Abstract
The heat shock protein 70 (Hsp70) family of molecular chaperones has important functions in maintaining proteostasis under stress conditions. Several Hsp70 isoforms, especially Hsp72 (HSPA1A), are dramatically upregulated in response to stress; however, it is unclear whether these family members have biochemical properties that are specifically adapted to these scenarios. The redox-active compound, methylene blue (MB), has been shown to inhibit the ATPase activity of Hsp72 in vitro and it promotes degradation of the Hsp72 substrate, tau, in cellular and animal models. Here, we report that MB irreversibly inactivates Hsp72 but not the nearly identical, constitutively expressed isoform, heat shock cognate 70 (Hsc70; HSPA8). Mass spectrometry results show that MB oxidizes Cys306, which is not conserved in Hsc70. Molecular models suggested that oxidation of Cys306 exposes Cys267 to modification and that both events contribute to loss of ATP binding in response to MB. Consistent with this model, mutating Cys267 and Cys306 to serine made Hsp72 largely resistant to MB in vitro and over-expression of the C306S mutant blocked MB-mediated loss of tau in a cellular model. Further, mutating Cys267 and Cys306 to the pseudo-oxidation mimic, aspartic acid, mirrored MB treatment: the C267D and C306D mutants had reduced ATPase activity in vitro and over-expression of the C267/306D double mutant significantly reduced tau levels in cells. Together, these results suggest that redox sensing by specific cysteine residues in Hsp72, but not Hsc70, may be an important component of the chaperone response to oxidative stress.
INTRODUCTION
Reactive oxygen species (ROS), such as free radicals and peroxides, are produced as the result of normal metabolic and signaling processes (Forman, et al., 2010; Giles, et al., 2003; Paulsen and Carroll, 2010). However, an abundance of ROS is also implicated in oxidative damage to lipids, nucleic acids and proteins, contributing to pathology in a number of diseases (Lin and Beal, 2006; Patten, et al., 2010; Waris and Ahsan, 2006). Thus, to control the accumulation of ROS, organisms are equipped with several scavengers, such as glutathione and ascorbate (Apel and Hirt, 2004), and redox-sensitive transcription factors, including HIF1α (Majmundar, et al., 2010), NF-κB (Morgan and Liu, 2011) and HSF1 (Zhang, et al., 2011), that coordinate cellular adaptation to ROS. Another important cellular response is to protect the proteome from acute denaturation and aggregation, which could cause proteotoxicity. This type of ROS protection is often provided by a molecular chaperone that contains a reactive redox sensor (e.g. cysteine residue) linked to a system for protecting other proteins from oxidative unfolding. For example, the prokaryotic heat shock protein 33 (Hsp33) contains cysteine residues that are selectively oxidized in response to redox stress, which induces a conformation with high chaperone activity (Ilbert, et al., 2007).
The heat shock protein 70 (Hsp70) family is a series of highly conserved molecular chaperones that are well known for their activity in maintaining global proteostasis. Hsp70s are involved in most steps in the life of a protein, including the folding of nascent polypeptides, protein trafficking and degradation (Hartl, et al., 2011). Members of the Hsp70 family specifically interact with unfolded substrates through a C-terminal, substrate-binding domain (SBD). In addition, Hsp70s also have a conserved N-terminal nucleotide-binding domain (NBD), which binds and hydrolyzes ATP. These two domains are allosterically coupled, such that ATP hydrolysis within the NBD causes conformational changes in the SBD that enhance affinity for unfolded substrates (Mayer, et al., 2000). In turn, binding of Hsp70s to unfolded proteins protects them against aggregation and assists with their refolding. However, if this process fails, Hsp70s are also involved in triage, shuttling misfolded proteins to the proteasome for turnover (Mayer and Bukau, 2005). Through these activities, Hsp70s have been linked to diseases associated with aberrant protein quality control, such as cancer and neurodegenerative disease (Evans, et al., 2010; Patury, et al., 2009). In addition, like Hsp33, Hsp70s have been linked to redox signaling based on its reactive cysteine residues (Liu, et al., 1996; Vignols, et al., 2003).
In humans, the cytosol contains at least six Hsp70 isoforms, including the constitutively expressed Hsc70 (HSPA8) and the major stress inducible isoform, Hsp72 (HSPA1A) (Hageman, et al., 2011). Hsp72 and Hsc70 have very high sequence similarity (85% identical and 94% similar). However, the levels of Hsp72 are typically low under normal conditions and they are only highly induced in response to stress, including redox imbalance (Lindquist, 1986). Thus, Hsp72 belongs to a subfamily of Hsp70s, including HSPA6, HSPA7 and HSPA4, which is characterized by elevated expression in response to stress. One important question is whether the different family members, such as the stress-inducible forms, have any specialized biochemical functions that might distinguish them from the constitutive ones.
