Abstract
Alcohol dependence remains among the most common substance abuse problems worldwide, and compulsive alcohol consumption is a significant public health concern. Alcohol is an addictive drug that alters brain function through interactions with multiple neurotransmitter systems. These neurotransmitter systems mediate the reinforcing effects of alcohol. Specifically, the serotonergic system is important in mediating alcohol reward, preference, dependence, and craving. In this review chapter, we first discuss the serotonin system as it relates to alcoholism, and then outline interactions between this system and other neurotransmitter systems. We emphasize the serotonin transporter and its possible role in alcoholism, then present several serotonergic receptors and discuss their contribution to alcoholism, and finally assess the serotonin system as a target for pharmacotherapy, with an emphasis on current and potential treatments.
I. Introduction
Alcohol dependence is among the most common substance abuse problems worldwide, and compulsive alcohol intake is a significant public health concern (cf. Refs. 1-6). Alcohol interacts with multiple neurotransmitter systems to alter brain function and produces an imbalance between inhibitory and excitatory neurotransmitter regulation. Altered neurotransmission is associated with the reinforcing effects of alcohol consumption, as well as the abnormal behaviors exhibited following acute alcohol intoxication. Chronic alcohol exposure induces adaptive changes in normal neurocircuitry that lead to dependence.7,8 A better understanding of the neurobiological impact of alcohol consumption will facilitate the development of novel intervention strategies that target both the prevention and treatment of alcohol dependence.
The serotonergic system plays a key role in the regulation of alcohol intake, reward, preference, and dependence.9-15 Deficient serotonin (5-hydroxytryptamine, 5-HT) neurotransmission has been associated with increased alcohol consumption and vulnerability to alcohol dependence.9,16-18 Acute alcohol exposure produces an increase in extracellular 5-HT levels, while chronic exposure causes an overall decrease in 5-HT neurotransmission as evidenced by lower levels of 5-hydroxyindoleacetic acid (5-HIAA), the primary metabolite of 5-HT, in cerebrospinal fluid of alcoholics (for review see Ref. 8). This reduction in extracellular 5-HT in a chronic alcohol exposure paradigm could be caused by accelerated 5-HT reuptake from the extracellular space through the serotonin transporter (5-HTT), or by dysfunctional 5-HT release from the raphe nuclei (for review see Ref. 8).
Alcohol was characterized in the past as a nonspecific drug; however, recent molecular and pharmacological studies have successfully defined specific protein targets. Several of these proteins belong to the serotonergic system, including 5-HT3, 5-HT1B, 5-HT1A receptors, and 5-HTT. Serotonergic projections originating in the raphe nuclei innervate many of the brain regions involved in the rewarding effects of alcohol and other drugs.19,20 Studies have shown that alcohol consumption affects the functionality and expression of 5-HTT21-23 and, thus, alters the removal of 5-HT from the postsynaptic cleft in these areas following intake. Furthermore, 5-HT autoreceptors and heterore-ceptors have been linked to the regulation of alcohol intake (for review see Ref. 24). For example, alcohol directly targets the 5-HT3 receptor,25 an excitatory ionotropic heteroreceptor often found on inhibitory GABA interneurons.26 It has been hypothesized that direct activation of the 5-HT3 receptor on GABA interneurons is at least partly responsible for acute intoxication, producing both excitatory and inhibitory effects which vary according to the neurocircuitry involved.26,27
Dopamine release in mesolimbic reward pathways is an established mechanism of the rewarding effects of alcohol.27 5-HT neurons originating in the raphe nuclei can mediate dopamine release in the ventral tegmental area (VTA) and the nucleus accumbens (NAc).7 Serotonergic projections also innervate other brain regions including the amygdala and the prefrontal cortex (PFC) (Fig. 1). Dysfunctional 5-HT1B heteroreceptors at the terminal of the NAc–VTA pathway may increase alcohol consumption by decreasing GABA release, and consequently increasing dopamine activation.19,24,28-32 In addition, the dorsal striatum is under the control of serotonergic neurotransmission and has been associated with obsessive tendencies related to addiction.8
FIG. 1.
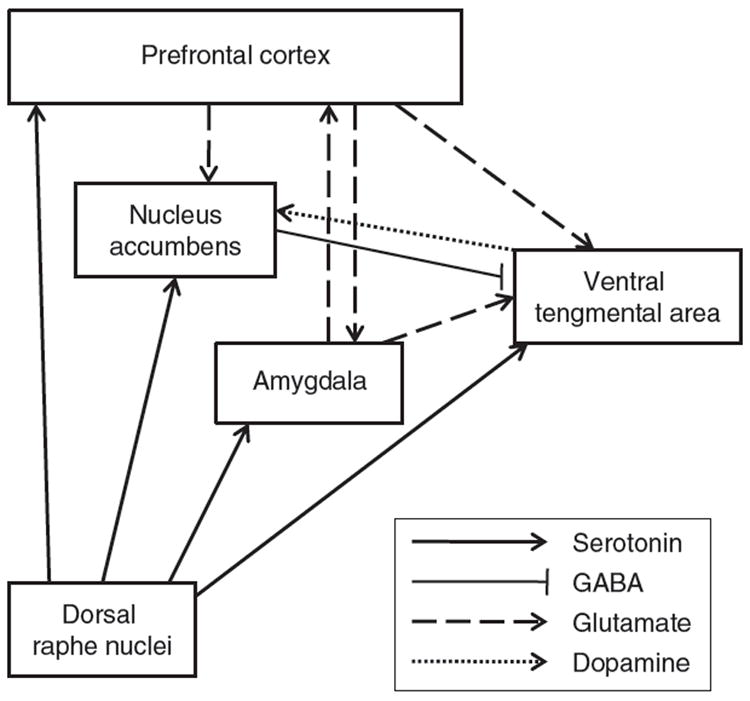
Schematic representation of the serotonergic neurocircuitry as it relates to the other neurotransmitter systems involved in alcohol dependence. Serotonergic neurons project to different reward brain regions such prefrontal cortex (PFC), nucleus accumbens (NAc), ventral tegmental area (VTA), and amygdala. The GABAergic NAc-VTA pathway contains 5-HT receptors at its terminal and controls the release of GABA, which in turn regulates the release of dopamine of the VTA-NAc pathway. The glutamatergic projections from the PFC targeting the NAc and VTA express 5-HT receptors at their terminals, which control the release of glutamate.
Pharmacological treatments for alcohol dependence have advanced over the years. However, there are still relatively few therapeutic drugs intended for the treatment of alcoholism. Currently, drugs that have been shown to effectively reduce alcohol intake include naltrexone (an opioid antagonist), acamprosate (glutamate-N-methyl D-aspartate (NMDA) and calcium channel dependent activity; the precise mechanism is unknown), and topiramate (γ-aminobutyric acid facilitator and glutamate function inhibitor).33-38 Drugs that target the serotonergic system in the context of alcohol dependence include selective serotonin reuptake inhibitors (SSRIs) that block 5-HTT, partial 5-HT1A receptor agonists, and 5-HT3 receptor antagonists.39,40
Treating alcohol addiction is difficult because of the highly heterogeneous nature of alcoholic populations. In attempts to remedy the tendency to group addicts as a whole, various subtypes of alcoholism have been defined. These include Babor’s type A and type B41 and Cloninger’s type I and type II.42 Type A alcoholics have less severe substance dependence, a later onset of addiction, and a lesser degree of comorbid psychological dysfunction,41 while type B alcoholics have earlier onset, a greater amount of stress, more childhood environmental risk factors, history of polydrug abuse, a greater potential for comorbid psychological dysfunction, and greater severity of dependence.43 Type I alcoholics are characterized by late-onset drinking and the presence of genetic and environmental risk factors.42 However, type II alcoholics are characterized by earlier onset, weak environmental influence, and a high frequency of antisocial and impulsive traits.42 Studies have suggested an association between type I or type A alcoholism and dopaminergic dysfunction, where an alcoholic user seeks the anxiety relieving effects of alcohol.44 Type B or type II alcoholism has been linked to deficient serotonergic neurotransmission and inherited biological risk factors.45
In this review chapter, we have discussed the role of the serotonergic system in alcoholism. The influence of genetic differences in 5-HTT and 5-HT receptor genotypes has been addressed to understand their involvement in alcohol craving. We discuss also the interactions between the serotonergic system and other neurotransmitters in the regulation of alcohol intake in animal models and clinics. Finally, we discuss the pharmacological implications of drugs that target the 5-HTT and the 5-HT receptors, which have been shown to be potential treatments for alcohol dependence.
II. The Role of 5-HTT in Alcohol-Directed Neuroadaptation, Intoxication Response, and Potential for Abuse and Dependence
5-HTT is a member of the SLC6 family of transporters.46 It is a transmembrane monoamine neurotransmitter sodium symporter that is sodium- and chloride-dependent and controls the concentration of 5-HT at both central and peripheral sites.47 The functionality of 5-HTT is dependent on the concentration of transporters, the relative affinity for 5-HT, and the rate at which the transporters remove 5-HT. 5-HTT has been examined as a potential mechanism by which chronic alcohol exposure decreases 5-HT neurotransmission in alcohol-dependent individuals.
A. Alcohol’s Influence on 5-HTT Expression, Function, and Region-Specific Neuroadaptations
Alcohol’s influence on 5-HTT mRNA and protein levels has been shown in numerous models of alcohol intake and dependence. In animal models, alcohol exposure induced increases in 5-HTT mRNA concentrations in central serotonergic brain regions, as well as in reward circuitry and information processing regions.48,49 A series of human postmortem autoradiography studies determined that chronic alcohol exposure decreases 5-HTT binding potential for ligands that mimic 5-HT.21-23 However, some positron-emission tomography (PET) scan findings have indicated that there is no difference in 5-HTT density in the brains of alcoholics.45,50
Chronic voluntary alcohol consumption increased 5-HTT mRNA levels in the dorsal and median raphe nuclei where 5-HT cell bodies are located in Wistar rats.49 However, when high alcohol-consuming rats were treated with the noncompetitive opioid antagonist naltrexone, 5-HTT gene expression in the aforementioned areas was significantly reduced, and voluntary alcohol consumption significantly decreased.49 This effect indicates that alcohol consumption did not alter 5-HTT expression exclusively in a direct way, and suggests that interactions between alcohol and neurotransmitter systems cause complex changes in adjacent pathways. Chronic voluntary alcohol consumption also increased 5-HTT protein levels in the raphe nuclei of living nonhuman primates.48 A single-photon emission computed tomography (SPECT) study, using the radioligand [123I]2β-carbomethoxy-3B-(4-iodophenyl)tropane ([123I]β-CIT), concluded that rhesus monkeys showing higher 5-HTT protein levels consumed greater amounts of alcohol. Interestingly, [123I]β-CIT binding was not found to be significantly increased in other brain regions. However, extracellular 5-HT in the raphe nuclei activates autoreceptors on serotonergic cell bodies and this region-specific change in 5-HT binding may indeed be indicative of 5-HT release from serotonergic projections in other brain regions.48,51
Postmortem autoradiography study has been used to examine the activity of 5-HTT in specific regions of the human brain. A study using [3H]citalopram reported lower 5-HTT binding in the anterior cingulate cortex of nonabstinent types I and II alcoholics.21 However, no significant change in 5-HTT density was observed in the superior frontal gyrus. Collectively, the frontal cortex plays an important role in motivation and learning, and low 5-HTT density in this area has been associated with depression and other psychiatric illnesses.21 A decrease in 5-HTT [3H]citalopram binding sites was also reported in the dorsal amygdala and the caudate body of the same postmortem brains as compared to the control group.22,23 No significant differences were seen, however, in the ventral amygdala, the rostral caudate, or the putamen of alcoholics. [3H]citalopram binding in the caudate putamen was decreased in type I alcoholics as compared to type II; however, no significant difference was found between the two groups in the dorsal amygdala.22 Alcohol was detected via blood sample analysis in the majority of the alcoholic subjects included in the above postmortem studies when the tissue was collected.21,22 Importantly, these findings indicate that brain areas are selectively affected in alcohol-dependent individuals, and that alcohol does not cause global decreases in brain 5-HTT.21
In vivo SPECT and the radioactive ligand [123I] β-CIT have also been tested in humans to measure 5-HTT availability based on binding potential. 5-HTT β-CIT binding was found to be lower in the dorsal brainstem, where high density of serotonergic neurons are located, of male alcoholics following 3–5 weeks of abstinence.52 The severity of this decrease was negatively correlated to longer lengths of lifetime drinking. An in vivo PET imaging study using the PET tracer [11C](+)McN5652 found corresponding decreases in 5-HTT distribution volumes in recovering and abstinent alcoholics.53 However, a different PET analysis employing the novel tracer, [11C]-3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile ([11C]DASB), disagreed with the previous postmortem, SPECT, and PET results.21-23,48,52 It is suggested that [11C]DASB targets 5-HTT with higher specificity, possesses a faster washout time, and enters the brain more readily than the SPECT ligand β-CIT or PET ligand [11C](+)McN5652, and therefore produced different results than those from past studies.50 Using [11C]DASB, no significant differences in 5-HTT binding potential were measured in the occipital cortex, thalamus, frontal cortex, amygdala, hippocampus, raphe nuclei, anterior cingulate, or caudate putamen in alcoholics compared to control subjects.50 These findings suggest that decreased 5-HT neurotransmission is not a direct result of lower 5-HTT density in alcohol-dependent individuals, as was previously hypothesized. These findings were confirmed in type II alcoholics using [11C]DASB.45
Moreover, it has been reported that alcohol exposure increases the expression, as well as the function, of 5-HTT in vivo in human dendritic cells.46 This pharmacological effect of alcohol may be responsible for a certain degree of the neuroimmune degeneration seen in alcoholic individuals who present weakened immune responses. Dendritic cells that were treated with alcohol had higher levels of cyclic AMP (cAMP) mRNA indicating that the cAMP signaling pathway mediates alcohol directed upregulation of 5-HTT expression. cAMP has also been shown to increase 5-HTT mRNA levels in human placental choriocarcinoma cells.54 In addition, 5-HTT transcription has been shown to be protein kinase-c dependent. 5-HTT expression increases in a dose-dependent manner as protein kinase-c is added to the cell culture medium.55
One explanation for contrasting findings across studies is sexual differences. Incongruence between male and female subjects is not uncommon. Studies demonstrated that male subjects showed a 30% decrease in [123I]β-CIT-5-HTT binding in the midbrain, but no significant change was seen in female subjects.52,56 Gender-specific differences in 5-HTT expression have also been reported in lymphoblast cell lines, with 5-HTT mRNA levels being lower in females as compared to males. Gender-specific epigenetic differences affect 5-HTT expression. Site-specific DNA modification affects gene expression in all mammalian cells. DNA methylation at the 5′ position of cytosine residues present in CpG islands—stretches of DNA that contain cytosine positioned directly next to guanine—has been consistently shown to alter gene expression.57 Higher levels of CpG island methylation at specific residues have been shown to decrease 5-HTT transcription. This is especially true for residues that are located close to the start site. In general, CpG island methylation is higher in males than in females58; this may explain some gender-based differences in 5-HTT expression results. Exposure to other types of drugs, variant endogenous 5-HT concentrations, and genotype also affect the outcome of binding experiments.50
B. Relationship Between Functional Polymorphisms in the 5-HTT Gene Promoter and Alcohol Dependence
The 5-HTT gene is located on chromosome 17q11.1–q12 at the SLC6A4 locus.59 A functional 5-HTT linked polymorphic region (5-HTTLPR) at the 5′ end alters 5-HTT transcription60 and has been examined in humans to help define a hereditary basis for the development of alcohol dependence. Two functional 5-HTTLPR polymorphisms are produced by an insertion/deletion mutation of 44 base pairs, and are denoted as the long (L) and short (S) alleles. Healthy individuals that possess two copies of the L version of the allele (LL) exhibit greater 5-HTT activity at the synaptic cleft and higher mRNA density in the raphe nuclei than those that possess one or more copies of the S allele (SS/LS).59,61 5-HTTLPR genotype affects an individual’s response to alcohol craving,62 strength of withdrawal symptoms,63,64 reaction upon first exposure,65,66 and alcohol neurotoxicity.59,67 The 5-HTTLPR has also been linked to antisocial behavior and impulsivity.59
The development of alcohol dependence may be partially due to a predisposing genetic influence. Numerous studies have searched for a concrete genetic relationship between the 5-HTT gene and alcohol dependence; however, because of the complex nature of alcohol-influenced neurotransmission, it has been difficult to reach a consensus. In addition to differences in pharmacological response to alcohol, alcoholics frequently present comorbidities with depression and anxiety, and there are physiological differences between women and men, as well as between different ethnic groups.
