Summary
The prefrontal cortex (PFC) regulates emotional responses, but it is unclear how PFC integrates diverse inputs to select the appropriate response. We therefore evaluated the contribution of basolateral amygdala (BLA) and ventral hippocampus (vHPC) inputs to fear signaling in the prelimbic (PL) cortex, a PFC region critical for the expression of conditioned fear. In conditioned rats trained to press for food, BLA inactivation decreased the activity of projection cells in PL, and reduced PL conditioned tone responses. In contrast, vHPC inactivation decreased activity of interneurons in PL, and increased PL conditioned tone responses. Consistent with hippocampal gating of fear after extinction, vHPC inactivation increased fear and PL pyramidal activity in extinguished, but not in conditioned, rats. These results suggest a prefrontal circuit whereby hippocampus gates amygdala-based fear. Thus, deficient hippocampal inhibition of PFC may underlie emotional disorders, especially in light of reduced hippocampal volume observed in depression and PTSD.
Regulation of emotions allows individuals to control otherwise automatic reactions to emotionally salient stimuli. Such executive control has long been associated with the prefrontal cortex (PFC), which is thought to integrate a diverse range of information necessary for selecting appropriate behavioral responses (Miller and Cohen, 2001). Here we studied the mechanisms underlying how the PFC integrates information using the well-characterized circuit of auditory fear conditioning (LeDoux, 2000; Maren and Quirk, 2004; Sotres-Bayon and Quirk, 2010), by evaluating the contribution of different inputs to PFC in behaving rats. In addition to the amygdala (LeDoux, 2000), the prelimbic (PL) prefrontal cortex is critical for expression of conditioned fear (Sierra-Mercado et al., 2011), and PL neurons show conditioning-induced increases in auditory responses (Burgos-Robles et al., 2009). Unlike the lateral amygdala, in which conditioned responses last only a few hundred milliseconds (Quirk et al., 1995), PL neurons exhibit sustained conditioned increases that mirror the time course of freezing to a tone (Burgos-Robles et al., 2009). This suggests that fear responses are initiated by the amygdala, but sustained by computations occurring in PL.
PL receives direct input from the basolateral amygdala (BLA) and the ventral hippocampus (vHPC), which has been implicated in contextual gating of fear responses (Bouton, 2002). Both BLA and vHPC innervate pyramidal neurons as well as inhibitory interneurons in PL (Carr and Sesack, 1996; Gabbott et al., 2002; Gabbott et al., 2006; Hoover and Vertes, 2007; McDonald, 1991), consistent with excitatory and inhibitory influences (Degenetais et al., 2003; Floresco and Tse, 2007; McDonald, 1991; Parent et al., 2010; Sun and Laviolette 2012; Tierney et al., 2004). It is not known, however, if PL integrates hippocampal and amygdala inputs in behaving rats.
We addressed this by combining multichannel recording in PL with local pharmacological inactivation in behaving rats subjected to auditory fear conditioning. We evaluated the effects of inactivation of BLA and vHPC on both spontaneous and tone-evoked activity of PL neurons. Inactivation of BLA reduced the firing rate of pyramidal neurons, and eliminated conditioned tone responses. In contrast, inactivation of vHPC reduced the firing rate of inhibitory interneurons and augmented conditioned tone responses. Consistent with vHPC gating of fear after extinction (Bouton, 2002; Hobin et al., 2006), inactivation of vHPC caused a return of fear responses and increased PL pyramidal cell activity in rats that had been extinguished.
Results
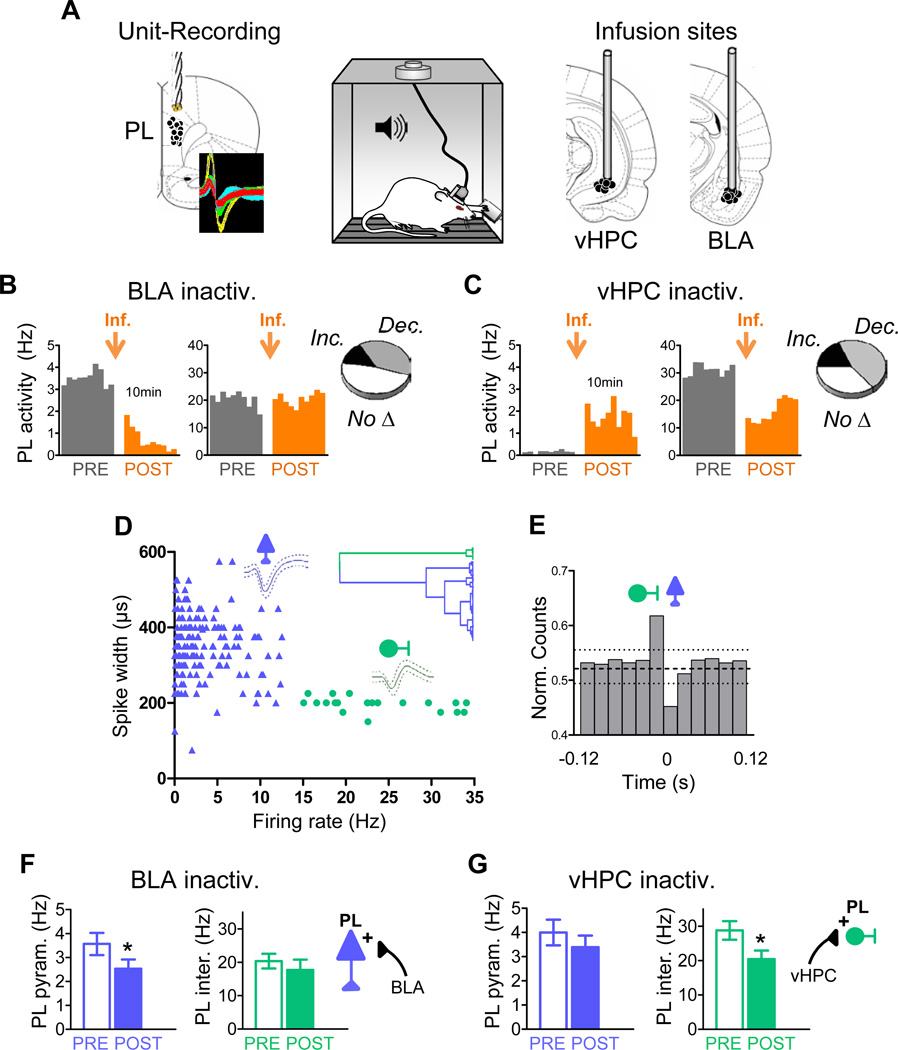
To evaluate fear signaling in PL, we conducted our experiments in conditioned rats, which show robust tone responses in PL (Burgos-Robles et al., 2009). Rats previously subjected to auditory fear conditioning were infused with the GABAA agonist muscimol into either BLA (n=7) or vHPC (n=7), while the activity of PL neurons was monitored through chronically implanted drives. Coronal brain drawings in figure 1A show the reconstruction of PL unit-recording sites as well as BLA and vHPC infusion sites.