Previously, we identified methylene blue (MB) as an inhibitor of the ATPase activity of Hsp72 in a high throughput chemical screen (Jinwal, et al., 2009). This compound has been shown to reduce the levels of some Hsp72 substrates, such as tau, polyglutamine fragments and Akt, in cells (Congdon, et al., 2012; Jinwal, et al., 2009; Koren, et al., 2010; Wang, et al., 2010). MB also improves cognitive functions in mouse models of Alzheimer’s disease (AD) (Congdon, et al., 2012; O'Leary, et al., 2010) and it has been explored in Phase IIb clinical trials in AD patients (Wischik, et al., 2008). Although MB is a highly promiscuous compound, it has an enviable safety record and is used clinically for multiple indications (Schirmer, et al., 2011). For these reasons, we decided to further explore the mechanism by which it might inactivate Hsp70s. Specifically, we hypothesized that MB might prevent ATP turnover in Hsp72 by oxidizing cysteine residues (Liu, et al., 1996; Oz, et al., 2009), because MB has been shown to oxidize sulfhydryls in other targets (Kelner and Alexander, 1985). Here, we report that Hsp72 is indeed oxidized by MB and that treatment with either MB or hydrogen peroxide irreversibly inactivates ATP turnover in vitro. Based on modeling studies and mutagenesis, this inhibition appears to be caused by oxidation-induced conformation changes in the NBD that block ATP binding. However, during the course of these studies we also made the unexpected observation that the constitutive Hsp70 family member, Hsc70, is entirely resistant to irreversible inhibition by MB. Mass spectrometry and point mutants revealed that two reactive cysteines, C267S and C306S, are sufficient to distinguish the special sensitivity of Hsp72 to MB in vitro and in cells. Interestingly, Cys306 is also highly conserved in the other stress-inducible Hsp70 isoforms, but it is absent from constitutive forms, supporting the idea that this residue is important in stress-related redox signaling. Thus, these finding suggest a major difference between closely related Hsp70 isoforms, which may be important in redox signaling and protecting cells from oxidative stress. Moreover, this work may provide insight into one mechanism by which MB reduces tau accumulation in cells, animals and AD patients.
RESULTS
MB and H2O2 irreversibly inhibits Hsp72 but not Hsc70
Because MB has been known to directly oxidize sulfhydryls (Kelner and Alexander, 1985), we hypothesized that the inhibition of Hsp70’s ATPase activity by MB (Jinwal, et al., 2009; Miyata, et al., 2011) may involve oxidation of cysteines. To test this idea, Hsp72 was treated with MB (200 µM) at 37 °C for 1 hour and then dialyzed to remove any remaining compound. The remaining ATPase activity in the MB-treated Hsp72 sample was then measured using a malachite green assay (Chang, et al., 2008). Because Hsp72 is a weak ATPase, the stimulatory co-chaperone, DnaJ, was added to enhance nucleotide turnover in these experiments. Using this approach, we found that MB-treated Hsp72 had dramatically reduced ATPase activity (Fig. 1a). Hsp72 was also inhibited by treatment with hydrogen peroxide (100 µM) under similar conditions, suggesting that the loss of activity may be due to oxidation (Fig 1a). Cysteine residues in proteins can be progressively oxidized to sulfenic, sulfinic and then sulfonic acids (Reddie and Carroll, 2008), but only sulfenic acids are readily reversible by glutathione or DTT (Poole, et al., 2004). The ATPase activity of MB-treated Hsp72 was only partially (~80%) recovered after exposure to DTT (1 mM) (Suppl. Fig 1a), suggesting that MB oxidizes the protein to a mixture of oxidation states.
Fig 1.
The ATPase activity of Hsp72, but not Hsc70, is sensitive to oxidation. (A) Purified Hsp72 (0.6 µM) was incubated with either MB, peroxide (H2O2) or a mock control and the remaining compound removed by extensive dialysis. The stimulation of ATPase activity by the model J co-chaperone, DnaJ, was then measured. Results are the average of three experiments performed in triplicate. Error bars are standard error of the mean (SEM). (B) Human Hsc70 is resistant to oxidation. Experiments were performed as described for panel A. (C) The locations of cysteine residues (red) in Hsp72 are shown using the crystal structures of the NBD (pdb # 3JXU; yellow) and the SBD (pdb # 1DKX; green). In the insets, identical residues are shown in yellow, conserved residues in gray and the positions of cysteines are red. Alignments were prepared in Vector NTI (Invitrogen). The chemical structure of methylene blue (MB) is shown. See Suppl. Fig. 2 for the expanded alignment.