Because of the intricacy of genotypic, phenotypic, and environmental influences, individual studies of genotype-related to alcohol dependence demonstrate disparate findings. Some studies have linked the short version of the 5-hydroxytryptamine-transporter-linked promoter region (HTTLRP) to alcohol addiction.68-72 Other studies have determined a significant relationship between the presence of the long allele and alcohol dependence.59,73-75 It is noteworthy, however, that other groups have demonstrated that there is no relationship between allelic make-up and alcohol dependence.63,71,76-82 To encompass a heterogeneous population and determine an overall consensus between studies, meta-analytic reviews have been performed. Two separate meta-analyses have determined that alcoholics are 15–18% more likely to possess an S allele.68,83 A survey of data from one meta-analysis of 2325 control subjects and 3489 subjects diagnosed with alcohol dependence68 demonstrated that S-allele carriers were 18% more likely to develop alcohol dependence. This amplified S frequency was stronger in alcohol-dependent individuals who reported an early onset of the problem of alcohol consumption and more severe alcohol dependence, and highest in those who also suffered from a co-occurring psychiatric disorder.68 These findings are especially applicable for type II or type B alcoholics. A second meta-analysis concerning the link between the 5-HTTLPR polymorphism and alcohol dependence included a total of 8050 subjects and concluded that individuals who had been clinically diagnosed with alcohol dependence were 15% more likely to possess one or more short alleles,83 and homozygous SS individuals were more likely to develop alcohol dependence than heterozygous individuals. The authors of this study suggested that due to the weakness of this association, it is important to interpret the results carefully and consider that 5-HTTLPR expression and the development of alcohol dependence may not be the result of a direct causal relationship. 5-HTTLRP genotypes are also related to behaviors such as reaction to stress, impulsivity, and low emotional regulation that influence the development of alcohol dependence.83
C. Relationship Between Functional Polymorphisms in the 5-HTT Gene Promoter and Alcohol Response, Alcohol-Directed Neuroadaptation
Studies have searched for specific genotype-centric differences in alcohol response to understand the etiology of alcohol abuse. It has been shown that L-allele carriers experience fewer negative side effects upon first exposure to alcohol65 and stronger cravings,62 which may increase susceptibility to alcohol abuse. S-allele carriers are also vulnerable to alcohol dependence as they build tolerance more efficiently,84,85 are more likely to binge drink,86 experience heightened withdrawal symptoms,63 and are statistically more likely to relapse.87
Lower intoxication upon first exposure to alcohol has been linked to increased availability of 5-HTT in nonhuman primates.88 This finding was also confirmed in young male, LL alcoholics, who self-reported lower intoxicative response upon first exposure.66 The long version of 5-HTTLPR has also been shown to affect alcohol craving in L-carriers who have lower synaptic 5-HT on the basis of self-reported assessments in the presence of cues.62 Craving measurements were higher in LL/LS alcoholics than in SS alcoholics, especially in those who reported a longer length of lifetime consumption.62 To further test this effect, craving level was assessed following tryptophan depletion, which mimics a transient 5-HT decrease. Acute tryptophan depletion caused a reduction in “urge to drink” in LL/LS compared to SS subjects. This effect is likely due to a brief reduction in extracellular 5-HT and a decrease in auto-receptor activation that transiently increases 5-HT neurotransmission and alleviates the severity of craving.
The expected availability of 5-HTT is dependent on genotype-related changes following chronic alcohol exposure. It has been suggested that LL alcoholics are more susceptible to alcohol neurotoxicity following chronic consumption than SS alcoholics.74 As predicted, LL control subjects have shown higher effective binding potential for [123I]β-CIT than SS subjects in the raphe nuclei, which is interpreted to indicate higher 5-HTT levels in this region. Furthermore, when compared to healthy LL controls, LL alcoholics presented a significant decrease in 5-HTT binding potential.74 This was not the case for SS alcoholics versus SS controls that had essentially equal 5-HTT binding. These data indicate that chronic alcohol consumption causes the availability of 5-HTT in the raphe nuclei to decrease substantially only in LL alcoholics.
Another factor concerning genotype-related response to alcohol is complex intersystem neural communication. Serotonergic neurotransmission directly influences mesolimbic dopamine activity via the 5-HT3 receptor.89-92 This interaction is of interest in alcoholics who carry the LL genotype and have greater 5-HTT synaptic activity than SS carriers. To determine whether genotype affects dopaminergic activation, growth hormone secretion was measured in LL, LS, and SS alcoholic subjects following apomorphine injections. LL individuals had a lower response to apomorphine administration than SS and LS individuals. The reduction in dopamine response suggests that 5-HT3 receptors located on dopamine neurons are less sensitive following chronic alcohol exposure.60
The alcohol-intoxication effect discussed above necessitates a different hypothesis as to why, statistically, chronically alcohol-dependent humans and animal models show deficient 5-HT neurotransmission overall. Prior to chronic alcohol exposure, LL subjects possess increased 5-HTT activity and, therefore, reduced synaptic 5-HT. However, in chronic alcohol consumption paradigms, LL alcoholics may actually possess reduced 5-HTT activity. It is probable that a decrease in presynaptic 5-HTT clearance may increase synaptic 5-HT activation of inhibitory autoreceptors. This feedback mechanism may cause an overall decrease in 5-HT release and an associated deficit in serotonergic neurotransmission.61,62
Following repeated alcohol exposure, S-carriers report lesser degrees of intoxication than L-carriers at equivalent blood alcohol concentrations.84,85 This may indicate that S-carriers develop alcohol tolerance more efficiently than L-carriers, as previous findings correlate persistently increased extracellular 5-HT with faster development of alcohol tolerance.84 Although SS individuals may experience lower alcohol craving than L-carriers, it has been shown that SS alcoholics are more likely to binge drink. This assertion was first made on the basis of an analysis of the drinking behavior and associated polymerase chain reaction (PCR) genotyping of female college students.86 SS female alcoholics who also possess the efficiently expressed version of the monoamine oxidase type A (MAO-A) promoter region reported even higher frequencies of binge drinking behavior. These 5-HTTLPR data were confirmed via platelet analyses of 5-HTT availability in a population that included both men and women. The corroborating study found that SS alcoholics consumed more alcohol and were, generally, younger than LL alcoholics.59 Whether genotype has an effect on withdrawal symptoms is somewhat unclear. Studies reported that there were no significant withdrawal-associated 5-HTTLPR genotypes in alcoholics.64 However, a subgroup of alcoholics who presented severe symptoms of withdrawal had a higher frequency of the S allele.63 Moreover, 5-HTT genotype has been examined in the context of relapse. Variant 5-HT neurotransmission has been associated with impulsivity and the ability to avoid instant-gratification patterns, which may impart difficulties in alcohol abstinence.87
Determining genetically linked differences in alcohol response is important when considering treatment. Studies that examined the therapeutic effects of ondansetron, a 5-HT3 receptor antagonist, hypothesized that the rewarding effects of alcohol are amplified in LL alcoholics treated with SSRIs as compared to SS alcoholics.93,94 Low synaptic 5-HT availability prior to alcohol consumption as a result of the LL genotype has been found to cause increased densities of 5-HT3 receptors on dopaminergic neurons. Thus, alcohol consumption is considered rewarding because it causes a greater increase in dopamine activity in LL alcoholics. SSRI treatment may potentiate this effect by causing an additional neuroadaptative increase in transporters. However, ondansetron would attenuate this effect by antagonizing 5-HT3 receptors. This theory was strengthened by a simulation of the rewarding properties of alcohol, which found that acute and chronic consumption caused increased dopamine responses that were accelerated in LL alcoholics treated with SSRIs and, conversely, in SS alcoholics treated with ondansetron.95
There are other contributing genetic factors that mediate 5-HTT expression apart from 5-HTTLPR long/short genotype and that warrant mention. One important genotypic variation is the existence of a second type of functional polymorphism present in the 5-HTT promoter region of the L allele, a single nucleotide A to G substitution at rs25531. An in vitro analysis in human cell lines showed that L alleles that contain this substitution (LG) cause 5-HTT mRNA to be expressed at levels similar to that in the S allele. Carriers of two L alleles that do not have this mutation (LA) transcribed 5-HTT mRNA at the highest levels.96 In addition, the in vivo binding potential of 5-HTT is greater in the putamen of healthy LA/LA individuals than in healthy LG/LG individuals according to a [11C]DASB PET scan study.97 This genotypic effect was strongest in subjects with European ancestry. Because this single nucleotide substitution is not considered in many previous genetic studies of alcohol dependence, some results may be less conclusive than predicted.
The 5-HTT promoter polymorphism is not the only genetic factor that affects 5-HTT protein expression. The promoter region contains a TATA-like domain and a series of transcription factor binding sites, including one for the transcription factor AP2 (TFAP2B).55,96 The transcription factor TFAP2B affects gene expression of 5-HTT as well, as MAO-A has been linked to severe alcoholism in females.98 A functional polymorphism alters the degree of TFAP2B expression. The presence of the higher functioning version of the TFAP2B allele has been linked to severe alcoholism in females.98
III. Serotonergic Receptors: Molecular, Pharmacological, and Physiological Aspects and Their Role in Alcohol Dependence
A series of 5-HT receptor subtypes have been shown to play a role in alcohol dependence, including the 5-HT3, the 5-HT1B, and the 5-HT1A receptors. These receptors are distributed throughout the nervous system and possess different molecular and pharmacological characteristics. Each of these receptors has been implicated in both alcohol intake and craving.
A. 5-HT3 Receptors
The 5-HT3 receptor is the only ligand-gated ion channel associated with 5-HT.99 Five subunits, each composed of four transmembrane domains, form a channel which is permeable to Na+, K+, and Ca2+ when the receptor is activated by 5-HT binding.99,100 This activation induces rapid depolarization that may increase the concentration of cytosolic Ca2+.99 Other agonists that activate 5-HT3 receptors are 2-methyl-5-HT, phenylbiguanide, and m-chlorophenylbiguanide.101,102 The 5-HT3 antagonists most studied include zacopride, MDL72222, tropisetron, ondansetron, and granisetron.
Alcohol administration has been shown to potentiate the 5-HT3 receptor,103-105 and this potentiation varies inversely with agonist concentration.104 Studies focusing on residue 294 of the 5-HT3 receptor revealed that mutation of this residue to threonine in the 5-HT3 subunit eliminates the alcohol potentiation function of this receptor.106 These studies suggest that 5-HT3 receptor in the central nervous system is a site of alcohol action.
The 5-HT3 receptor is localized in cortical and subcortical brain regions.107,108 Although there is very low 5-HT3 receptor density in the VTA and NAc, electrophysiology and microdialysis studies indicate that 5-HT3 receptors play a role in regulating the activity of VTA dopaminergic neurons and their projections to the NAc.89-92 Systemic administration of 5-HT3 receptor antagonists has been shown to effectively reduce alcohol intake in rats in free-choice conditions.109,110
B. 5-HT1B Receptors
The 5-HT1B receptor (human analog 5-HT1Dβ) is coupled to a G protein and contains seven transmembrane domains, one of which is composed of eight amino acids and acts as the ligand binding site.111-113 Studies investigating 5-HT1B receptor signal transduction indicate that these receptors are coupled to an intermediate inhibitory G protein. When activated, this G protein acts as a second messenger, inducing a decrease in the activity of adenylyl cyclase.114-118
The 5-HT1B receptor has been implicated in several physiological functions, including locomotor activity, drug abuse reinforcement, migraine, aggressive behavior, depression, and anxiety states (for review see Ref. 24). 5-HT1B receptors are serotonergic autoreceptors located at the terminal (for review see Ref. 24); their activation inhibits the presynaptic release of 5-HT.119-122 Studies investigating the distribution of 5-HT1B receptors in the central nervous system have demonstrated that high densities of these receptors are located in the globus pallidus, ventral striatum, substantia nigra, and dorsal subiculum.24,28,123-126 Moderate expression of the 5-HT1B receptor has been found in the caudate putamen, hippocampus, entopeduncular nucleus, periaqueductal gray, superior colliculus, and some regions of the cerebellum. 5-HT1B receptors are located on the terminals of VTA neurons that project to the NAc, amygdala complex, and frontal cortex.127 The VTA sends dopaminergic projections to the central nucleus of the amygdala, the bed nucleus of the stria terminalis, and to the NAc.128,129 Collectively, these regions form a reward circuit for drugs of abuse, including alcohol, and are central in the development of addiction/dependence.130-132 This complex is called “the extended amygdala,” and is connected to the raphe nuclei, the locus coeruleus, the hippocampus, and the ventral pallidum.133-137 A large number of studies using pharmacological, molecular, and genetic manipulations have suggested that 5-HT1B receptors modulate the reinforcement and intoxication effects of alcohol and that these receptors are key players in regulating alcohol intake.24,138-145
The alcohol intake phenotype has been shown to be linked in mice to chromosome 9 where the 5-HT1B receptor gene is located, and knockout mice lacking 5-HT1B receptors tend to have increased alcohol intake versus controls.139,146 Moreover, the role of 5-HT1B receptors in alcohol dependence has been investigated in studies using gene manipulation. Microinjections of the viral vector-herpes simplex virus (HSV) carrying 5-HT1B receptors in the NAc shell increased alcohol intake in Long–Evans rats.138 Manipulation of 5-HT1B receptors in brain assists in determining the neurocircuitry involving this receptor in alcohol intake. Importantly, a clinical study demonstrated that alcohol dependence is associated with upregulation of 5-HT1B receptor levels in the ventral striatum.147
Numerous single nucleotide mutations have been identified in the 5′ untranslated region, the 3′ untranslated region, and the coding region of the 5-HT1B receptor gene.148 To date, 16 polymorphisms in the 5-HT1B receptor gene have been reported.64 Many of these polymorphisms are functional, and some have been shown to alter the expression of 5-HT1B receptor. Two specific mutations examined in the context of alcohol dependence are the G861C polymorphism and the A-161T polymorphism. The resultant associations between these genotypic changes and alcohol dependence have been inconsistent, and no clear relationship between these polymorphisms and the propensity for alcoholism has been established. Studies have revealed that the 861 G > C polymorphism of the 5-HT1B receptor gene (human analog 5-HT1Dβ) was found to be associated with antisocial alcoholism.149-151 The A-161T polymorphism has been shown to be more associated with alcoholism in individuals who have concurrent anxiety or depression versus those that are antisocial.152 It is noteworthy that Han Chinese alcoholics classified as antisocial were more likely to possess the A allele, while alcoholics that were classified as depressed or anxious were more likely to possess the T allele.152 However, the same study reported that no significant A-161T genotypic differences exist between control group and the collective of alcohol-dependent individuals.152
Understanding the pharmacology of 5-HT1B receptors is an important step in determining the physiological role of these receptors. Pharmacological studies have demonstrated that RU 24969 and trifluoromethyl-phenylpiperazine possess high affinity for 5-HT1B receptor binding sites in rodents, and that serotonergic agonist 5-CT has high affinity for both 5-HT1D and 5-HT1B receptor binding sites.153-155 Another agonist, CP 93129, demonstrates stronger affinity for 5-HT1B receptor binding sites over 5-HT1D receptors,156 whereas sumatriptan has a higher affinity for 5-HT1D receptor binding than for 5-HT1B receptors.28,157 Although the compounds tested are not highly selective to 5-HT1B receptors, it is important to note that the pharmacological manipulations of these receptors in animal models have contributed largely to understanding the role of 5-HT1B receptors in the regulation of alcohol intake. Studies that tested partial and selective 5-HT1B agonists have shown that these compounds when administered i.p. reduced alcohol intake in animals.143-145,158 Some of these agonists are discussed in the Section V.B.2.
