Synopsis
As with any radiologic imaging test, there are a number of potential interpretive pitfalls at CT colonography (CTC) that need to be recognized and handled appropriately. Perhaps the single most important step in learning to avoid most of these diagnostic traps is simply to be aware of their existence. With a little experience, most of these potential pitfalls will be easily recognized. This review will systematically cover the key pitfalls confronting the radiologist at CTC interpretation, primarily dividing them into those related to technique and those related to underlying anatomy. Tips and pointers for how to effectively handle these potential pitfalls are included.
Keywords: CT colonography, Virtual colonoscopy, Pitfalls, Technique, Polyps
Introduction
CT colonography (CTC) has rapidly evolved into a highly effective minimally invasive test for detecting colorectal polyps and cancers.1,2 With attention to detail and proper technique in terms of colonic preparation, distention, scanning, and interpretation,3,4 excellent results can and should be expected. However, even when proven state-of-the-art techniques are consistently applied, there are a number of potential pitfalls that may be encountered at interpretation. When suboptimal techniques are applied, the number and severity of interpretive pitfalls can rapidly multiply, underscoring the need for high quality practice standards. At first glance, the laundry list of potential pitfalls at CTC may seem rather daunting (Table 1). However, some of these “pitfalls” pose little challenge once they are fully appreciated. These can largely be divided into two main categories: pitfalls related to technical factors, and pitfalls related to specific anatomic features. With proper awareness, these potential pitfalls can be effectively managed so as to minimize any negative impact on diagnostic performance. Common interpretive pitfalls will be reviewed, with illustrations to demonstrate the typical appearances. A more extensive review with hundreds of illustrations can be found in our dedicated referenced textbook.5
Table 1.
Interpretive Pitfalls at CTC
Potential pitfalls related to technique
|
Potential pitfalls related to anatomy
|
POTENTIAL PITFALLS RELATED TO TECHNIQUE
Retained Solid Fecal Material
Residual stool represents a fundamental diagnostic challenge for CTC interpretation, even when cathartic agents are employed. Although laxatives and lavages generally remove the major bulk of fecal volume, residual adherent debris can closely mimic the appearance of soft tissue polyps, especially if not tagged by oral contrast. Unlike formed stool, which typically contains foci of near-air density, smaller particulate fecal matter can more closely approximate uniform soft tissue attenuation. This underscores the critical need for oral contrast tagging, which is highly effective for internally labeling otherwise nonspecific residual adherent stool (Figs. 1 and 2), thus allowing for clear distinction from true soft tissue polyps.6,7
FIGURE 1. Tagged adherent stool simulating a sessile polyp on 3D.
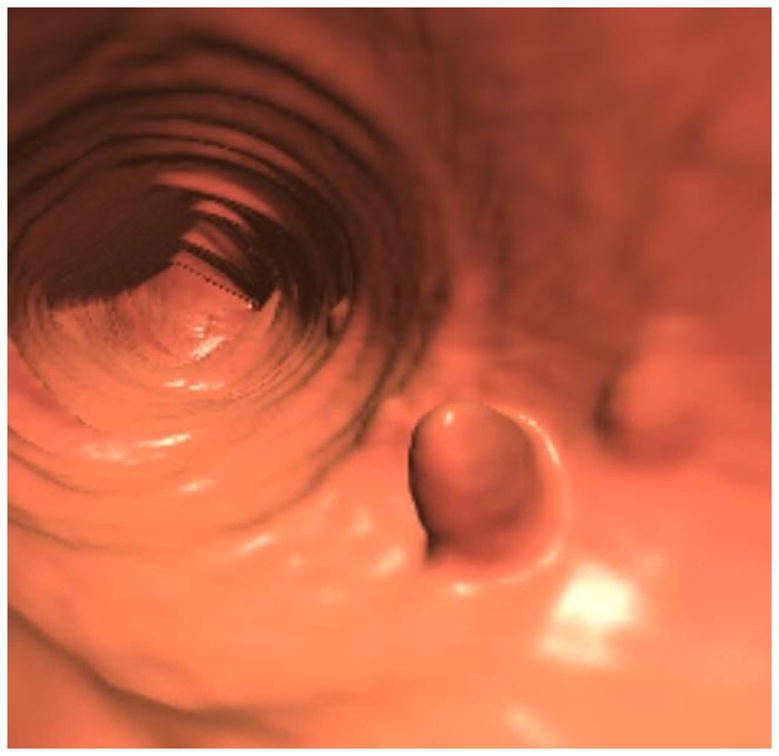
3D endoluminal CTC image (A) shows a polypoid lesion, as well as smaller adjacent diminutive foci. Both 3D translucency rendering (B) and 2D correlation (C) show dense internal contrast tagging, easily excluding a polyp. Note that the adherent stool is nondependent on this prone 2D view, which could simulate a true lesion if untagged.
(From Pickhardt PJ, Kim DH. Potential pitfalls at CTC Interpretation, In: CT colonography: principles and practice of virtual colonoscopy. Philadelphia: Saunders; 2010, with permission)
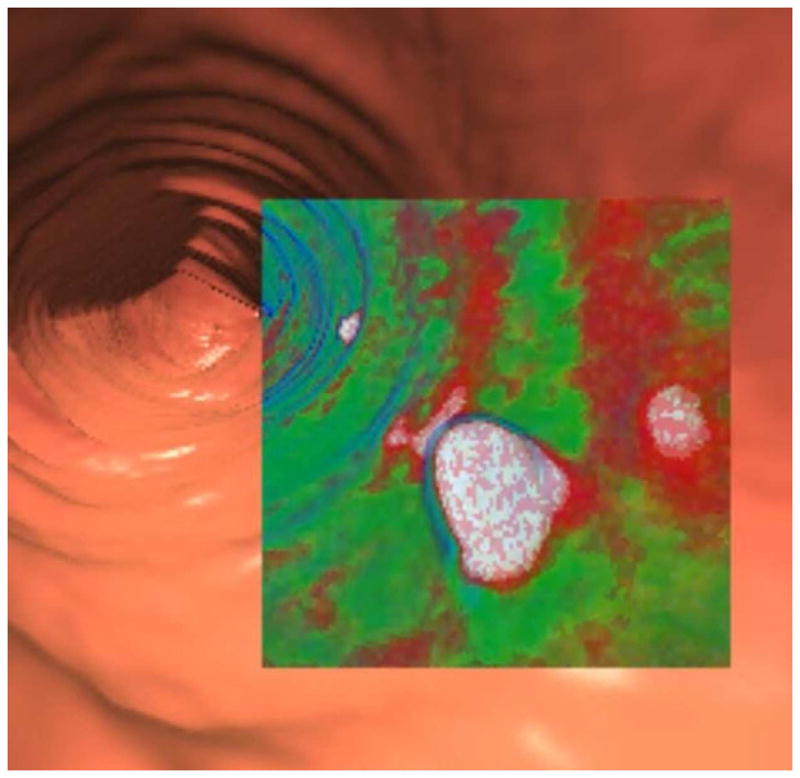
FIGURE 2. Tagged stool mimicking a flat polyp on 3D.
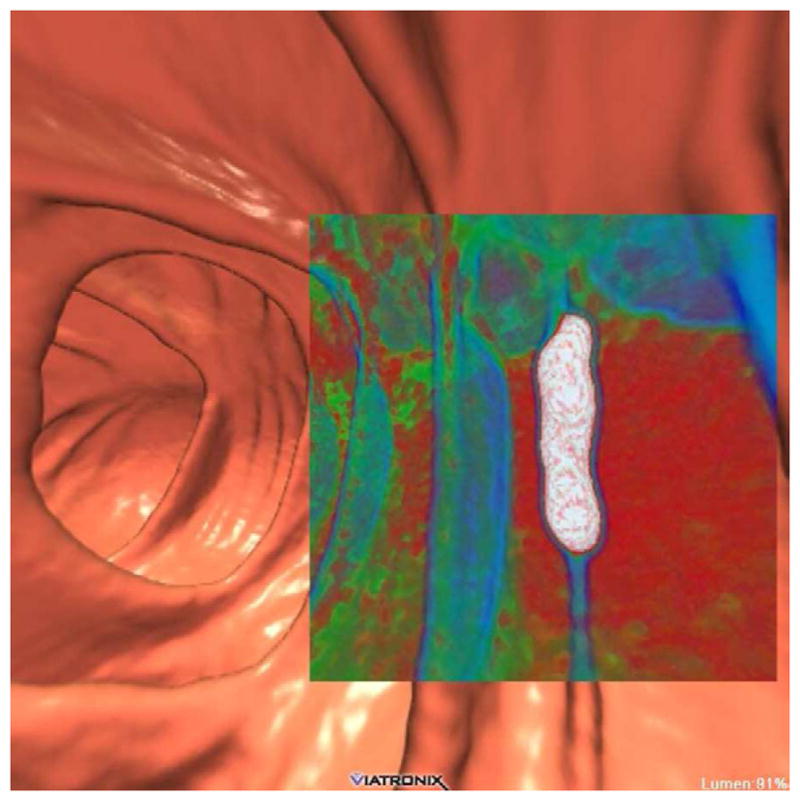
3D endoluminal CTC image (A) shows an elongated flat lesion on a colonic fold. However, both 3D with translucency rendering (B) and coronal 2D correlation (C) show dense internal contrast tagging, excluding a flat polyp. Care must be taken in such cases to ensure a true flat soft tissue polyp does not lie deep to the contrast.
We discovered early on that, although diatrizoate (Gastrograffin) is extremely valuable for its cathartic-like effect and ability to uniformly opacify luminal fluid, barium sulfate (2% w/v) is much more effective for internal tagging of solid residue. As such, we continue to employ both of these contrast agents in our CTC bowel preparation. At primary 3D evaluation, translucency rendering can rapidly demonstrate internal tagging of fecal material (Figs. 1 and 2),8 which can reduce the number of time-consuming 2D correlations. Nonetheless, it is the 2D display that provides the most definitive assessment for equivocal findings seen on 3D.
In our experience, a false positive interpretation due to residual stool is extremely rare when using our dedicated cathartic preparation with the dual contrast tagging regimen. Untagged stool, however, continues to be a major issue at same-day completion CTC following incomplete optical colonoscopy (OC). Although diatrizoate is typically administered in this setting, it lacks the ability of 2% barium given the evening before in terms of solid stool tagging. Incomplete tagging of solid stool will likely be an important issue facing non-cathartic approaches as well.9
Retained Luminal Fluid
Untagged residual luminal fluid can obscure even large polyps and masses when they are submerged. Early on, some advocated for the routine use of intravenous contrast to help identify submerged lesions,10 but we have found it much simpler, safer, and probably more effective to “enhance” the surrounding fluid with oral contrast instead.3 As noted above, the iodinated contrast agents undoubtedly tag luminal fluid more effectively and homogeneously than the barium preparations.7,11 Therefore, we continue to believe that both barium and iodinated contrasts agents are extremely useful in tandem for adequate tagging of both solid and liquid residuals, respectively.3 In cases where a relevant colorectal lesion is submerged under opacified fluid, soft tissue windowing may completely obscure the finding, necessitating the wider “polyp window” (W: 2000 HU, L: 0 HU) for detection (Fig. 3).
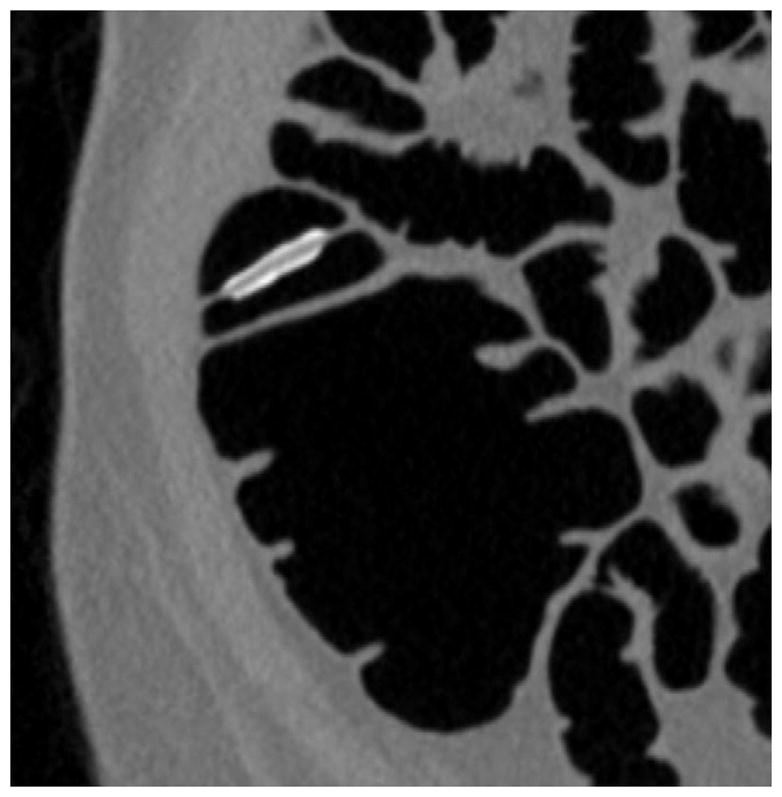
FIGURE 3. Flat lesion obscured by densely opacified fluid on soft tissue windows.
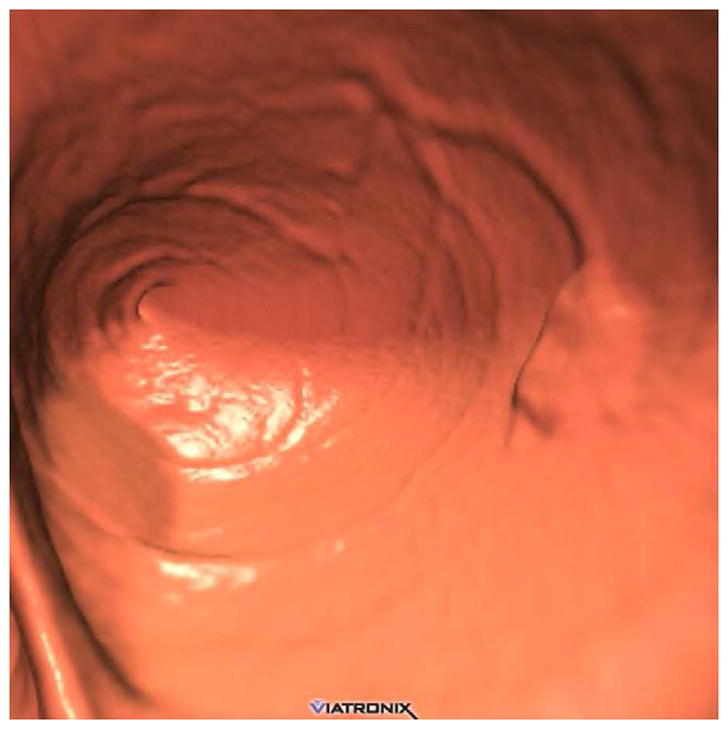
Prone transverse 2D CTC image with polyp windowing (A) shows a flat cecal polyp (arrowhead), which is submerged under opacified fluid but is nonetheless detectable. On the soft tissue window setting (B), however, the lesion is obscured by the dense surrounding fluid. This windowing phenomenon is also the reason why 2D lesion measurement must take place on the wider polyp window setting. 3D endoluminal CTC image (C) in the supine position shows the flat lesion outlined by air. The lesion was confirmed at subsequent OC (D) and proved to be a tubulovillous adenoma.
Although we initially performed electronic cleansing on the tagged fluid prior to interpretation very early on in our CTC experience, we discontinued this practice in 2003 due to the troublesome artifacts that were introduced (discussed later).7 Instead, we prefer to concentrate on the submerged regions on the 2D evaluation. Due to the complementary shifting of luminal fluid between supine and prone positions, it is extremely rare to have a significant polyp completely submerged on both views with our standard CTC preparation.12 The most common scenario where we may still encounter larger volumes of residual luminal fluid is in the setting of same-day tagging following failed OC. In this setting, we administer 30 ml of diatrizoate once the patient has adequately recovered from sedation, and wait up to 2–3 hours prior to scanning. In the future, a better approach might be to give diatrizoate as part of the original OC preparation, which would allow for a reduction in the amount of cathartic needed and also provide fluid tagging for CTC in the event of an incomplete OC examination.13
Inadequate luminal distention
Inadequate luminal distention impacts both 2D and 3D evaluation at CTC. The minimum requirement for a diagnostic CTC evaluation is to have all segments at least partially distended on at least one view. Due to the complementary nature of supine and prone positioning in terms of preventing luminal collapse, non-diagnostic examinations are fortunately uncommon (<1% of cases in our experience). Cases with focal or segmental partial but incomplete collapse may be suboptimal but are often diagnostic. Compared with excellent luminal distention, such cases generally require more scrutiny, as the luminal narrowing is compounded by dynamic thickening of the colonic folds, which makes interpretation more challenging. However, because relevant colorectal lesions require detection on just one view, confirmation on the lesser quality view is generally achievable even in cases of inadequate distention (Fig. 4).
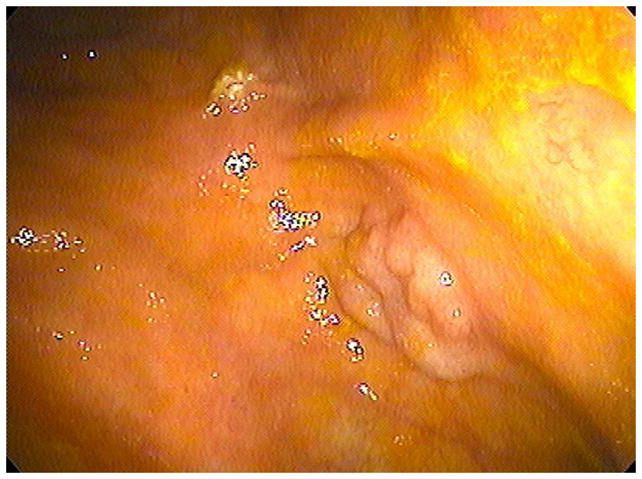
FIGURE 4. Confirmation of polyp on poorly-distended colonic segment.
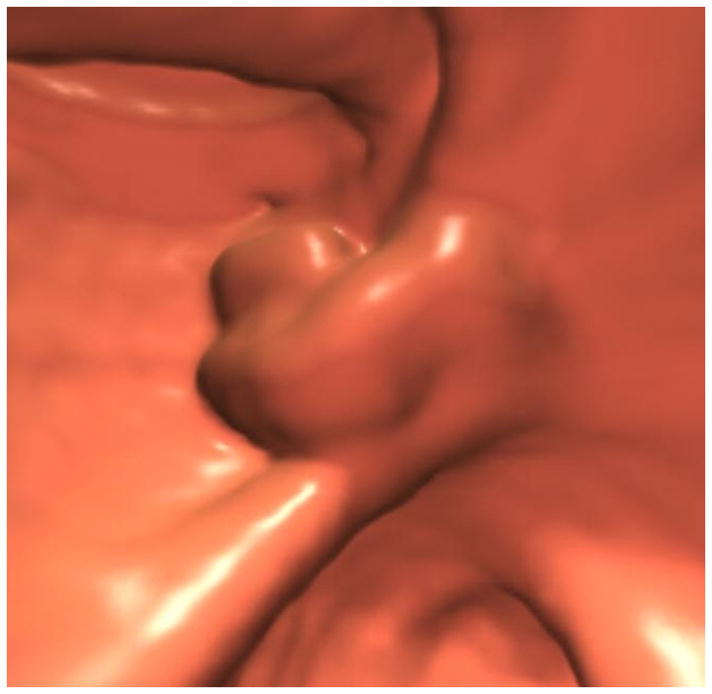
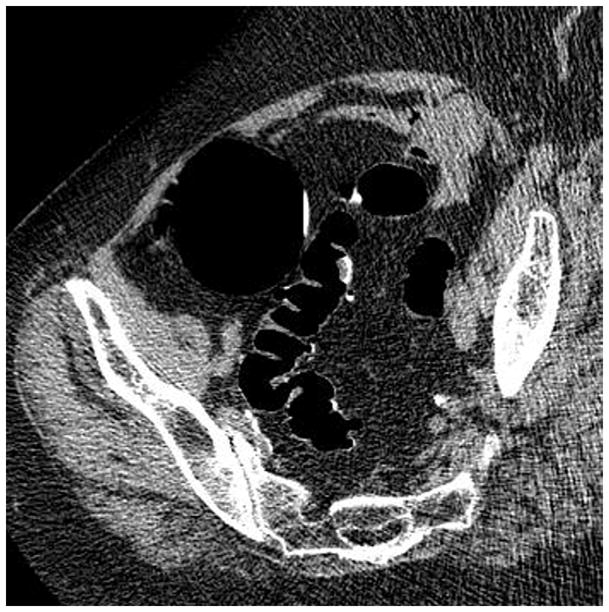
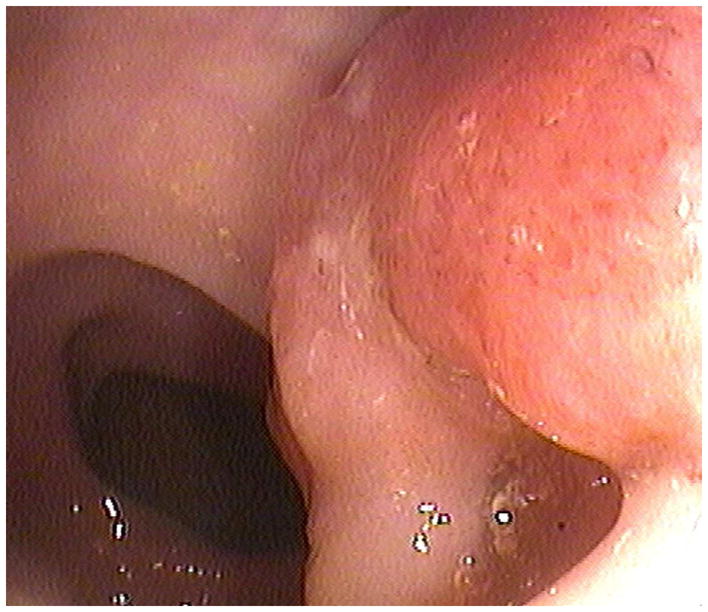
Supine 2D CTC images (A and B) show long-segment collapse of the sigmoid colon, largely obscuring a 15-mm polyp (arrows), which is easily identified on the alternate position (C and D). This proved to be a tubular adenoma after resection at OC (E).