Initially as a control, the effects of MB on the ATPase activity of Hsc70 were evaluated. Unexpectedly, we found that MB had no effect on Hsc70 (Fig. 1b). The chemical differences between these two well-conserved (85% identical and 94% similar) Hsp70 isoforms were then more closely examined. Sequence alignments of the human proteins showed that Hsp72 has five cysteine residues (three in the NBD and two in the SBD) whereas Hsc70 has only four (two each in the NBD and SBD). Thus, one difference between these isoforms is that Hsp72 has a unique Cys306, which is an asparagine in Hsc70 (Fig. 1c). The sequences of a number of inducible or constitutive human Hsp70 family members were then examined, revealing that Cys306 is exclusively found in stress inducible Hsp70s but not in any of the constitutively expressed family members (Suppl. Fig 2a). Moreover, C267 is conserved in 4 of 5 inducible family members and only 2 of 5 constitutive forms (Suppl. Fig. 2a), suggesting that both of these residues may be important. Together, these results suggest that MB might selectively compromise ATPase by oxidizing at least one cysteine in Hsp72, but not Hsc70.
MB oxidizes Cys306 of Hsp72
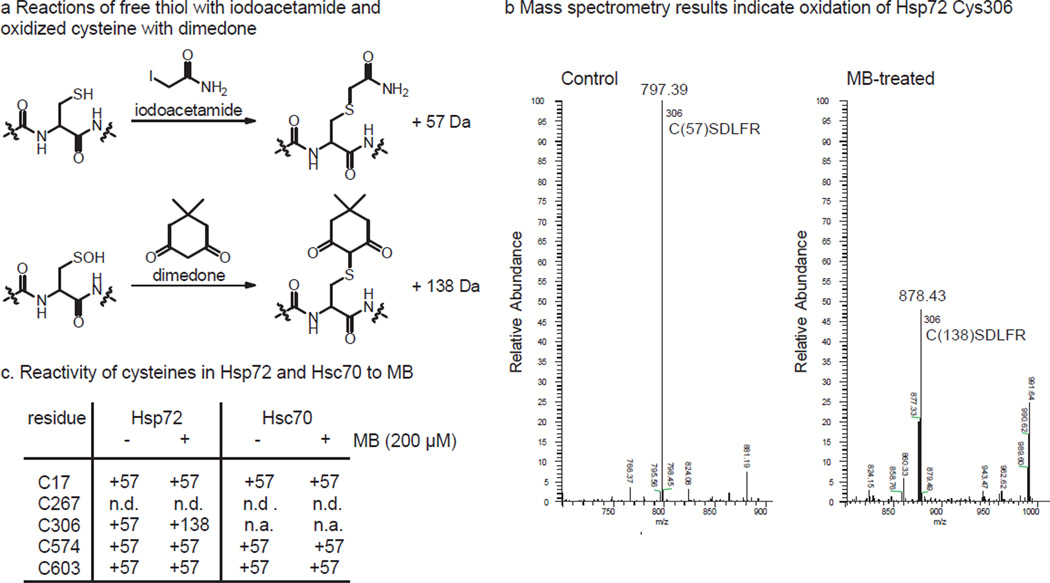
To map which cysteines in Hsp72 were oxidized by MB, a well-established mass spectrometry (MS) method using dimedone (5,5-dimethyl-1,3-cyclohexanedione) was employed. Briefly, dimedone is known to react with oxidized cysteines to form stable thioethers that are readily observed in the MS spectra as a +138 Da shift in molecular mass (Fig. 2a). Accordingly, MB-treated Hsp72 was treated with dimedone and unreacted thiols were capped with carbamidomethyl groups. Subsequent trypsin digestions and analysis by LC-tandem MS/MS revealed that the mass of the fragment containing Cys306 in Hsp72 was oxidized (Fig. 2b). No other residues were modified, although it is important to note that the fragment containing Cys267 was not detected (likely because the nearby region is highly charged) (Fig. 2c). As expected from the ATPase experiments, Hsc70 treated with MB was resistant to modification by dimedone (Fig. 2c and Suppl. Fig 2b). Thus, Hsp72 differs from Hsc70 in reactivity of its cysteines at positions 306 and perhaps 267.
Fig 2.
Hsp72 is oxidized by MB at specific cysteine residues. (a) Schematic of the specific reactions of iodoacetimide with free thiol and dimedone with sulfenic acid, producing a mass shift of +57 or +138 Da, respectively. (b) Select region of the MS/MS spectra focused on the region including the C306 fragment (CSDLFR 306–311). This fragment is 797.39 Da in the mock treated control (indicating iodoacetamide capping) and 878.43 Da in the MB treated (indicating dimedone conjugation). (c) Summary of the mass spectrometry findings, showing that MB only oxidized C306 in Hsp72. n.d. = not detected. n.a = residue not conserved. See Suppl. Fig. 1 for the DTT reversibility.