C. 5-HT1A Receptors
Like the 5-HT1B receptor, the 5-HT1A receptor is a G-coupled transmem-brane protein with seven domains.159 Activation of 5-HT1A receptors inhibits adenylyl cyclase in rodents.160-162 Studies using radioligand binding sites demonstrated a high density of 5-HT1A receptors present in the limbic system including the hippocampus, lateral septum, entorhinal cortex, amygdala, and raphe nuclei.163,164 5-HT1A receptors are located on the dendrites of serotonergic neurons (autoreceptors), as well as on postsynaptic membranes of nonserotonergic neurons (heteroreceptors).165-167 As such, 5-HT1A receptors play a role in the regulation of 5-HT release,168,169 as well as in the regulation of the release of other neurotransmitters including noradrenaline and dopamine.170,171 5-HT1A receptors are involved in several physiological functions including sexual behavior, sleep, regulation of body temperature, and pain.172-175 Moreover, 5-HT1A receptors are involved in psychiatric disorders including anxiety164,176 and depression.177,178 Importantly, studies conducted in animals and humans have demonstrated a role of 5-HT1A receptors in alcohol-drinking behavior.179-182
Pharmacological studies suggest that there are several selective agonists for 5-HT1A receptors. Among these are 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), ipsapirone, gepirone, buspirone, and tandospirone.183 5-HT1A receptor antagonists include spiperone,184 (–)pindolol, (–)propranolol,160,185 and (–)tertatolol.186,187 Studies of alcohol-preferring rats demonstrated deficits in 5-HT transmission and a compensatory upregulation of 5-HT1A receptors.188 Moreover, 5-HT1A receptors have been linked to alcohol consumption and alcohol withdrawal symptoms.189,190 A large body of evidence using pharmacological investigations has demonstrated the involvement of 5-HT1A receptors in alcohol dependence.179,181,182,191,192 The role of 5-HT1A receptor agonists in alcohol intake is discussed further in the Section V.B.3.
IV. Interactions Between the Serotonergic System and Other Neurotransmitter Systems in the Modulation of Alcohol Consumption
Alcohol abuse is a disorder characterized by a disruption of several neurotransmitter systems, including 5-HT. 5-HT interacts with and modulates other neurotransmitter systems (Fig. 1) to reinforce the hedonic effects of alcohol, some of which are discussed below.
A. Dopaminergic System
It is well established that enhanced dopaminergic neurotransmission arising from the VTA and terminating in the NAc is involved in reward130 and that acute alcohol exposure increases dopaminergic neurotransmission.193,194 A large portion of the VTA consists of dopaminergic projection neurons that are under the inhibitory control of GABAergic interneurons.195 The VTA and the NAc receive substantial innervations from the dorsal raphe serotonergic neurons32 (Fig. 1) and 5-HT is believed to modulate neurons in the VTA through several receptors including 5-HT1B, 5-HT2, and 5-HT3 receptors.
The 5-HT1B receptor is known to function both as an autoreceptor and as a heteroreceptor to inhibit presynaptic neurotransmitter release.196 An increase in 5-HT1B activation via the selective 5-HT1B agonist, CP93129, within the VTA has been shown to disinhibit dopamine neurons by inhibiting GABA release, thereby increasing extracellular dopamine and decreasing extracellular GABA in both the NAc and the VTA.197 Systemic alcohol-induced enhancements of dopamine in the VTA and the NAc were significantly reduced by local administration (into the VTA) of the selective high affinity 5-HT1B receptor antagonist SB216641 but not the 5-HT1D/1A receptor antagonist BRL 15572.198 Furthermore, the 5-HT1B receptor agonists, m-chlorophenylpiperazine (mCPP) and Trifluoromethylphenyl piperazine (TFMPP), dose-dependently suppressed alcohol intake39,144,199 presumably because activation of this receptor increased dopamine neurotransmission in the VTA and the NAc.
Although we did not emphasize the role of 5-HT2 receptors in alcohol dependence in the review chapter, we briefly cover the interaction between 5-HT2 and the dopaminergic system as it relates to alcohol-drinking behavior. 5-HT2 receptor modulation has been shown to affect several aspects of dopaminergic neurotransmission, although with less consistency than the 5-HT1B receptor modulation. In vitro applications of the 5-HT2 receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) potentiated the inhibitory effects of dopamine acting at D2 receptors on dopaminergic cell bodies in the VTA, and this effect was reversed by the selective 5-HT2 antagonist ketanserin.200 In agreement with these results, local infusion of the 5-HT2 antagonist ritanserin into the medial PFC of rats increased extracellular dopamine in this area.201 However, infusion of DOI directly into the NAc increased extracellular dopamine only in the posterior NAc whereas coperfusion with a 5-HT2 antagonist completely blocked the DOI-stimulated dopamine release.202 These differential effects could be due to the nonselectivity of the 5-HT2 agonists/antagonist. For instance, it has been demonstrated that 5-HT2A and 5-HT2B/2C receptors exert different functions in the modulation of dopamine release.203 In vivo electrical stimulation of the dorsal raphe nuclei followed by the selective blockade of the 5-HT2A receptor subtype by SR 46349B significantly reduced dopamine release into the NAc whereas application of the 5-HT2B/C receptor antagonist SB 206553 significantly enhanced dopamine release in the NAc.203 Furthermore, administration of the selective 5-HT2C antagonist SB242084 into the medial PFC decreased dopamine outflow into the NAc following morphine administration.204 These studies suggest that there are complex interactions between 5-HT and other neurotransmitter systems that differ depending on which brain region is examined.
Several lines of evidence have demonstrated that 5-HT2 receptor antagonists reduce alcohol consumption. For example, the 5-HT2 antagonist ritanserin was shown to be effective in reducing alcohol intake in Wistar rats under free choice conditions.205 Additionally, in several alcohol-preferring rat lines, the 5-HT2A antagonist amperozide was shown to be effective in reducing alcohol intake although it also reduced total fluid intake.206,207 Moreover, it has been shown that direct self-coadministration of the selective 5-HT2A antagonist R-96544 and alcohol directly into the posterior VTA significantly reduced operant responding to alcohol alone in this area.208 5-HT could, therefore, be acting on different subtypes of 5-HT2 receptors within reward areas to modulate dopamine release and thereby alcohol consumption.
The 5-HT3 receptor has also been reported to alter dopaminergic neuro-transmission in central reward regions. In vivo application of the 5-HT3 agonist CPBG has been shown to increase extracellular dopamine in the NAc90 and the VTA,89,209 suggesting an action of the 5-HT3 receptor activation on both terminal and somatodendritic dopamine release. Local perfusion of the 5-HT3 antagonist ICS 205-930 in both VTA and NAc in combination with an i.p. injection of alcohol prevented the alcohol-induced increase in extracellular dopamine in these regions.89,90 Consistent with the fact that increased dopamine mediates the reinforcing properties of alcohol, 5-HT3 antagonists have been shown to decrease voluntary alcohol consumption in rats under 24 h free-choice conditions.110,210 Furthermore, in Wistar rats that readily self-administer alcohol directly into the posterior VTA, self-coadministration of ICS 205-930 and alcohol abolishes this operant response, which suggests that 5-HT3 receptors within the posterior VTA are necessary for alcohol self-administration.211
B. Glutamatergic/GABA Systems
It is well established that alcohol has a direct effect on NMDA receptors and that it prevents NMDA-mediated calcium influx in a noncompetitive manner (for review, see Ref. 212). These effects have been observed in several brain regions, including the cerebral cortex, NAc, amygdala, hippocampus, and VTA.212 In vivo microdialysis studies have shown that acute alcohol exposure significantly reduces basal glutamate levels in the NAc whereas chronic exposure increases extracellular glutamate in the NAc and NMDA sensitivity in the striatum.213-215 Activation of the NAc via glutamatergic mechanisms is implicated in the reinforcing effects of alcohol. For instance, direct infusion of the mGLUR5 antagonist MPEP, or the mGLUR2/3 agonist LY379268, into the NAc reduced alcohol self-administration in alcohol-preferring rats.216 These results suggest that decreased glutamatergic activity in the NAc reduces alcohol-drinking behavior.
Excitatory glutamatergic efferents from the PFC to the NAc are strongly believed to contribute to drug addiction (for review see Ref. 217). The PFC is composed primarily of glutamatergic pyramidal neurons which are under the control of local GABAergic interneurons (for review see Ref. 218). 5-HT has been strongly implicated in modulating glutamatergic neurotransmission within the PFC and the NAc. For instance, studies have demonstrated that activation of presynaptic 5-HT1B receptors on glutamatergic terminals located in the NAc blocks glutamate mediated EPSPs on medium spiny neurons in the NAc.219 The PFC is highly enriched in 5-HT1A receptors,220 and 5-HT primarily down-regulates fast spiking interneuronal activity via 5-HT1A receptors in this region, disinhibiting glutamatergic pyramidal neurons.221 Furthermore, 5-HT has been shown to excite striatal cholinergic and fast spiking GABAergic interneurons, which in turn inhibit glutamatergic input to projecting medium spiny neurons.222-224 Thus, 5-HT has various effects in different regions by potentiating pyramidal neurons in the PFC and inhibiting medium spiny neurons in the NAc. Interestingly, it has been shown that Sardinian alcohol-preferring rats displayed lower 5-HT and 5-HIAA in the frontal cortex than their nonpreferring counterparts but no changes in 5-HT or metabolites were found in the NAc.225 This 5-HT imbalance could act as a mechanism by which alcohol is reinforcing to some animals and not to others. Although these findings are not consistent across high alcohol preferring rat strains,226,227 they do share similarities in that the overall consensus is a reduction in 5-HT in high-alcohol-preferring models compared to their nonpreferring counterparts.
C. Endocannabinoid System
Endocannabinoids act as retrograde messengers in the central nervous system and have been implicated in the reinforcing effects of drugs of abuse and feeding behavior. 2-Arachidonoylglycerol (2-AG) and anandamide (AEA) are the predominant endocannabinoids within the central nervous system and have their neurological effects by acting on presynaptic cannabinoid 1 (CB1) receptors (for review see Ref. 228). For instance, studies have demonstrated that CB1−/− C57BL/6J knockout mice consumed significantly less alcohol than their CB1+/+ counterparts in a free choice paradigm whereas food and water intake remained relatively unchanged.229 Endogenous cannabinoids are thought to be synthesized on demand in the postsynaptic neuron and mediated by postsynaptic calcium influx. They are then released in a retrograde fashion and bind to presynaptic CB1 receptors.230 CB1 receptors in the NAc are primarily located on excitatory presynaptic terminals where their activation results in the inactivation of presynaptic voltage gated calcium channels and subsequent neurotransmitter release.231,232 Thus, an increase in 2-AG or AEA within this region may significantly decrease the excitatory input the NAc receives from key reward regions such as the PFC, amygdala, and hippocampus.
Moreover, acute systemic administration of alcohol dose-dependently was found to increase 2-AG levels in the NAc of alcohol-naïve rats and this effect was potentiated and prolonged in rats with a history of alcohol consumption.233 As a consequence of chronic alcohol use, prolonged elevations in brain endo-cannabinoid levels decrease CB1 receptor binding.234 Since both systemic alcohol administration and voluntary consumption are associated with increases in 2-AG and AEA in key reward regions,233,235,236 and CB1 knockout mice display reduced alcohol-drinking behavior,229 activation of CB1 receptors may be important for mediating alcohol-seeking behavior. Indeed, several reports have shown that administration of the selective CB1 receptor antagonist SR141716A reduces alcohol consumption in both rat235,237 and mouse238 models whereas intra-NAc administration of CB1 receptor agonists increased alcohol consumption.239
The endocannabinoid system has been shown to interact with other neurotransmitter systems involved in alcohol consumption both in vitro and in vivo. It has been shown that male Wistar rats treated i.p. with SR141716A display increased 5-HT and 5HIAA both in the medial PFC and NAc as measured by microdialysis.240 Furthermore, studies have demonstrated that stimulation of CB1 receptors in mouse brain cortical slices inhibited subsequent 5-HT release,241 whereas other researchers have shown that administration of SR141716A dose-dependently increased 5-HT, dopamine, and their respective metabolites in the forebrain of shrews.242 Additionally, acute alcohol-induced increases in dopamine in the NAc of wild type mice were abolished by pretreatment with SR141716A whereas no effect on extracellular dopamine was found in CB1−/− mice, implicating that the alcohol-induced increase in dopamine was mediated by activation of CB1 receptors.229
5-HT has been shown to interact with the endogenous cannabinoid system in a reciprocal fashion. For example, activation of CB1 receptors by AEA inhibits glutamate release in the dorsal raphe, whereas depolarization of dorsal raphe 5-HT neurons triggers endocannabinoid release which further mediates the depolarization-induced suppression of excitation.243 In agreement, activation of 5-HT2 receptors by 5-HT in the inferior olive induced endocannabinoid release which decreased the probability of glutamate release from the presynaptic terminal.244 Furthermore, it was demonstrated in vitro that 5-HT2A receptor activation triggered an increase in 2-AG release through the activation of phosphatidylinositol-specific phospholipase C.245 Thus, 5-HT may, in part, modulate other neurotransmitter systems by acting to stimulate endocannabinoid release.
V. Serotonergic System as a Potential Therapeutic Target in Alcohol Dependence/Addiction
Treating alcohol dependence is challenging because of its complexity. Investigations testing naltrexone (a noncompetitive opioid antagonist) to treat alcohol dependence have shown that in heavy-drinking subjects, naltrexone administration attenuated relapse during the treatment period. However, a study investigating the long-term effects of naltrexone showed that individuals doubled their drinking over the 6 months following termination of naltrexone treatment.246 This suggests that the therapeutic effect of naltrexone is abolished once the treatment is completed. Although, naltrexone demonstrated significantly greater efficacy compared to placebo during the drug administration period, once the treatment was discontinued, the frequency of heavy drinking days gradually increased in patients suffering from alcoholism.247
Studies have shown that SSRIs effectively maintain the attenuation of alcohol intake achieved during treatment for at least 6 months after pharmacotherapy in type A alcoholics.248 As suggested above, subgroups of alcoholics may respond differently to treatment with serotonergic medication. Although SSRIs-treatment may be associated with a specific subtype of alcoholism, experimental studies have reported that SSRIs reduce alcohol intake in some alcoholics249-253; see also Table I. Several types of 5-HT receptors have been shown to play a role in alcohol-drinking behavior and are potential treatment targets, including 5-HT3, 5-HT1B, and 5-HT1A receptors143,144,151,181,192,254; see also Table II. We discussed here the possible outcomes of the pharmacotherapy for the treatment of alcohol dependence with regard to drugs that target the 5-HTT and the 5-HT receptors (Tables I and II).