(From reference 5, with permission)
For cases in which complete focal collapse persists at the same point on both supine and prone displays, a decubitus view will usually allow for diagnostic assessment.14 The region of non-diagnostic evaluation from focal collapse typically accounts for just a tiny fraction of the total colonic length. Because the sigmoid and descending colon account for the vast majority of such cases, typically related to underlying diverticular disease (as discussed later on), a right lateral decubitus view is typically performed (Fig. 5). Online assessment of the 2D images for adequate left-sided distention during CTC examination should be made by the technologist at the CT console because the scout view alone can be unreliable or misleading.15 Depending upon the specific patient population, a decubitus rate of approximately 10% can be expected, although the frequency will generally be increased in the setting of an incomplete OC, among other factors.14
FIGURE 5. Right lateral decubitus position for salvaging adequate luminal distention.
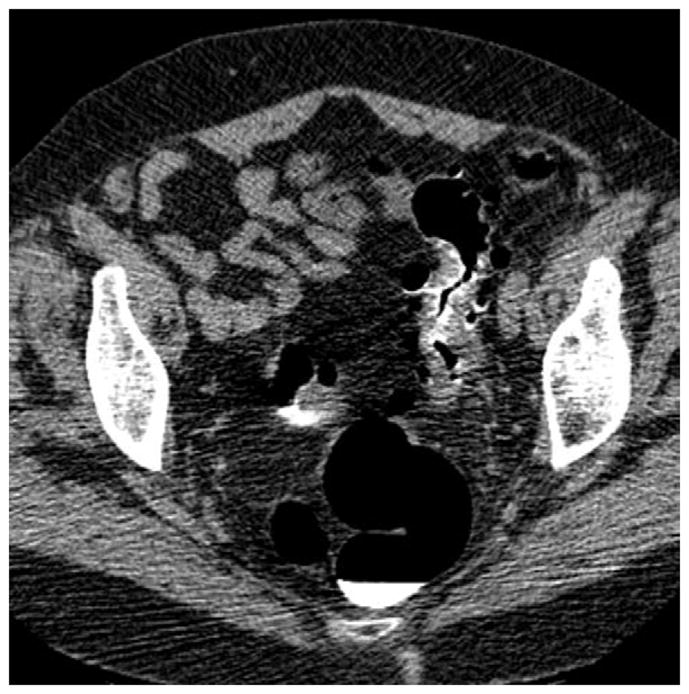
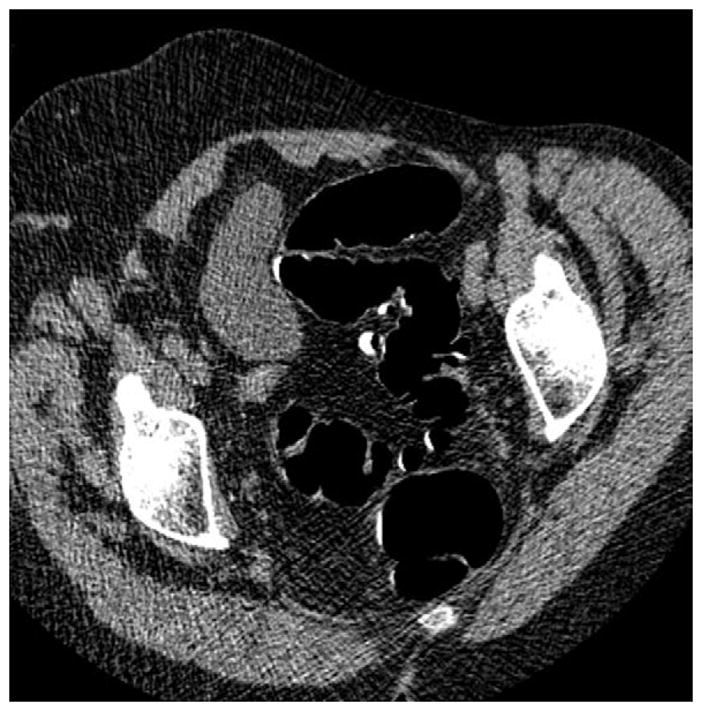
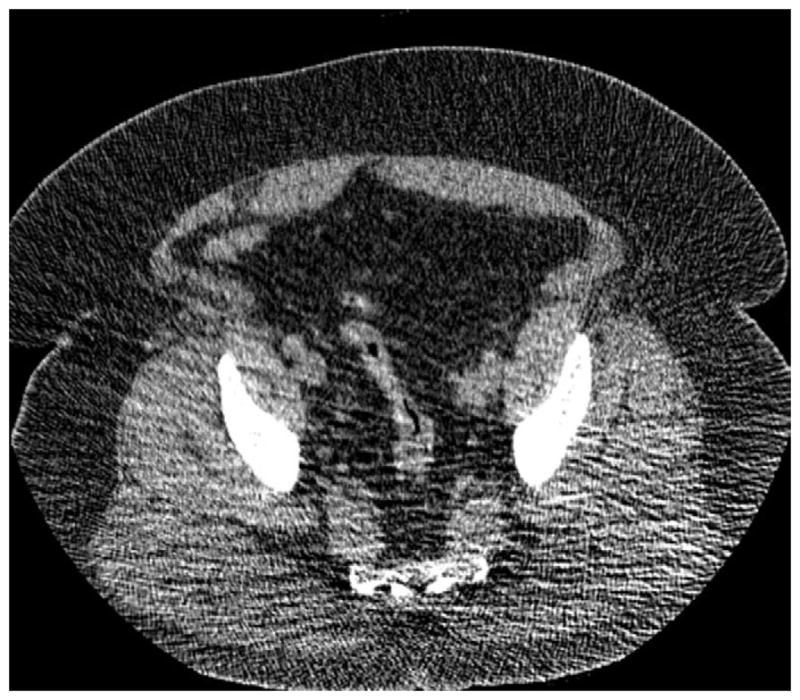
Supine 2D CTC image (A) shows long-segment collapse of the sigmoid colon, related to diverticular disease. The prone view had a similar appearance (not shown). Luminal distention on the decubitus view (C), however, was excellent and allowed for a diagnostic examination.
We have not found spasmolytics to be necessary in our CTC practice, since less than 1% of cases will have a persistent area of luminal collapse in our experience. Additional reasons include that glucagon is generally not effective, Buscopan is not available in the U.S., and a number of these difficult cases will actually represent fixed pathologic strictures. For cases of truly non-diagnostic evaluation in the sigmoid or descending colon, unsedated same-day flexible sigmoidoscopy may be performed to complete the screening evaluation.
Automated low-pressure CO2 delivery provides for adequate distention on a more consistent basis than manual room air insufflations, and should be considered the standard of care.16 However, the lower pressures and increased resorption with CO2 can also result in partial collapse, particularly in the transverse colon in the prone position and rectosigmoid junction in the supine position. This issue is exacerbated in morbidly obese patients, where the low-pressure CO2 cannot overcome extracolonic pressures. In such cases, decubitus positioning or even conversion to manual room air may be necessary on occasion (Fig. 6).
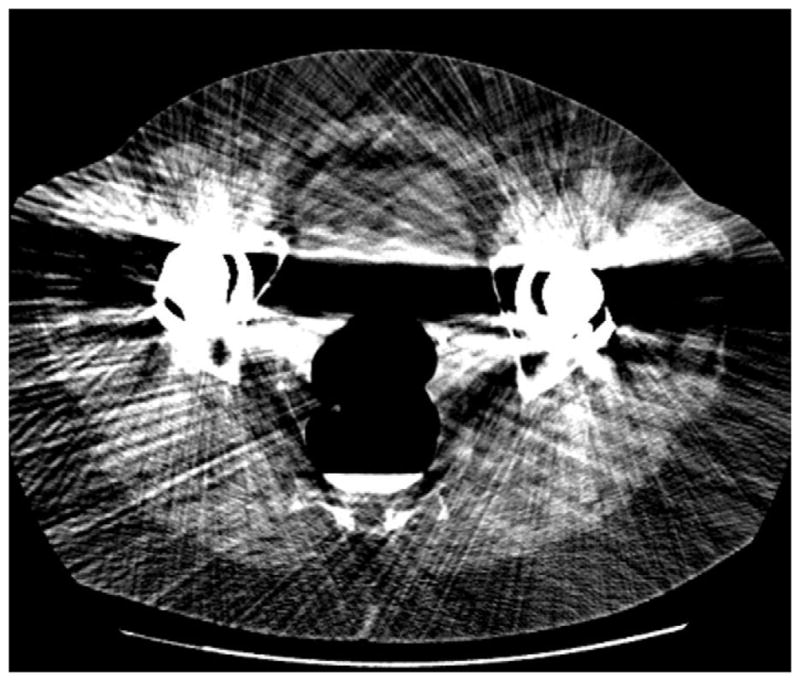
FIGURE 6. Decubitus position in morbidly obese individual.
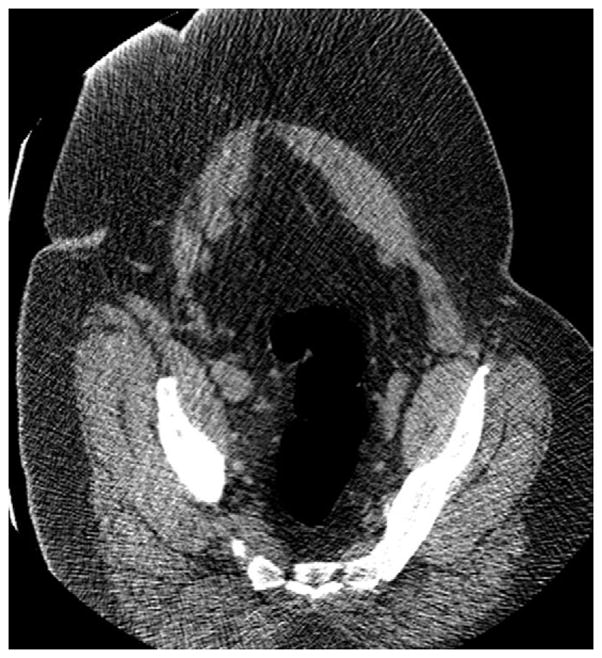
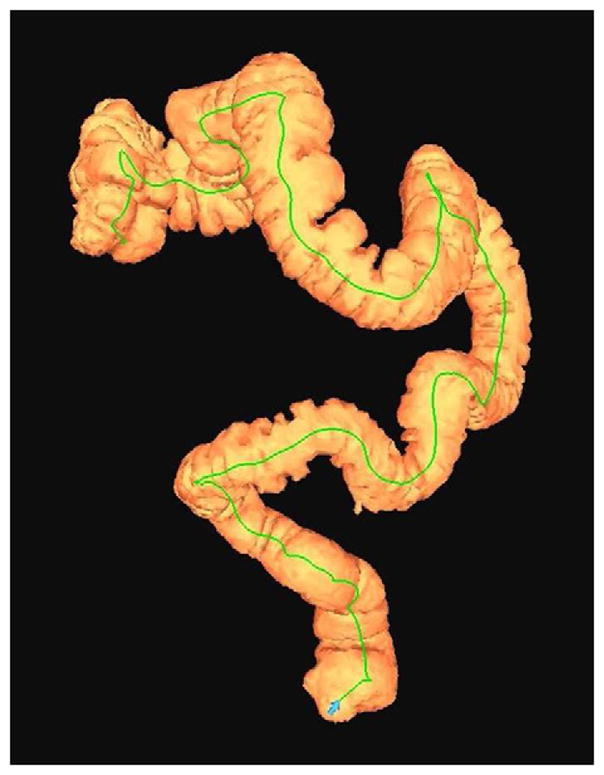
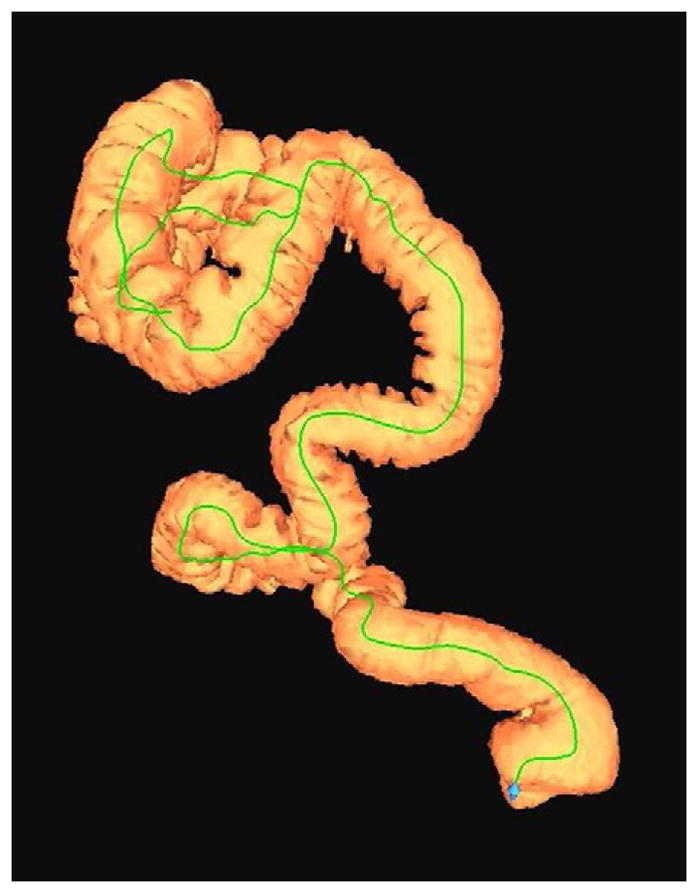
Supine 2D CTC image (A) in a 350-lb patient shows complete collapse of the sigmoid colon. An equilibrium pressure of 20 mm Hg was utilized for the automated CO2. Decubitus positioning (B) and increase to 25 mm Hg resulted in good luminal distention of this segment, as shown by frontal (C) and lateral (D) 3D colon maps.
Imaging Artifacts and Distortion
There are a wide variety of potential artifacts related to CT scanning, image reconstruction, and post-processing that can result in interpretive challenges. Most radiologists with ample experience in body CT interpretation, including advanced visualization techniques, will be adept at handling most of these imaging artifacts. The larger concern stems from potential interpretation by non-radiologists with minimal CTC training and little or no familiarity with either general CT interpretation or the basic physics of medical imaging. Although CAD has been advanced as a way to compensate for inadequate training, this notion ignores the fact that poor specificity would lead to an unacceptable false-positive rate.
Artifacts related to respiratory and other patient motion are much less common with multi-detector CT (MDCT) scanners having 16 or more channels, due to shorter acquisition times. However, we have noticed a common artifact at CTC relating to the active intra-scan flow of opacified luminal fluid, which we refer to as the “dense waterfall” sign.17 This artifact is characterized by alternating bright and dark curvilinear bands, which can cause substantial distortion on both 2D and 3D images (Fig. 7). Dynamic spilling of the opacified fluid between differential air-fluid levels is almost always apparent. Beam-hardening artifacts related to metallic objects such as spinal hardware or hip prostheses are accentuated by the low-dose CTC technique (Fig. 8). Polyp windowing reduces the impact of the beam-hardening artifact, and may allow for lesion detection. Novel reconstruction algorithms should reduce the effect of beam hardening in the near future.
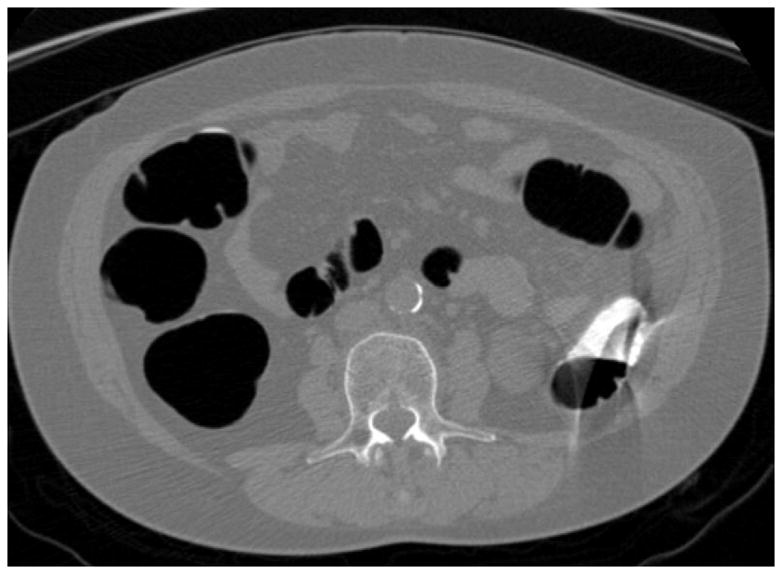
FIGURE 7. Artifact related to actively flowing luminal fluid (the “dense waterfall” sign).
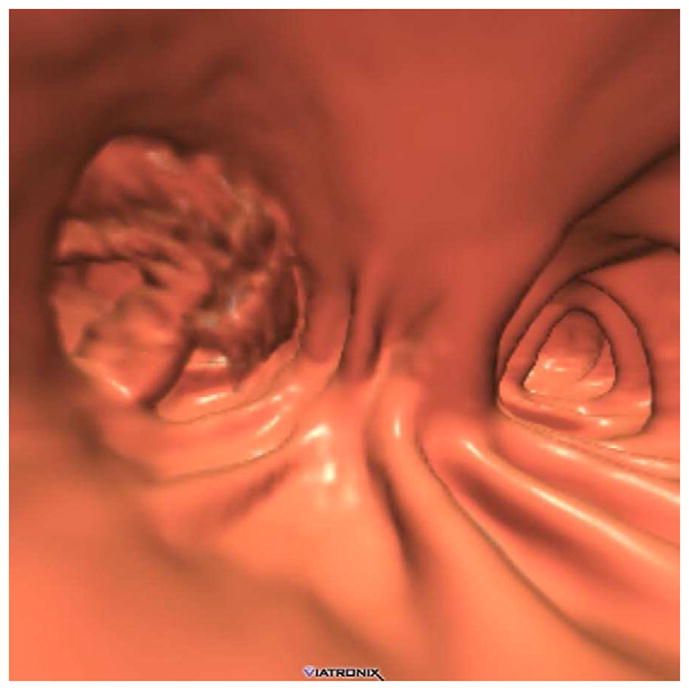
Prone 2D CTC image (A) shows multiple arciform streaks off the air-fluid level in the descending colon, which is caused by the intra-scan flow of fluid. Note the lack of motion or artifacts elsewhere on the image. The artifacts are also evident on the 3D endoluminal view (B).