Hsp72 Cys to Ser mutations confer resistance to MB
The mass spectrometry studies suggested that MB-based oxidation of specific cysteines might be responsible for the compound’s effects on Hsp72’s ATPase activity. To test this idea, Cys306, Cys267 or both residues were mutated to serine by site-directed mutagenesis and the resulting mutant proteins were purified. These substitutions did not change the global structure (Suppl. Fig. 3) or ATPase activity of the mutant chaperones (Fig. 3a–c). However, the ATPase activities of C267S (Fig. 3a) and C306S (Fig. 3b) were partially resistant to MB (50 µM) and the double mutant (C267/306S) was completely resistant (Fig. 3c). Thus, MB appeared to exert its inhibitory effect on Hsp72 ATPase activity via oxidation of these cysteines and both residues appeared to be involved.
Fig 3.
Serine mutants of Hsp72 are resistant to MB treatment in ATPase and cell-based assays. (a) C267S mutation confers partial resistance to MB (50 µM). (b) C306S confers partial resistance to MB (50 µM). (c) C267, 306S double mutation confers resistance to MB (50 µM). All of the ATPase experiments were performed at least twice in triplicate and error bars are SEM. (d) Over-expression of the C306S mutation blocks MB-mediated clearance of tau. HeLaC3 cells were transfected with vector, Flag-tagged WT Hsp72 or C306S Hsp72 mutant for 48 hours and then treated with MB for 10 minutes. Samples were analyzed by western blot and the results are representative of experiments performed in duplicate. See Suppl. Fig. 3 for the CD spectra of the mutants.
MB is known to reduce tau levels in cells through a mechanism dependent on Hsp72 (Jinwal, et al., 2009). Thus, the over-expression of the C306S, C267S and C267/306S mutants may de-sensitize cells to MB. In fact, when HeLaC3 cells were stably transfected with Hsp72 C306S, MB no longer reduced the levels of phosphorylated (pS396/404) or total tau (Fig. 3d). Together, these results strongly suggest that MB acts on Hsp72 by oxidizing Cys267 and Cys306, and that this activity is important in regulating the levels of tau.
C267D mutation causes a conformational change and disrupts nucleotide binding
To gain some insight into the structural basis of these observation, the NBD of Hsp72 containing a C267D mutation was modeled using Robetta (Kim, et al., 2004), using the structure of human Hsp72 NBD in the ADP form (3JXU) as a starting point (Wisniewska, et al., 2010). An aspartic acid substitution was used because it sterically and electronically mimics a sulfinic acid (Permyakov, et al., 2011), one of the possible oxidation states sampled by MB-treated cysteine residues. A comparison of the structures of the wild type Hsp72 NBD and the C267D Hsp72 NBD showed that they were globally very similar, with an RMSD of 1.55Å and a TM-score of 0.94 (Fig. 4a). However, several residues that are specifically involved in nucleotide binding were predicted to be significantly shifted. For example, Gly339, which makes a hydrogen bond interaction with the alpha phosphate group of ADP, was shifted away from the nucleotide by 1.0 Å, likely preventing the formation of this important bond (Fig. 4b). Further, Arg272, which interacts with the adenine ring by pi-stacking, is significantly pulled away by regional rotations in the backbone (Fig. 4c). Collectively, these changes and others (Suppl. Table 1) resulted in side chain displacements totaling more than 25Å in the nucleotide binding cleft. Very similar results were seen with the C306D mutant, suggesting that these residues might both contribute to conformational rearrangements (Suppl. Fig. 4a). Interestingly, oxidation of C306 is predicted to swivel residue C267 into the solvent-exposed cleft above nucleotide in the NBD (Suppl. Fig. 4b), perhaps making it more accessible to oxidation by MB. Together, these in silico observations suggest that oxidation of Cys267 and Cys306 in Hsp72 might damage nucleotide binding and inactivate ATP turnover. Further, these results suggest that sequential oxidation of C306 and then C267 may reinforce and promote these conformational changes (Suppl. Fig. 4c).
Fig 4.
Modeling of Hsp72 C267D reveals structural changes in residues that contact nucleotide. (a) Overall alignment of the NBDs of Hsp72 (yellow: pdb entry 3JXU) and Hsp72 C267D Model (red: modeled from template 3JXU using Robetta Server). In the C267D model, (b) Gly339 is shifted away from the a-phosphate of nucleotide and (c) Arg272 is shifted away from the adenine ring. In total, C267D caused structural changes totaling 25Å (see Suppl. Table 1). Similar results were seen in the C306D mutant (see Suppl. Fig. 4). (d) Purified C267D and C306D mutants do not bind nucleotide. Purified mutants and wild type Hsp72 (5 µM) were treated with 1 mL of ATP-agarose, washed with 3 mL of Buffer A (25 mM HEPES, 10 mM KCl, 5 mM MgCl2, pH 7.5) and flowthrough (FT) collected. Following three additional washes, the remaining protein was collected in the eluent (E) by washing with 3 mL of Buffer A containing 3 mM ATP. Fractions were analyzed on 1–20% Tris-Tricine gels using a polyclonal anti-Hsp72 antibody (Enzo). See Suppl. Table 1 for details.