Figure 1. Basolateral amygdala and ventral hippocampus differentially modulate the spontaneous activity of prelimbic pyramidal neurons and interneurons after conditioning.
(A) Conditioned rats previously trained to press a lever to obtain food were implanted with unit recording electrodes in the prelimbic (PL) prefrontal cortex and locally infused with muscimol (MUS) in the basolateral amygdala (BLA) or ventral hippocampus (vHPC). Coronal drawings show the location of recording electrodes (left) and cannula injector tips (right). Inset shows example extracellular waveforms.
(B & C) Histograms show ten minutes of spontaneous PL firing rate of representative neurons before (grey) and after (orange) MUS infusion (orange arrow) into either the BLA (B) or vHPC (C) (bin width = 1 min). Delay between pre- and post-infusion recordings was between 2 h and several days. Insets illustrate the proportion of PL cells that significantly increased rate, decreased rate, or showed no significant changes.
(D) Classification of PL neurons into putative pyramidal excitatory cells (blue) and interneurons (green) based on waveform duration and firing rate. Dendrogram inset shows the unsupervised cluster analysis that identified two main clusters.
(E) Averaged cross-correlation analysis for 10 pairs of simultaneously recorded PL interneurons and pyramidal cells, illustrating inhibitory interactions between interneurons (reference cell, firing at 0) and pyramidal cells (binned spike count normalized to overall firing rate) (bin width = 20 ms). Dashed horizontal line indicates mean; dotted lines indicate 95% confidence intervals.
(F) BLA inactivation significantly decreased activity of PL pyramidal neurons (blue bars) without affecting activity of PL interneurons (green bars).
(G) vHPC inactivation significantly decreased the activity of PL interneurons without affecting activity of pyramidal neurons. In this and subsequent figures, error bars illustrate s.e.m.; *p<0.05.
Differential contributions of BLA and vHPC to spontaneous activity in PL
We initially characterized the effects of input inactivation on spontaneous activity of PL cells, while rats that were conditioned pressed a bar for food in the conditioning chamber. Inactivation of either BLA or vHPC after conditioning yielded significant increases and decreases in firing rate of individual PL neurons (paired Student's t-test, P<0.05). The proportions of different responses were similar for the two inputs (Figure 1B and 1C insets). We noted, however, that cells with high firing rate tended to decrease their rate after vHPC inactivation (Figure 1C right), but not after BLA inactivation (Figure 1B right). Given that cells with high firing rate are often interneurons, we divided the PL neurons into putative pyramidal cells and interneurons by carrying out an unsupervised cluster analysis of the cells based on their firing rates and spike widths (Letzkus et al., 2011)(see Methods for details). This procedure yielded two main clusters (Figure 1D). Consistent with previous reports, one cluster contained a majority of neurons with low firing rate (<15 Hz) and broad spike waveform (>225 µs; putative excitatory pyramidal neurons), while the other contained neurons with high firing rates (>15 Hz) and narrow spike waveforms (<225 µs; putative inhibitory interneurons). Of 194 PL neurons, 174 (89.7 %) were classified as putative pyramidal neurons, and 20 (10.3 %) were classified as putative interneurons. Similar proportions of interneurons in the rat prefrontal cortex have been previously reported (Homayoun and Moghaddam, 2007). In support of our classification, we observed significant inhibitory interactions between putative inhibitory neurons and putative pyramidal cells, as evidenced by cross-correlation analyses (n=10 of 88 pairs of neurons; Figure 1E).
Classification of PL neurons into pyramidal cells and interneurons revealed dissociable effects of BLA and vHPC inputs. We limited our analysis to PL cells that exhibited significant changes in firing rate following input inactivation (P< 0.05, n=103/187). This additional restriction eliminated the few putative pyramidal cells that fell below the firing rate/waveform length criterion. Inactivation of BLA significantly decreased the firing rate of pyramidal neurons (n=52; Wilcoxon test: Z=2.57, P=0.010), without affecting interneuron activity (n=6; Wilcoxon test: Z=1.36, P=0.17) (Figure 1F), suggesting that BLA input to PL is largely excitatory in conditioned rats pressing for food. In contrast, inactivation of vHPC significantly decreased the firing rate of interneurons (n=7; Wilcoxon test: Z=2.36, P=0.01), without affecting pyramidal cell activity (n=43; Wilcoxon test: Z=1.48, P=0.13) (Figure 1G). This suggests that vHPC inputs are capable of triggering feed-forward inhibition of PL neurons by exciting local interneurons. This difference between BLA and vHPC inputs could not be predicted from prior anatomical (Carr and Sesack, 1996; Gabbott et al., 2002; Gabbott et al., 2006; Hoover and Vertes, 2007; McDonald, 1991) or physiological (Degenetais et al., 2003; Floresco and Tse, 2007; Laviolette et al., 2005; Tierney et al., 2004) studies.