TABLE I.
Involvement of SSRIs and Other Antidepressants in Alcohol Dependence or Craving
| Type of SSRIs and other antidepressants | Effectiveness of SSRI or antidepressant in alcohol dependence or craving | Long-term effect after withdrawal of medication | References |
|---|---|---|---|
| Fluoxetine | Reduces alcohol consumption in type A but not type B alcoholics Reduces anxiety and alleviates depression state during alcohol withdrawal in alcoholics | Not determined | 276,280,281,283 |
| Sertraline | Reduces alcohol consumption in type A but not type B alcoholics | The effects of the drug can last for at least 6 months after the completion of the pharmacotherapy | 248,285,311 |
| Escitalopram | Reduces alcohol consumption in alcoholics but it is less effective than fluoxetine or sertraline | Not determined | 284,287 |
| Zimelidine | Induced increases in the days of abstinence and decreased the number of drinks consumed in alcoholics | Not determined | 250,251 |
| Tianeptine | Acute drug treatment was more effective than chronic treatment in the improvement of depression state and anxiety after alcohol withdrawal in alcoholics | Not determined | 288,290,292,293 |
TABLE II.
Role of 5-HT Receptors in the Attenuation of Alcohol Consumption
| Type of 5-HT receptors | Effect of selective 5-HT receptor agonist or antagonist in animal model in alcohol intake | Effect of selective 5-HT receptor agonist or antagonist in clinics in alcohol consumption | References |
|---|---|---|---|
| 5-HT1A receptors | Agonists, 8-OH-DPAT, buspirone, and NDO-008 reduced alcohol intake in Wistar rats | Partial agonist, buspirone, reduced depressive state and the number of days in desire to drink alcohol | 179-181,308,310 |
| 5-HT1B receptors | Agonists, CP-94253, mCPP, and CGS-12066B reduced alcohol intake in Wistar rats | Not determined | 142,145,158,294 |
| 5-HT3 receptors | Antagonists reduced alcohol intake in rats | Antagonist, ondansetron, reduced alcohol intake in type B but not type A alcoholics | 94,182,294,297,300,301 |
A. Serotonin Transporter as Potential Pharmacological Therapeutic Target for the Treatment of Alcohol Dependence
Dysfunction of the serotonergic system has been considered for many years to be the cause of mood disorders including anxiety and depression.255-257 Studies have shown that some alcoholic patients show symptoms of depression.258,259 Low 5-HT turnover may lead to behavioral impulsivity and aggression, as well as early onset of alcohol intake.16,260-263 Genetic and imaging studies have demonstrated that reduced 5-HTT availability is associated with anxiety disorder and depressive state in alcoholic patients suffering from major depression.52,264-267 In accordance, studies in animal models have shown a link between 5-HT deficit and aggression that together are associated with excessive alcohol intake,139,268 alcohol consumption, and measurable behavioral depression in selected alcohol-preferring AA rats269 and in fawn-hooded rats.270 Increase in alcohol intake is due to the fact that these animals develop tolerance to the depressant effects of alcohol and possess faster alcohol metabolism.
In clinical studies, individuals that consume alcohol heavily present dysfunctional serotonergic neurotransmission.18 Lower cerebrospinal fluid concentrations of the 5-HT metabolite 5-HIAA have been found in abstinent alcoholics.271-273 Interestingly, low concentrations of 5-HIAA in cerebrospinal fluid were found to be associated with type II alcoholism.263,274 SSRIs have been used to treat alcohol addiction in subgroups of alcoholics.52,275 Moreover, studies have suggested that there might be an association between alcohol consumption and hyposerotonergic activity in humans.9,18,276 Decreased 5-HT turnover rate has been associated with reduced response to excessive alcohol consumption.277 Moderate reductions in alcohol consumption were found in alcoholics treated with SSRIs.278 It is noteworthy that findings related to SSRIs have been variable between alcoholics.279 We discussed here the variability in SSRIs efficacy in type A and B alcoholics.
1. Effects of Fluoxetine on Alcohol Consumption
Fluoxetine is an SSRI that was found to decrease the quantity of daily alcoholic drinks and decrease total drinks over a period of 14 days, with no significant effect on days of abstinence, in some alcoholics.249 However, fluoxetine was not found to be effective for the treatment of a heterogeneous group of alcohol-dependent individuals.280 In addition, fluoxetine was effective only in improving depressive symptoms in alcoholics with comorbid depression.281 Importantly, in the absence of a comorbid mood or anxiety disorder, fluoxetine is not to be used to maintain abstinence or reduce drinking in high-risk/severity alcoholics (type B).279
Moreover, studies have suggested that fluoxetine is effective in relieving alcohol withdrawal symptoms. Fluoxetine reduces anxiety and alleviates depressive state during alcohol withdrawal.282 In addition, independently from craving, fluoxetine at antidepressant doses is able to prevent short-term relapses in alcoholics.283 In animal models, fluoxetine inhibited locomotor hyperactivity, agitation, increased stereotyped behavior, and tremors associated with withdrawal.284 This later study suggests that the inhibitory effects of fluoxetine on the signs of alcohol withdrawal were specific and may not be related to other effects including sedation and muscle relaxation.
2. Effects of Sertraline on Alcohol Consumption
Sertraline is another SSRI that has been clinically shown to reduce alcohol consumption. Studies have investigated the effects of sertraline in alcohol-dependent patients with and without lifetime histories of depression. The findings revealed that there was no antidepressive effect of sertraline; however, sertraline was effective in reducing alcohol intake in subjects without a lifetime history of depression.275 It has also been revealed that sertraline treatment reduced alcohol intake in type A alcoholics but not in type B.285 Further studies indicate that type A alcoholics demonstrated a consistent benefit from sertraline that lasted for at least 6 months after the completion of pharmacotherapy.248 In contrast, sertraline has not been found to be efficacious in type B alcoholics during treatment or after. Sertraline was actually shown to increase alcohol intake in type B alcoholics 6 months after withdrawal from the medication. It is suggested that there might be a deficit in 5-HT synthesis in the brains of type B alcoholics,248 resulting in a neuroadaptative upregulation of some 5-HT receptors. Thus, treating type B alcoholics with SSRIs may induce neuronal overstimulation because of increased 5-HT at the synaptic cleft and resulting in increased alcohol intake.93,286
3. Effects of Escitalopram on Alcohol Consumption
Escitalopram, an active analog of citalopram, is another SSRI. Studies in rat models revealed that escitalopram was less effective than fluoxetine in reducing withdrawal symptoms.287 Unlike fluoxetine, escitalopram did not prevent withdrawal effects including locomotor hyperactivity, agitation, and audiogenic seizures.284 These results show that escitalopram has a limited ability to ameliorate alcohol dependence and alcohol withdrawal in rat models.287
4. Effects of Zimelidine on Alcohol Consumption
The effect of zimelidine, another SSRI, on alcohol consumption was tested in nondepressed healthy heavy drinkers without regard for subtype. Zimelidine treatment was associated with increased length of abstinence and decreased the number of drinks consumed daily.251 In a follow-up study, the authors demonstrated that ~ 50% of the subjects responded to the treatment, 35% partially responded, and 10–15% were nonresponsive to the treatment.250 This suggests that this study may have individuals from various alcoholic subtypes, which may have led to differential findings.
5. Effects of Tianeptine on Alcohol Consumption
Tianeptine, a unique tricyclic antidepressant, in contrast to SSRIs, actually increases 5-HT reuptake.288 In animal models, tianeptine decreases alcohol intake289 and reverses the anxiogenic effects of alcohol withdrawal in rats.290 Moreover, tianeptine was suggested to be a potent pharmacologically active drug in the ethanol withdrawal syndrome in rats (for review see Ref. 291). In clinics, patients treated with tianeptine have shown long-term improvement in depression and anxiety after alcohol withdrawal.292 Moreover, tianeptine has been shown to be effective in preventing alcohol intake in alcoholics suffering from depression.293 Together, these findings suggest that tianeptine might be used for the treatment of alcohol dependence associated with depression.
B. Serotonin Receptors as Potential Pharmacological Targets for the Treatment of Alcohol Dependence
1. Role of 5-HT3 Receptors in Pharmacotherapy of Alcohol Dependence
Rodents treated with 5-HT3 receptor antagonists have shown reduction in alcohol consumption.182,294 Further, 5-HT3 receptor antagonists have been shown to block both alcohol- and morphine-induced dopamine releases in the mesolimbic system.89,90,295 These findings demonstrate the importance of the 5-HT3 receptor in alcohol-mediated activation of the dopamine reward pathways in the mesolimbic system, even though there are low densities of the receptor in this system.296 5-HT3 receptor antagonist administration in the NAc was found to be effective in reducing the initiation and in the maintenance of free-choice alcohol intake. However, this effect was abolished after a 2-week deprivation period.297 Moreover, chronic alcohol consumption reduced 5-HT transmission in the NAc by reducing basal 5-HT release; however, a 2-week period of alcohol deprivation led to an elevation of 5-HT release compared to control groups.298
Ondansetron is the only 5-HT3 receptor antagonist that has been shown to be beneficial for the treatment of alcohol dependence, and has been clinically demonstrated to reduce alcohol preference and craving in some alcoholic subtypes. In a 6-week double-blind clinical trial study, ondansetron treatment was associated with the attenuation of alcohol consumption in male alcoholics in a dose-dependent manner.299 Additionally, in a 12-week double-blind clinical trial, ondansetron was found to be effective at reducing alcohol intake only in early-onset alcoholism or type B.93 Further study confirmed the efficacy of ondansetron in type B alcoholics showing severe psychological problems related to alcohol addiction.300 The effectiveness of ondansetron treatment in type B but not type A alcoholics has been confirmed by other studies as well.301 It is important to note that type B alcoholics with apparent serotonergic deficits respond best to drugs that block 5-HT3 receptors.93,302 However, type A alcoholics who have a normal serotonergic system may respond best to drugs that effect the uptake of 5-HT.301
2. Role of 5-HT1B Receptors in Pharmacotherapy for Alcohol Dependence
There is a large body of evidence demonstrating the importance of 5-HT1B receptors in alcohol intake in animal models. Early studies suggested that 5-HT1B receptor knockout mice consumed alcohol at an increased rate,139-141 while later studies suggested no difference from wild type.303 The disparity between these findings might be due to the doses of alcohol tested, exposure paradigm, and environmental factors influencing alcohol intake. Differences in alcohol intake in 5-HT1B receptor knockout mice may suggest that phenotypical abnormalities attributed to the knockout mutation may exist.304,305
Pharmacological studies using rodents have investigated the role of 5-HT1B receptors in alcohol intake. Administration of CP-94253 (i.p.), a partial 5-HT1B receptor agonist, reduced alcohol self-administration142 and its aggression-heightening effects.145,158 It should be taken into consideration that CP-94253 is a nonselective 5-HT1B receptor agonist and thus the effects of this drug may involve other receptors. Other drugs targeting 5-HT1B receptors have been studied in animal models of alcoholism. For example, the 5-HT1B receptor agonist mCPP was shown to reduce alcohol intake in the cologne alcohol-addicted (cAA) rat model of alcoholism.143 Moreover, administration of CGS12066B (i.p.), another partial 5-HT1B receptor agonist, reduced oral alcohol self-administration in Wistar rats.144 Lastly, administration of the 5-HT1B receptor agonist, anpirtoline (i.p.), significantly decreased alcohol intake in Swiss Webster mice.145 Together, data generated from pharmacological studies and mice lacking 5-HT1B receptors suggest an important role for these receptors in alcohol intake in animal models. Studies are warranted to develop new selective compounds targeting 5-HT1B receptors as therapeutic drugs for the treatment of alcohol dependence in humans.
3. Role of 5-HT1A Receptors in Pharmacotherapy of Alcohol Dependence
Several 5-HT1A receptor agonists have been tested in animal models to demonstrate the role of this receptor in alcohol dependence. For example, studies reported that the 5-HT1A receptor agonist buspirone reduces alcohol intake in rats and monkeys.179,180 Treatment with another 5-HT1A receptor agonist, ipsapirone, induced a dose-dependent reduction in alcohol preference and intake in rats.181,182 A study using Wistar rats tested three 5-HT1A receptor agonists, 8-OH-DPAT, buspirone, and NDO-008 on alcohol preference.191 Treatment with these agonists induced a significant reduction in alcohol consumption in the high preference group but not in the low preference group of rats. These preclinical studies suggest that 5-HT1A receptor agonists may be effective in reducing alcohol intake.
Postmortem studies of alcoholics have shown that blood alcohol concentration is correlated with a downregulation in 5-HT1A receptor binding sites in frontal-parietal cortical and hippocampal brain regions.306,307 Moreover, the 5-HT1A receptor agonist, buspirone, has been shown to be effective in the treatment of comorbid anxiety and depressive features in alcoholics.308-310 Studies have investigated 51 patients diagnosed with generalized anxiety disorder and depressive symptoms with concomitant alcohol abuse features.310 These studies revealed that the buspirone metabolite 1-pyrimidinylpiperazine (1-PP) improved anxiety and depressive symptoms, and reduced the number of alcohol-seeking days. Buspirone was also found to reduce alcohol craving, and lead to reduced anxiety and improvement of depressive symptoms in another study.308 Together, these studies suggest that targeting the 5-HT1A receptor with drugs such as buspirone may improve alcohol craving in patients suffering from alcohol dependence with features of anxiety disorder and depression.
VI. Conclusions
The serotonergic system plays an important role in the modulation of alcohol-drinking behavior, dependence, and withdrawal. Serotonergic dysregulation is associated with increased alcohol consumption and vulnerability to alcohol dependence. 5-HTT and 5-HT receptors, including 5-HT1A, 5-HT1B, and 5-HT3 receptors, are involved in alcohol-related changes in serotonergic neurotransmission.
Animal and clinical studies have demonstrated that chronic alcohol consumption alters 5-HTT function and expression. It is noteworthy that there is a possible genetic relationship between the 5-HTT gene and alcohol dependence. There are two functional polymorphic regions in this gene that appear to contribute to the hereditary basis for the development of alcohol dependence. The development of alcohol dependence may be due partly to a genetic predisposition involving 5-HTT polymorphisms. Targeting 5-HTT with SSRIs has been shown to be a promising pharmacotherapy in some alcoholics. It is noteworthy that SSRIs have been clinically shown to be effective only in type A alcoholics.
Pharmacological studies have demonstrated that several 5-HT receptors including 5-HT1A, 5-HT1B, and 5-HT3 receptors are involved in alcohol dependence. Interestingly, the 5-HT3 antagonist, ondansetron was found to be effective in type B alcoholics but not in type A. Moreover, the 5-HT1A receptor agonist, buspirone, is a potential therapeutic drug for the treatment of alcohol craving in patients suffering from anxiety disorder and depression. Finally, we suggest that clinical tests targeting the 5-HT1B receptor with new selective drugs should be considered as a potential therapy for the treatment of alcohol dependence associated with anxiety disorder and depressive state.
Acknowledgments
The authors thank the National Institute of Health for their support (R21AA017735, YS; R21AA016115, YS). The authors also thank Mr. Jordan Bolyard for editing this review chapter.