Cys to Asp mutations “phenocopies” MB treatment in vitro and in cells
To test this model, Cys267 and Cys306 of Hsp72 were mutated to the pseudo-oxidation residue, Asp, and the resulting mutant proteins were purified. Initial attempts to purify the mutants on ATP agarose immediately revealed that they had significantly reduced affinity for nucleotide (Fig. 4d), consistent with the models. Switching to size exclusion, the mutants were purified and found to have normal circular dichroism (CD) spectra (Suppl. Fig. 4a), suggesting that the global structure was not significantly disrupted by the Asp mutations. However, partial proteolysis showed that the mutants were more prone to digestion (Suppl. Fig. 4b), suggesting that they may be more flexible. Interestingly, MB-treated wild type Hsp72 is also prone to proteolysis (Suppl. Fig 4b), further enforcing the similarities between the Asp point mutants and the oxidized wild type.
To further explore this possibility, the enzymatic activities of the Hsp72 pseudo-oxidation mutants were examined using ATPase assays, showing that the C267D and C306D mutants had dramatically decreased enzymatic activity (Fig. 6a). These mutants essentially behaved like Hsp72 that had been treated with MB. We next tested the ability of Hsp72 and the mutants to refold denatured firefly luciferase. These experiments showed that Hsp72 treated with MB and the C267D, C306D and C267/306D double mutants all had reduced refolding activity (Suppl. Fig. 4c). Taken together, these studies suggest that the Asp mutants phenocopy some aspects of MB-treated Hsp72.
Fig 6.
Pseudo-oxidation mutants phenocopy MB treatment. (A) The DnaJ-stimulated ATPase activities of purified Hsp72 mutants were reduced, resembling MB-treated wild type. These experiments were performed at least three times using two independently prepared samples. Error bars represent the standard error of the mean (SEM). (B) Over-expression of wild type Hsp72 had little effect on tau levels, but the C306D and C267/306D mutants reduced tau. The gels show two independent replicates and the quantification of band intensities includes SEM. See Suppl. Fig. 4 for the characterization of the mutants.
In HeLaC3 cells, over-expression of the C306D mutant produced modest (~40%) reductions in total tau levels and no significant effect on phosphorylated tau (Fig. 5b). Over-expression of the double mutant (C267/306D) substantially (>70%) reduced both tau and phosphorylated tau levels (Fig. 6b). These findings provide strong support for MB acting through oxidation of specific cysteine residues in Hsp72 to reduce tau levels.
Fig 5.
Homology model of Hsp72 C306D NBD. (a) Pseudo-oxidation of residue 306 does not produce global changes in the NBD fold, similar to what was seen with the C267D mutant (see Fig 4). Green is C306D. Yellow is wild type (PDB 3JXU). The C306D and C267D models are nearly identical (see Fig. 4), with C306D also causing an ~25 Å total displacement of residues associated with nucleotide binding. (b) Close up that illustrates how C306D increases the solvent exposure of Cys267, potentially enhancing its oxidation. C267 moves by ~3 Å in the C306D model. (c) Model for initial oxidation at Cys306, leading to synergistic oxidation of C267 and reduced ATP binding.
DISCUSSION
One “arm” of the cellular response to redox stress likely involves the acute protection of proteins from oxidation-induced unfolding and aggregation (Ilbert, et al., 2007). Here, we were specifically interested in understanding how the Hsp70 family of chaperones, especially the stress inducible forms, might be linked to these types of redox responses. We found that Hsp72, but not Hsc70, was sensitive to oxidation by either MB or peroxide. A recent large-scale proteomic study positively identified Hsp72 as sensitive to oxidation by peroxide in HeLa cells (Leonard, et al., 2009), consistent with this finding. Our mass spectrometry, modeling and point mutagenesis results suggest that oxidation of Hsp72 occurs selectively at two cysteine residues, Cys267 and Cys306. Importantly, Cys306 is uniquely conserved in the stress-inducible Hsp70 family members and is absent from the constitutive ones (see Suppl. Fig. 2). Although it might be initially surprising for such highly homologous proteins to have different biochemical properties, Goldfarb, et al. recently demonstrated that Hsp72 and Hsc70 have opposing effects on the surface expression of murine epithelial sodium channel (Goldfarb, et al., 2006), further suggesting that even highly conserved Hsp70 isoforms can sometimes have distinct functions. Together, our findings suggest that Hsp72, and possibly other stress-inducible Hsp70 family members, are specially adapted for sensing and responding to redox stress. Based on the observed effects on tau stability, this redox response may involve switching the triage decision to favor degradation of misfolded Hsp72 substrates, perhaps clearing the cytosol of folding intermediates that are particularly prone to oxidative damage.