Differential contributions of BLA and vHPC to tone-evoked activity in PL
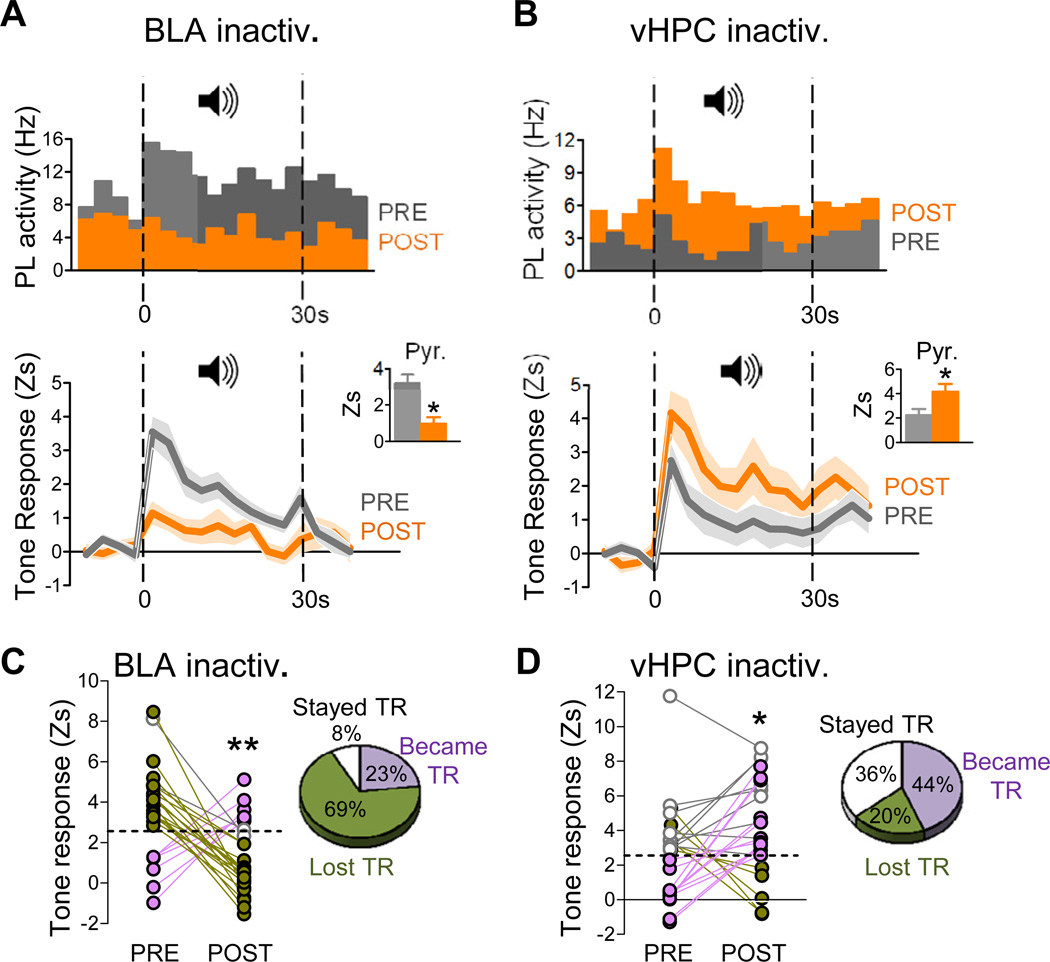
We next assessed the effect of input inactivation on tone responses of PL neurons, tested after conditioning. Conditioned tone tests occurred from 2 h to several days post conditioning (see Methods for details). Unilateral inactivation of either BLA or vHPC did not alter tone evoked freezing in conditioned rats (Figure S1). We intentionally used unilateral inactivation so that any changes in tone-evoked activity in PL could not be attributed to changes in freezing. We evaluated the effects of input inactivation on the magnitude of tone responses as indicated by responses in the first 3 s bin after tone onset. We limited our analysis to cells showing significant tone responses (Z> 2.58; P<0.01) either before or after input inactivation. We found that 20% of PL cells (n=34/172) were tone responsive prior to input inactivation, similar to our previous report (Burgos-Robles et al., 2009). Inactivation of BLA and vHPC produced opposite effects. Averaging over all tone responsive neurons (n=26/78, 33%), BLA inactivation significantly decreased tone responsiveness (t25=3.52 (paired); P=0.002) (see Figure 2A, top, bottom). This effect was due to decreased tone responses of pyramidal neurons (t21=2.81 (paired); P=0.011) (Figure 2A bottom inset) and interneurons (t3=4.11 (paired); P=0.03) (Figure S2). In contrast to BLA, vHPC inactivation increased tone responses (for example see Figure 2B top). Averaging over all tone responsive PL neurons (n=25/95, 26%), vHPC inactivation significantly increased tone responsiveness (t24=-2.26 (paired); P=0.03) (Figure 2B bottom). This effect was due to increased tone responses of pyramidal neurons (t18=2.12 (paired); P=0.048) (Figure 2B bottom inset), and not to interneurons (t5=0.75 (paired); P=0.48) (Figure S2). These opposing effects of BLA and vHPC inactivation could be detected as early as 300 milliseconds after tone onset.
Figure 2. Conditioned tone responses in PL neurons were decreased by BLA inactivation, but increased by vHPC inactivation.
(A) Upper: Peri-event time histogram of a representative PL tone-responsive neuron before (grey) and after (orange) BLA inactivation (bin width = 3 s), showing reduction in tone response with no change in spontaneous activity (Hz; pre-tone). Data represent the average of two conditioned tones before/after input inactivation. Lower: Group data showing that BLA inactivation significantly reduced tone responsiveness of PL neurons, as indicated by z-scores (P<0.001). Data represent averaged conditioned tone presentations over repeated days. Shaded areas represent s.e.m. Inset shows decreased PL tone responses were due in part to decreased pyramidal cell activity.
(B) Upper: Peri-event time histogram of a representative PL neuron that increased its tone response following inactivation of vHPC. Lower: Group data showing that vHPC inactivation significantly increased tone responsiveness of PL neurons, as indicated by z-scores (P<0.05). Inset shows that increased PL tone responses were due to increased pyramidal cell activity.
(C) Line plots tracking the tone responses (TRs) of individual PL neurons classified as significantly tone responsive (Z> 2.58; P<0.01; dotted line) before and/or after inactivation. Pie charts illustrate the percentage of PL neurons that lost TR (green), became TR (purple), or remained TR (white) after inactivation. The majority of PL neurons lost their tone response (green) after BLA inactivation.
(D) In contrast to BLA, inactivation of vHPC caused the majority of PL neurons to maintain their tone response (white) or become tone responsive (purple). *p<0.05; **p<0.01.
See also Figure 2S.
To evaluate within-cell changes, we tracked the tone responses (TRs) of each cell before and after inactivation in conditioned rats. Cells were classified as significantly tone responsive if they fired > 2.58 s.d., (P<0.01) above baseline rate within the first 3 s bin. Inactivation of BLA caused the majority of the 26 PL cells to lose their TRs, a small proportion to become TR, and some to remain TR (Figure 2C). This suggests that BLA is the major route by which conditioned tones can influence PL. In contrast to BLA, inactivation of vHPC resulted in most of the 25 PL neurons either becoming TR or remaining TR, with a smaller number losing their TR (Figure 2D). Thus, despite the typical heterogeneity of single-cell responses, the pattern of responses we observed supports the idea that BLA communicates conditioned responses to PL, whereas vHPC gates those responses.