References
- 1.Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136–40. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- 2.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–76. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: present findings and future research needs. J Gastroenterol Hepatol. 2008;23(Suppl. 1):S2–8. doi: 10.1111/j.1440-1746.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- 4.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–30. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan EV, Zahr NM. Neuroinflammation as a neurotoxic mechanism in alcoholism: commentary on “Increased MCP-1 and microglia in various regions of human alcoholic brain”. Exp Neurol. 2008;213:10–7. doi: 10.1016/j.expneurol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Ross S, Peselow E. The neurobiology of addictive disorders. Clin Neuropharmacol. 2009;32:269–76. doi: 10.1097/wnf.0b013e3181a9163c. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee S, Das SK, Vaidyanathan K, Vasudevan DM. Consequences of alcohol consumption on neurotransmitters—an overview. Curr Neurovasc Res. 2008;5:266–72. doi: 10.2174/156720208786413415. [DOI] [PubMed] [Google Scholar]
- 9.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- 10.McBride WJ, Murphy JM, Gatto GJ, Levy AD, Lumeng L, Li TK. Serotonin and dopamine systems regulating alcohol intake. Alcohol Alcohol. 1991;(Suppl. 1):411–6. [PubMed] [Google Scholar]
- 11.Rezvani AH, Overstreet DH, Janowsky DS. Genetic serotonin deficiency and alcohol preference in the fawn hooded rats. Alcohol Alcohol. 1990;25:573–5. [PubMed] [Google Scholar]
- 12.Roy A, Virkkunen M, Linnoila M. Reduced central serotonin turnover in a subgroup of alcoholics? Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:173–7. doi: 10.1016/0278-5846(87)90056-x. [DOI] [PubMed] [Google Scholar]
- 13.Sellers EM, Higgins GA, Sobell MB. 5-HT and alcohol abuse. Trends Pharmacol Sci. 1992;13:69–75. doi: 10.1016/0165-6147(92)90026-3. [DOI] [PubMed] [Google Scholar]
- 14.Uzbay IT, Usanmaz SE, Akarsu ES. Effects of chronic ethanol administration on serotonin metabolism in the various regions of the rat brain. Neurochem Res. 2000;25:257–62. doi: 10.1023/a:1007579705230. [DOI] [PubMed] [Google Scholar]
- 15.Uzbay IT, Usanmaz SE, Tapanyigit EE, Aynacioglu S, Akarsu ES. Dopaminergic and serotonergic alterations in the rat brain during ethanol withdrawal: association with behavioral signs. Drug Alcohol Depend. 1998;53:39–47. doi: 10.1016/s0376-8716(98)00102-1. [DOI] [PubMed] [Google Scholar]
- 16.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–6. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 17.Hammoumi S, Payen A, Favre JD, Balmes JL, Benard JY, Husson M, et al. Does the short variant of the serotonin transporter linked polymorphic region constitute a marker of alcohol dependence? Alcohol. 1999;17:107–12. doi: 10.1016/s0741-8329(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 18.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994;36:326–37. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 19.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BA. Role of the serotonergic system in the neurobiology of alcoholism—implications for treatment. CNS Drugs. 2004;18:1105–18. doi: 10.2165/00023210-200418150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Mantere T, Tupala E, Hall H, Sarkioja T, Rasanen P, Bergstrom K, et al. Serotonin transporter distribution and density in the cerebral cortex of alcoholic and nonalcoholic comparison subjects: a whole-hemisphere autoradiography study. Am J Psychiatry. 2002;159:599–606. doi: 10.1176/appi.ajp.159.4.599. [DOI] [PubMed] [Google Scholar]
- 22.Storvik M, Tiihonen J, Haukijarvi T, Tupala E. Lower serotonin transporter binding in caudate in alcoholics. Synapse. 2006;59:144–51. doi: 10.1002/syn.20228. [DOI] [PubMed] [Google Scholar]
- 23.Storvik M, Haukijarvi T, Tupala E, Tiihonen J. Correlation between the SERT binding densities in hypothalamus and amygdala in Cloninger type 1 and 2 alcoholics. Alcohol Alcohol. 2008;43:25–30. doi: 10.1093/alcalc/agm157. [DOI] [PubMed] [Google Scholar]
- 24.Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–82. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Oz M, Stewart RR, Peoples RW, Weight FF. Volatile general anaesthetic actions on recombinant nACh alpha 7, 5-HT3 and chimeric nACh alpha 7-5-HT3 receptors expressed in Xenopus oocytes. Br J Pharmacol. 1997;120:353–5. doi: 10.1038/sj.bjp.0700934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovinger DM. 5-HT3 receptors and the neural actions of alcohols: an increasingly exciting topic. Neurochem Int. 1999;35:125–30. doi: 10.1016/s0197-0186(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 28.Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, et al. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–86. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 29.Bruinvels AT, Palacios JM, Hoyer D. Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:569–82. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- 30.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 31.Hoyer D, Martin G. 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology. 1997;36:419–28. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 32.Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–38. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- 33.Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999;281:1318–25. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- 34.Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 35.Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–41. [PubMed] [Google Scholar]
- 36.Litten RZ, Allen JP. Advances in development of medications for alcoholism treatment. Psychopharmacology (Berl) 1998;139:20–33. doi: 10.1007/s002130050686. [DOI] [PubMed] [Google Scholar]
- 37.Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–52. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- 38.Swift RM. Drug therapy for alcohol dependence. N Engl J Med. 1999;340:1482–90. doi: 10.1056/NEJM199905133401907. [DOI] [PubMed] [Google Scholar]
- 39.Wilson AW, Neill JC, Costall B. An investigation into the effects of 5-HT agonists and receptor antagonists on ethanol self-administration in the rat. Alcohol. 1998;16:249–70. doi: 10.1016/s0741-8329(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 40.Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babor TF, Dolinsky ZS, Meyer RE, Hesselbrock M, Hofmann M, Tennen H. Types of alcoholics: concurrent and predictive validity of some common classification schemes. Br J Addict. 1992;87:1415–31. doi: 10.1111/j.1360-0443.1992.tb01921.x. [DOI] [PubMed] [Google Scholar]
- 42.Cloninger CR, Sigvardsson S, Bohman M. Type I and type II alcoholism: an update. Alcohol Health Res World. 1996;20:18–23. [PMC free article] [PubMed] [Google Scholar]
- 43.Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, et al. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- 44.Tupala E, Hall H, Halonen P, Tiihonen J. Cortical dopamine D2 receptors in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Synapse. 2004;54:129–37. doi: 10.1002/syn.20071. [DOI] [PubMed] [Google Scholar]
- 45.Martinez D, Slifstein M, Gil R, Hwang DR, Huang YY, Perez A, et al. Positron emission tomography imaging of the serotonin transporter and 5-HT1A receptor in alcohol dependence. Biol Psychiatry. 2009;65:175–80. doi: 10.1016/j.biopsych.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babu DK, Diaz A, Samikkannu T, Rao KV, Saiyed ZM, Rodriguez JW, et al. Upregulation of serotonin transporter by alcohol in human dendritic cells: possible implication in neuroimmune deregulation. Alcohol Clin Exp Res. 2009;33:1731–8. doi: 10.1111/j.1530-0277.2009.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang CIA, Lewis RJ. Emerging structure-function relationships defining monoamine NSS transporter substrate and ligand affinity. Biochem Pharmacol. 2010;79:1083–91. doi: 10.1016/j.bcp.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 48.Heinz A, Jones DW, Gorey JG, Bennet A, Suomi SJ, Weinberger DR, et al. Serotonin transporter availability correlates with alcohol intake in non-human primates. Mol Psychiatry. 2003;8:231–4. doi: 10.1038/sj.mp.4001214. [DOI] [PubMed] [Google Scholar]
- 49.Oliva JM, Manzanares J. Gene transcription alterations associated with decrease of ethanol intake induced by naltrexone in the brain of Wistar rats. Neuropsychopharmacology. 2007;32:1358–69. doi: 10.1038/sj.npp.1301237. [DOI] [PubMed] [Google Scholar]
- 50.Brown AK, George DT, Fujita M, Liow JS, Ichise M, Hibbeln J, et al. PET [C-11]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol Clin Exp Res. 2007;31:28–32. doi: 10.1111/j.1530-0277.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 51.Baumgarten HG, Grozdanovic Z. Psychopharmacology of central serotonergic systems. Pharmacopsychiatry. 1995;28(Suppl. 2):73–9. doi: 10.1055/s-2007-979623. [DOI] [PubMed] [Google Scholar]
- 52.Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, et al. Reduced central serotonin transporters in alcoholism. Am J Psychiatry. 1998;155:1544–9. doi: 10.1176/ajp.155.11.1544. [DOI] [PubMed] [Google Scholar]
- 53.Szabo Z, Owonikoko T, Peyrot M, Varga J, Mathews WB, Ravert HT, et al. Positron emission tomography imaging of the serotonin transporter in subjects with a history of alcoholism. Biol Psychiatry. 2004;55:766–71. doi: 10.1016/j.biopsych.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 54.Ramamoorthy S, Cool DR, Mahesh VB, Leibach FH, Melikian HE, Blakely RD, et al. Regulation of the human serotonin transporter. Cholera toxin-induced stimulation of serotonin uptake in human placental choriocarcinoma cells is accompanied by increased serotonin transporter mRNA levels and serotonin transporter-specific ligand binding. J Biol Chem. 1993;268:21626–31. [PubMed] [Google Scholar]
- 55.Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism—basic research and clinical implications. J Neural Transm. 1997;104:1005–14. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- 56.Heinz A, Jones DW, Bissette G, Hommer D, Ragan P, Knable M, et al. Relationship between cortisol and serotonin metabolites and transporters in alcoholism [correction of alcolholism] Pharmacopsychiatry. 2002;35:127–34. doi: 10.1055/s-2002-33197. [DOI] [PubMed] [Google Scholar]
- 57.Strathdee G, Sim A, Brown R. Control of gene expression by CpG island methylation in normal cells. Biochem Soc Trans. 2004;32:913–5. doi: 10.1042/BST0320913. [DOI] [PubMed] [Google Scholar]
- 58.Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:543–9. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson BA, Javors MA, Roache JD, Seneviratne C, Bergeson SE, Ait-Daoud N, et al. Can serotonin transporter genotype predict serotonergic function chronicity, and severity of drinking? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:209–16. doi: 10.1016/j.pnpbp.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Budde H, Sander T, Wernicke C, Muller A, Gallinat J, Schmidt LG, et al. Serotonin transporter promoter polymorphism and dopaminergic sensitivity in alcoholics. J Neural Transm. 2010;117:133–8. doi: 10.1007/s00702-009-0331-9. [DOI] [PubMed] [Google Scholar]
- 61.Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155:207–13. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- 62.Ait-Daoud N, Roache JD, Dawes MA, Liu L, Wang XQ, Javors MA, et al. Can serotonin transporter genotype predict craving in alcoholism? Alcohol Clin Exp Res. 2009;33:1329–35. doi: 10.1111/j.1530-0277.2009.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohnke MD, Kolb W, Lutz U, Maurer S, Batra A. The serotonin transporter promotor polymorphism 5-HTTLPR is not associated with alcoholism or severe forms of alcohol withdrawal in a German sample. Psychiatr Genet. 2006;16:227–8. doi: 10.1097/01.ypg.0000218629.11375.b0. [DOI] [PubMed] [Google Scholar]
- 64.Lee YS, Choi SW, Han DH, Kim DJ, Joe KH. Clinical manifestation of alcohol withdrawal symptoms related to genetic polymorphisms of two serotonin receptors and serotonin transporter. Eur Addict Res. 2009;15:39–46. doi: 10.1159/000173008. [DOI] [PubMed] [Google Scholar]
- 65.Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin Exp Res. 2001;25:487–95. [PubMed] [Google Scholar]
- 66.Schuckit MA, Mazzanti C, Smith TL, Ahmed U, Radel M, Iwata N, et al. Selective genotyping for the role of 5-HT2A, 5-HT2C, and GABA alpha 6 receptors and the serotonin transporter in the level of response to alcohol: a pilot study. Biol Psychiatry. 1999;45:647–51. doi: 10.1016/s0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- 67.Javors MA, Seneviratne C, Roache JD, Ait-Daoud N, Bergeson SE, Walss-Bass MC, et al. Platelet serotonin uptake and paroxetine binding among allelic genotypes of the serotonin transporter in alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:7–13. doi: 10.1016/j.pnpbp.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- 69.Gorwood P. Eating disorders, serotonin transporter polymorphisms and potential treatment response. Am J Pharmacogenomics. 2004;4:9–17. doi: 10.2165/00129785-200404010-00002. [DOI] [PubMed] [Google Scholar]
- 70.Konishi T, Calvillo M, Leng AS, Lin KM, Wan YJ. Polymorphisms of the dopamine D2 receptor, serotonin transporter, and GABA(A) receptor beta(3) subunit genes and alcoholism in Mexican-Americans. Alcohol. 2004;32:45–52. doi: 10.1016/j.alcohol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Saiz PA, Garcia-Portilla MP, Florez G, Arango C, Corcoran P, Morales B, et al. Differential role of serotonergic polymorphisms in alcohol and heroin dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:695–700. doi: 10.1016/j.pnpbp.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 72.Sander T, Harms H, Lesch KP, Dufeu P, Kuhn S, Hoehe M, et al. Association analysis of a regulatory variation of the serotonin transporter gene with severe alcohol dependence. Alcohol Clin Exp Res. 1997;21:1356–9. [PubMed] [Google Scholar]
- 73.Gokturk C, Schultze S, Nilsson KW, von Knorring L, Oreland L, Hallman J. Serotonin transporter (5-HTTLPR) and monoamine oxidase (MAOA) promoter polymorphisms in women with severe alcoholism. Arch Womens Ment Health. 2008;11:347–55. doi: 10.1007/s00737-008-0033-6. [DOI] [PubMed] [Google Scholar]
- 74.Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–9. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 75.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 76.Choi IG, Kee BS, Son HG, Ham BJ, Yang BH, Kim SH, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenase, dopamine and serotonin transporters in familial and non-familial alcoholism. Eur Neuropsychopharmacol. 2006;16:123–8. doi: 10.1016/j.euroneuro.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Dick DM, Plunkett J, Hamlin D, Nurnberger J, Jr, Kuperman S, Schuckit M, et al. Association analyses of the serotonin transporter gene with lifetime depression and alcohol dependence in the Collaborative Study on the Genetics of Alcoholism (COGA) sample. Psychiatr Genet. 2007;17:35–8. doi: 10.1097/YPG.0b013e328011188b. [DOI] [PubMed] [Google Scholar]
- 78.Foley PF, Loh EW, Innes DJ, Williams SM, Tannenberg AE, Harper CG, et al. Association studies of neurotransmitter gene polymorphisms in alcoholic Caucasians. Ann NY Acad Sci. 2004;1025:39–46. doi: 10.1196/annals.1316.005. [DOI] [PubMed] [Google Scholar]
- 79.Gorwood P, Batel P, Ades J, Hamon M, Boni C. Serotonin transporter gene polymorphisms, alcoholism, and suicidal behavior. Biol Psychiatry. 2000;48:259–64. doi: 10.1016/s0006-3223(00)00840-4. [DOI] [PubMed] [Google Scholar]
