardive and idiopathic oromandibular dystonia: a clinical comparison. J Neurol Neurosurg Psychiatry. 2000;68:186–90. doi: 10.1136/jnnp.68.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres RJ, Mateos FA, Molano J, Gathoff BS, O’Neill JP, Gundel RM, et al. Molecular basis of hypoxanthine-guanine phosphoribosyl-transferase deficiency in thirteen Spanish families. Hum Mutat. 2000;15:383. doi: 10.1002/(SICI)1098-1004(200004)15:4<383::AID-HUMU17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- van Bogaert P, Ceballos I, Desguerre I, Telvi L, Kamoun P, Ponsot G. Lesch-Nyhan syndrome in a girl. J Inherit Metab Dis. 1992;15:790–1. doi: 10.1007/BF01800022. [DOI] [PubMed] [Google Scholar]
- Visser JE, Baer PR, Jinnah HA. Lesch-Nyhan syndrome and the basal ganglia. Brain Res Rev. 2000;32:449–75. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Visser JE, Harris JC, Barabas G, Eddey GE, Jinnah HA. The motor disorder of classic Lesch-Nyhan disease. Nucleosides Nucleotides Nucleic Acids. 2004;23:8–9. doi: 10.1081/NCN-200027432. [DOI] [PubMed] [Google Scholar]
- Wada Y, Arakawa T, Loizumi K. Lesch-Nyhan syndrome: autopsy findings and in vitro study of incorporation of 14C-8-inosine into uric acid, guanosine-monophosphate and adenosine-monophosphate in the liver. Tohoku J Exp Med. 1968;95:253–60. doi: 10.1620/tjem.95.253. [DOI] [PubMed] [Google Scholar]
- Warzok R, Schwesinger G, Knapp A, Seidlitz F. Neuropathologische Befunde beim Lesch-Nyhan Syndrom. Zbl Allg Pathol. 1982;126:95–104. [PubMed] [Google Scholar]
- Watts RW, Harkness RA, Spellacy E, Taylor NF. Lesch-Nyhan syndrome: growth delay, testicular atrophy and a partial failure of the 11 beta-hydroxylation of steroids. J Inherit Metab Dis. 1987;10:210–23. doi: 10.1007/BF01800063. [DOI] [PubMed] [Google Scholar]
- Watts RWE, McKeran RO, Brown E, Andrews TM, Griffiths MI. Clinical and biochemical studies on treatment of Lesch-Nyhan syndrome. Arch Dis Child. 1974;49:693–702. doi: 10.1136/adc.49.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts RWE, Spellacy E, Gibbs DA, Allsop J, McKeran RO, Slavin GE. Clinical, post-mortem, biochemical and therapeutic observations on the Lesch-Nyhan syndrome with particular reference to the neurological manifestions. Q J Med. 1982;201:43–78. [PubMed] [Google Scholar]
- Wong CJ. School of Medicine Thesis. University of California; San Diego, CA: 1988. Radiology of Lesch-Nyhan disease. [Google Scholar]
- Wong DF, Harris JC, Naidu S, Yokoi F, Marenco S, Dannals RF, et al. Dopamine transporters are markedly reduced in Lesch-Nyhan disease in vivo. Proc Natl Acad Sci USA. 1996;93:5539–43. doi: 10.1073/pnas.93.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]