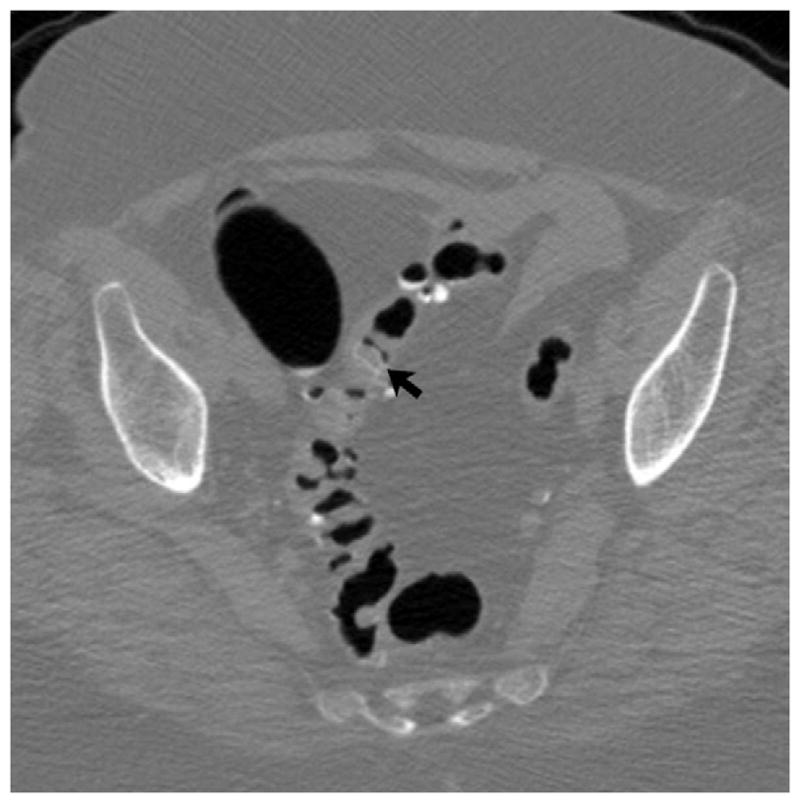
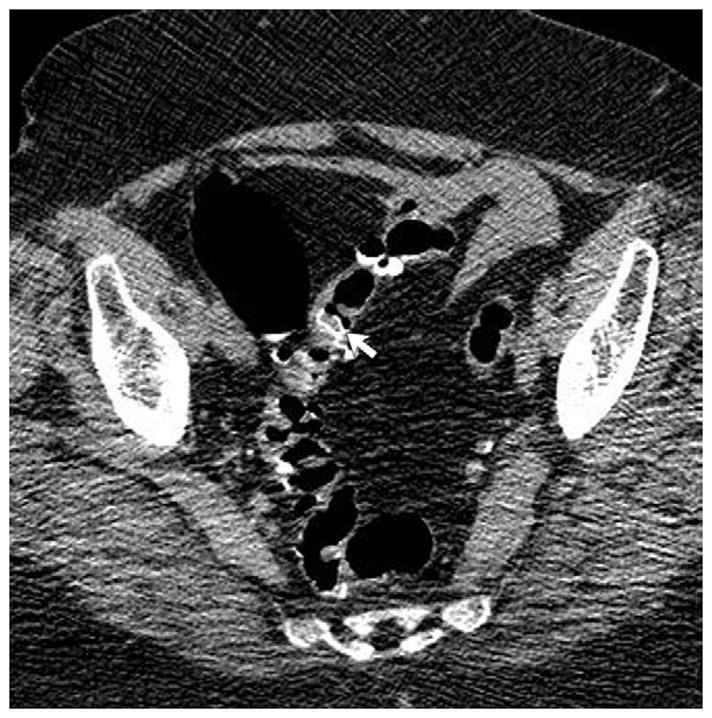
FIGURE 8. Beam-hardening artifact from bilateral total hip arthroplasties.
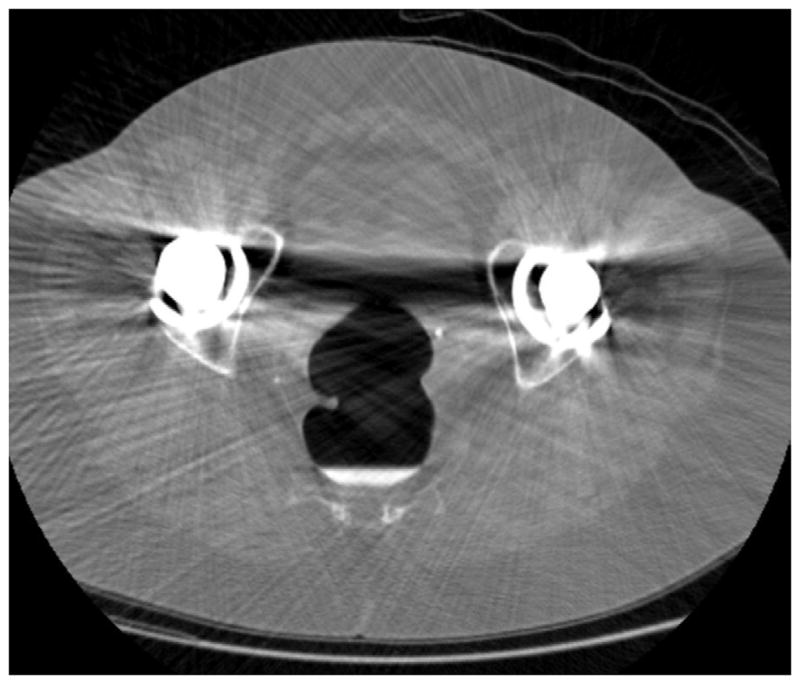
2D CTC images through the pelvis (A and B) show marked beam-hardening artifact, which appears more severe on the soft tissue windows. Streak artifact across the rectum is also apparent on the 3D endoluminal view (C).
A number of important artifacts result from post-processing of the MDCT source data. Chief among these are the geometric distortions that are introduced in creating nonstandard 3D displays, such as the 360° virtual dissection view.18 The primary motivation for developing these alternate views is to reduce the time needed to visualize the entire luminal surface. However, geometric distortion is the unavoidable trade-off, which can greatly compromise polyp recognition. Polyp size is also difficult to judge on the 360° virtual diss ection view, since areas are either stretched or compressed to fit a standard width. We prefer a milder form of distortion that simply consists of widening the field-of-view angle to 120° on the more stable 3D endoluminal view. By decreasing the number of fly-throughs from four down to two on well-distended cases, one can decrease interpretation time without introducing troublesome spatial distortion.19,20
Another important potential source of post-processing artifacts come from digital fluid subtraction or “electronic cleansing” of tagged luminal fluid.7 Because of volume averaging effects at the interfaces between luminal gas, tagged fluid, and the colonic wall, pseudopolyps are a common result of the digital subtraction process. Submerged semi-solid stool that approaches soft tissue density can appear polypoid as well. Interrogating these pseudopolyps will not only increase interpretation time, it also has the potential to decrease specificity if such lesions are mistaken for true pathology. With our current bowel preparation, the fluid level almost never overlaps between supine and prone positioning.12 Therefore, primary 3D evaluation without digital fluid extraction generally covers the entire luminal surface, and navigation is much faster without the troublesome artifacts. Furthermore, true lesions are detectable on 2D within tagged fluid pools without subtraction. As digital fluid subtraction techniques continue to improve, the various artifacts will perhaps be minimized or eliminated.
Low-dose CT Technique
Concerns regarding potential harms from radiation dose exposure related to CT have increased recently in the U.S. Although any health risks related to CT-level doses in adults are too small to measure,21 it nonetheless behooves radiology as a specialty to minimize dose to the lowest levels possibly that maintain diagnostic accuracy. Fortunately for CTC, there are a number of factors that further reduce the concern for radiation. The study is generally performed on older adults and excludes most of the chest. Furthermore, the inherent characteristics of the colon wall-air interface allow for substantial dose reduction at CTC compared with standard abdominal CT imaging. However, at extremely low dose levels, image noise can become an issue even for CTC, especially with the use of traditional filtered back projection for image reconstruction. Noise is especially problematic when reading thin images in obese patients, particularly when viewing soft tissue windows. It is critical not to mistake the image noise within a true lesion on 2D soft tissue windowing (or 3D translucency rendering) as low-attenuation heterogeneity from stool or fat. Image noise is much better tolerated on the wider 2D polyp windows. On the 3D endoluminal view, image noise from low-dose technique manifests as surface mottling when mild to luminal streaks when severe (Fig. 9).
FIGURE 9. Ultra-low dose CTC using newer iterative reconstruction algorithms.
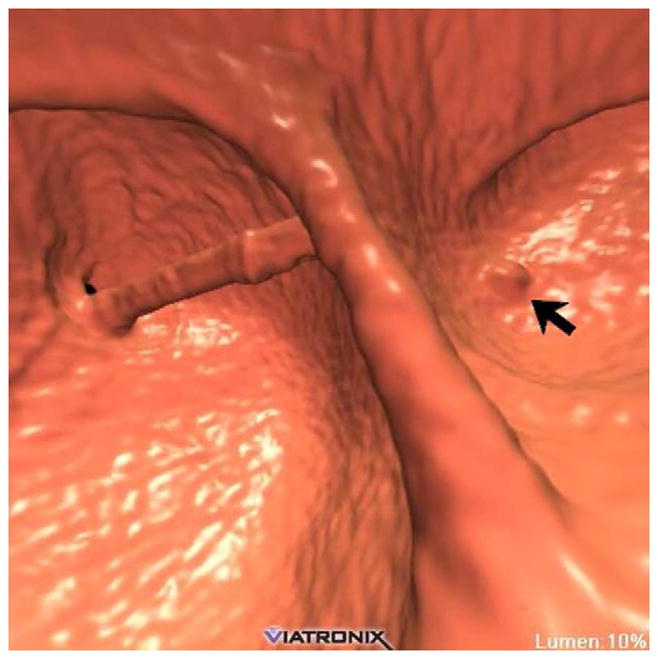
3D endoluminal CTC image (A) from ultra-low-dose scan (0.3 mSv) reconstructed with traditional filtered back projection (FBP) technique shows significant image noise. The rectal catheter is visible but the rectal polyp is largely obscured. When the same CT image data are reconstructed with a newer iterative reconstruction algorithm (B), the polyp (arrow) becomes much more conspicuous.
With a fixed mA low-dose technique, image noise can be accentuated inferiorly due to the bony pelvis but may be unnecessarily low for the upper abdomen. Tube current modulation can avoid this discordance by boosting the mA only as needed to maintain a static noise level. Some CTC protocols target more aggressive dose reduction on the prone view since much of the information is redundant to the supine view. Perhaps the most significant dose reduction will come from the implementation of the newer iterative reconstructions techniques, some of which allow for routine sub-mSv scanning for CTC (Fig. 9).22,23 Some of these newer techniques will need to be validated prior to widespread clinical implementation.
Contrast coating of polyps
One potential pitfall that has actually become a useful interpretive asset is the tendency for true soft tissue polyps, particularly flat and villous lesions, to demonstrate a thin surface coating of adherent positive oral contrast (Fig. 10).24 This contrast etching is generally not seen with the surrounding normal colonic mucosa or adherent stool. On 2D, contrast coating of polyps is easy to distinguish from internal tagging of stool, which is a critical distinction. In effect, this thin surface coating of contrast serves as a beacon for polyp detection. One notable pitfall is the fact that small coated polyps may mimic tagged stool on the 3D translucency view, since the underlying soft tissue signature may be obscured by the overlying shell of contrast.
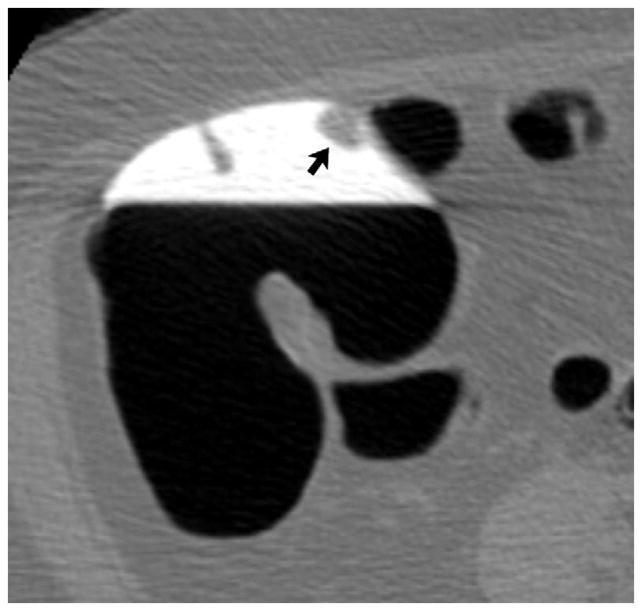
FIGURE 10. Large contrast-coated tubulovillous adenoma involving the ileocecal valve.
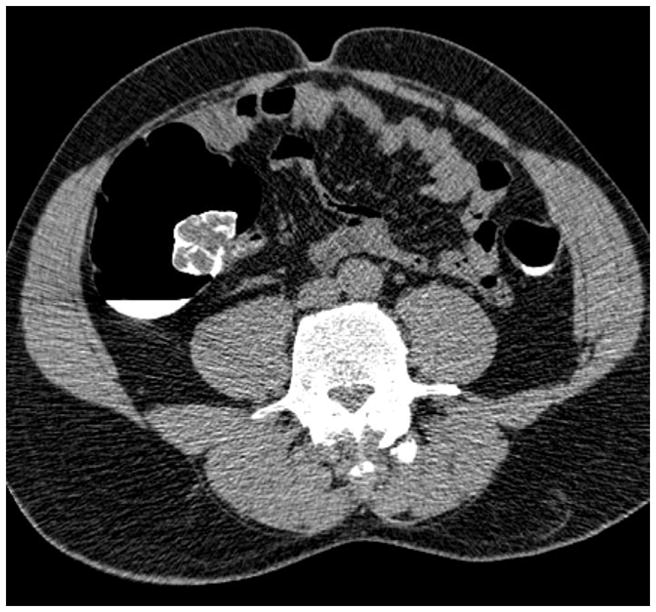
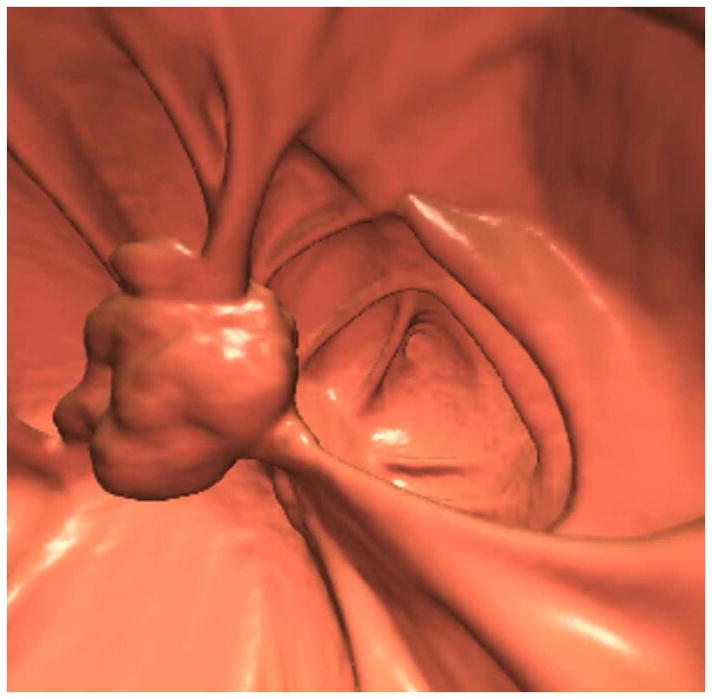
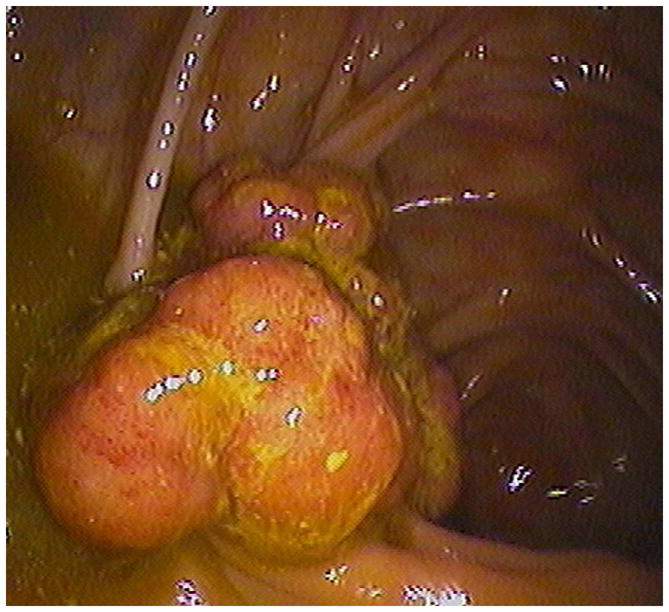
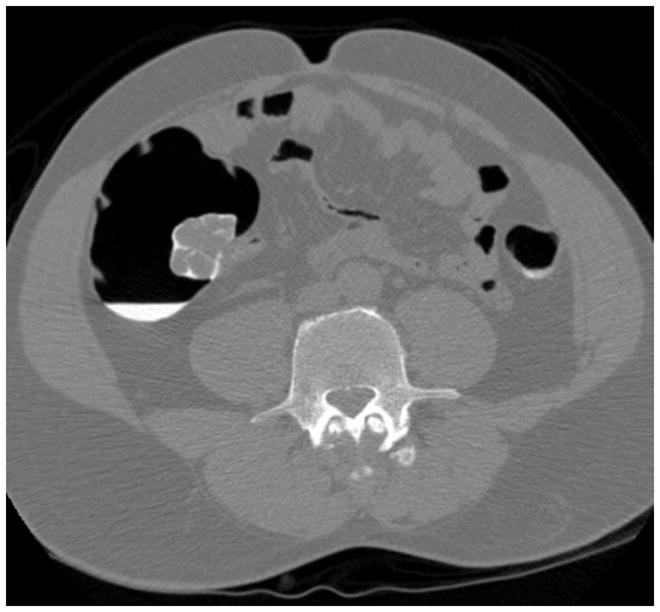
Transverse 2D CTC images with polyp (A) and soft tissue (B) window settings show a multi-lobulated mass occupying the expected location of the ileocecal valve. Note the distinct contrast etching that outlines the surfaces of the lesion. 3D endoluminal (C) and OC (D) images show the mass, which involved the ileocecal valve.
(From reference 5, with permission)
2D-only Polyp Detection
Early CTC studies relied on polyp detection by using only the 2D images, with 3D endoluminal imaging reserved for “problem solving”,25–27 which may largely explain the often disappointing performance. The search pattern for detecting polyps on 2D, especially small 6–9 mm lesions, is simply too onerous to maintain acceptable performance. In contrast, lesion conspicuity at 3D is greatly enhanced but requires adequate CTC software for execution. Subsequent CTC trials adding primary 3D detection alongside 2D review showed markedly improved sensitivity for polyps.1,28 In fact, when a large subset of the DoD cases were retrospectively interpreted using only 2D for initial detection (and 3D for problem solving) by radiologists with more CTC experience and training than those involved in the original trial, there was a significant drop-off in sensitivity that paralleled the earlier 2D trials.29 In practice, the ease of polyp detection at 3D compared with 2D is quite obvious to anyone who has interpreted more than a few CTC exams.
Polyp Measurement
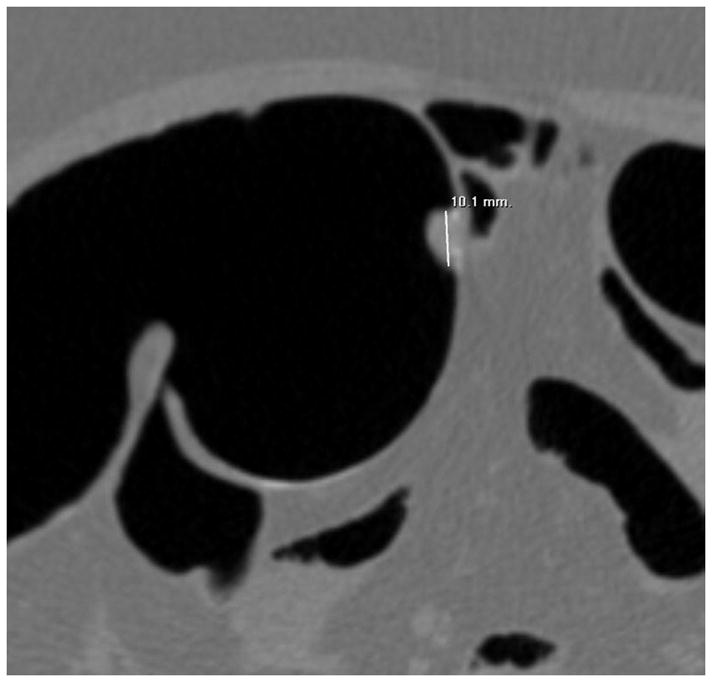
Although CTC is the most accurate method available for polyp measurement,30 inaccurate size assessment remains an important potential pitfall because it could lead to inappropriate patient management. In our experience, a CTC measurement that combines both 2D and 3D assessment can closely match a very careful OC measurement.31 2D measurement of polyps should be carried out on the wider “polyp” window setting (W: 2000 HU, L: 0 HU), since the lesion will be undersized on the soft tissue window setting (Fig. 11). 2D polyp size will also be artifactually decreased for lesions submerged under densely opacified fluid (Fig. 11). It is important to recognize that 2D polyp measurement on the standard orthogonal views (i.e., transverse, coronal, and sagittal) will under-size lesions whenever the long axis of the polyp does not align perfectly with an orthogonal plane (Fig. 12).32 This is a more relevant issue for lesions with an irregular or elongated morphology. Polyp measurement on the 3D endoluminal view can also be problematic if care is not taken to optimize the vantage point. Incorrect caliper placement, partially submerged polyps, and thick contrast coating of polyps can also lead to erroneous 3D measurement. Over-sizing diminutive lesions on 3D is a common pitfall that can lead to over-aggressive management if not carefully correlated with the 2D polyp size. Over time, one can generally learn to appreciate lesions that are clearly diminutive in size without the need to formally measure each one.