What is the molecular mechanism linking oxidation of Hsp72 to a loss of ATPase activity? We propose a model in which oxidation of the unique and solvent exposed Cys306 leads to re-arrangement of Cys267, such that this residue is now also sensitive to oxidation (see Suppl. Fig. 4). Thus, although Hsc70 has Cys267, it lacks the critical initiator (i.e. residue Cys306) and, therefore, treatment with either MB or peroxide does not inactivate it (see Fig. 1). This model further suggests that oxidation of both Cys267 and Cys306 causes numerous, subtle re-arrangements in residues that normally contact nucleotide (see Fig. 4 and Suppl. Fig. 4). These changes destabilize a number of key contacts, including hydrogen bonds with ATP, hydrogen binds with the phosphate, and a number of hydrophobic interactions (Suppl. Table 1). Consistent with this idea, the purified mutants, C267D and C306D, lacked the ability to bind ATP in vitro (see Fig. 4). Thus, we propose a model in which a cascade of oxidations severely damages the binding of Hsp72 to nucleotide. How this re-arrangement favors degradation of bound substrates, such as tau, is not yet clear.
Another goal of this work was to better understand the mechanism by which MB reduces tau levels in cellular and mouse models of AD (Jinwal, et al., 2009; O'Leary, et al., 2010). Although MB is clearly a promiscuous compound that lacks a classic “drug-like” profile, it is remarkably non-toxic and, thus, it is used in humans for the treatment of a variety of indications, including inherited and acute methemoglobinemia, prevention of urinary tract infections, ifosfamid-induced neurotoxicity, vasoplegic adrenaline resistant shock and pediatric malaria (Schirmer, et al., 2011). Because of MB’s particular promise as an AD therapeutic, we were interested in understanding whether any of its effects on tau accumulation may be mediated by oxidation of Hsp72. In this work, we found that mutating Hsp72 Cys267 and/or Cys306 to serine blocked the ability of MB to reduce tau levels in cells (see Fig 3d). This is an important finding because it strongly links Hsp72 oxidation to effects on tau accumulation. To further enforce this idea, over-expression of the corresponding C267/306D pseudo-oxidation mutant was a strong “dominant negative” and it dramatically reduced tau levels (see Fig. 5). Together, these results suggest that Hsp72 oxidation is one important way by which MB reduces tau accumulation in AD models. These findings may aid in the discovery of additional AD therapeutics that take advantage of this under-explored mechanism.
Treatments with high levels of MB are tolerated in cells (Yaglom, et al., 2007) and, moreover, it is relatively non-toxic in humans (Schirmer, et al., 2011). On first glance, the global inactivation of Hsp70s might be hypothesized to be acutely and dramatically toxic, given the proposed roles of this chaperone family in “housekeeping” activities. However, another point of view is that the stress-inducible Hsp72 isoform is specifically concerned with degrading only the substrates that have been damaged or misfolded in response to stress (Pratt and Toft, 2003), protecting the cytosol from the accumulation of proteotoxic intermediates. In normal cells, the levels of Hsp72 and such misfolded substrates may be low due to the action of other components of the protein quality controls system. Thus, MB may have low toxicity because of its unusual selectivity for the stress-inducible Hsp70 family members. This is an important finding in the continued search for Hsp70-modifying compounds with low toxicity and favorable therapeutic benefits. Together, these findings provide one potential mechanism by which MB has activity in tau-related diseases, such as AD.
MATERIALS AND METHODS
Proteins and reagents
Unless otherwise specified, reagents were purchased from Sigma (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). Human Hsp72, Hsc70 and E. coli DnaJ were purified according to published schemes (Chang, et al., 2010). Site directed mutagenesis primers were designed based on previous reports (Zheng, et al., 2004) and mutagenesis of Hsp72 was carried out following the user manual for the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The Hsp72 C267S, C306S and C267/306S mutants were expressed and purified using the same protocol as Hsp72 (42). The Hsp72 C267D and C306D mutants were expressed as previously described (42) and purified using nickel-nitrilotriacetic acid His·Bind® resin (Novagen, Darmstadt, Germany), then buffer exchanged into a 25mM HEPES buffer (10mM KCl, 5mM MgCl2 pH 7.5).
Oxidation of Hsp72/Hsc70
Protein (10 µM) and MB (5 mM) were incubated at 37 °C for 1 hour. For H2O2 oxidation, protein sample (10 µM) was incubated with 1 mM H2O2 at 37 °C for 1 hour. Treated protein samples were subsequently dialyzed against buffer A (100 mM Tris-HCl, pH 7.4, 20 mM KCl, 6 mM MgCl2) at 4 °C.
ATPase activity
ATPase activity was measured according to the previously published method (Chang, et al., 2008). Briefly, malachite green-based assays were used to measure phosphate release from purified Hsp72, Hsc70 or mutants (1 µM). Reactions were initiated with 1 mM ATP, performed for 60 minutes and quenched before measuring absorbance. Absorbance readings were converted to pmol of ATP using a phosphate standard curve.