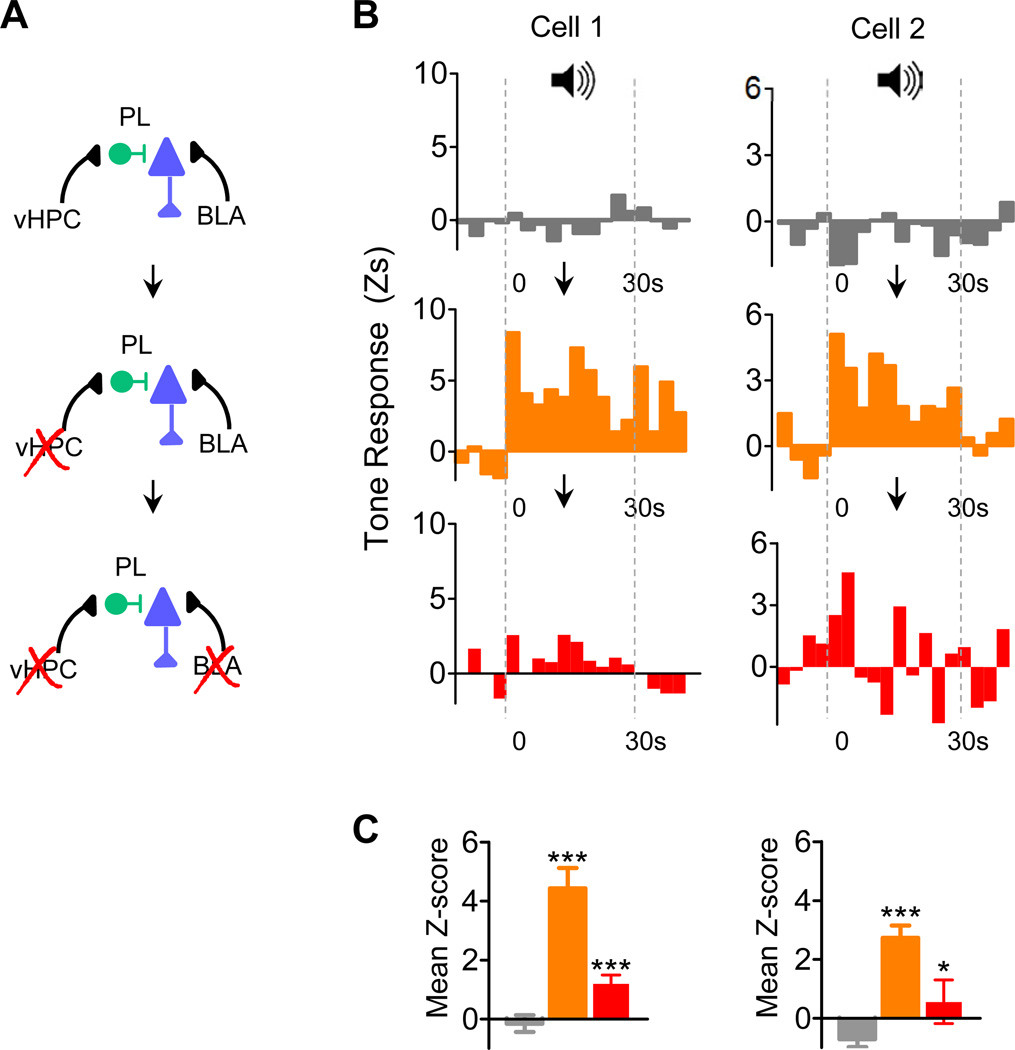
To test whether these effects of BLA and vHPC converge onto single PL cells, we implanted a subset of rats with cannulas in both structures. Out of 10 PL neurons tested, we found 3 that were TR and 7 that were not TR. Notably, 2 of these 7 non-TR cells (from different rats) revealed evidence of BLA and vHPC convergence. Figure 3B shows the tone responses of these neurons, which were not initially TR (top panel), but became significantly TR after vHPC inactivation (middle panel). These conditioned responses likely originated in the amygdala, because they were significantly reduced by subsequent BLA inactivation (lower panel) (F(2,29) = 28.03; and F(2,29) =10.53; Ps<0.001). Post hoc analysis of both cells revealed that tone responses increased after vHPC (Tukey Ps<0.001) and decreased after BLA inactivation (Tukey Ps<0.05) (Figure 3C). Thus, a latent fear signal in PL became apparent only after vHPC inactivation and was eliminated after BLA inactivation.
Figure 3. Examples of interaction between vHPC and BLA inputs within single PL neurons from conditioned rats.
(A) Experimental design: PL neuron activity without brain inactivation(s) (top), PL neuron activity with vHPC inactivation (middle) and PL neuron activity with vHPC + BLA inactivations (bottom) (B) Peri-event time histograms showing the tone responses of two PL neurons (left and right) recorded prior to any manipulation (top, grey), after vHPC inactivation (middle; orange), and after vHPC + BLA inactivation (bottom; red). Note that these two PL neurons were not originally TR, but became TR after vHPC inactivation. Downward pointing arrows represent succession of events. Subsequent inactivation of BLA eliminated the latent tone responses.
(C) Mean z-scores (10 bins) show that vHPC inactivation increased tone responses significantly, while subsequent BLA inactivation of the same cells decreased tone responses significantly. Tukey post hoc before inactivation (grey) vs. after vHPC inactivation (orange), and vHPC inactivation vs. BLA inactivation (red): *p<0.05; ***p<0.001.
vHPC inhibits fear after, but not before, extinction
Our electrophysiological findings suggest that the vHPC can inhibit PL responses to fear signals emanating from the amygdala. If true, then vHPC inactivation should increase fear. However, most previous studies of vHPC have shown the opposite, namely, that vHPC inactivation decreases conditioned freezing (Maren and Holt, 2004; Sierra-Mercado et al., 2011). These studies were performed in rats that were conditioned, but not extinguished. The hippocampus mediates contextual regulation of fear after extinction, when the meaning of the conditioned tone is ambiguous (Bouton, 2002). In agreement with a role of the hippocampus in ambiguous environmental conditions (Tsetsenis et al., 2007), we predicted that the vHPC-PL circuit would be recruited after extinction. In addition to freezing, we measured the rate of bar pressing for food which detects moderate levels of fear, even in the absence of freezing (Mast et al., 1982).