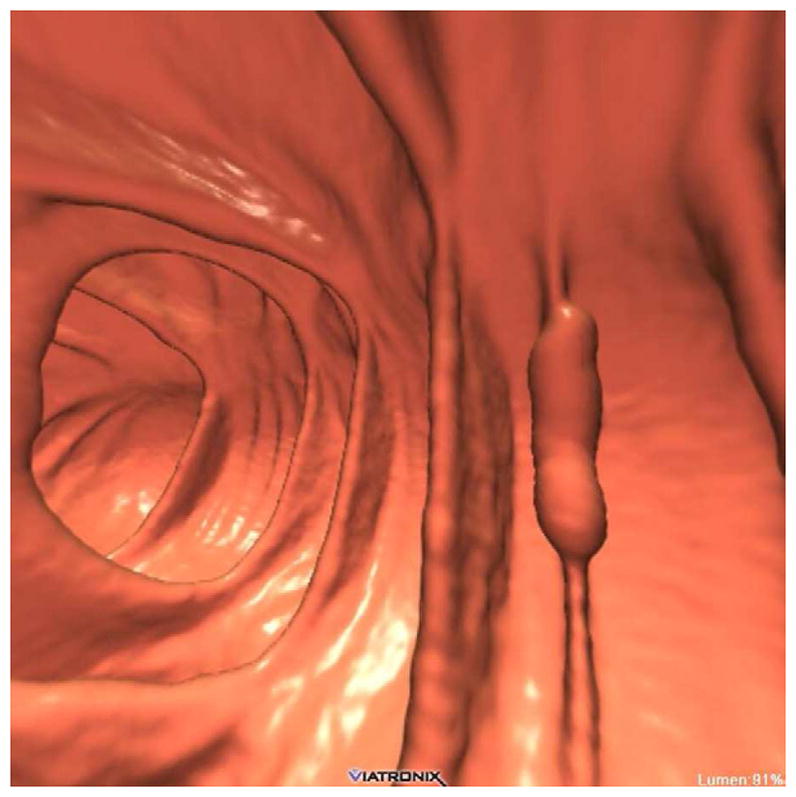
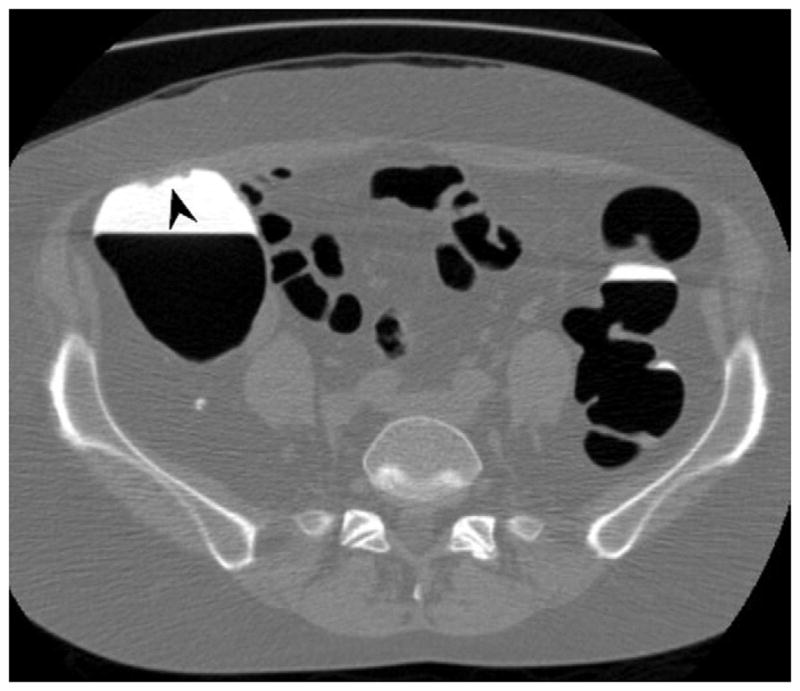
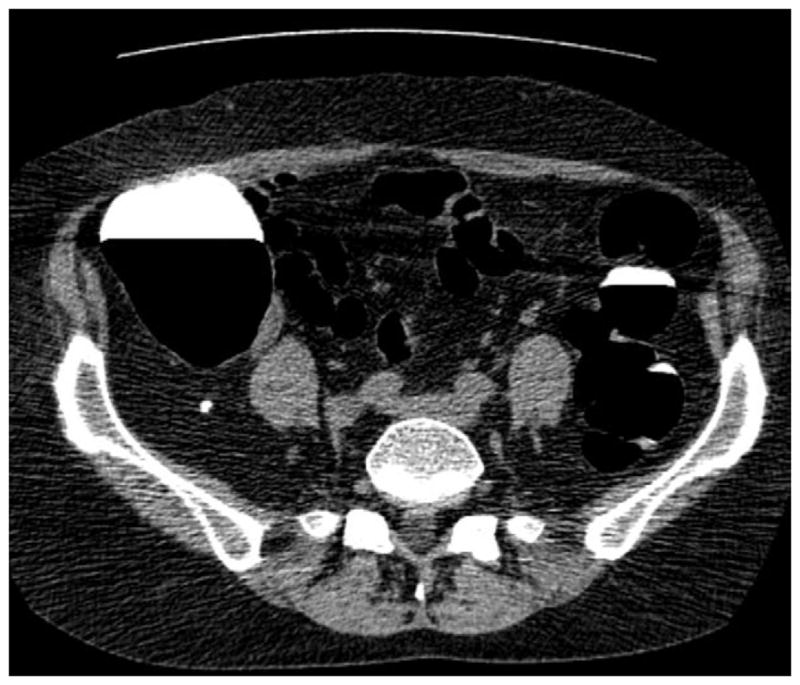
FIGURE 11. Apparent decrease in polyp size on soft tissue window setting.
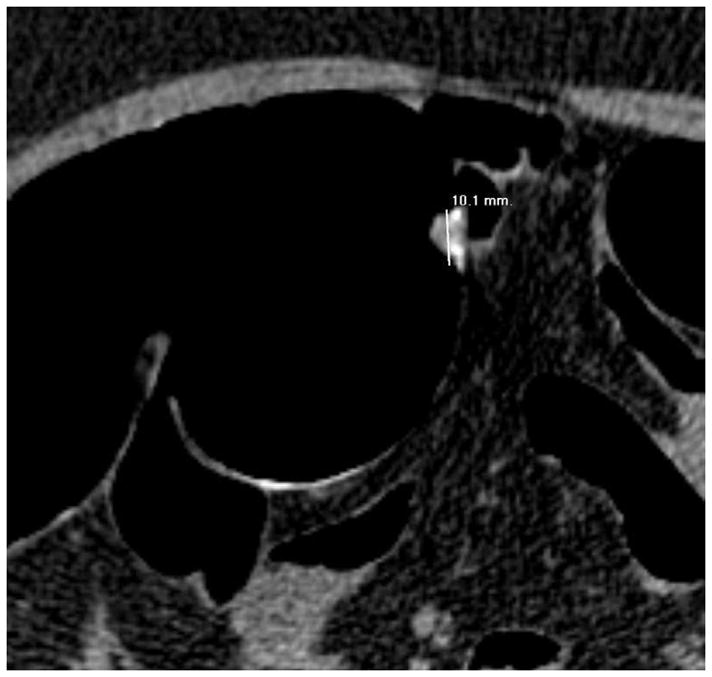
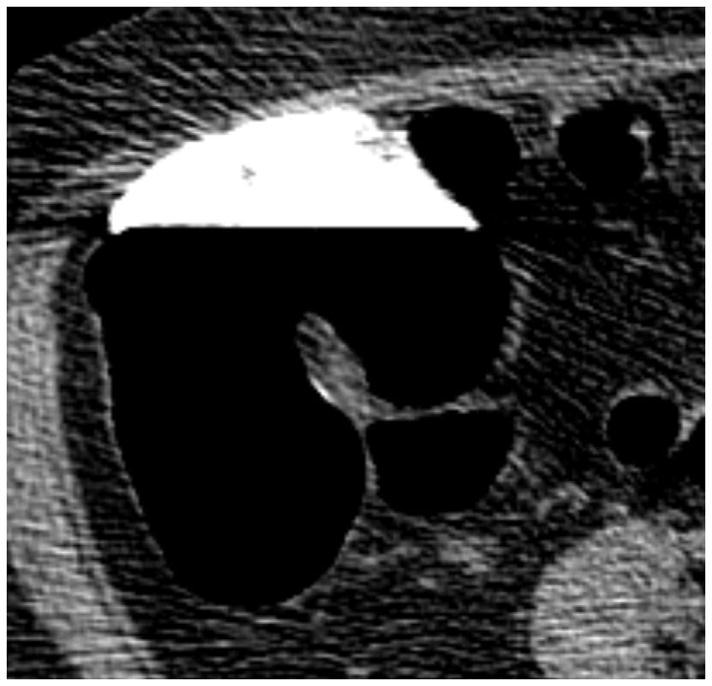
Supine transverse 2D CTC image (A) with polyp window setting (2000/0) shows a 10-mm sessile polyp in the cecum (calipers). On a soft tissue window setting (B, 350/40), the polyp appears to decrease in size to less than 10-mm. Polyp measurement on soft tissue windows could lead to inappropriate management. On the prone 2D CTC images (C and D), the polyp (arrow) is submerged under densely opacified fluid, which further decreases the apparent polyp size. Note how the lesion is barely perceptible on the soft tissue window setting (D).
(From reference 5, with permission)
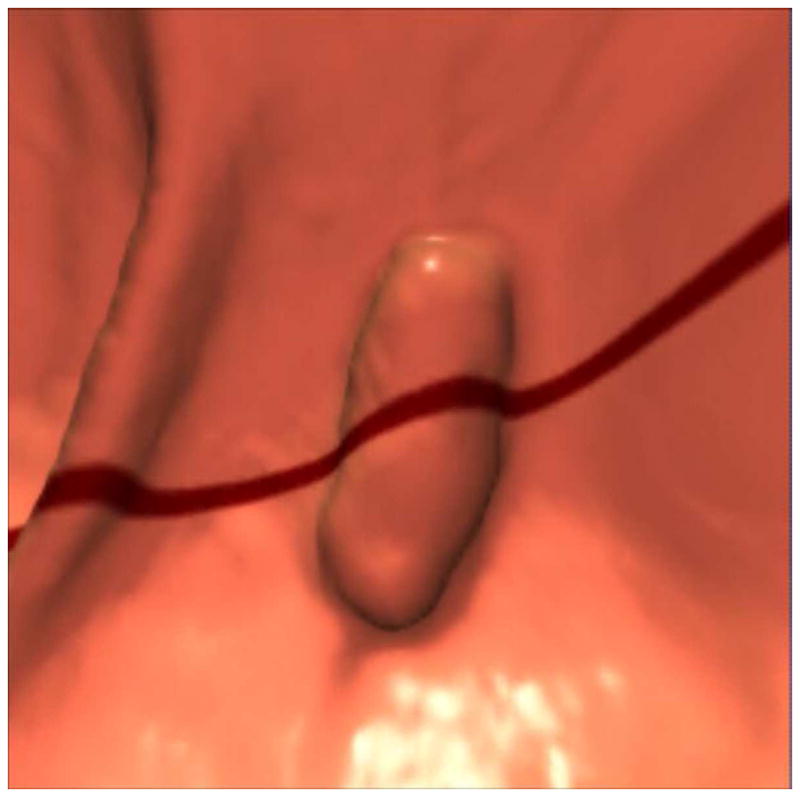
FIGURE 12. 2D versus 3D CTC measurement of an elongated polyp.
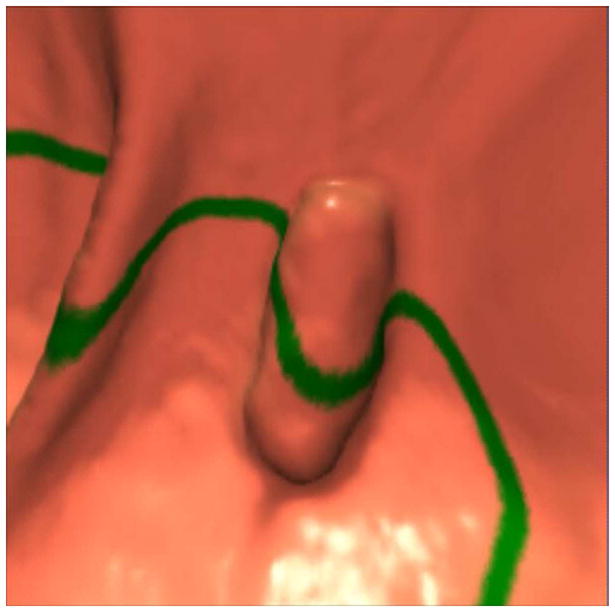
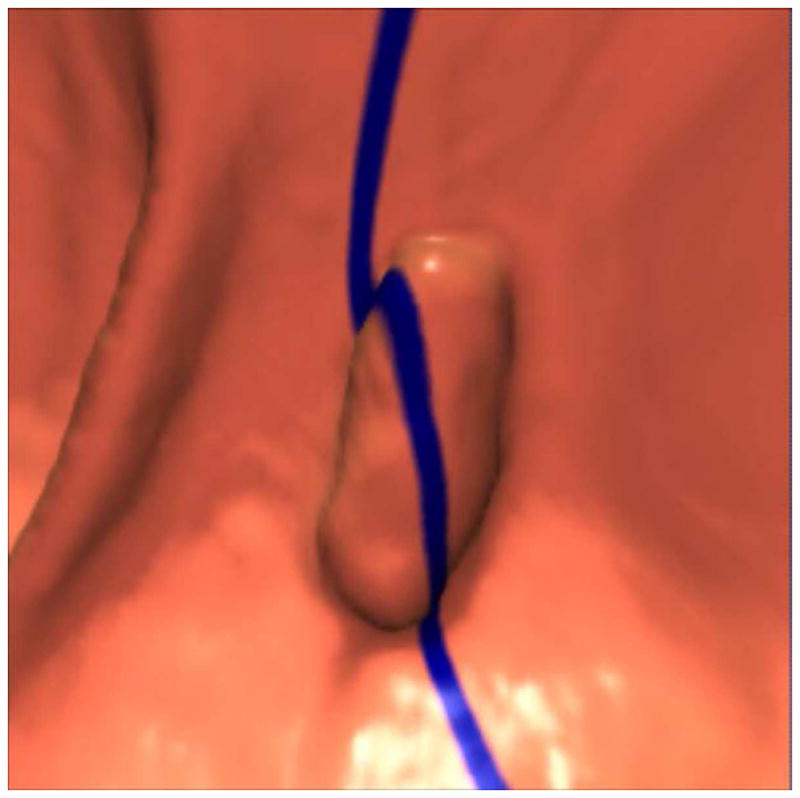
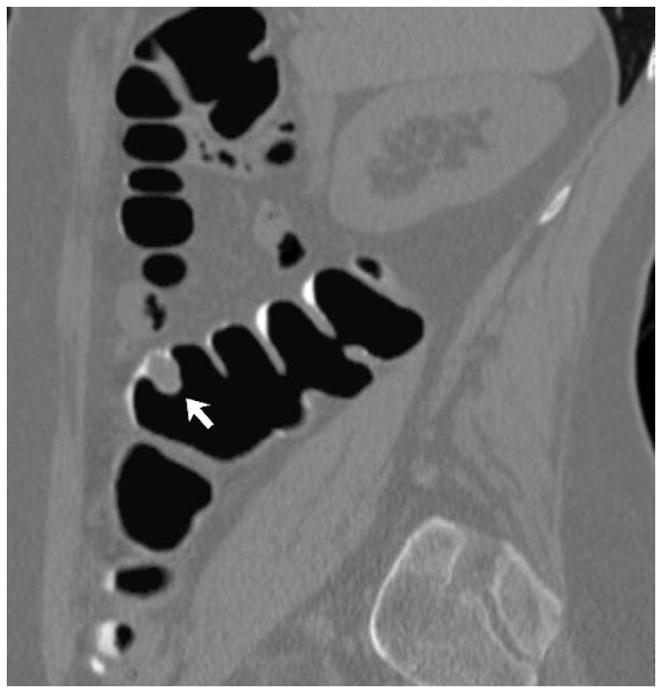
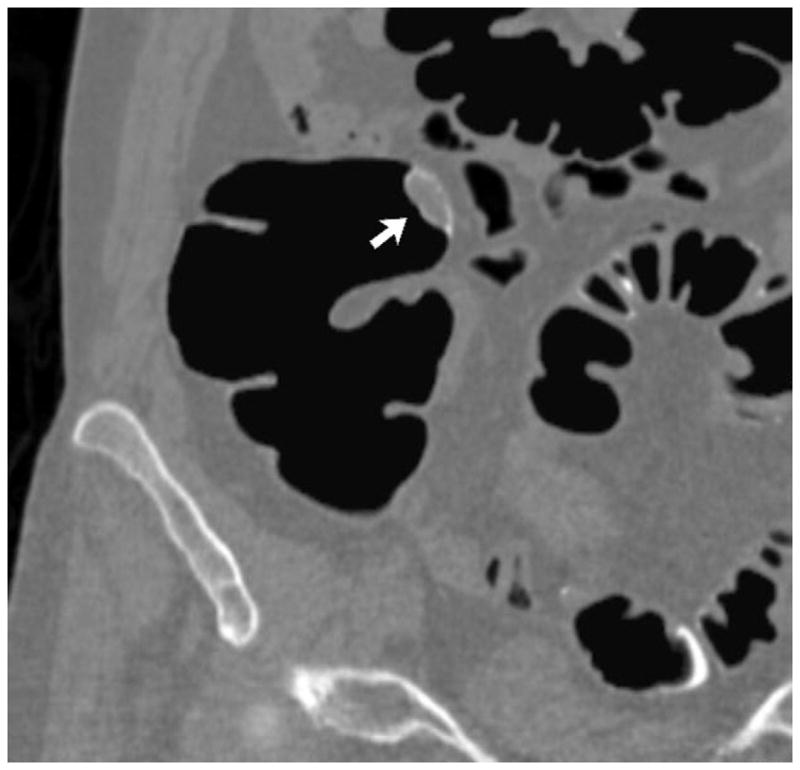
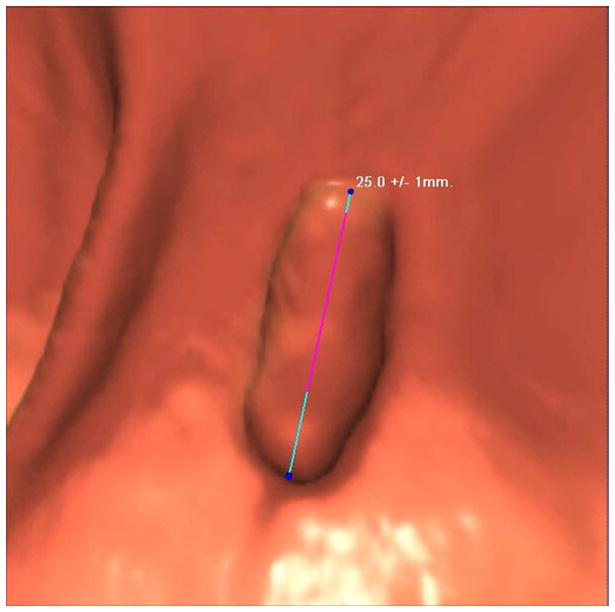
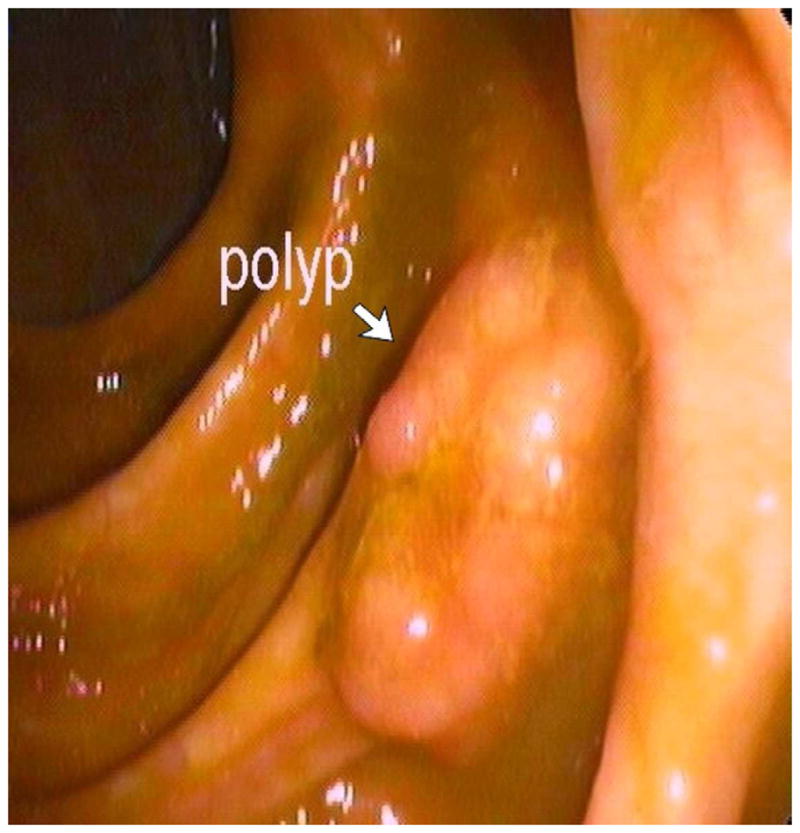
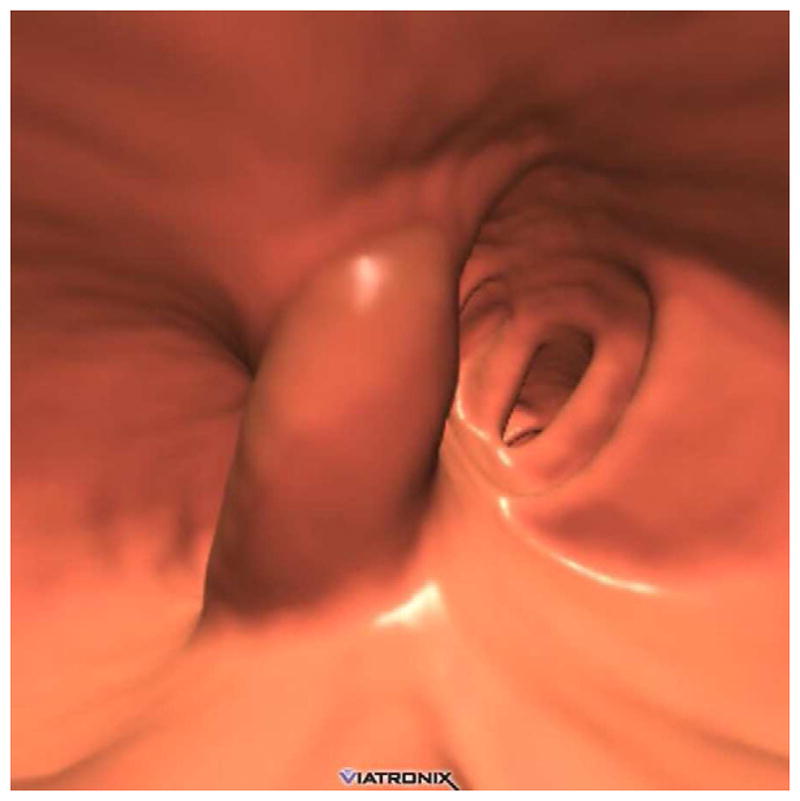
3D endoluminal CTC images (A–C) show an elongated polyp with the transverse (A), sagittal (B), and coronal (C) 2D planes as colored lines through the polyp, corresponding to the 2D transverse (D), sagittal (E), and coronal (F) images (arrows). Even with the “optimized” coronal 2D measurement (C and F), the polyp is significantly undersized, whereas the 3D measurement (G) corresponds to the actual long axis of the polyp, and correlated best with OC (H).