Preparation of dimedone-modified Hsp70s
MB-treated or untreated Hsp70s (10 µM) were incubated with 5 mM dimedone (5,5-dimethyl-1,3-cyclohexanedione) in buffer A (100 mM Tris-HCl, pH 7.4, 20 mM KCl, 6 mM MgCl2) at room temperature for 1 hour. The samples were analyzed by SDS-PAGE and stained with colloidal Coomassie blue (Invitrogen, Carlsbad, CA). Bands corresponding to Hsp72 were excised and stored at −20 °C until use.
Mass spectrometry
In-gel digestion was performed as previously described (Brady, et al., 2010). After reduction (10 mM DTT) and alklylation (65 mM iodoacetamide) of the free cysteines at room temperature for 30 minutes, proteins were digested overnight with trypsin (Promega). Resulting peptides were resolved on a nano-capillary reverse phase column (Picofritcolumn, New Objective) using a 1% acetic acid/acetonitrile gradient at 300 nL/min and subjected to LC-tandem MS using LTQ Orbitrap XL mass spectrometer. MS/MS spectra were searched against the database considering either carbamidomethyl- or dimedone-modified cysteine.
Supplementary Material
Significance.
The heat shock protein 70 (Hsp70) family of molecular chaperones plays a central role in proteostasis, especially in the response to stress. These proteins have been implicated in regulating the stability and turnover of a number of important substrates, including the microtubule-associated protein tau (MAPT/tau). Tau aberrantly accumulates in fifteen neurodegenerative disorders, including Alzheimer’s disease (AD). Accordingly, there is great interest in finding ways of using the Hsp70s to restore balance in this system and reduce tau accumulation. We found that the stress-inducible Hsp70, Hsp72, is sensitive to oxidation by peroxide or methylene blue (MB), while the constitutive Hsc70 is resistant. Mutation of key cysteines in Hsp72 to serine rendered the chaperone resistant to MB in vitro and over-expression of this mutant partially suppressed MB-mediated degradation of tau in cells. Together, these results suggest that oxidation of Hsp72 is one mechanism by which MB reduces tau levels. These results are particularly interesting because this compound has recently undergone clinical evaluation as a tau-reducing therapy in Alzheimer’s disease (AD). These results suggest that oxidation of Hsp72 might be one mechanism to explain the activity of this clinical candidate.
ACKNOWLEDGEMENTS
The authors thank S. Leonard for helpful conversations. This work was supported by an Alzheimer’s Association grant and NIH grants R00AG031291 and R01NS073899 to C.A.D. In addition, A.D.T. was supported by GM007863, S.S. was supported by GM008353 and this work was further supported by NIH grant R01NS059690 to J.E.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Brady GF, Galbán S, Liu X, Basrur V, Gitlin JD, Elenitoba-Johnson KS, Wilson TE, Duckett CS. Regulation of the copper chaperone CCS by XIAP-mediated ubiquitination. Mol Cell Biol. 2010;30:1923–1936. doi: 10.1128/MCB.00900-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Bertelsen EB, Wisen S, Larsen EM, Zuiderweg ER, Gestwicki JE. High-throughput screen for small molecules that modulate the ATPase activity of the molecular chaperone DnaK. Anal Biochem. 2008;372:167–176. doi: 10.1016/j.ab.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Chang L, Thompson AD, Ung P, Carlson HA, Gestwicki JE. Mutagenesis reveals the complex relationships between ATPase rate and the chaperone activities of Escherichia coli heat shock protein 70 (Hsp70/DnaK) J Biol Chem. 2010;285:21282–21291. doi: 10.1074/jbc.M110.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Wu J, Myeku N, Figueroa Y, Herman M, Marinec P, Gestwicki J, Dickey C, Yu W, Duff K. Phenothiazine induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8 doi: 10.4161/auto.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53:4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles NM, Watts AB, Giles GI, Fry FH, Littlechild JA, Jacob C. Metal and redox modulation of cysteine protein function. Chem Biol. 2003;10:677–693. doi: 10.1016/s1074-5521(03)00174-1. [DOI] [PubMed] [Google Scholar]
- Goldfarb SB, Kashlan OB, Watkins JN, Suaud L, Yan W, Kleyman TR, Rubenstein RC. Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels. Proc Natl Acad Sci U S A. 2006;103:5817–5822. doi: 10.1073/pnas.0507903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J, van Waarde MA, Zylicz A, Walerych D, Kampinga HH. The diverse members of the mammalian HSP70 machine show distinct chaperone-like activities. Biochem J. 2011;435:127–142. doi: 10.1042/BJ20101247. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Ilbert M, Horst J, Ahrens S, Winter J, Graf PC, Lilie H, Jakob U. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat Struct Mol Biol. 2007;14:556–563. doi: 10.1038/nsmb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinwal UK, Miyata Y, Koren J, 3rd, Jones JR, Trotter JH, Chang L, O'Leary J, Morgan D, Lee DC, Shults CL, et al. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29:12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner MJ, Alexander NM. Methylene blue directly oxidizes glutathione without the intermediate formation of hydrogen peroxide. J Biol Chem. 1985;260:15168–15171. [PubMed] [Google Scholar]