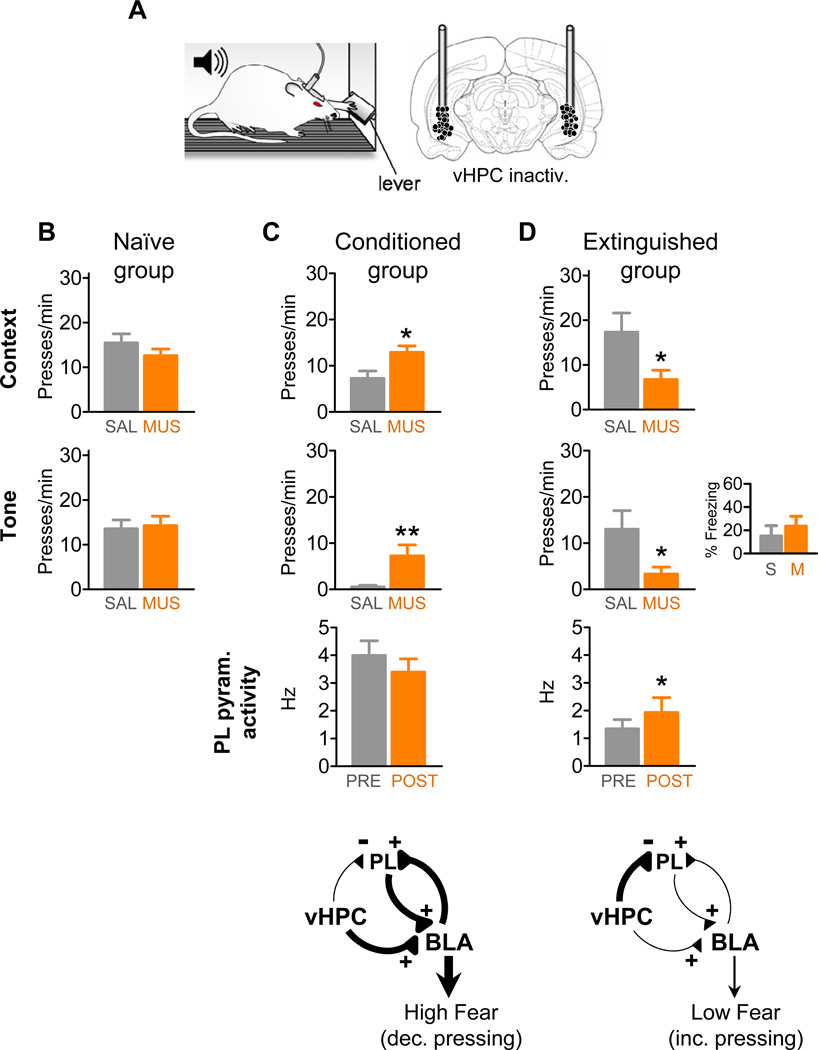
To assess the effects of vHPC inactivation before and after extinction, we compared the infusion of either saline or muscimol into vHPC in three groups: 1) naive controls, which were never conditioned, 2) conditioned rats that were conditioned on day 1 and tested on day 2 (without extinction), and 3) extinguished rats that were conditioned on day 1, extinguished on day 2, and tested for extinction retrieval on day 3. All testing was performed in the same chamber, with rats pressing a bar for food (Figure 4A). vHPC inactivation in the naïve group (n=10) had no effect on press rates compared to saline-infused rats (n=9), both prior to any tones (t17=1.13 (unpaired); P=0.27) or during tone presentations (t17=0.23 (unpaired); P=0.82) (Figure 4B). Thus, vHPC inactivation (on its own) does not affect hunger or motivation to press for food.
Figure 4. Inactivation of vHPC induced moderate fear and increased PL activity after, but not before, extinction.
(A) Rats trained to press a bar to obtain food were locally infused with saline (SAL) or muscimol (MUS) to temporarily inactivate the vHPC. Coronal drawing shows the location of cannula injector tips in vHPC. Press rates prior to the onset of the first tone were used as a measure of fear to the context, and rates during the tone as a measure of fear to the tone.
(B) Inactivation of vHPC in naïve rats did not affect pressing during pre-tone or tone periods.
(C) Inactivation of vHPC in conditioned rats increased pre-tone and tone pressing (decreased fear), but had no effect on spontaneous firing rate of PL pyramidal cells.
(D) In contrast, inactivation of vHPC in extinguished rats significantly reduced pre-tone and tone pressing (increased fear) and increased the firing rate of PL pyramidal cells. Press rates following vHPC inactivation resembled pre-extinction rates in the conditioned group (see 4C); however, freezing was not increased (inset).
Diagrams in the bottom of (C) and (D) suggest the underlying circuit recruited under different conditions. Following conditioning, heightened BLA activity (driven in part by vHPC input) controls PL activity. Following extinction, reduced excitatory drive emanating from BLA is balanced by increased inhibition of PL by vHPC. *p<0.05; **p<0.01.
See also Figure 3S.
Agreeing with previous results (Maren and Holt, 2004; Sierra-Mercado et al., 2011), vHPC inactivation in conditioned rats (n=14) decreased fear responses, as evidenced by increased pressing prior to any tones (t26=2.66 (unpaired); P=0.013) and during tone presentations (t24=2.84 (unpaired); P=0.009), compared to the saline-treated group (n=14) (Figure 4C). In contrast, vHPC inactivation in extinguished rats (n=11) increased fear, as evidenced by reduced pressing, both prior to any tones (pre tone; t19=2.29 (unpaired); P=0.033) as well as during tones (t19=2.32 (unpaired); P=0.032) (Figure 4D), compared to the saline-treated group (n=10). Agreeing with a previous report (Hobin et al., 2006), however, freezing was not increased (t19=0.68 (unpaired); P=0.50), suggesting a moderate level of fear. Thus, extinguished (but not naïve) rats require the vHPC to maintain low levels of fear, when food conflicts with potentially dangerous stimuli. In addition, extinguished rats showed an increased suppression ratio, confirming that the tone triggered increased fear (extinction group: t19=2.107 (unpaired) P=0.048; no-extinction group: t15=2.81 (unpaired); P=0.013) (Figure S3). Finally, an additional no-extinction control run on the same day as the extinction group (Day 3) also showed decreased fear (Figure S3), confirming that it is extinction rather than the mere passage of time that switched the effects of vHPC inactivation.
Consistent with a PL mechanism of action for the increased fear following extinction, vHPC inactivation in extinguished rats increased the spontaneous activity of PL putative pyramidal neurons (n=12 cells from 3 rats, Wilcoxon test: Z=2.04, P=0.04) (Figure 4D), and decreased the activity of a putative PL interneuron (from 24 Hz to 9 Hz). vHPC inactivation had no significant effect on PL tone responses after extinction (n=8 cells from 2 rats, first bin: t7=1.97 (paired), P=0.09) or spontaneous activity after conditioning (Figure 4C). Thus, vHPC inactivation can have opposite effects on fear expression, depending on whether or not extinction has taken place. Our findings indicate that vHPC inhibits fear expression via the PL after, but not before, extinction.