(From reference 5, with permission)
For suspected polyps approaching 5–6 mm or greater detected at CTC, we recommend performing a careful combined 2D and 3D size assessment. For most polyps, the 2D and 3D measurements will be within 1 mm of each other. Unless the lesion is at or near a critical size threshold (i.e., 6 mm and 10 mm), the final reported size is not a major issue. However, if the lesion is bordering one of these two important thresholds, or there is a relatively large discrepancy between the 2D and 3D assessment, then a judgment call is necessary to determine the most appropriate size to report. Polyp size need only be reported to the nearest mm. Beyond CTC, OC with use of a calibrated probe is probably the next best method, whereas visual estimation at OC is less accurate, and pathologic ex vivo measurement is least accurate of all.30,31,33 Given its ability to better assess interval change in polyp size, volume assessment will likely play an increasingly important role in the future.34–36
POTENTIAL PITFALLS RELATED TO ANATOMY
Thickened Colonic Folds
Fold thickening at CTC is largely due to inadequate luminal distention, underlying diverticular disease, or a combination of the two. At 2D evaluation, it can be challenging to distinguish an unimportant thickened fold from relevant pathology. However, as long as the lumen is at least partially distended, primary 3D evaluation can generally make this distinction, as the smooth, uniform, and often circumferential nature of incidental fold thickening is readily apparent (Fig. 13). Atypical thickened folds will occasionally need confirmation at OC; biopsy is sometimes necessary, but often yields only normal mucosa. Without the associated features of contrast coating or a lobulated contour, we have found that the diagnostic yield of sending isolated “thick folds” to OC is too low to justify. Other causes of apparent focal soft tissue thickening at 2D CTC include complex or convergent folds, redundant colonic segments with subclinical twisting, and dynamic spasm or collapse, all of which are best appreciated on the 3D endoluminal view. These pitfalls must be distinguished from true flat lesions, which are discussed below.
FIGURE 13. Persistently thickened fold at CTC.
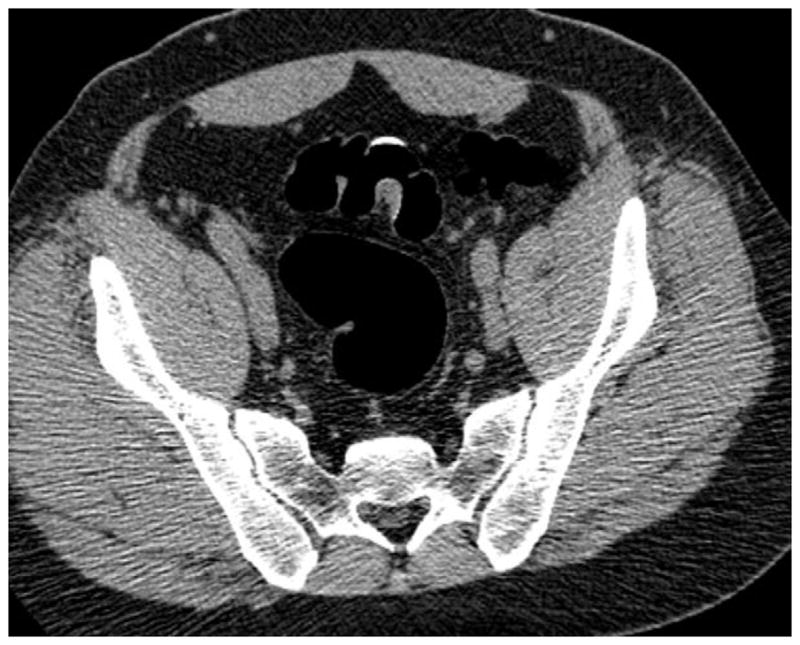
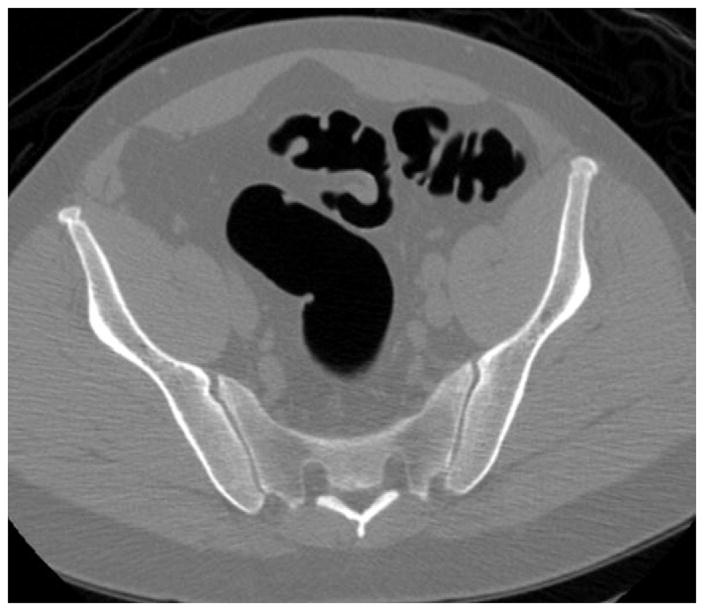
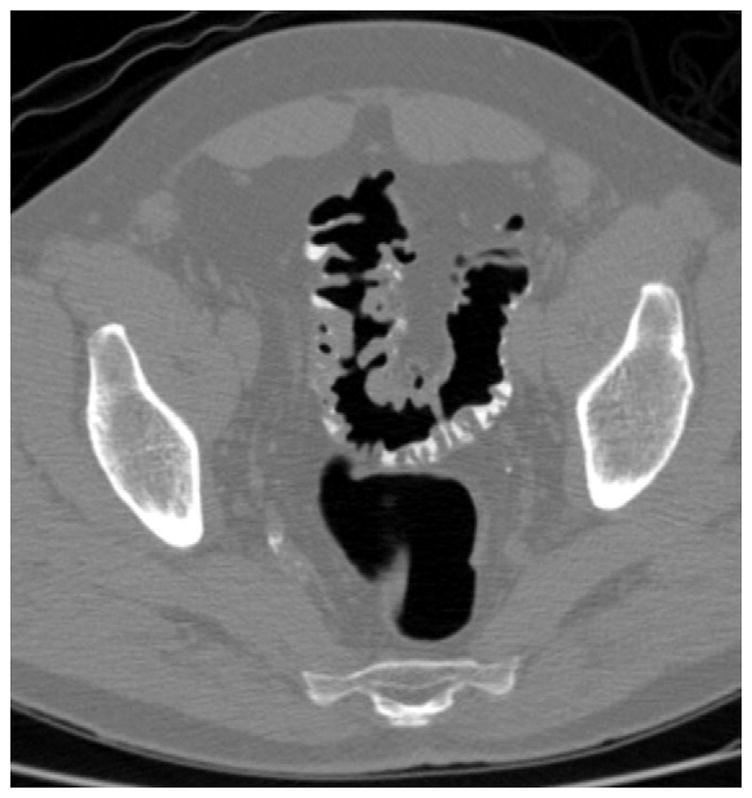
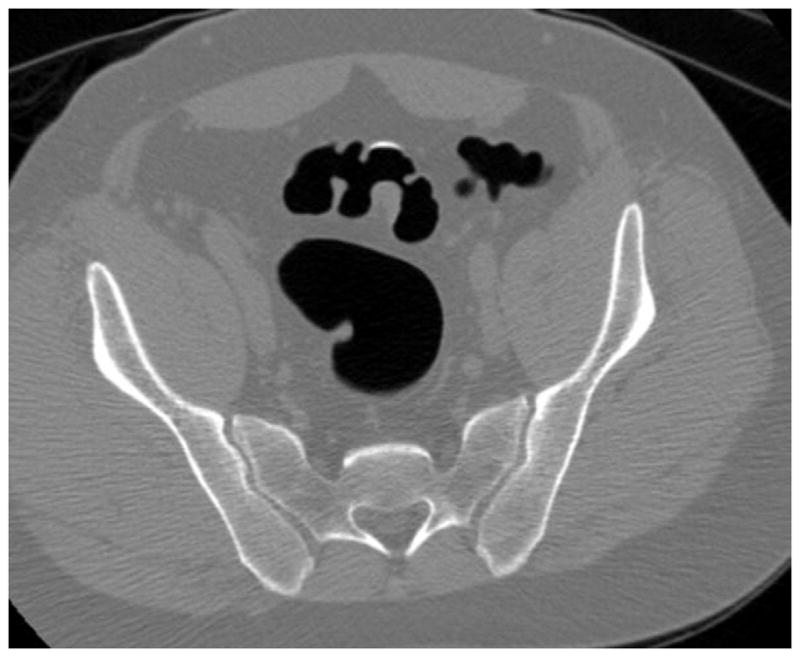
Prone (A and B) and supine (C and D) CTC images show a thickened sigmoid fold that appears almost mass-like at 2D. The smooth thickening appears to be related to a point of slight twisting or torsion. Note the fat extending into the fold on B, which excludes an infiltrating cancer.
Diverticular Disease
The prevalence of diverticular disease exceeds 50% in most adult populations, heavily favoring the sigmoid colon, followed by the descending colon.37 The pathologic features are characterized by myochosis with elastin deposition, thickening of the circular smooth muscle, shortening of the tenia, decreased compliance, and luminal narrowing. From these pathologic features, and combined with its high prevalence, diverticular disease not surprisingly represents the leading cause of nondiagnostic segmental evaluation at CTC. At 2D evaluation, evaluating segments with advanced diverticular disease can be a daunting task (Fig. 14), as diffuse fold thickening combines with luminal narrowing. However, as long as luminal narrowing does not impede 3D endoluminal evaluation (Fig. 15), diagnostic performance may not be adversely affected.37 For cases with persistent complete or near-complete luminal collapse on all views, a pathologic diverticular stricture must be considered (Fig. 16).
FIGURE 14. Sigmoid diverticular disease at CTC.
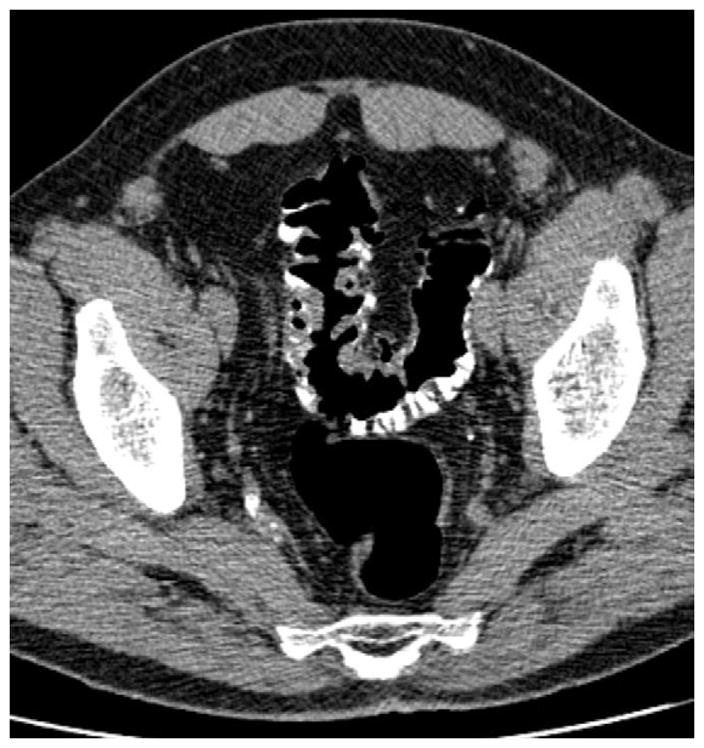
Supine 2D CTC images (A and B) show wall thickening and luminal narrowing related to advanced sigmoid diverticulosis. This appearance can make it challenging to exclude superimposed neoplastic pathology. 3D evaluation can be very valuable in this setting
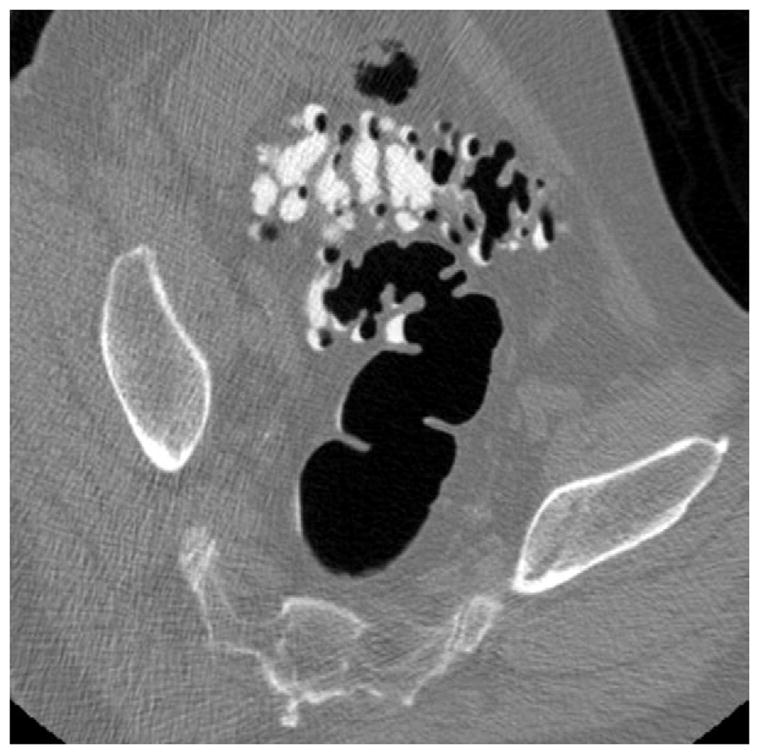
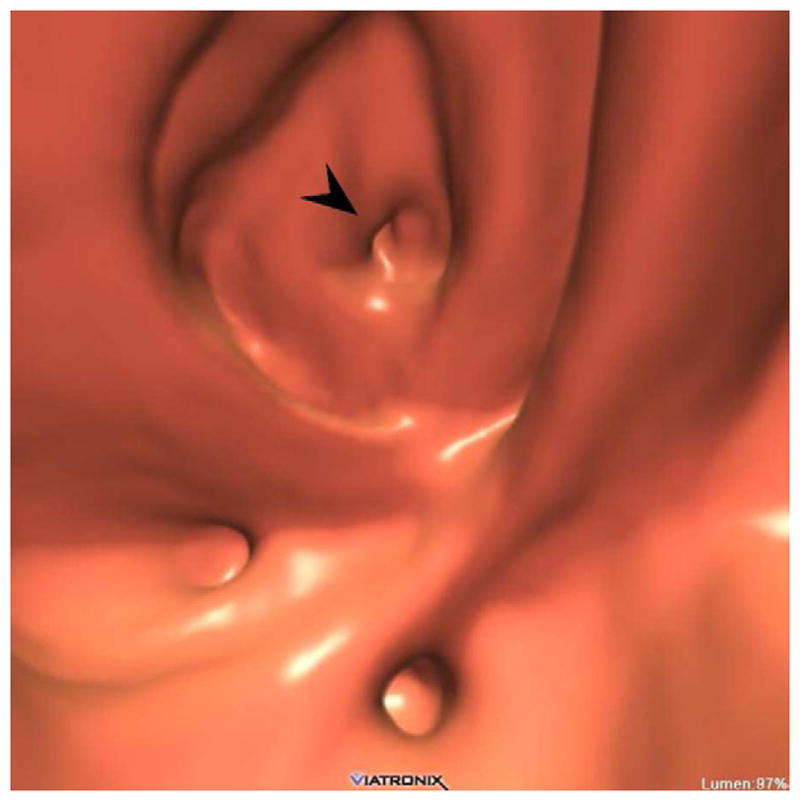
FIGURE 15. Diverticular disease at CTC – 2D versus 3D evaluation.