- 80.Kranzler H, Lappalainen J, Nellissery M, Gelernter J. Association study of alcoholism subtypes with a functional promoter polymorphism in the serotonin transporter protein gene. Alcohol Clin Exp Res. 2002;26:1330–5. doi: 10.1097/01.ALC.0000030840.48315.40. [DOI] [PubMed] [Google Scholar]
- 81.Mokrovic G, Matosic A, Hranilovic D, Stefulj J, Novokmet M, Oreskovic D, et al. Alcohol dependence and polymorphisms of serotonin-related genes: association studies. Coll Antropol. 2008;32(Suppl. 1):127–31. [PubMed] [Google Scholar]
- 82.Thompson MD, Gonzalez N, Nguyen T, Comings DE, George SR, O’Dowd BF. Serotonin transporter gene polymorphisms in alcohol dependence. Alcohol. 2000;22:61–7. doi: 10.1016/s0741-8329(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 83.McHugh RK, Hofmann SG, Asnaani A, Sawyer AT, Otto MW. The serotonin transporter gene and risk for alcohol dependence: a meta-analytic review. Drug Alcohol Depend. 2010;108:1–6. doi: 10.1016/j.drugalcdep.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turker T, Sodmann R, Goebel U, Jatzke S, Knapp M, Lesch KP, et al. High ethanol tolerance in young adults is associated with the low-activity variant of the promoter of the human serotonin transporter gene. Neurosci Lett. 1998;248:147–50. doi: 10.1016/s0304-3940(98)00347-4. [DOI] [PubMed] [Google Scholar]
- 85.Fromme K, de Wit H, Hutchison KE, Ray L, Corbin WR, Cook TA, et al. Biological and behavioral markers of alcohol sensitivity. Alcohol Clin Exp Res. 2004;28:247–56. doi: 10.1097/01.alc.0000113420.28472.25. [DOI] [PubMed] [Google Scholar]
- 86.Herman AI, Kaiss KM, Ma R, Philbeck JW, Hasan A, Dasti H, et al. Serotonin transporter promoter polymorphism and monoamine oxidase type a VNTR allelic variants together influence alcohol binge drinking risk in young women. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:74–8. doi: 10.1002/ajmg.b.30135. [DOI] [PubMed] [Google Scholar]
- 87.Pinto E, Reggers J, Gorwood P, Boni C, Scantamburlo G, Pitchot W, et al. The short allele of the serotonin transporter promoter polymorphism influences relapse in alcohol dependence. Alcohol Alcohol. 2008;43:398–400. doi: 10.1093/alcalc/agn015. [DOI] [PubMed] [Google Scholar]
- 88.Heinz A, Higley JD, Gorey JG, Saunders RC, Jones DW, Hommer D, et al. In vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. Am J Psychiatry. 1998;155:1023–8. doi: 10.1176/ajp.155.8.1023. [DOI] [PubMed] [Google Scholar]
- 89.Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–74. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- 90.Campbell AD, McBride WJ. Serotonin-3 receptor and ethanol-stimulated dopamine release in the nucleus accumbens. Pharmacol Biochem Behav. 1995;51:835–42. doi: 10.1016/0091-3057(95)00050-7. [DOI] [PubMed] [Google Scholar]
- 91.Minabe Y, Ashby CR, Jr, Schwartz JE, Wang RY. The 5-HT3 receptor antagonists LY 277359 and granisetron potentiate the suppressant action of apomorphine on the basal firing rate of ventral tegmental dopamine cells. Eur J Pharmacol. 1991;209:143–50. doi: 10.1016/0014-2999(91)90162-j. [DOI] [PubMed] [Google Scholar]
- 92.Rasmussen K, Stockton ME, Czachura JF. The 5-HT3 receptor antagonist zatosetron decreases the number of spontaneously active A10 dopamine neurons. Eur J Pharmacol. 1991;205:113–6. doi: 10.1016/0014-2999(91)90781-k. [DOI] [PubMed] [Google Scholar]
- 93.Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963–71. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- 94.Johnson BA, Ait-Daoud N. Neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Psychopharmacology (Berl) 2000;149:327–44. doi: 10.1007/s002130000371. [DOI] [PubMed] [Google Scholar]
- 95.Stoltenberg SF. Serotonergic agents and alcoholism treatment: a simulation. Alcohol Clin Exp Res. 2003;27:1853–9. doi: 10.1097/01.ALC.0000098876.94384.0A. [DOI] [PubMed] [Google Scholar]
- 96.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Praschak-Rieder N, Kennedy JL, Wilson AA, Houle S, Willeit M, Boovariwala A, et al. A novel functional polymorphism within 5-HTTLPR is associated with higher putamen serotonin transporter density in healthy subjects. Biol Psychiatry. 2006;59:213S. [Google Scholar]
- 98.Nordquist N, Gokturk C, Comasco E, Nilsson KW, Oreland L, Hallman J. Transcription factor AP2 beta involved in severe female alcoholism. Brain Res. 2009;1305:S20–6. doi: 10.1016/j.brainres.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 99.Peters JA, Malone HM, Lambert JJ. Recent advances in the electrophysiological characterization of 5-HT3 receptors. Trends Pharmacol Sci. 1992;13:391–7. doi: 10.1016/0165-6147(92)90119-q. [DOI] [PubMed] [Google Scholar]
- 100.Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–9. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- 101.Kilpatrick GJ, Butler A, Hagan RM, Jones BJ, Tyers MB. [3H] GR67330, a very high affinity ligand for 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:22–30. doi: 10.1007/BF00178967. [DOI] [PubMed] [Google Scholar]
- 102.Tadipatri S, Feniuk W, Saxena PR. Rabbit isolated renal artery contractions by some tryptamine derivatives, including 2-methyl-5-HT, are mediated by a 5-HT1-like receptor. Br J Pharmacol. 1992;107:322–8. doi: 10.1111/j.1476-5381.1992.tb12745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jenkins A, Franks NP, Lieb WR. Actions of general anaesthetics on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br J Pharmacol. 1996;117:1507–15. doi: 10.1111/j.1476-5381.1996.tb15314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–70. [PubMed] [Google Scholar]
- 105.Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther. 1994;271:898–905. [PubMed] [Google Scholar]
- 106.Sessoms-Sikes JS, Hamilton ME, Liu LX, Lovinger DM, Machu TK. A mutation in transmembrane domain II of the 5-hydroxytryptamine(3A) receptor stabilizes channel opening and alters alcohol modulatory actions. J Pharmacol Exp Ther. 2003;306:595–604. doi: 10.1124/jpet.103.050542. [DOI] [PubMed] [Google Scholar]
- 107.Barnes JM, Barnes NM, Champaneria S, Costall B, Naylor RJ. Characterisation and autoradiographic localisation of 5-HT3 receptor recognition sites identified with [3H]-(S)-zacopride in the forebrain of the rat. Neuropharmacology. 1990;29:1037–45. doi: 10.1016/0028-3908(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 108.Gehlert DR, Gackenheimer SL, Wong DT, Robertson DW. Localization of 5-HT3 receptors in the rat brain using [3H]LY278584. Brain Res. 1991;553:149–54. doi: 10.1016/0006-8993(91)90242-n. [DOI] [PubMed] [Google Scholar]
- 109.Fadda F, Garau B, Marchei F, Colombo G, Gessa GL. MDL 72222, a selective 5-HT3 receptor antagonist, suppresses voluntary ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 1991;26:107–10. doi: 10.1093/oxfordjournals.alcalc.a045088. [DOI] [PubMed] [Google Scholar]
- 110.McKinzie DL, Eha R, Cox R, Stewart RB, Dyr W, Murphy JM, et al. Serotonin3 receptor antagonism of alcohol intake: effects of drinking conditions. Alcohol. 1998;15:291–8. doi: 10.1016/s0741-8329(97)00132-8. [DOI] [PubMed] [Google Scholar]
- 111.Findlay J, Eliopoulos E. Three-dimensional modelling of G protein-linked receptors. Trends Pharmacol Sci. 1990;11:492–9. doi: 10.1016/0165-6147(90)90050-i. [DOI] [PubMed] [Google Scholar]
- 112.Hibert MF, Trumpp-Kallmeyer S, Bruinvels A, Hoflack J. Three-dimensional models of neurotransmitter G-binding protein-coupled receptors. Mol Pharmacol. 1991;40:8–15. [PubMed] [Google Scholar]
- 113.Trumpp-Kallmeyer S, Hoflack J, Bruinvels A, Hibert M. Modeling of G-protein-coupled receptors: application to dopamine, adrenaline, serotonin, acetylcholine, and mammalian opsin receptors. J Med Chem. 1992;35:3448–62. doi: 10.1021/jm00097a002. [DOI] [PubMed] [Google Scholar]
- 114.Bouhelal R, Smounya L, Bockaert J. 5-HT1B receptors are negatively coupled with adenylate cyclase in rat substantia nigra. Eur J Pharmacol. 1988;151:189–96. doi: 10.1016/0014-2999(88)90799-6. [DOI] [PubMed] [Google Scholar]
- 115.Schoeffter P, Hoyer D. How selective is GR 43175? Interactions with functional 5-HT1A, 5-HT1B, 5-HT1C and 5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:135–8. doi: 10.1007/BF00169219. [DOI] [PubMed] [Google Scholar]
- 116.Hamblin MW, Metcalf MA. Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor. Mol Pharmacol. 1991;40:143–8. [PubMed] [Google Scholar]
- 117.Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci USA. 1992;89:3020–4. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seuwen K, Magnaldo I, Pouyssegur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988;335:254–6. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- 119.Fink K, Zentner J, Gothert M. Subclassification of presynaptic 5-HT autoreceptors in the human cerebral cortex as 5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:451–4. doi: 10.1007/BF00172785. [DOI] [PubMed] [Google Scholar]
- 120.Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–95. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- 121.Grimaldi B, Fillion MP, Bonnin A, Rousselle JC, Massot O, Fillion G. Immunocytochemical localization of neurons expressing 5-HT-moduline in the mouse brain. Neuropharmacology. 1997;36:1079–87. doi: 10.1016/s0028-3908(97)00099-3. [DOI] [PubMed] [Google Scholar]
- 122.Hjorth S, Suchowski CS, Galloway MP. Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995;19:170–6. doi: 10.1002/syn.890190304. [DOI] [PubMed] [Google Scholar]
- 123.Boulenguez P, Segu L, Chauveau J, Morel A, Lanoir J, Delaage M. Biochemical and pharmacological characterization of serotonin-O-carboxymethylglycyl[125I]iodotyrosinamide, a new radioiodinated probe for 5-HT1B and 5-HT1D binding sites. J Neurochem. 1992;58:951–9. doi: 10.1111/j.1471-4159.1992.tb09348.x. [DOI] [PubMed] [Google Scholar]
- 124.Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–30. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 125.Sari Y, Lefevre K, Bancila M, Quignon M, Miquel MC, Langlois X, et al. Light and electron microscopic immunocytochemical visualization of 5-HT1B receptors in the rat brain. Brain Res. 1997;760:281–6. doi: 10.1016/s0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- 126.Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, et al. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 127.Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- 128.Palacios JM, Waeber C, Hoyer D, Mengod G. Distribution of serotonin receptors. Ann NY Acad Sci. 1990;600:36–52. doi: 10.1111/j.1749-6632.1990.tb16871.x. [DOI] [PubMed] [Google Scholar]
- 129.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–80. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 130.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- 131.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–76. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 132.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 133.Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–67. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 134.Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978;180:509–32. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- 135.Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–91. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- 136.Krettek JE, Price JL. Projections from the amygdala to the perirhinal and entorhinal cortices and the subiculum. Brain Res. 1974;71:150–4. doi: 10.1016/0006-8993(74)90199-1. [DOI] [PubMed] [Google Scholar]
- 137.Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- 138.Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38:73–9. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, et al. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- 140.Risinger FO, Bormann NM, Oakes RA. Reduced sensitivity to ethanol reward, but not ethanol aversion, in mice lacking 5-HT1B receptors. Alcohol Clin Exp Res. 1996;20:1401–5. doi: 10.1111/j.1530-0277.1996.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 141.Risinger FO, Doan AM, Vickrey AC. Oral operant ethanol self-administration in 5-HT1b knockout mice. Behav Brain Res. 1999;102:211–5. doi: 10.1016/s0166-4328(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 142.Maurel S, De Vry J, Schreiber R. 5-HT receptor ligands differentially affect operant oral self-administration of ethanol in the rat. Eur J Pharmacol. 1999;370:217–23. doi: 10.1016/s0014-2999(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 143.Maurel S, De Vry J, De Beun R, Schreiber R. 5-HT2A and 5-HT2C/5-HT1B receptors are differentially involved in alcohol preference and consummatory behavior in cAA rats. Pharmacol Biochem Behav. 1999;62:89–96. doi: 10.1016/s0091-3057(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 144.Tomkins DM, O’Neill MF. Effect of 5-HT(1B) receptor ligands on self-administration of ethanol in an operant procedure in rats. Pharmacol Biochem Behav. 2000;66:129–36. doi: 10.1016/s0091-3057(00)00232-x. [DOI] [PubMed] [Google Scholar]
- 145.Miczek KA, de Almeida RM. Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology (Berl) 2001;157:421–9. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- 146.Crabbe JC, Belknap JK, Buck KJ. Genetic animal models of alcohol and drug abuse. Science. 1994;264:1715–23. doi: 10.1126/science.8209252. [DOI] [PubMed] [Google Scholar]
- 147.Hu J, Henry S, Gallezot JD, Ropchan J, Neumaier JF, Potenza MN, et al. Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. 2010;67:800–3. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Duan J, Sanders AR, Molen JE, Martinolich L, Mowry BJ, Levinson DF, et al. Polymorphisms in the 5’-untranslated region of the human serotonin receptor 1B (HTR1B) gene affect gene expression. Mol Psychiatry. 2003;8:901–10. doi: 10.1038/sj.mp.4001403. [DOI] [PubMed] [Google Scholar]
- 149.Hasegawa Y, Higuchi S, Matsushita S, Miyaoka H. Association of a polymorphism of the serotonin 1B receptor gene and alcohol dependence with inactive aldehyde dehydrogenase-2. J Neural Transm. 2002;109:513–21. doi: 10.1007/s007020200042. [DOI] [PubMed] [Google Scholar]
- 150.Lappalainen J, Dean M, Charbonneau L, Virkkunen M, Linnoila M, Goldman D. Mapping of the serotonin 5-HT1D beta autoreceptor gene on chromosome 6 and direct analysis for sequence variants. Am J Med Genet. 1995;60:157–61. doi: 10.1002/ajmg.1320600214. [DOI] [PubMed] [Google Scholar]
- 151.Lappalainen J, Long JC, Eggert M, Ozaki N, Robin RW, Brown GL, et al. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55:989–94. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- 152.Lee SY, Lin WW, Huang SY, Kuo PH, Wang CL, Wu PL, et al. The relationship between serotonin receptor 1B polymorphisms A-161T and alcohol dependence. Alcohol Clin Exp Res. 2009;33:1589–95. doi: 10.1111/j.1530-0277.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- 153.Hamblin MW, Metcalf MA, McGuffin RW, Karpells S. Molecular cloning and functional characterization of a human 5-HT1B serotonin receptor: a homologue of the rat 5-HT1B receptor with 5-HT1D-like pharmacological specificity. Biochem Biophys Res Commun. 1992;184:752–9. doi: 10.1016/0006-291x(92)90654-4. [DOI] [PubMed] [Google Scholar]
- 154.Middlemiss DN. Blockade of the central 5-HT autoreceptor by beta-adrenoceptor antagonists. Eur J Pharmacol. 1986;120:51–6. doi: 10.1016/0014-2999(86)90638-2. [DOI] [PubMed] [Google Scholar]
- 155.Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacol Rev. 1992;44:401–58. [PubMed] [Google Scholar]
- 156.Macor JE, Burkhart CA, Heym JH, Ives JL, Lebel LA, Newman ME, et al. 3-(1, 2, 5, 6-Tetrahydropyrid-4-yl)pyrrolo[3, 2-b]pyrid-5-one: a potent and selective serotonin (5-HT1B) agonist and rotationally restricted phenolic analogue of 5-methoxy-3-(1, 2, 5, 6-tetrahydropyrid-4-yl)indole. J Med Chem. 1990;33:2087–93. doi: 10.1021/jm00170a007. [DOI] [PubMed] [Google Scholar]