Discussion
We have identified a circuit in behaving rats, whereby PL integrates information from BLA and vHPC to regulate fear responses. Inactivation of BLA decreased activity of PL pyramidal neurons and eliminated conditioned tone responses. In contrast, vHPC inactivation decreased activity of PL inhibitory interneurons and increased conditioned tone responses. Consistent with vHPC gating of fear after extinction, vHPC inactivation caused a return of moderate fear and increased PL activity. Together, these findings suggest that the vHPC reduces fear after extinction, by inhibiting cortical responsiveness to amygdala input.
Amygdala contribution to fear signaling in prelimbic cortex
Because conditioned neural responses were virtually eliminated by BLA inactivation, we conclude that BLA is the source of fear-related input to PL. This agrees with prior findings in anesthetized rats showing that BLA input is necessary for olfactory conditioned responses in PL (Laviolette et al., 2005). Direct projections from BLA to PL are prominent (Hoover and Vertes, 2007), but we cannot rule out indirect projections, via central nucleus, to catecholamine nuclei in the brainstem (McGaugh, 2004), cholinergic basal forebrain nuclei (Gozzi et al., 2010), or auditory cortex (Armony et al., 1998; Letzkus et al., 2011). Our findings disagree with Garcia et al. (1999) who suggested, based on permanent lesions and multiunit recording, that BLA projections inhibit prefrontal fear signals. Rather, our findings provide physiological support for the notion that ascending projections from the amygdala interrupt cortical information processing so that the animal attends to dangerous stimulus in the environment (Armony et al., 1998). Consistent with our findings, amygdala activation of the cortex has been shown to induce risk assessment (Gozzi et al., 2010), sustained attention (Holland and Gallagher, 1999), and moderate fear or vigilance (Davis and Whalen, 2001).
Hippocampal contribution to fear signaling in prelimbic cortex
Our findings suggest that vHPC can reduce or even prevent fear signaling in PL. This is consistent with behavioral and electrophysiological evidence that the hippocampus gates fear responses via the PFC (Hobin et al., 2003; Sotres-Bayon et al., 2004). For example, stimulation-induced depression of the HPC-PFC pathway impairs extinction (Hugues and Garcia, 2007). Hippocampal inhibition of PL also agrees with data from anaesthetized rats showing that stimulation of vHPC consistently activates interneurons prior to pyramidal cells in PL (Tierney et al., 2004). The vHPC also projects to the BLA (Orsini et al., 2011) and could conceivably inhibit PL tone responses via feed-forward inhibition of BLA efferent to PL. Arguing against this, however, is our observation that interneurons local to PL are modulated by vHPC, and that vHPC and BLA manipulations were able to differentially modulate tone responses of single neurons.
Moreover, existing evidence suggests that the hippocampus excites rather than inhibits the BLA. The HPC-BLA pathway shows long-term potentiation (Maren and Fanselow, 1995) and inactivating the hippocampus decreases conditioned tone responses of BLA neurons (Maren and Hobin, 2007). Direct projections from vHPC to BLA may promote responding to unambiguous danger cues. Indeed, “fear cells” in BLA receive input from vHPC (Herry et al., 2008). Consistent with this, inactivation of vHPC prior to extinction increased pressing (decreased fear) (present study), and was previously shown to reduce conditioned freezing (Maren and Holt, 2004; Sierra-Mercado et al., 2011).
We suggest that the hippocampal inhibition of spontaneous activity of PL becomes behaviorally apparent only after extinction. After extinction, amygdala output is reduced (Amano et al., 2010; Herry et al., 2008). The reduced excitatory drive of PL emanating from BLA is augmented by increased inhibition of PL by vHPC (see circuit diagrams of Fig 4). The vHPC-induced increase of PL activity appears only after extinction because PL activity before extinction is at a ceiling level. Thus, hippocampal projections to PL could effectively modulate behavioral responses to cues made ambiguous by prior extinction training. Additionally, fear-promoting and fear-inhibiting functions of vHPC may be mediated by either distinct subsets of hippocampal neurons (Tronson et al., 2009) or local circuits in PL-BLA, differentially engaged by conditioning or extinction.
Ambiguity and vigilance
Our behavioral task was optimized to induce and detect moderate levels of fear suggestive of vigilance (readiness for danger). Suppression of bar pressing is more sensitive than freezing (Mast et al., 1982), and is able to reveal fear below the threshold for a full “fight or flight” response. Our use of the same context for conditioning and extinction prevented the rat from using context to disambiguate the meaning of the extinguished cue. Under such conditions, hippocampal activity is known to be required to disambiguate cues (Bouton, 2002; Tsetsenis et al., 2007) and prefrontal activity is required to switch between memory strategies (Rich and Shapiro, 2009) and select between conflicting motivations (Granon and Changeux, 2012).
Individuals suffering from mood and anxiety disorders tend to interpret ambiguous situations as threatening, leading to a state of hypervigilance. Individuals with high anxiety (Kim et al., 2011) or post-traumatic stress disorder (PTSD) (Milad et al., 2009) show hyperactivity in the dorsal anterior cingulate cortex (dACC), a homologue of rodent PL (Milad et al., 2007). Consistent with the inhibitory function of vHPC we describe, emotional disorders associated with heightened vigilance, such as PTSD, depression, and schizophrenia, are all accompanied by a reduction in the volume of the anterior hippocampus (Bremner et al., 2000; Gilbertson et al., 2002; McCarley et al., 1999), a homologue of rodent vHPC. This is consistent with the notion that the hippocampus is necessary to keep fear and vigilance under control (Tsetsenis et al., 2007). Notably, in PTSD, increased activity in dACC is correlated with decreased activity in the anterior hippocampus (Milad et al., 2009). Thus, deficient hippocampal inhibition of the prefrontal cortex may put individuals at risk for anxiety disorders (Shin et al., 2009), and may even constitute a premorbid risk factor (Linnman et al., 2012). Targeting the hippocampal-dACC gating circuit, for example with transcranial magnetic stimulation or methods to promote neurogenesis in the anterior hippocampus (Sahay and Hen, 2007), may help treat a wide range of disorders characterized by deficits in emotional regulation.