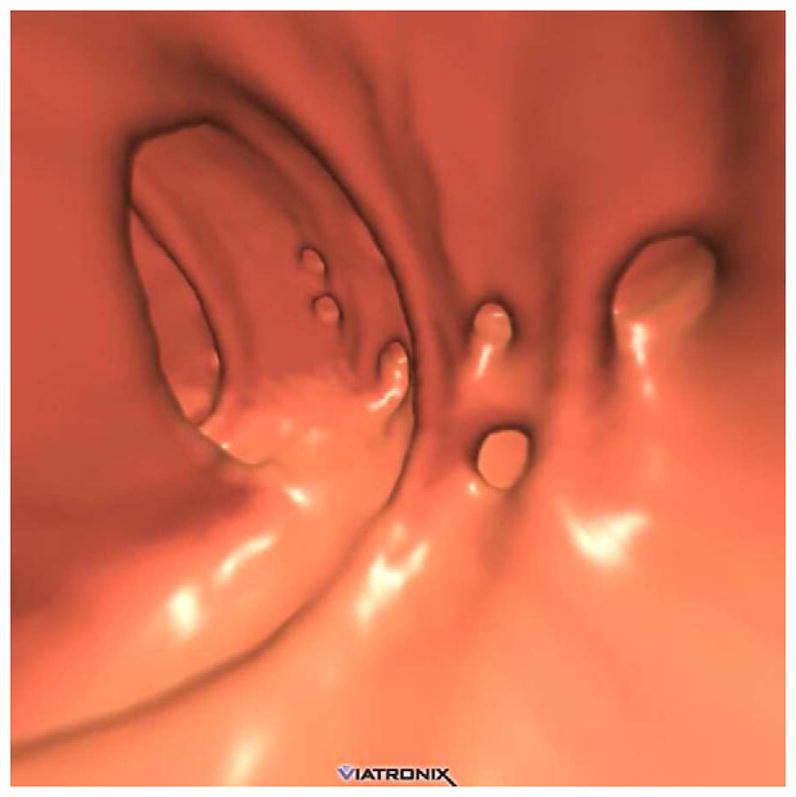
2D (A) and 3D (B) CTC images show innumerable sigmoid diverticula. Exclusion of superimposed polyps is a much simpler task on the 3D endoluminal view.
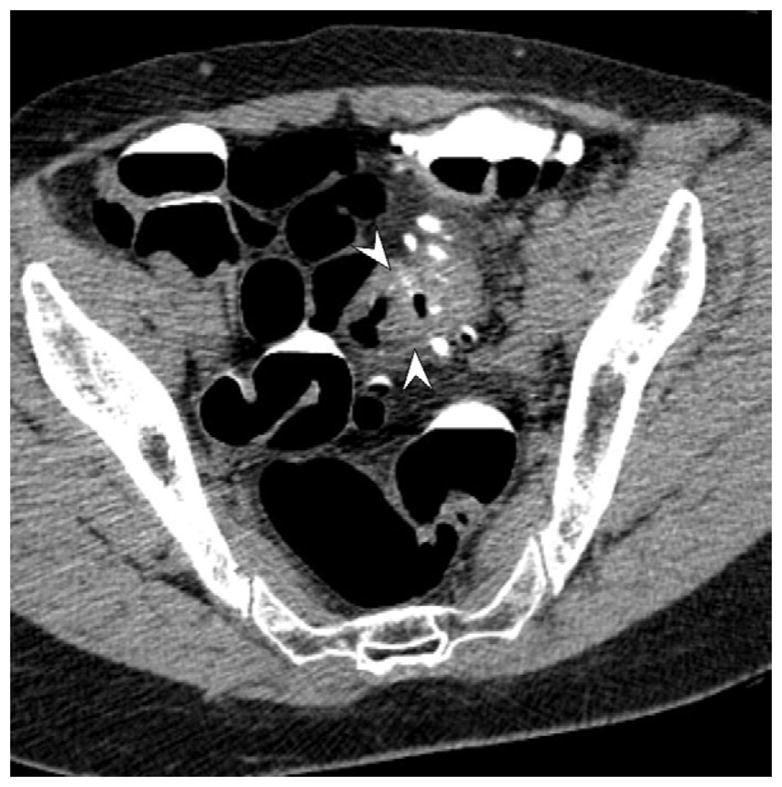
FIGURE 16. Nondiagnostic luminal distention related to a diverticular stricture.
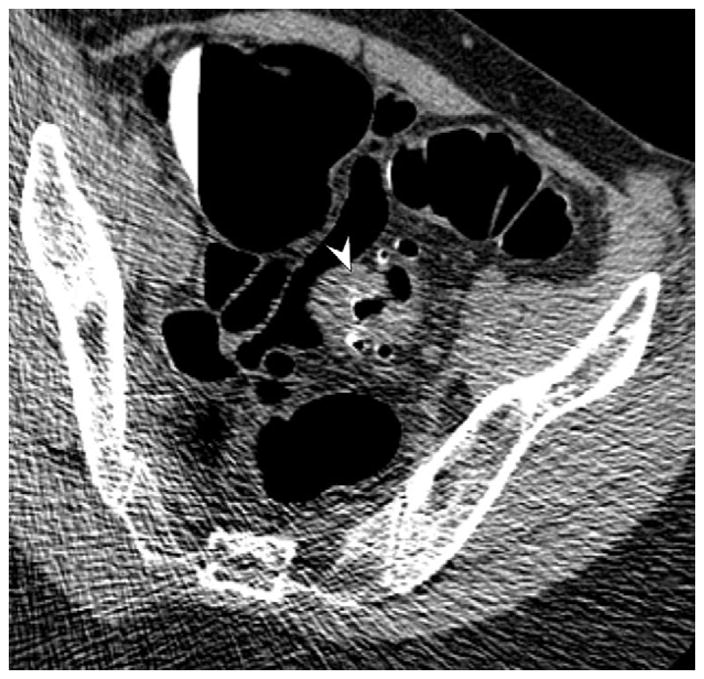
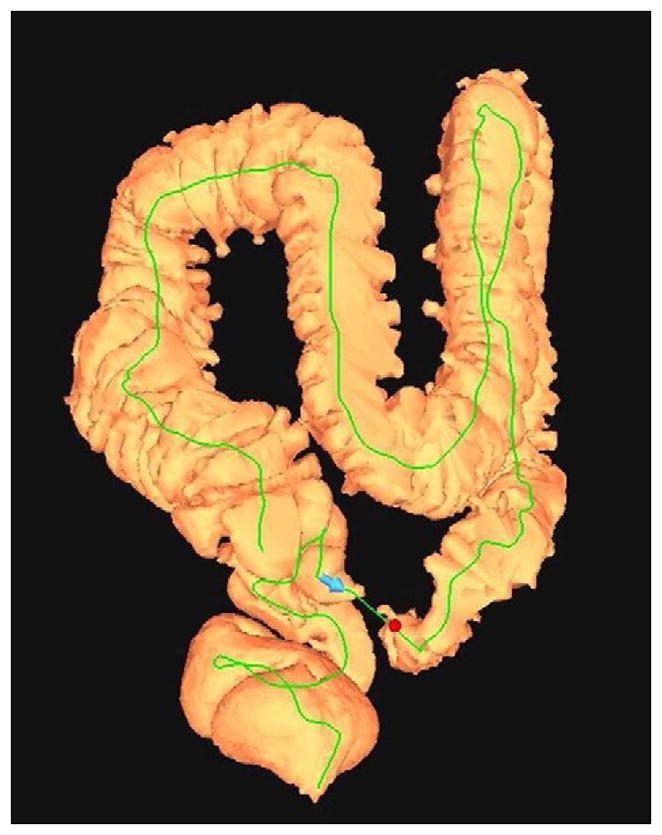
Supine (A and B) and decubitus (C) CTC images show an area of persistent wall thickening and luminal narrowing (arrowheads) in the setting of advanced sigmoid diverticular disease. The prone images had a similar appearance (not shown). The 3D colon map shows the site of this persistent stenosis (arrow and red dot), which proved to be a diverticular stricture.
Unlike barium enema examination, the diverticula themselves cause little problem at CTC interpretation. However, there are additional pitfalls related to diverticular disease beyond fold thickening and inadequate luminal distention. Stool-filled or “impacted” diverticula, which contain inspissated debris, often bulge or prolapse slightly into the colonic lumen, creating a polypoid appearance on the 3D endoluminal view. Exclusion of a true polyp on the 2D display (or 3D translucency rendering) is straightforward, rendering such “lesions” more of a nuisance than a diagnostic dilemma. Frankly inverted diverticula are relatively rare but represent a related pitfall. Prolapsing mucosal polyps represent redundant colonic mucosa in the setting of sigmoid diverticular disease. These non-neoplastic lesions can be difficult to differentiate from neoplastic disease.38
Flat Lesions
Flat lesions represent a subset of sessile polyps that, as the name implies, have a “nonpolypoid” plaque-like morphology (Fig. 1). Although the traditional definition was a lesion height that is less than half the width, a preferred definition for flat polyps less than 3 cm in size is an elevation above the mucosal surface that does not exceed 3 mm.39 Given their intrinsic morphology, flat lesions are less conspicuous and therefore more challenging to initially detect at CTC (and OC). Nonetheless, the sensitivity of combined 3D-2D polyp detection with contrast tagging appears to be satisfactory.40 The importance of both contrast tagging for 2D (given the propensity for flat lesions to coat) and 3D endoluminal evaluation for flat lesion detection cannot be overstated. In our clinical screening experience, more large flat advanced adenomas were detected at primary CTC screening compared with primary OC screening.41 Of note, histologically-advanced and/or centrally-depressed flat lesions are relatively rare in our screening population. In fact, the majority of flat lesions detected (or missed) at CTC are hyperplastic.42,43 The relative increase in flat hyperplastic lesions is likely due in part to their tendency to flatten out when the colonic lumen is distended at CTC.44,45 Most occult polyps at CTC (i.e., missed lesions that cannot be identified even retrospectively) represent flat hyperplastic polyps.43,46,47 Not only do flat lesions constitute an important source of potential false negatives at CTC, they are a common cause of potential false positives at CTC (Fig. 2).48 Although some of these CTC “false positives” could actually represent OC false negatives, this still underscores the importance of not overcalling flat lesions at CTC.
The prevalence and clinical relevance of flat colonic lesions have been the source of great debate. A clear distinction must be made between relatively flat lesions, and completely flat or depressed lesions, which are quite rare.49 In general, multiple studies have shown that, for a given size, polypoid lesions greatly outnumber flat lesions and more often harbor aggressive histology.49–51 For larger flat masses (ie, ≥3 cm), the terms “carpet lesion” and “laterally spreading tumor” are applied (Fig. 17).52 Carpet lesions have a strong predilection for the rectum and cecum.53 Despite their large linear size, they have a relatively low rate of malignancy but frequently demonstrate villous features, with or without high-grade dysplasia.52,53 Although classic carpet lesions are less conspicuous than obvious polypoid or annular colorectal masses, they are nonetheless detectable at CTC due to the fixed distortion of folds, rolled-up edges, and lobulated appearance (Fig. 17).
FIGURE 17. Large cecal carpet lesion (laterally spreading tumor).
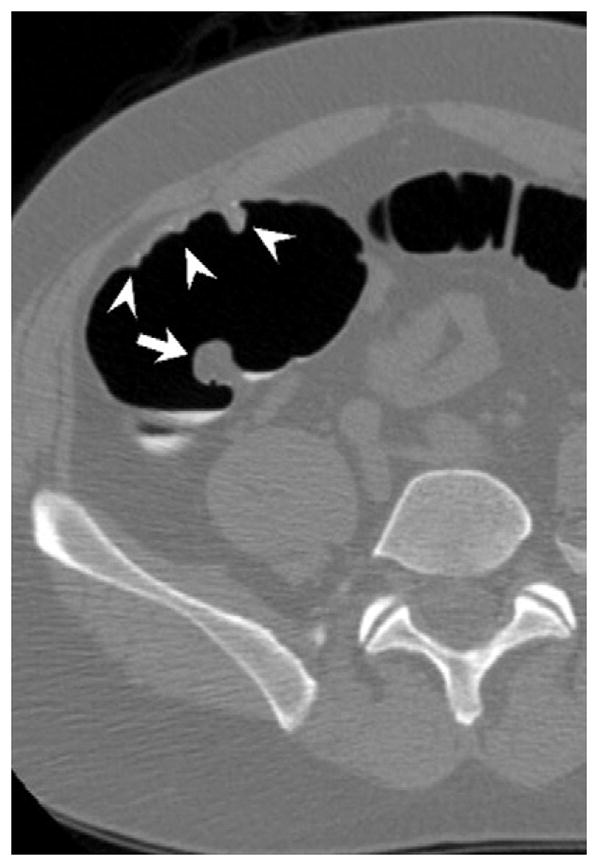
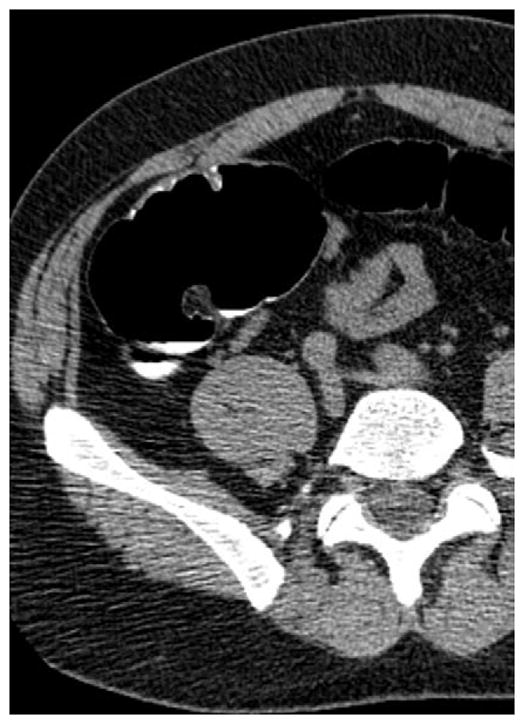
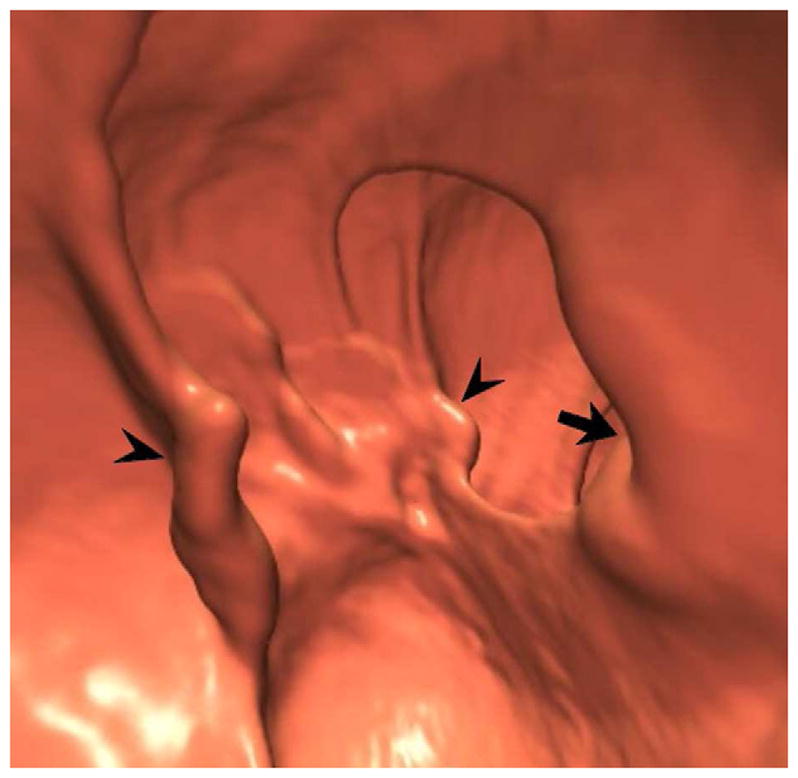
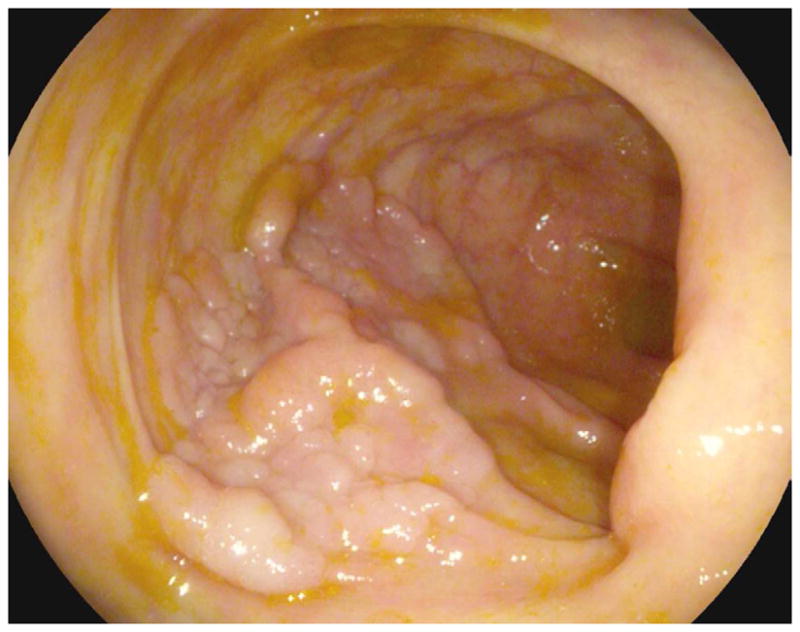
Supine transverse 2D (A and B) and 3D endoluminal (C) CTC images show a large flat soft tissue mass (arrowheads) opposite the ileocecal valve (arrow) that has a somewhat lobulated appearance and results in fold distortion on 3D. Note the contrast coating portions of the lesion on B. This carpet lesion was confirmed at same-day optical colonoscopy (D) and proved to be a tubulovillous adenoma. Most non-flat lesions of this large size would be malignant.
Submucosal and Extrinsic Lesions
Broadly speaking, “submucosal” lesions can include anything deep to the mucosal surface, including both intramural (Fig. 18) and extrinsic lesions (Fig. 19). Although a wide variety of neoplastic54 and non-neoplastic55 causes for a submucosal luminal impression exist at CTC, only a relatively small subset might potentially be confused for a mucosal-based soft tissue polyp or mass. 2D correlation at CTC will often divulge the nature of an extrinsic impression seen on the 3D endoluminal view, avoiding any potential misdiagnosis. The same cannot be said for luminal examinations like BE and OC. At CTC, specific definitive diagnosis can be made in the case of submucosal lipomas, pneumatosis, and extrinsic impression from extracolonic structures. The presence of inwardly displaced but uninterrupted folds at 3D endoluminal CTC strongly suggests extrinsic impression (Fig. 19).
FIGURE 18. Submucosal venous bleb simulating a flat polyp at CTC.
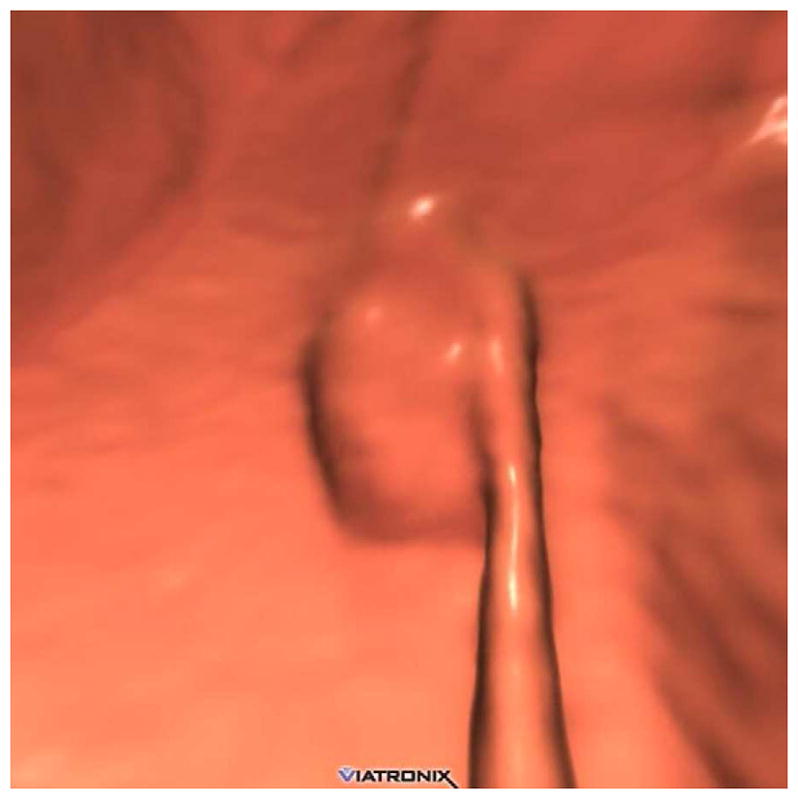
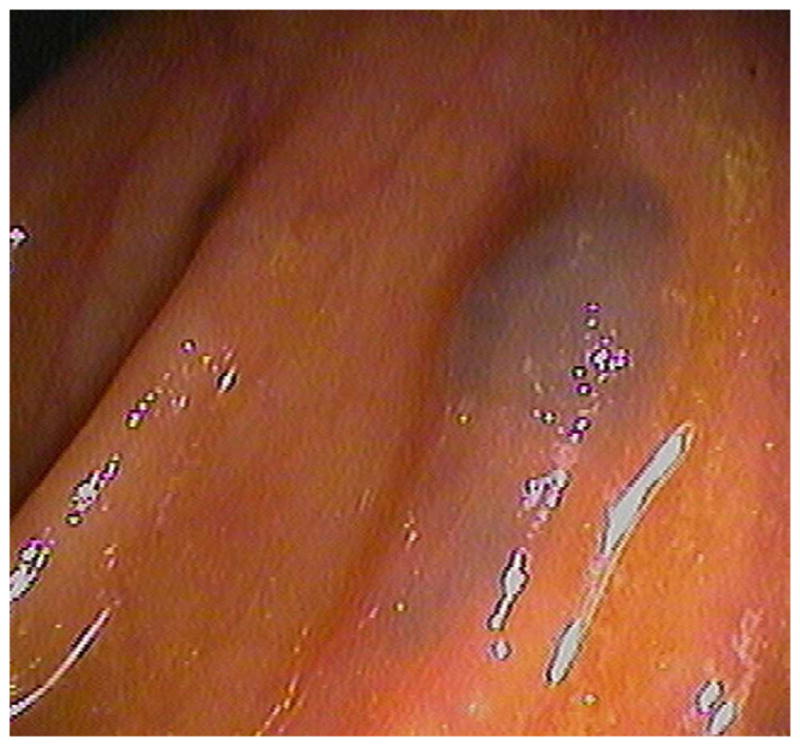
3D endoluminal CTC image (A) shows a flat plaque-like lesion adjacent to a colonic fold, which appeared to be soft tissue attenuation on 2D correlation (not shown). At subsequent OC (B), however, the lesion proved to be a submucosal venous bleb.