- Kim DE, Chivian D, Baker D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004;32:W526–W531. doi: 10.1093/nar/gkh468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren J, 3rd, Jinwal UK, Jin Y, O'Leary J, Jones JR, Johnson AG, Blair LJ, Abisambra JF, Chang L, Miyata Y, et al. Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J Biol Chem. 2010;285:2498–2505. doi: 10.1074/jbc.M109.057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard SE, Reddie KG, Carroll KS. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem Biol. 2009;4:783–799. doi: 10.1021/cb900105q. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Liu Q, Levy EJ, Chirico WJ. N-Ethylmaleimide inactivates a nucleotide-free Hsp70 molecular chaperone. J Biol Chem. 1996;271:29937–29944. doi: 10.1074/jbc.271.47.29937. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Schröder H, Rüdiger S, Paal K, Laufen T, Bukau B. Multistep mechanism of substrate binding determines chaperone activity of Hsp70. Nat Struct Biol. 2000;7:586–593. doi: 10.1038/76819. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Koren J, Kiray J, Dickey CA, Gestwicki JE. Molecular chaperones and regulation of tau quality control: strategies for drug discovery in tauopathies. Future Med Chem. 2011;3:1523–1537. doi: 10.4155/fmc.11.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary JC, 3rd, Li Q, Marinec P, Blair LJ, Congdon EE, Johnson AG, Jinwal UK, Koren J, 3rd, Jones JR, Kraft C, et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener. 2010;5:45. doi: 10.1186/1750-1326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer's disease. Biochem Pharmacol. 2009;78:927–932. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–S367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- Patury S, Miyata Y, Gestwicki JE. Pharmacological targeting of the Hsp70 chaperone. Curr Top Med Chem. 2009;9:1337–1351. doi: 10.2174/156802609789895674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permyakov SE, Zernii EY, Knyazeva EL, Denesyuk AI, Nazipova AA, Kolpakova TV, Zinchenko DV, Philippov PP, Permyakov EA, Senin II. Oxidation mimicking substitution of conservative cysteine in recoverin suppresses its membrane association. Amino Acids. 2011 doi: 10.1007/s00726-011-0843-0. [DOI] [PubMed] [Google Scholar]
- Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Schirmer RH, Adler H, Pickhardt M, Mandelkow E. "Lest we forget you--methylene blue…". Neurobiol Aging. 2011;32 doi: 10.1016/j.neurobiolaging.2010.12.012. 2325.e2327-2316. [DOI] [PubMed] [Google Scholar]
- Vignols F, Mouaheb N, Thomas D, Meer Y. Redox control of Hsp70-co-chaperone interaction revealed by expression of a thioredoxin-like Arabidopsis protein. J. Biol. Chem. 2003;278:4516–4523. doi: 10.1074/jbc.M210080200. [DOI] [PubMed] [Google Scholar]
- Wang AM, Morishima Y, Clapp KM, Peng HM, Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Inhibition of hsp70 by methylene blue affects signaling protein function and ubiquitination and modulates polyglutamine protein degradation. J Biol Chem. 2010;285:15714–15723. doi: 10.1074/jbc.M109.098806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C, Betham P, Wischik D, Seng K. Tau aggregation inhibitor (TAI) therapy with Rember™ arrests disease progression in mild and moderate Alzheimer's disease over 50 weeks. Alzhimer's and Dementia. 2008:T167. [Google Scholar]
- Wisniewska M, Karlberg T, Lehtiö L, Johansson I, Kotenyova T, Moche M, Schüler H. Crystal structures of the ATPase domains of four human Hsp70 isoforms: HSPA1L/Hsp70-hom, HSPA2/Hsp70-2, HSPA6/Hsp70B', and HSPA5/BiP/GRP78. PLoS One. 2010;5:e8625. doi: 10.1371/journal.pone.0008625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaglom JA, Gabai VL, Sherman MY. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007;67:2373–2381. doi: 10.1158/0008-5472.CAN-06-3796. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ahn YH, Benjamin IJ, Honda T, Hicks RJ, Calabrese V, Cole PA, Dinkova-Kostova AT. HSF1-Dependent Upregulation of Hsp70 by Sulfhydryl-Reactive Inducers of the KEAP1/NRF2/ARE Pathway. Chem Biol. 2011;18:1355–1361. doi: 10.1016/j.chembiol.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Baumann U, Reymond JL. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 2004;32:e115. doi: 10.1093/nar/gnh110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.