Methods
Subjects
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 270–320g were individually housed and handled as described previously (Burgos-Robles et al., 2009; Sierra-Mercado et al., 2011). Food was restricted to 18 g/day of standard laboratory rat chow until rats reached 85% of their free-feeding weight. Rats were trained to press a bar for food on a variable interval schedule of reinforcement (VI-60). Pressing maintains a constant level of activity against which freezing could be reliably measured, and provides a measure of moderate levels of fear (Mast et al., 1982; Sierra-Mercado et al., 2011). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Puerto Rico – School of Medicine in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Surgery
After bar-press training, rats were anesthetized with intraperitoneal injections of a mixture of ketamine (80 mg/kg)-xylazine (10 mg/kg) and were stereotaxically implanted with a 26-gauge guide cannula (Plastics One, Roanoke, VA) aimed at either BLA (3.00 mm posterior, 5.00 mm lateral, 7.5 mm ventral to bregma) and/or vHPC (6.00 mm posterior, 5.00 mm lateral, 6.0 mm ventral to bregma). Cannulas were fixed to the skull with acrylic dental cement and stainless steel screws. Stainless steel obturators (33-gauge) were inserted into guide cannulas to maintain it unobstructed until infusions were made. Rats were implanted with bilateral cannulas for behavioral experiments, and a separate group of rats were implanted with unilateral cannulas for single-unit experiments (ipsilateral to the recording electrode). Therefore, rats with unilateral implanted cannulas were also surgically implanted with chronic microdrives for recording extracellular action potentials from single cells, as described previously (Burgos- Robles et al., 2009). The electrode bundle contained in a cannula was aimed at the PL (3.0 mm anterior, 0.5–0.8 mm lateral, and 4.00mm ventral to skull to bregma). Before electrodes were implanted, the tip of each wire was plated with gold by passing a cathodal current of 1 µA while cables were submerged in gold solution. Gold-plating allowed reducing electrode impedance to a range of 250–350 kΩ. Immediately after surgery, all rats were given a triple antibiotic and an analgesic (buprenorphine; 0.05 mg/kg, i.m.). Rats were allowed 5–7 days to recover from surgery, and then acclimated to recording procedures while electrodes were driven in steps of 44 µm until clear extracellular waveforms were isolated.
Behavior
Auditory fear conditioning and extinction was performed in standard operant chambers (Coulbourn Instruments, Allentown, PA) located inside sound-attenuating boxes (MED Associates, Burlington, VT). The floor of the chambers consisted of stainless steel bars that delivered a scrambled electric footshock. Between experiments, shock grids and floor trays were cleaned with soap and water, and the walls were cleaned with wet paper towels. Rats received five habituation tones (30 s, 4 Hz, 78 dB) immediately followed by fear conditioning consisting of five (unit-recording experiments) or seven (behavior-infusion experiments) tone presentations that co-terminated with footshocks (0.5 s, 0.5 mA). For behavior-infusion experiments, one day after conditioning, rats received extinction training which consisted of twenty presentations of tone alone. The next day, rats were tested for extinction memory with two presentations of tone alone. The interval between successive tones was variable with an average of 3 min. For the unit-recording experiments, at the end of the conditioning phase, rats were transported to their homecages, and 2 h later they were brought back to the same operant chamber and received additional test tones. Post conditioning test occurred from 2 h to several days post conditioning. A subset of rats received up to three additional test tone sessions. Also a subset of rats received extinction training and the next day were tested for extinction memory.
Behavior was recorded with digital video cameras (Micro Video Products, Bobcaygeon, Ontario, Canada). In behavioral experiments freezing was quantified from digitized video images using commercially available software (Freezescan, Clever Systems, Reston, VA). In unit-recording experiments, total seconds freezing during the tone presentations were scored by an observer blinded with respect to the experiment. Freezing was defined as the absence of all movement except for those related to breathing. In addition to freezing, suppression of bar-pressing was also used as a measure of conditioned fear (Sierra-Mercado et al., 2011). We found no appreciable contextual freezing in any phase, probably due to the competing motivation to press for food. We therefore used the rate of pre-tone pressing as a measure of contextual fear. Freezing and bar pressing during the tone was used as a measure of cued fear. A suppression ratio comparing pre-tone press rates with tone press rates was calculated as follows: (pretone − tone)/(pretone + tone). A value of 0 represents no suppression (low fear), whereas a value of +1 represents complete suppression of bar pressing (high fear). Group comparisons for both freezing and pressing were analyzed using two-tailed unpaired or paired Student’s t-tests (STATISTICA; Statsoft, Tulsa, OK).
Pharmacological inactivations
Muscimol (MUS) is a GABAA receptor agonist used to enhance neuronal inhibition, thereby temporarily and reversibly inactivating the target structures. Before infusions, obturators were removed and injectors were placed into the guide cannula. Injectors’ tips extended 1.0 mm beyond the guide cannula. On the day prior to the experiment, injectors were passed without infusion, and rats were acclimated to handling. MUS or saline vehicle (SAL) was administered 30 min prior to bar pressing and tone alone presentations in non-conditioned (naïve) rats, prior to a conditioning memory test, or prior to an extinction memory test. MUS or SAL was infused into BLA (0.11 nmol/0.5 ul at 0.2 ul/min) or vHPC (2.2 nmol/0.25 ul at 0.16 ul/min)(as in Sierra-Mercado et al., 2011). Injectors were left in place for 1 min after infusion to allow the drug to diffuse.
Multichannel unit-recording and unit-analysis
Individual neurons were recorded extracellularly using a microdrive, while rats pressed for food. The microdrive consisted of an electrode bundle of 16 microwires (25 µm diameter, nickel chromium iron wires; Stablohm 675; California Fine Wire Company, Grover Beach, CA) attached to a cannula and fastened to Mill-Max pin connectors cemented to an acrylic cement board and stainless steel screws. Extracellular waveform signals exceeding a voltage threshold were amplified (gain X 100), digitized at 40 kHz using a Multichannel Acquisition Processor system (Plexon, Dallas, TX), and stored on a disk for further off-line analysis. Waveforms were recorded during 10 min periods of spontaneous activity, as well as during pre-tone, tone and post-tone periods, each lasting 30 s. Single units were isolated using principle component analysis and template matching (Offline Sorter; Plexon, Dallas, TX). We applied semi-automated (automated followed by manual correction) processing techniques to sort spikes from single units in clusters. Automated processing involved using a valley-seeking scan algorithm (Offline Sorter; Plexon, Dallas, TX), one channel at a time, and then evaluated using sort quality metrics. For manual verification of automated clustering techniques a cluster was considered to be generated from a single neuron if the cluster was distinct from clusters for other units in principal component space. In addition, the cluster had to exhibit a clear refractory period (>1 ms). Only stable clusters of single units during recording were considered for analysis. Timestamps of neural spiking and flags for the occurrence of tones were imported to NeuroExplorer (NEX Technologies, Littleton, MA) for analysis. Once cells in PL were well-isolated, we assessed the effects of BLA and vHPC inactivations on PL activity in conditioned rats while pressing a bar to obtain food. For each cell, spontaneous and tone-evoked PL activity was recorded before and after unilateral vHPC or BLA inactivation with muscimol. To detect whether a particular neuron significantly changed its rate after infusion, firing rates of each cell were separated into bins of 1 min for the 10 min session and compared before and after inactivations (paired Student’s t-test, two tails). After recording a pre/post inactivation session at a given location, the electrode drive was advanced in 80 µm increments until new cells were found, and the experiment was repeated. A single rat was allowed to receive up to three inactivation sessions separated by at least two days.