FIGURE 19. Extrinsic impression related to an adjacent small bowel loop.
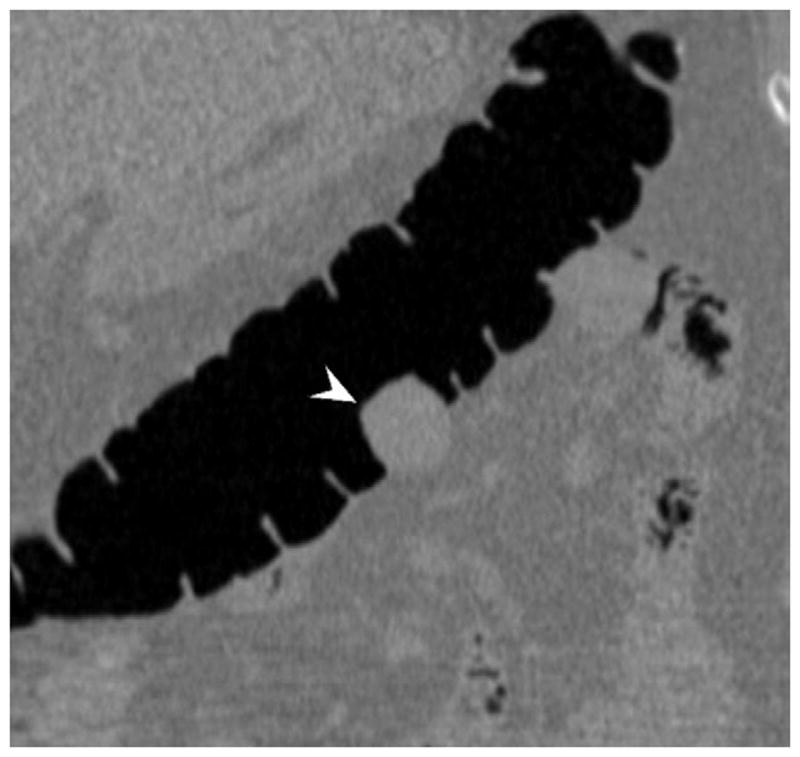
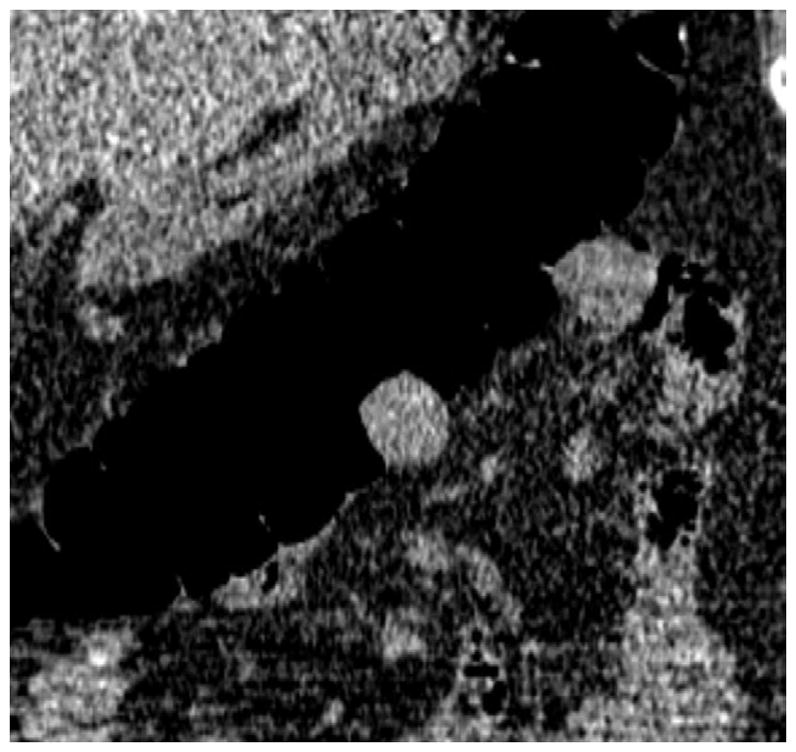
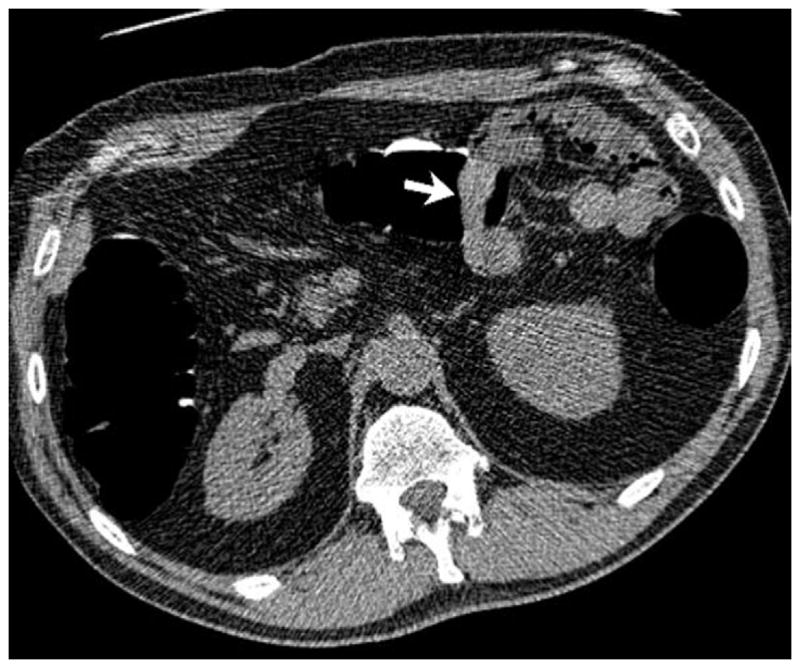
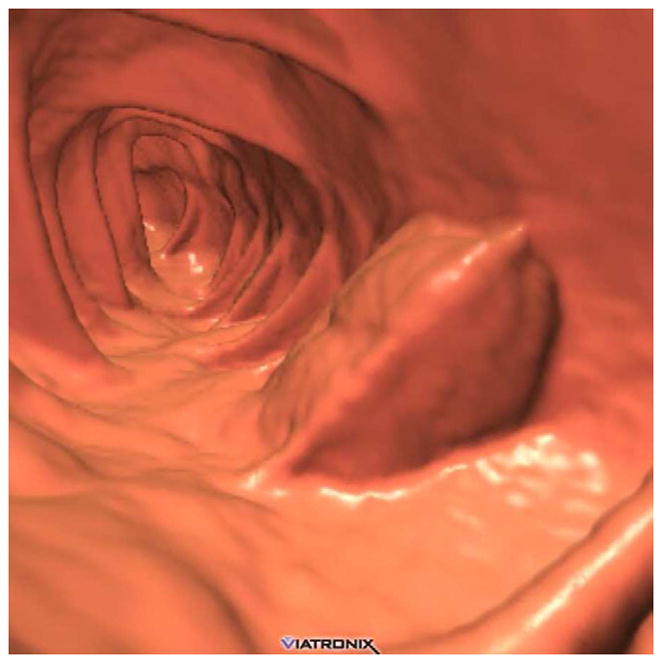
Coronal 2D CTC images (A and B) show a focal soft tissue “mass” (arrowhead) involving the transverse colon. Transverse 2D image (C), however, shows the small bowel loop (arrow) extending across the adjacent colon. At 3D (D), the preservation of the overlying colonic fold is a sign that the lesion is caused by extrinsic impression.
Anorectal Pitfalls
There are a number of findings specific to the anorectum that deserve attention because they are relatively common and can mimic neoplastic disease.6 Most incidental findings will not require further evaluation, whereas some may require correlation with digital rectal examination, anoscopy, proctoscopy, or flexible sigmoidoscopy. The first key is awareness of anorectal-specific pathology. In many ways, the anorectal region represents the most important source of pitfalls at CTC, since common incidental findings may distract the reader from important underlying pathology, which may be relatively subtle due its specific location. The anorectal pitfalls that seem to cause the most trouble at CTC interpretation are hypertrophied anal papillae, internal hemorrhoids, the rectal balloon catheter, and low rectal tumors. A variety of other anorectal pathology is much less commonly encountered.6 Of note, because the anal canal itself is not properly evaluated at CTC, digital rectal examination should still be performed as part of the colorectal screening process.
Hypertrophied anal papillae represent focal fibrous protrusions at the dentate line that essentially represent internal skin tags. These typically have a polypoid sessile appearance and measure 5–6 mm or less, but can rarely attain a larger size and become pedunculated. The key to recognition for a typical anal papilla is its constant anatomic location at the anorectal junction, virtually always abutting the rectal catheter at its lowest visualized point. In comparison, low-lying rectal polyps will almost always show at least some separation from the anorectal junction.
Internal hemorrhoids are a relatively common finding and represent dilated vascular structures above the dentate line. Internal hemorrhoids may present clinically with bleeding or prolapse. When advanced or thrombosed, internal hemorrhoids may appear polypoid or mass-like at CTC (Fig. 20). Circumferential involvement around the rectal catheter is often seen in prominent cases. The soft tissue fullness from internal hemorrhoids often appears prominent on transverse 2D images but is often less mass-like on other 2D planes or the 3D endoluminal view, and may change with patient position. In contrast to hemorrhoids, rectal varices have a tubular, serpiginous appearance.
FIGURE 20. Interpretive pitfalls related to the anorectal, ileocecal valve, and appendiceal regions within the same case.
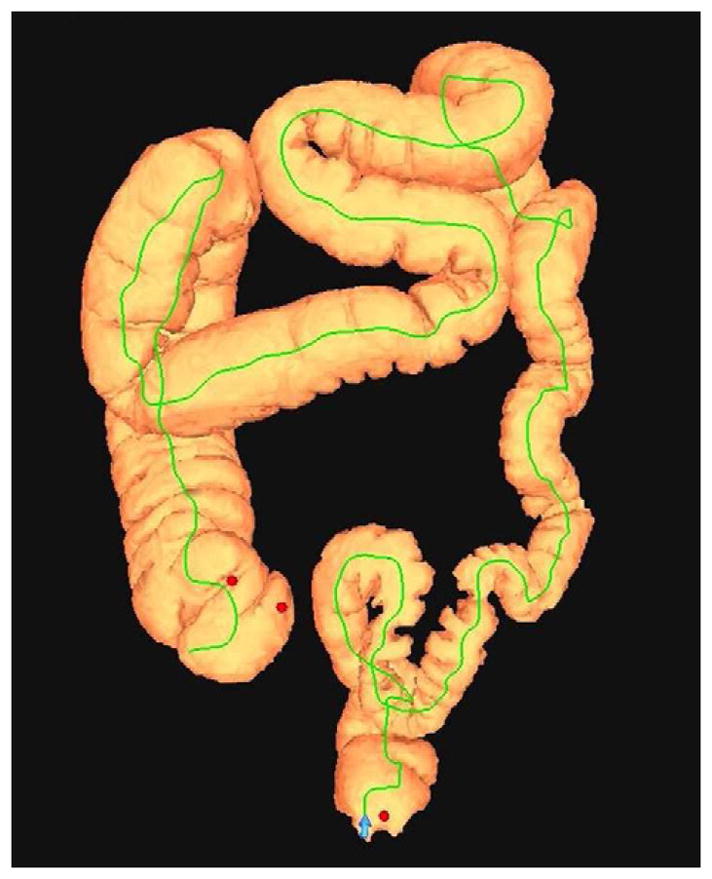
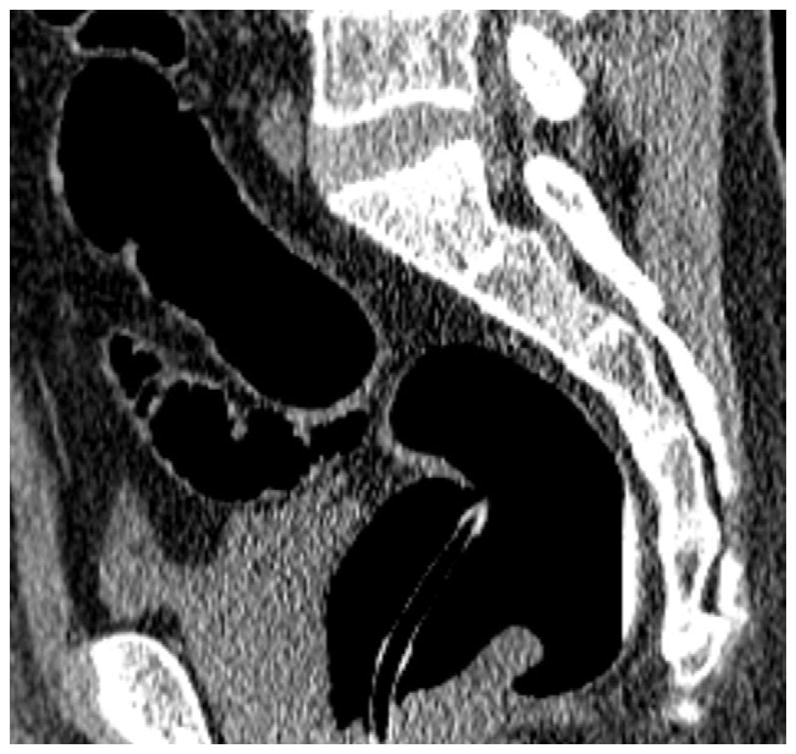
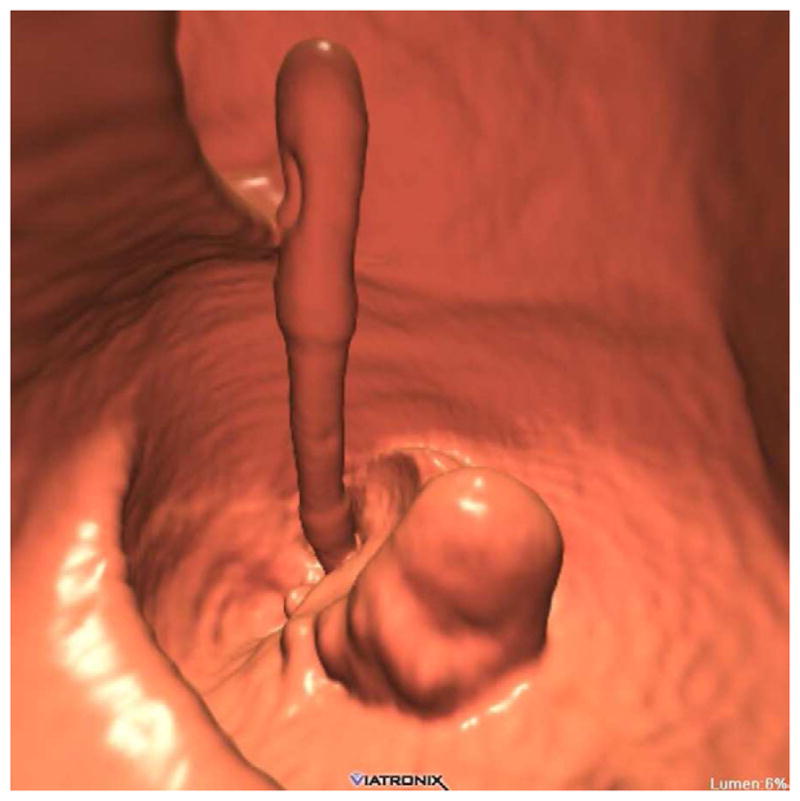
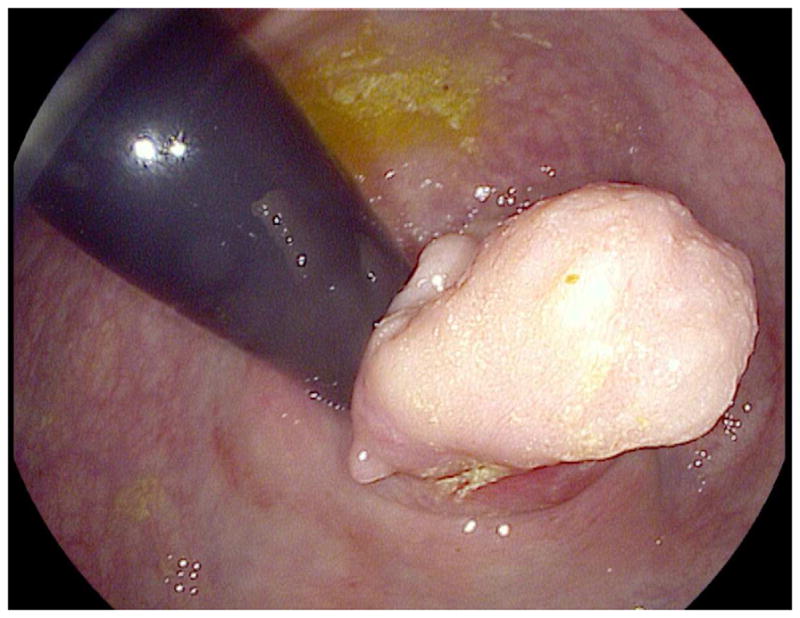
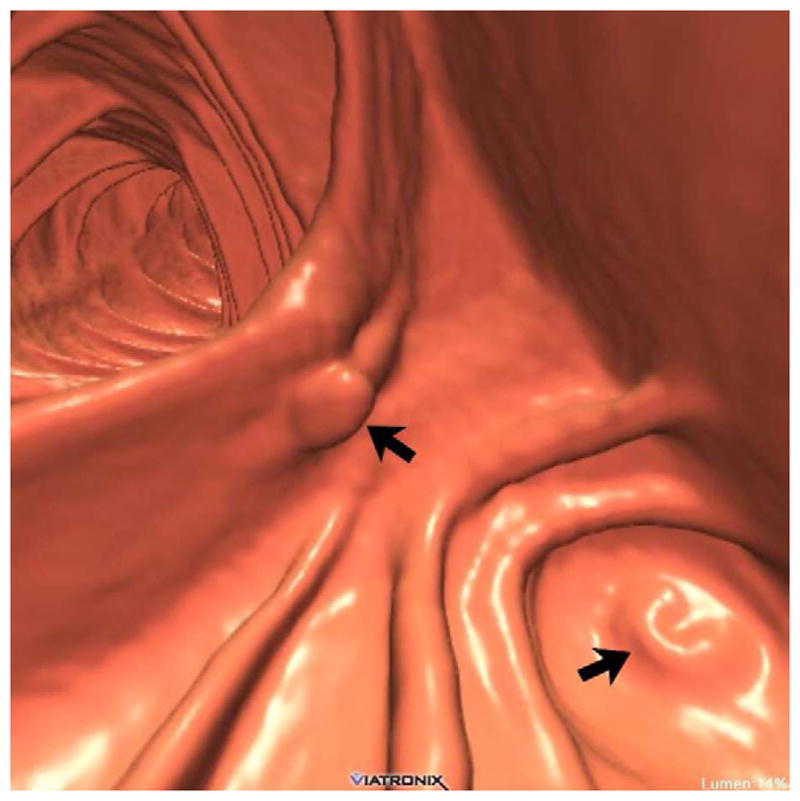
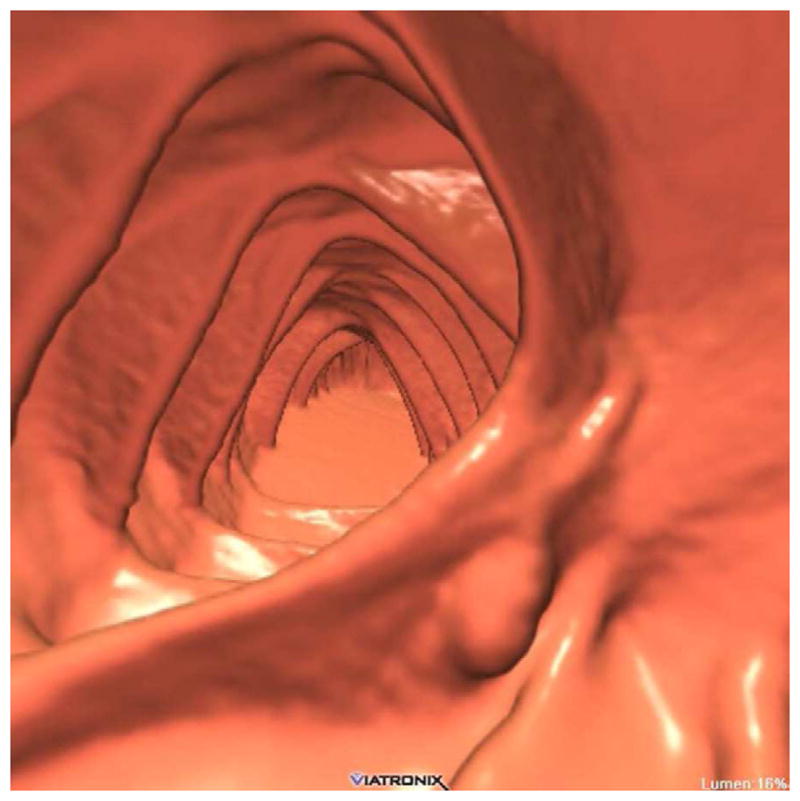
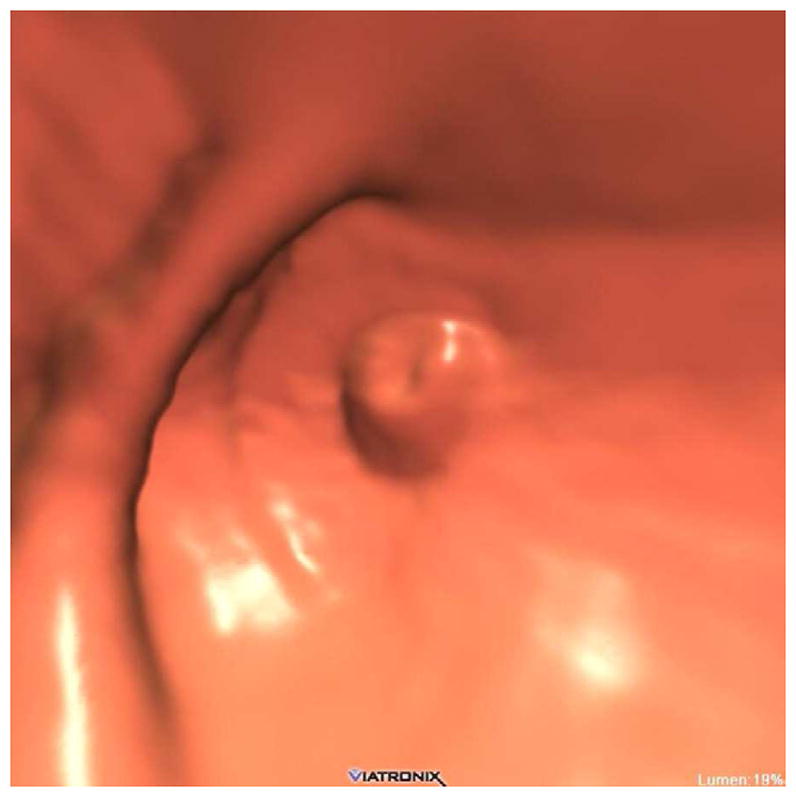
The 3D colon map shows three bookmarks (red dots) denoting focal findings in the regions of the anorectum, ileocecal valve, and the appendiceal orifice. Sagittal 2D (B and C) and 3D endoluminal (D) CTC images show an unusual 3–4 cm soft tissue mass extending up from the anorectal region. Note the mass effect upon the lesion from the adjacent balloon on the rectal catheter. The mass was confirmed at OC (E) but endoscopic biopsies were inconclusive. After transanal excision, a hemorrhoid with organizing thrombosis was confirmed. Focal abnormalities (arrows) were also noted at the ileocecal valve (F and G) and the appendiceal orifice (F and H). At OC, an inflammatory polyp on the ileocecal valve and a small inverted appendiceal stump were confirmed.