- 157.Waeber C, Moskowitz MA. [3H]sumatriptan labels both 5-HT1D and 5-HT1F receptor binding sites in the guinea pig brain: an autoradiographic study. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:263–75. doi: 10.1007/BF00168556. [DOI] [PubMed] [Google Scholar]
- 158.Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT(1B) receptor agonist CP-94, 253. Psychopharmacology (Berl) 1999;146:391–9. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- 159.Dohlman HG, Caron MG, Lefkowitz RJ. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987;26:2657–64. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- 160.Adrien J, Lanfumey L, Gozlan H, Fattaccini CM, Hamon M. Biochemical and electrophysiological evidence for an agonist action of CM 57493 at pre- and postsynaptic 5-hydroxytryptamine1A receptors in brain. J Pharmacol Exp Ther. 1989;248:1222–30. [PubMed] [Google Scholar]
- 161.Bockaert J, Dumuis A, Bouhelal R, Sebben M, Cory RN. Piperazine derivatives including the putative anxiolytic drugs, buspirone and ipsapirone, are agonists at 5-HT1A receptors negatively coupled with adenylate cyclase in hippocampal neurons. Naunyn Schmiedebergs Arch Pharmacol. 1987;335:588–92. doi: 10.1007/BF00169129. [DOI] [PubMed] [Google Scholar]
- 162.Schoeffter P, Hoyer D. Centrally acting hypotensive agents with affinity for 5-HT1A binding sites inhibit forskolin-stimulated adenylate cyclase activity in calf hippocampus. Br J Pharmacol. 1988;95:975–85. doi: 10.1111/j.1476-5381.1988.tb11728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bruning G, Kaulen P, Schneider U, Baumgarten HG. Quantitative autoradiographic distribution and pharmacological characterization of (3H)buspirone binding to sections from rat, bovine and marmoset brain. J Neural Transm. 1989;78:131–44. doi: 10.1007/BF01252499. [DOI] [PubMed] [Google Scholar]
- 164.Glaser T, Traber J. Buspirone: action on serotonin receptors in calf hippocampus. Eur J Pharmacol. 1983;88:137–8. doi: 10.1016/0014-2999(83)90404-1. [DOI] [PubMed] [Google Scholar]
- 165.Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, et al. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5, 7-dihy-droxytryptamine-treated rats. J Neurosci. 1986;6:3474–82. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Verge D, Daval G, Patey A, Gozlan H, el Mestikawy S, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol. 1985;113:463–4. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- 167.Weissmann-Nanopoulos D, Mach E, Magre J, Demassey Y, Pujol JF. Evidence for the localization of 5HT1A binding sites on serotonin containing neurons in the raphe dorsalis and raphe centralis nuclei of the rat brain. Neurochem Int. 1985;7:1061–72. doi: 10.1016/0197-0186(85)90156-1. [DOI] [PubMed] [Google Scholar]
- 168.Hamon M, Fattaccini CM, Adrien J, Gallissot MC, Martin P, Gozlan H. Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptamine1A agonists with potential anxiolytic properties. J Pharmacol Exp Ther. 1988;246:745–52. [PubMed] [Google Scholar]
- 169.Sprouse JS, Aghajanian GK. Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology. 1988;27:707–15. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- 170.Benloucif S, Galloway MP. Facilitation of dopamine release in vivo by serotonin agonists: studies with microdialysis. Eur J Pharmacol. 1991;200:1–8. doi: 10.1016/0014-2999(91)90658-d. [DOI] [PubMed] [Google Scholar]
- 171.Schechter LE, Bolanos FJ, Gozlan H, Lanfumey L, Haj-Dahmane S, Laporte AM, et al. Alterations of central serotoninergic and dopaminergic neurotransmission in rats chronically treated with ipsapirone: biochemical and electrophysiological studies. J Pharmacol Exp Ther. 1990;255:1335–47. [PubMed] [Google Scholar]
- 172.Ahlenius S, Larsson K. Antagonism by pindolol, but not betaxolol, of 8-OH-DPAT-induced facilitation of male rat sexual behavior. J Neural Transm. 1989;77:163–70. doi: 10.1007/BF01248929. [DOI] [PubMed] [Google Scholar]
- 173.Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–13. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- 174.Tissier MH, Lainey E, Fattaccini CM, Hamon M, Adrien J. Effects of ipsapirone, a 5-HT1A agonist, on sleep/wakefulness cycles: probable post-synaptic action. J Sleep Res. 1993;2:103–9. doi: 10.1111/j.1365-2869.1993.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 175.Zemlan FP, Behbehani MM, Murphy RM. Serotonin receptor subtypes and the modulation of pain transmission. Prog Brain Res. 1988;77:349–55. doi: 10.1016/s0079-6123(08)62801-0. [DOI] [PubMed] [Google Scholar]
- 176.Bohm C, Placchi M, Stallone F, Gammans RE, Alms DR, Shrotriya RC, et al. A double-blind comparison of buspirone, clobazam, and placebo in patients with anxiety treated in a general practice setting. J Clin Psychopharmacol. 1990;10:38S–NaN. doi: 10.1097/00004714-199006001-00008. [DOI] [PubMed] [Google Scholar]
- 177.Chaput Y, de Montigny C. Effects of the 5-hydroxytryptamine receptor antagonist, BMY 7378, on 5-hydroxytryptamine neurotransmission: electrophysiological studies in the rat central nervous system. J Pharmacol Exp Ther. 1988;246:359–70. [PubMed] [Google Scholar]
- 178.Jolas T, Haj-Dahmane S, Kidd EJ, Langlois X, Lanfumey L, Fattaccini CM, et al. Central pre- and postsynaptic 5-HT1A receptors in rats treated chronically with a novel antidepressant, cericlamine. J Pharmacol Exp Ther. 1994;268:1432–43. [PubMed] [Google Scholar]
- 179.Collins DM, Myers RD. Buspirone attenuates volitional alcohol intake in the chronically drinking monkey. Alcohol. 1987;4:49–56. doi: 10.1016/0741-8329(87)90060-7. [DOI] [PubMed] [Google Scholar]
- 180.Privette TH, Hornsby RL, Myers RD. Buspirone alters alcohol drinking induced in rats by tetrahydropapaveroline injected into brain monoaminergic pathways. Alcohol. 1988;5:147–52. doi: 10.1016/0741-8329(88)90012-2. [DOI] [PubMed] [Google Scholar]
- 181.Svensson L, Fahlke C, Hard E, Engel JA. Involvement of the serotonergic system in ethanol intake in the rat. Alcohol. 1993;10:219–24. doi: 10.1016/0741-8329(93)90039-q. [DOI] [PubMed] [Google Scholar]
- 182.Meert TF. Effects of various serotonergic agents on alcohol intake and alcohol preference in Wistar rats selected at two different levels of alcohol preference. Alcohol Alcohol. 1993;28:157–70. [PubMed] [Google Scholar]
- 183.Richardson BP, Hoyer D. Selective agonists and antagonists at 5-hydroxytryptamine receptor subtypes. In: Paoletti R, editor. Serotonin: From Cell Biology to Pharmacology and Therapeutics. Dordrecht: Kluwer Academic Publishers; 1990. pp. 265–76. [Google Scholar]
- 184.Lum JT, Piercey MF. Electrophysiological evidence that spiperone is an antagonist of 5-HT1A receptors in the dorsal raphe nucleus. Eur J Pharmacol. 1988;149:9–15. doi: 10.1016/0014-2999(88)90035-0. [DOI] [PubMed] [Google Scholar]
- 185.Sprouse JS, Aghajanian GK. (-)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur J Pharmacol. 1986;128:295–8. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- 186.Jolas T, Haj-Dahmane S, Lanfumey L, Fattaccini CM, Kidd EJ, Adrien J, et al. (-)Tertatolol is a potent antagonist at pre- and postsynaptic serotonin 5-HT1A receptors in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:453–63. doi: 10.1007/BF00166735. [DOI] [PubMed] [Google Scholar]
- 187.Prisco S, Cagnotto A, Talone D, De Blasi A, Mennini T, Esposito E. Tertatolol, a new beta-blocker, is a serotonin (5-hydroxytryptamine1A) receptor antagonist in rat brain. J Pharmacol Exp Ther. 1993;265:739–44. [PubMed] [Google Scholar]
- 188.Wong DT, Threlkeld PG, Lumeng L, Li TK. Higher density of serotonin-1A receptors in the hippocampus and cerebral cortex of alcohol-preferring P rats. Life Sci. 1990;46:231–5. doi: 10.1016/0024-3205(90)90109-5. [DOI] [PubMed] [Google Scholar]
- 189.Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–9. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Overstreet DH, Knapp DJ, Breese GR. Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol Clin Exp Res. 2007;31:1473–81. doi: 10.1111/j.1530-0277.2007.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kostowski W, Dyr W. Effects of 5-HT-1A receptor agonists on ethanol preference in the rat. Alcohol. 1992;9:283–6. doi: 10.1016/0741-8329(92)90067-k. [DOI] [PubMed] [Google Scholar]
- 192.Long TA, Kalmus GW, Bjork A, Myers RD. Alcohol intake in high alcohol drinking (HAD) rats is suppressed by FG5865, a novel 5-HT1A agonist/5-HT2 antagonist. Pharmacol Biochem Behav. 1996;53:33–40. doi: 10.1016/0091-3057(95)00195-6. [DOI] [PubMed] [Google Scholar]
- 193.Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–28. [PubMed] [Google Scholar]
- 194.Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–56. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 195.Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- 196.Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol. 2000;58:1271–8. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- 197.Yan QS, Zheng SZ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in regulation of mesolimbic dopaminergic neuronal activity via GABA mechanisms: a study with dual-probe microdialysis. Brain Res. 2004;1021:82–91. doi: 10.1016/j.brainres.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 198.Yan QS, Zheng SZ, Feng MJ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in ethanol-induced increases in mesolimbic dopaminergic transmission. Brain Res. 2005;1060:126–37. doi: 10.1016/j.brainres.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 199.Higgins GA, Tomkins DM, Fletcher PJ, Sellers EM. Effect of drugs influencing 5-HT function on ethanol drinking and feeding behaviour in rats: studies using a drinkometer system. Neurosci Biobehav Rev. 1992;16:535–52. doi: 10.1016/s0149-7634(05)80195-2. [DOI] [PubMed] [Google Scholar]
- 200.Brodie MS, Bunney EB. Serotonin potentiates dopamine inhibition of ventral tegmental area neurons in vitro. J Neurophysiol. 1996;76:2077–82. doi: 10.1152/jn.1996.76.3.2077. [DOI] [PubMed] [Google Scholar]
- 201.Pehek EA. Local infusion of the serotonin antagonists ritanserin or ICS 205, 930 increases in vivo dopamine release in the rat medial prefrontal cortex. Synapse. 1996;24:12–8. doi: 10.1002/(SICI)1098-2396(199609)24:1<12::AID-SYN2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 202.Bowers BJ, Henry MB, Thielen RJ, McBride WJ. Serotonin 5-HT(2) receptor stimulation of dopamine release in the posterior but not anterior nucleus accumbens of the rat. J Neurochem. 2000;75:1625–33. doi: 10.1046/j.1471-4159.2000.0751625.x. [DOI] [PubMed] [Google Scholar]
- 203.De Deurwaerdere P, Spampinato U. Role of serotonin(2A) and serotonin(2B/2C) receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. J Neurochem. 1999;73:1033–42. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- 204.Leggio GM, Cathala A, Neny M, Rouge-Pont F, Drago F, Piazza PV, et al. In vivo evidence that constitutive activity of serotonin2C receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus accumbens: differential effects of inverse agonist versus antagonist. J Neurochem. 2009;111:614–23. doi: 10.1111/j.1471-4159.2009.06356.x. [DOI] [PubMed] [Google Scholar]
- 205.Panocka I, Massi M. Long-lasting suppression of alcohol preference in rats following serotonin receptor blockade by ritanserin. Brain Res Bull. 1992;28:493–6. doi: 10.1016/0361-9230(92)90052-y. [DOI] [PubMed] [Google Scholar]
- 206.Lankford MF, Bjork AK, Myers RD. Differential efficacy of serotonergic drugs FG5974, FG5893, and amperozide in reducing alcohol drinking in P rats. Alcohol. 1996;13:399–404. doi: 10.1016/0741-8329(96)00061-4. [DOI] [PubMed] [Google Scholar]
- 207.Overstreet DH, McArthur RA, Rezvani AH, Post C. Selective inhibition of alcohol intake in diverse alcohol-preferring rat strains by the 5-HT2A antagonists amperozide and FG 5974. Alcohol Clin Exp Res. 1997;21:1448–54. [PubMed] [Google Scholar]
- 208.Ding ZM, Toalston JE, Oster SM, McBride WJ, Rodd ZA. Involvement of local serotonin-2A but not serotonin-1B receptors in the reinforcing effects of ethanol within the posterior ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2009;204:381–90. doi: 10.1007/s00213-009-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu W, Thielen RJ, Rodd ZA, McBride WJ. Activation of serotonin-3 receptors increases dopamine release within the ventral tegmental area of Wistar and alcohol-preferring (P) rats. Alcohol. 2006;40:167–76. doi: 10.1016/j.alcohol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149:217–24. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- 211.Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology (Berl) 2003;165:252–9. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- 212.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–31. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- 214.Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–40. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- 215.Yan QS, Reith ME, Yan SG, Jobe PC. Effect of systemic ethanol on basal and stimulated glutamate releases in the nucleus accumbens of freely moving Sprague-Dawley rats: a microdialysis study. Neurosci Lett. 1998;258:29–32. doi: 10.1016/s0304-3940(98)00840-4. [DOI] [PubMed] [Google Scholar]
- 216.Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–22. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 218.Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev. 2003;41:203–28. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 219.Muramatsu M, Lapiz MD, Tanaka E, Grenhoff J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur J Neurosci. 1998;10:2371–9. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- 220.Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–53. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–22. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Blomeley C, Bracci E. Excitatory effects of serotonin on rat striatal cholinergic interneurones. J Physiol. 2005;569:715–21. doi: 10.1113/jphysiol.2005.098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Blomeley CP, Bracci E. Serotonin excites fast-spiking interneurons in the striatum. Eur J Neurosci. 2009;29:1604–14. doi: 10.1111/j.1460-9568.2009.06725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Devoto P, Colombo G, Stefanini E, Gessa GL. Serotonin is reduced in the frontal cortex of Sardinian ethanol-preferring rats. Alcohol Alcohol. 1998;33:226–9. doi: 10.1093/oxfordjournals.alcalc.a008386. [DOI] [PubMed] [Google Scholar]
- 226.Casu MA, Pisu C, Lobina C, Pani L. Immunocytochemical study of the forebrain serotonergic innervation in Sardinian alcohol-preferring rats. Psychopharmacology. 2004;172:341–51. doi: 10.1007/s00213-003-1663-z. [DOI] [PubMed] [Google Scholar]
- 227.Strother WN, Lumeng L, Li TK, McBride WJ. Dopamine and serotonin content in select brain regions of weanling and adult alcohol drinking rat lines. Pharmacol Biochem Behav. 2005;80:229–37. doi: 10.1016/j.pbb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 228.Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–77. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- 229.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 230.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–8. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 231.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–80. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 232.Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–12. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 233.Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009;111:37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–8. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 235.Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Malinen H, Lehtonen M, Hyytia P. Modulation of brain endocannabinoid levels by voluntary alcohol consumption in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2009;33:1711–20. doi: 10.1111/j.1530-0277.2009.01008.x. [DOI] [PubMed] [Google Scholar]