Cell-type classification of neurons into putative pyramidal cells and interneurons was performed using a hierarchical unsupervised cluster analysis (Letzkus et al., 2011). This analysis was performed on firing rate (Hz) and spike waveform width (µs) based on Euclidean distance using Ward’s method (XLSTAT, Addinsoft, New York, NY). To further validate this cell-type classification we performed averaged and normalized cross-correlations in pairs of neurons. This analysis revealed a short latency inhibitory interaction between putative interneurons taken as a reference and putative pyramidal cells recorded simultaneously. We used the total number of spikes recorded to normalize spikes counts. To evaluate significance of cross-correlations during spontaneous activity between a reference and target neuron, mean firing rate with 95% confidence limits of the target neuron was calculated. Short latency inhibitory cross-correlograms were considered to be significant if the number of action potentials of the target neuron (−20 ms to 20 ms) fell outside the 95% confidence limits. Firing rates of different putative cell types before and after BLA and vHPC inactivations were compared with non-parametric two-tailed Wilcoxon matched-pairs tests (STATISTICA; Statsoft, Tulsa, OK).
Conditioned tone responses were calculated by normalizing firing rate (z-scores) during the tone relative to pre-tone activity (Burgos-Robles et al., 2009). In brief, we divided the 30 s tone into 10 3 second bins. A z-score for each of these bins was calculated, relative to 10 pre-tone bins of equal duration. Neurons were considered tone responsive if the first 3 s bin following tone onset exceeded a z-score of >2.58 (P<0.01, two tails). Only excitatory conditioned tone responses were included. Tone responses represent the average of two trials during fear expression test after conditioning or extinction. For analysis of successive inactivations of vHPC and BLA in the same PL neuron, we used repeated-measures ANOVA followed by Tukey post hoc analysis (STATISTICA; Statsoft, Tulsa, OK).
Histology
Upon completion of all experiments, rats were transcardially perfused with 0.9% saline solution followed by 10% buffered formalin. To assist with electrode placement, a microlesion was made by passing anodal current (20 µA for 20 s) through the wires to deposit iron in the tissue. Brains were extracted and fixed in a 30% sucrose/ 10% formalin solution, and 6% of ferrocyanide to stain the iron deposits. Injector’s cannula and electrode placements were verified by cutting coronal sections 40 µm thick, mounted on slides and staining for Nissl bodies with cresyl violet. Location of the tips of the injectors and electrode marking microlesions were reconstructed onto atlas coronal templates.
Supplementary Material
Highlights.
-
►
In behaving rats, BLA excites projection cells and vHPC excites interneurons in PL
-
►
BLA promotes fear-signaling in PL, whereas vHPC inhibits it
-
►
vHPC inhibits fear expression after, but not before, extinction
-
►
vHPC gates fear expression via the PL after, but not before, extinction
Acknowledgements
We thank C. Bravo-Rivera, and K. Quiñones-Laracuente for technical assistance. We also thank. M.R. Milad and J.P. Johansen for helpful comments on the manuscript. This work was supported by National Institutes of Health grants R01-MH058883 and R01-MH081975, by National Center for Research Resources award U54 RR026139, and by the National Institute on Minority Health and Health Disparities award 8U54MD 007587-03 to GJQ, a Fellowship from the Consejo Nacional de Ciencia y Tecnologia to FS-B, an R36-MH089296 to DS-M, and COR program T34-MH19134 to EP-D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. J. Neurosci. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am. J. of Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J. Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J. Comp. Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: an in vivo intracellular recording study. Cereb. Cortex. 2003;13:782–792. doi: 10.1093/cercor/13.7.782. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J. Neurosci. 2007;27:2045–2057. doi: 10.1523/JNEUROSCI.5474-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott P, Headlam A, Busby S. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res. 2002;946:314–322. doi: 10.1016/s0006-8993(02)02487-3. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Jain A, Giovanelli A, Bertollini C, Crestan V, Schwarz AJ, Tsetsenis T, Ragozzino D, Gross CT, Bifone A. A neural switch for active and passive fear. Neuron. 2010;67:656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Granon S, Changeux JP. Deciding between conflicting motivations: What mice make of their prefrontal cortex. Beh. Brain Res. 2012;229:419–426. doi: 10.1016/j.bbr.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J. Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts contextspecific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cog. Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn. Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb. Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J. Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR. Resting Amygdala and Medial Prefrontal Metabolism Predicts Functional Activation of the Fear Extinction Circuit. Am. J. of Psychiatry. 2012 doi: 10.1176/appi.ajp.2011.10121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J. Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn. Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav. Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Mast M, Blanchard RJ, Blanchard DC. The relationship of freezing and response suppression in a CER situation. Psychol. Rec. 1982;32:151–167. [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol. Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol. Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J. Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro. Cereb. Cortex. 2010;20:393–403. doi: 10.1093/cercor/bhp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci. 2009;29:7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, Goetz JM, Fischman AJ, Rauch SL, Pitman RK. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch. of Gen. Psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn. Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Laviolette SR. Inactivation of the basolateral amygdala during opiate reward learning disinhibits prelimbic cortical neurons and modulates associative memory extinction. Psychopharm. (Berl) 2012;4:645–661. doi: 10.1007/s00213-012-2665-5. [DOI] [PubMed] [Google Scholar]
- Tierney PL, Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur. J. Neurosci. 2004;20:514–524. doi: 10.1111/j.1460-9568.2004.03501.x. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, Radulovic J. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J. Neurosci. 2009;29:3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat. Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.