- 237.Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, et al. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–30. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- 238.Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 239.Malinen H, Hyytia P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–83. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 240.Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, et al. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–53. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- 242.Darmani NA, Janoyan JJ, Kumar N, Crim JL. Behaviorally active doses of the CB1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacol Biochem Behav. 2003;75:777–87. doi: 10.1016/s0091-3057(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 243.Haj-Dahmane S, Shen RY. Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J Pharmacol Exp Ther. 2009;331:186–96. doi: 10.1124/jpet.109.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Best AR, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J Neurosci. 2008;28:6508–15. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Parrish JC, Nichols DE. Serotonin 5-HT(2A) receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem. 2006;99:1164–75. doi: 10.1111/j.1471-4159.2006.04173.x. [DOI] [PubMed] [Google Scholar]
- 246.O’Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, et al. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–24. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- 247.Anton RF, Moak DH, Latham PK, Waid LR, Malcolm RJ, Dias JK, et al. Posttreatment results of combining naltrexone with cognitive-behavior therapy for the treatment of alcoholism. J Clin Psychopharmacol. 2001;21:72–7. doi: 10.1097/00004714-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 248.Dundon W, Lynch KG, Pettinati HM, Lipkin C. Treatment outcomes in type A and B alcohol dependence 6 months after serotonergic pharmacotherapy. Alcohol Clin Exp Res. 2004;28:1065–73. doi: 10.1097/01.alc.0000130974.50563.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Naranjo CA, Kadlec KE, Sanhueza P, Woodley-Remus D, Sellers EM. Fluoxetine differentially alters alcohol intake and other consummatory behaviors in problem drinkers. Clin Pharmacol Ther. 1990;47:490–8. doi: 10.1038/clpt.1990.62. [DOI] [PubMed] [Google Scholar]
- 250.Naranjo CA, Sellers EM, Lawrin MO. Modulation of ethanol intake by serotonin uptake inhibitors. J Clin Psychiatry. 1986;47:16–22. [PubMed] [Google Scholar]
- 251.Naranjo CA, Sellers EM, Roach CA, Woodley DV, Sanchez-Craig M, Sykora K. Zimelidine-induced variations in alcohol intake by nondepressed heavy drinkers. Clin Pharmacol Ther. 1984;35:374–81. doi: 10.1038/clpt.1984.46. [DOI] [PubMed] [Google Scholar]
- 252.Naranjo CA, Sellers EM, Sullivan JT, Woodley DV, Kadlec K, Sykora K. The serotonin uptake inhibitor citalopram attenuates ethanol intake. Clin Pharmacol Ther. 1987;41:266–74. doi: 10.1038/clpt.1987.27. [DOI] [PubMed] [Google Scholar]
- 253.Naranjo CA, Sullivan JT, Kadlec KE, Woodley-Remus DV, Kennedy G, Sellers EM. Differential effects of viqualine on alcohol intake and other consummatory behaviors. Clin Pharmacol Ther. 1989;46:301–9. doi: 10.1038/clpt.1989.142. [DOI] [PubMed] [Google Scholar]
- 254.Roache JD, Wang Y, Ait-Daoud N, Johnson BA. Prediction of serotonergic treatment efficacy using age of onset and Type A/B typologies of alcoholism. Alcohol Clin Exp Res. 2008;32:1502–12. doi: 10.1111/j.1530-0277.2008.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Artigas F. Pindolol, 5-hydroxytryptamine, and antidepressant augmentation. Arch Gen Psychiatry. 1995;52:969–71. doi: 10.1001/archpsyc.1995.03950230083012. [DOI] [PubMed] [Google Scholar]
- 256.Barr LC, Goodman WK, McDougle CJ, Delgado PL, Heninger GR, Charney DS, et al. Tryptophan depletion in patients with obsessive-compulsive disorder who respond to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:309–17. doi: 10.1001/archpsyc.1994.03950040053007. [DOI] [PubMed] [Google Scholar]
- 257.Mann JJ, Malone KM, Psych MR, Sweeney JA, Brown RP, Linnoila M, et al. Attempted suicide characteristics and cerebrospinal fluid amine metabolites in depressed inpatients. Neuropsychopharmacology. 1996;15:576–86. doi: 10.1016/S0893-133X(96)00102-9. [DOI] [PubMed] [Google Scholar]
- 258.Miguel-Hidalgo JJ, Rajkowska G. Comparison of prefrontal cell pathology between depression and alcohol dependence. J Psychiatr Res. 2003;37:411–20. doi: 10.1016/s0022-3956(03)00049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Weissman MM, Myers JK. Clinical depression in alcoholism. Am J Psychiatry. 1980;137:372–3. doi: 10.1176/ajp.137.3.372. [DOI] [PubMed] [Google Scholar]
- 260.Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44:573–88. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- 261.Kruesi MJ, Rapoport JL, Hamburger S, Hibbs E, Potter WZ, Lenane M, et al. Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Arch Gen Psychiatry. 1990;47:419–26. doi: 10.1001/archpsyc.1990.01810170019003. [DOI] [PubMed] [Google Scholar]
- 262.Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–14. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 263.Virkkunen M, Linnoila M. Serotonin in early onset, male alcoholics with violent behaviour. Ann Med. 1990;22:327–31. doi: 10.3109/07853899009147915. [DOI] [PubMed] [Google Scholar]
- 264.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 265.Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–8. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- 266.Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, et al. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch Gen Psychiatry. 1998;55:936–40. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- 267.Rosenthal NE, Mazzanti CM, Barnett RL, Hardin TA, Turner EH, Lam GK, et al. Role of serotonin transporter promoter repeat length polymorphism (5-HTTLPR) in seasonality and seasonal affective disorder. Mol Psychiatry. 1998;3:175–7. doi: 10.1038/sj.mp.4000360. [DOI] [PubMed] [Google Scholar]
- 268.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–8. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 269.Kiianmaa K, Stenius K, Sinclair JD. Determinants of alcohol preference in the AA and ANA rat lines selected for differential ethanol intake. Alcohol Alcohol. 1991;1(Suppl. 1):115–20. [PubMed] [Google Scholar]
- 270.Overstreet DH, Rezvani AH, Janowsky DS. Genetic animal models of depression and ethanol preference provide support for cholinergic and serotonergic involvement in depression and alcoholism. Biol Psychiatry. 1992;31:919–36. doi: 10.1016/0006-3223(92)90118-j. [DOI] [PubMed] [Google Scholar]
- 271.Ballenger JC, Goodwin FK, Major LF, Brown GL. Alcohol and central serotonin metabolism in man. Arch Gen Psychiatry. 1979;36:224–7. doi: 10.1001/archpsyc.1979.01780020114013. [DOI] [PubMed] [Google Scholar]
- 272.Banki CM. Factors influencing monoamine metabolites and tryptophan in patients with alcohol dependence. J Neural Transm. 1981;50:89–101. doi: 10.1007/BF01249132. [DOI] [PubMed] [Google Scholar]
- 273.Borg S, Kvande H, Liljeberg P, Mossberg D, Valverius P. 5-Hydroxyindoleacetic acid in cerebrospinal fluid in alcoholic patients under different clinical conditions. Alcohol. 1985;2:415–8. doi: 10.1016/0741-8329(85)90106-5. [DOI] [PubMed] [Google Scholar]
- 274.File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–5. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pettinati HM. The use of selective serotonin reuptake inhibitors in treating alcoholic subtypes. J Clin Psychiatry. 2001;62(20):26–31. [PubMed] [Google Scholar]
- 276.Naranjo CA, Knoke DM. The role of selective serotonin reuptake inhibitors in reducing alcohol consumption. J Clin Psychiatry. 2001;62(Suppl. 20):18–25. [PubMed] [Google Scholar]
- 277.Wrase J, Reimold M, Puls I, Kienast T, Heinz A. Serotonergic dysfunction: brain imaging and behavioral correlates. Cogn Affect Behav Neurosci. 2006;6:53–61. doi: 10.3758/cabn.6.1.53. [DOI] [PubMed] [Google Scholar]
- 278.Naranjo CA, Poulos CX, Bremner KE, Lanctot KL. Fluoxetine attenuates alcohol intake and desire to drink. Int Clin Psychopharmacol. 1994;9:163–72. doi: 10.1097/00004850-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 279.Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20:1534–41. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 280.Kranzler HR, Del Boca F, Korner P, Brown J. Adverse effects limit the usefulness of fluvoxamine for the treatment of alcoholism. J Subst Abuse Treat. 1993;10:283–7. doi: 10.1016/0740-5472(93)90076-e. [DOI] [PubMed] [Google Scholar]
- 281.Kranzler HR, Burleson JA, Korner P, Del Boca FK, Bohn MJ, Brown J, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152:391–7. doi: 10.1176/ajp.152.3.391. [DOI] [PubMed] [Google Scholar]
- 282.Romeo E, Pompili E, di Michele F, Pace M, Rupprecht R, Bernardi G, et al. Effects of fluoxetine, indomethacine and placebo on 3 alpha, 5 alpha tetrahydroprogesterone (THP) plasma levels in uncomplicated alcohol withdrawal. World J Biol Psychiatry. 2000;1:101–4. doi: 10.3109/15622970009150572. [DOI] [PubMed] [Google Scholar]
- 283.Janiri L, Gobbi G, Mannelli P, Pozzi G, Serretti A, Tempesta E. Effects of fluoxetine at antidepressant doses on short-term outcome of detoxified alcoholics. Int Clin Psychopharmacol. 1996;11:109–17. [PubMed] [Google Scholar]
- 284.Uzbay IT, Sag Lam E, Kayir H, Celik T, Beyazyurek M. Effects of fluoxetine on ethanol withdrawal syndrome in rats. J Psychiatr Res. 2004;38:445–50. doi: 10.1016/j.jpsychires.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 285.Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24:1041–9. [PubMed] [Google Scholar]
- 286.Lee MA, Meltzer HY. Neuroendocrine responses to serotonergic agents in alcoholics. Biol Psychiatry. 1991;30:1017–30. doi: 10.1016/0006-3223(91)90122-3. [DOI] [PubMed] [Google Scholar]
- 287.Saglam E, Kayir H, Celik T, Uzbay T. Effects of escitalopram on ethanol withdrawal syndrome in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1027–32. doi: 10.1016/j.pnpbp.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 288.Mennini T, Mocaer E, Garattini S. Tianeptine, a selective enhancer of serotonin uptake in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:478–82. doi: 10.1007/BF00169302. [DOI] [PubMed] [Google Scholar]
- 289.Daoust M, Compagnon P, Legrand E, Mocaer E. Tianeptine, a specific serotonin uptake enhancer, decreases ethanol intake in rats. Alcohol Alcohol. 1992;27:15–7. [PubMed] [Google Scholar]
- 290.File SE, Andrews N, al-Farhan M. Anxiogenic responses of rats on withdrawal from chronic ethanol treatment: effects of tianeptine. Alcohol Alcohol. 1993;28:281–6. [PubMed] [Google Scholar]
- 291.Uzbay IT. Serotonergic anti-depressants and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2008;43:15–24. doi: 10.1093/alcalc/agm145. [DOI] [PubMed] [Google Scholar]
- 292.Malka R, Loo H, Ganry H, Souche A, Marey C, Kamoun A. Long-term administration of tianeptine in depressed patients after alcohol withdrawal. Br J Psychiatry. 1992;160(Suppl. 15):66–71. [PubMed] [Google Scholar]
- 293.Favre JD, Guelfi-Sozzi C, Delalleau B, Loo H. Tianeptine and alcohol dependence. Eur Neuropsychopharmacol. 1997;7(Suppl. 3):S347–51. doi: 10.1016/s0924-977x(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 294.Tomkins DM, Le AD, Sellers EM. Effect of the 5-HT3 antagonist ondansetron on voluntary ethanol intake in rats and mice maintained on a limited access procedure. Psychopharmacology (Berl) 1995;117:479–85. doi: 10.1007/BF02246222. [DOI] [PubMed] [Google Scholar]
- 295.Imperato A, Angelucci L. 5-HT3 receptors control dopamine release in the nucleus accumbens of freely moving rats. Neurosci Lett. 1989;101:214–7. doi: 10.1016/0304-3940(89)90533-8. [DOI] [PubMed] [Google Scholar]
- 296.McBride WJ, Chernet E, Russell RN, Wong DT, Guan XM, Lumeng L, et al. Regional CNS densities of monoamine receptors in alcohol-naive alcohol-preferring P and -nonpreferring NP rats. Alcohol. 1997;14:141–8. doi: 10.1016/s0741-8329(96)00117-6. [DOI] [PubMed] [Google Scholar]
- 297.Rodd-Henricks ZA, McKinzie DL, Edmundson VE, Dagon CL, Murphy JM, McBride WJ, et al. Effects of 5-HT(3) receptor antagonists on daily alcohol intake under acquisition, maintenance, and relapse conditions in alcohol-preferring (P) rats. Alcohol. 2000;21:73–85. doi: 10.1016/s0741-8329(00)00083-5. [DOI] [PubMed] [Google Scholar]
- 298.McBride WJ, Lovinger DM, Machu T, Thielen RJ, Rodd ZA, Murphy JM, et al. Serotonin-3 receptors in the actions of alcohol, alcohol reinforcement, and alcoholism. Alcohol Clin Exp Res. 2004;28:257–67. doi: 10.1097/01.alc.0000113419.99915.da. [DOI] [PubMed] [Google Scholar]
- 299.Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MB. Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol Clin Exp Res. 1994;18:879–85. doi: 10.1111/j.1530-0277.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 300.Johnson BA, Roache JD, Ait-Daoud N, Zanca NA, Velazquez M. Ondansetron reduces the craving of biologically predisposed alcoholics. Psychopharmacology (Berl) 2002;160:408–13. doi: 10.1007/s00213-002-1002-9. [DOI] [PubMed] [Google Scholar]
- 301.Kranzler HR, Pierucci-Lagha A, Feinn R, Hernandez-Avila C. Effects of ondansetron in early- versus late-onset alcoholics: a prospective, open-label study. Alcohol Clin Exp Res. 2003;27:1150–5. doi: 10.1097/01.ALC.0000075547.77464.76. [DOI] [PubMed] [Google Scholar]
- 302.Johnson BA. Serotonergic agents and alcoholism treatment: rebirth of the subtype concept–an hypothesis. Alcohol Clin Exp Res. 2000;24:1597–601. [PubMed] [Google Scholar]
- 303.Bouwknecht JA, Hijzen TH, van der Gugten J, Maes RA, Hen R, Olivier B. Ethanol intake is not elevated in male 5-HT(1B) receptor knockout mice. Eur J Pharmacol. 2000;403:95–8. doi: 10.1016/s0014-2999(00)00527-6. [DOI] [PubMed] [Google Scholar]
- 304.Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–81. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 305.Phillips TJ, Hen R, Crabbe JC. Complications associated with genetic background effects in research using knockout mice. Psychopharmacology (Berl) 1999;147:5–7. doi: 10.1007/s002130051128. [DOI] [PubMed] [Google Scholar]
- 306.Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- 307.Gross-Isseroff R, Biegon A. Autoradiographic analysis of [3H]imipramine binding in the human brain postmortem: effects of age and alcohol. J Neurochem. 1988;51:528–34. doi: 10.1111/j.1471-4159.1988.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 308.Bruno F. Buspirone in the treatment of alcoholic patients. Psychopathology. 1989;22(Suppl. 1):49–59. doi: 10.1159/000284626. [DOI] [PubMed] [Google Scholar]
- 309.Malec TS, Malec EA, Dongier M. Efficacy of buspirone in alcohol dependence: a review. Alcohol Clin Exp Res. 1996;20:853–8. doi: 10.1111/j.1530-0277.1996.tb05263.x. [DOI] [PubMed] [Google Scholar]
- 310.Tollefson GD, Lancaster SP, Montague-Clouse J. The association of buspirone and its metabolite 1-pyrimidinylpiperazine in the remission of comorbid anxiety with depressive features and alcohol dependency. Psychopharmacol Bull. 1991;27:163–70. [PubMed] [Google Scholar]
- 311.Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001;21:143–53. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]