The rectal catheter is a constant finding in the anorectal region at CTC. The associated balloon is nearly invisible except for the mass effect it exerts, often creating pseudolesions through contact with fluid or the rectal wall. The real issue with the rectal catheter at CTC, however, is its potential for obscuring a pathologic lesion. Although this is of particular concern with the use of large-caliber catheters with large retention cuffs, it remains an issue with the CTC-specific small flexible balloon catheters.56 To minimize the risk of effacing true pathology in the anorectal region, we advocate the practice of deflating the balloon immediately prior to obtaining the second CTC series (typically the prone).
Of greatest concern among anorectal pitfalls if the possibility of missing a low rectal tumor or anal cancer. Low rectal polyps and masses can mimic some of the aforementioned incidental anorectal lesions, and can also be effaced by the balloon catheter. Careful attention to this area should be considered a routine part of CTC interpretation. Additional anorectal pathology that can be encountered at CTC is discussed in more detail elsewhere.5
Ileocecal Valve Pitfalls
Given its polypoid or mass-like appearance, confident assessment of the ileocecal valve at CTC seems to be an initial concern for many novice readers.57 The valve itself is easy to identify given its constant anatomic location relative to the cecum and terminal ileum. However, there is a relatively wide range of normal appearances to the ileocecal valve, ranging from a bulbous papillary or polypoid appearance to a more labial appearance. The overall morphology of the valve is much easier to appreciate on the 3D endoluminal view compared with 2D projections, where it is much more difficult to exclude a superimposed polyp. As with the anorectal region, it is good practice to specifically interrogate the ileocecal valve in each case, assessing both its morphology and composition. A diffusely fatty valve or focal fatty projection on 3D translucency rendering or 2D evaluation is reassuring since a focal soft tissue abnormality can be easily excluded. A focal soft tissue protuberance on or adjacent to a fatty valve is suspicious for a true polyp (Fig. 20). A mass involving or replacing the valve itself can present a more challenging problem (Fig. 10). The ileocecal valve is a relatively common location to see a drip of contrast, which can superficially mimic a polyp on the 3D display. With time and experience, recognizing the range of normal and knowing when to suspect an abnormality on or near the valve eventually become easier tasks.
Appendiceal Pitfalls
As with the ileocecal valve, the vermiform appendix represents another anatomic structure that can give rise to a number of unique findings at CTC interpretation, most notably false polyps and appendiceal neoplasms. As part of our general intake form, we obtain a surgical history on all patients when scheduling the CTC examination. Knowledge of prior appendectomy is useful because an inverted appendiceal stump can mimic a true cecal polyp at CTC, and even at OC (Fig. 20).58 In some cases, confident distinction between stump and true polyp is not possible and endoscopic evaluation is unavoidable. In patients without a history of appendectomy, partial invagination or rarely even complete intussusception may give rise to an intraluminal polypoid lesion. In these cases, continuity of the remainder of the appendix in its expected location prevents misdiagnosis. The appendiceal orifice is usually easily identified at CTC, allowing for detection of true polyps that are adjacent to but separate from the appendix. The most common primary tumor of the appendix to present as an incidental imaging finding in adults is a benign mucinous adenoma, which manifests as a mucocele from cystic dilatation of the appendix.59,60 In most cases, OC confirmation of a CTC-detected appendiceal mucocele is not necessary and may even be negative or lead to confusion, given the extraluminal nature of appendiceal tumors. Surgery is indicated for appendiceal mucoceles since almost all lesions are neoplastic (and mucinous) and are considered at least potentially malignant. CTC represents an ideal study for both detection and preoperative assessment of these tumors.
Summary
In this review, we have covered a wide array of potential pitfalls at CTC interpretation. We have tried to indicate which pitfalls can be avoided altogether, as well as those that cannot always be avoided but should be recognized as such to prevent mismanagement. In actual practice, the true challenges may come from cases in which more than one pitfall intersects on the same case. Fortunately, the built-in redundancy of 2D and 3D CTC interpretation allows for ample opportunity for accurate lesion detection in most cases. With proper attention to technique, including patient preparation, colonic distention, and scanning protocol, in addition to a combined 2D-3D interpretive strategy, the vast majority of these potential pitfalls can be handled appropriately.
Key Points.
A wide array of potential pitfalls exist for interpretation of CTC
Interpretive pitfalls at CTC can be divided in those related to technique and those related to anatomic considerations, although considerable overlap exists
Recognition and understanding of the major interpretive pitfalls are the most important steps in avoiding many of them
Robust preparation, distention, scanning, and interpretation techniques will greatly minimize or avoid many pitfalls at CTC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 2.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal Cancer: CT Colonography and Colonoscopy for Detection--Systematic Review and Meta-Analysis. Radiology. 2011;259:393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189:290–8. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 4.Pickhardt PJ, Kim DH. CT colonography (virtual colonoscopy): a practical approach for population screening. Radiol Clin North Am. 2007;45:361–75. doi: 10.1016/j.rcl.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Pickhardt PJ, Kim DH. CT colonography: principles and practice of virtual colonoscopy. Philadelphia: Saunders; 2010. Potential pitfalls at CTC Interpretation. [Google Scholar]
- 6.Pickhardt PJ. Differential diagnosis of polypoid lesions seen at CT colonography (virtual colonoscopy) Radiographics. 2004;24:1535–56. doi: 10.1148/rg.246045063. [DOI] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Choi JHR. Electronic cleansing and stool tagging in CT colonography: Advantages and pitfalls with primary three-dimensional evaluation. American Journal of Roentgenology. 2003;181:799–805. doi: 10.2214/ajr.181.3.1810799. [DOI] [PubMed] [Google Scholar]
- 8.Pickhardt PJ. Translucency rendering in 3D endoluminal CT colonography: A useful tool for increasing polyp specificity and decreasing interpretation time. American Journal of Roentgenology. 2004;183:429–36. doi: 10.2214/ajr.183.2.1830429. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PJ. Colonic preparation for computed tomographic colonography: Understanding the relative advantages and disadvantages of a noncathartic approach. Mayo Clinic Proceedings. 2007;82:659–61. doi: 10.4065/82.6.659. [DOI] [PubMed] [Google Scholar]
- 10.Morrin MM, Farrell RJ, Kruskal JB, Reynolds K, McGee JB, Raptopoulos V. Utility of intravenously administered contrast material at CT colonography. Radiology. 2000;217:765–71. doi: 10.1148/radiology.217.3.r00nv42765. [DOI] [PubMed] [Google Scholar]
- 11.Zalis ME, Perumpillichira JJ, Magee C, Kohlberg G, Hahn PF. Tagging-based, electronically cleansed CT colonography: Evaluation of patient comfort and image readability. Radiology. 2006;239:149–59. doi: 10.1148/radiol.2383041308. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt P, Kim D, Taylor A, Husain S. Complementary shifting of luminal fluid between supine and prone positioning at CT colonography: implications for 3D mucosal coverage. 8th International VC Symposium; 2007; Boston, MA. 2007. [Google Scholar]
- 13.Lawrence E, Pickhardt P. Comparison of High-Volume PEG Lavage with Low-Volume CT Colonography Bowel Preparations Utilizing Oral Contrast at Optical Colonoscopy. American Journal of Roentgenology. 2009:192. [Google Scholar]
- 14.Buchach CM, Kim DH, Pickhardt PJ. Performing an additional decubitus series at CT colonography. Abdominal Imaging. 2011;36:538–44. doi: 10.1007/s00261-010-9666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi M, Taylor AJ, VonBerge JL, Bartels CM, Pickhardt PJ. Can the CT scout reliably assess for adequate colonic distention at CT colonography? American Journal of Roentgenology. 2005;184:21–2. [Google Scholar]
- 16.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. American Journal of Roentgenology. 2006;186:1491–6. doi: 10.2214/AJR.05.0416. [DOI] [PubMed] [Google Scholar]
- 17.Boyce CJ, Vetter JR, Pickhardt PJ. MDCT artifact related to the intra-scan gravitational flow of opacified luminal fluid (the “Dense Waterfall” sign) Abdominal Imaging. 2011 doi: 10.1007/s00261-011-9731-z. [DOI] [PubMed] [Google Scholar]
- 18.Pickhardt PJ. Pictorial review of colonic polyp and mass distortion and recognition with the CT virtual dissection technique (invited commentary) Radiographics. 2010:30. doi: 10.1148/rg.e42. [DOI] [PubMed] [Google Scholar]
- 19.Pickhardt PJ, Taylor AJ, Gopal DV. Surface visualization at 3D endoluminal CT colonography: Degree of coverage and implications for polyp detection. Gastroenterology. 2006;130:1582–7. doi: 10.1053/j.gastro.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Pickhardt PJ, Schumacher C, Kim DH. Polyp Detection at 3-Dimensional Endoluminal Computed Tomography Colonography: Sensitivity of One-Way Fly-Through at 120 Degrees Field-of-View Angle. Journal of Computer Assisted Tomography. 2009;33:631–5. doi: 10.1097/RCT.0b013e31819778ea. [DOI] [PubMed] [Google Scholar]
- 21.Radiation risk in perspective: position statement of the Health Physics Society. Health Physics Society; Adopted January 1996, revised July 2010. [Google Scholar]
- 22.Lubner MG, Pickhardt PJ, Tang J, Chen G-H. Reduced Image Noise at Low-Dose Multidetector CT of the Abdomen with Prior Image Constrained Compressed Sensing Algorithm. Radiology. 2011;260:248–56. doi: 10.1148/radiol.11101380. [DOI] [PubMed] [Google Scholar]
- 23.Flicek KT, Hara AK, Silva AC, Wu Q, Peter MB, Johnson CD. Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: A pilot study. AJR Am J Roentgenol. 2010;195:126–31. doi: 10.2214/AJR.09.3855. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor SD, Summers RM, Choi JR, Pickhardt PJ. Oral contrast adherence to polyps on CT colonography. Journal of Computer Assisted Tomography. 2006;30:51–7. doi: 10.1097/01.rct.0000191686.35968.f1. [DOI] [PubMed] [Google Scholar]
- 25.Cotton PB, Durkalski VL, Benoit PC, et al. Computed tomographic colonography (virtual colonoscopy) - A multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. Jama-Journal of the American Medical Association. 2004;291:1713–9. doi: 10.1001/jama.291.14.1713. [DOI] [PubMed] [Google Scholar]
- 26.Johnson CD, Harmsen WS, Wilson LA, et al. Prospective blinded evaluation of computed tomographic colonography for screen detection of colorectal polyps. Gastroenterology. 2003;125:311–9. doi: 10.1016/s0016-5085(03)00894-1. [DOI] [PubMed] [Google Scholar]
- 27.Rockey DC, Poulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305–11. doi: 10.1016/S0140-6736(05)17784-8. [DOI] [PubMed] [Google Scholar]
- 28.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–8. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 29.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT Colonography. American Journal of Roentgenology. 2007;189:1451–6. doi: 10.2214/AJR.07.2291. [DOI] [PubMed] [Google Scholar]
- 30.Park SH, Choi EK, Lee SS, et al. Polyp measurement reliability, accuracy, and discrepancy: Optical colonoscopy versus CT colonography with pig colonic specimens. Radiology. 2007;244:157–64. doi: 10.1148/radiol.2441060794. [DOI] [PubMed] [Google Scholar]
- 31.Barancin C, Pickhardt P, Kim D, et al. Prospective Blinded Comparison of Polyp Size on Computed Tomography Colonography and Endoscopic Colonoscopy. Clin Gastroenterol Hepatol. 2011 doi: 10.1016/j.cgh.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Pickhardt PJ, Lee AD, McFarland EG, Taylor AJ. Linear polyp measurement at CT colonography: in vitro and in vivo comparison of two-dimensional and three-dimensional displays. Radiology. 2005;236:872–8. doi: 10.1148/radiol.2363041534. [DOI] [PubMed] [Google Scholar]
- 33.Barancin C, Pickhardt PJ, Kim DH, et al. Prospective blinded study of polyp size on CT colonography and various endoscopoic measures. Gastrointestinal Endoscopy. 2008;67:AB305-AB. [Google Scholar]
- 34.Yeshwant SC, Summers RM, Yao JH, Brickman DS, Choi JR, Pickhardt PJ. Polyps: Linear and volumetric measurement at CT colonography. Radiology. 2006;241:802–11. doi: 10.1148/radiol.2413051534. [DOI] [PubMed] [Google Scholar]
- 35.Pickhardt PJ, Lehman VT, Winter TC, Taylor AJ. Polyp volume versus linear size measurements at CT colonography: Implications for noninvasive surveillance of unresected colorectal lesions. American Journal of Roentgenology. 2006;186:1605–10. doi: 10.2214/AJR.05.0760. [DOI] [PubMed] [Google Scholar]
- 36.Blake ME, Soto JA, Hayes RA, Ferrucci JT. Automated volumetry at CT colonography: A phantom study. Academic Radiology. 2005;12:608–13. doi: 10.1016/j.acra.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Sanford MF, Pickhardt PJ. Diagnostic performance of primary 3-dimensional computed tomography colonography in the setting of colonic diverticular disease. Clinical Gastroenterology and Hepatology. 2006;4:1039–47. doi: 10.1016/j.cgh.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Tendler DA, Aboudola S, Zacks JF, O’Brien MJ, Kelly CP. Prolapsing mucosal polyps: an underrecognized form of colonic polyp--a clinicopathological study of 15 cases. Am J Gastroenterol. 2002;97:370–6. doi: 10.1111/j.1572-0241.2002.05472.x. [DOI] [PubMed] [Google Scholar]
- 39.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: A consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 40.Pickhardt PJ, Choi JR, Nugent PA, Hwang I, Schindler WR. Flat lesions at virtual and optical colonoscopy: Prevalence, histology, and sensitivity for detection in an asymptomatic screening population. American Journal of Roentgenology. 2004;182:74–5. [Google Scholar]
- 41.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. New England Journal of Medicine. 2007;357:1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 42.Fidler JL, Johnson CD, MacCarty RL, Welch TJ, Hara AK, Harmsen WS. Detection of flat lesions in the colon with CT colonography. Abdominal Imaging. 2002;27:292–300. doi: 10.1007/s00261-001-0171-z. [DOI] [PubMed] [Google Scholar]
- 43.Pickhardt PJ, Choi JR, Hwang I, Schindler WR. Nonadenomatous polyps at CT colonography: Prevalence, size distribution, and detection rates. Radiology. 2004;232:784–90. doi: 10.1148/radiol.2323031614. [DOI] [PubMed] [Google Scholar]
- 44.Waye JD, Bilotta JJ. Rectal Hyperplastic Polyps - Now You See Them, Now You Dont - a Differential Point. American Journal of Gastroenterology. 1990;85:1557–9. [PubMed] [Google Scholar]
- 45.Summers RM, Liu J, Yao J, Brown L, Choi JR, Pickhardt PJ. Automated Measurement of Colorectal Polyp Height at CT Colonography: Hyperplastic Polyps Are Flatter Than Adenomatous Polyps. American Journal of Roentgenology. 2009;193:1305–10. doi: 10.2214/AJR.09.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornett D, Barancin C, Roeder B, et al. Findings on optical colonoscopy after positive CT colonography exam. Am J Gastroenterol. 2008;103:2068–74. doi: 10.1111/j.1572-0241.2008.01919.x. [DOI] [PubMed] [Google Scholar]
- 47.MacCarty RL, Johnson CD, Fletcher JG, Wilson LA. Occult colorectal polyps on CT colonography: Implications for surveillance. American Journal of Roentgenology. 2006;186:1380–3. doi: 10.2214/AJR.05.0031. [DOI] [PubMed] [Google Scholar]
- 48.Pickhardt PJ, Wise SM, Kim DH. Positive predictive value for polyps detected at screening CT colonography. European Radiology. 2010 doi: 10.1007/s00330-009-1704-z. [DOI] [PubMed] [Google Scholar]
- 49.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. Jama-Journal of the American Medical Association. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien MJ, Winawer SJ, Zauber AG, et al. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenterol Hepatol. 2004;2:905–11. doi: 10.1016/s1542-3565(04)00392-1. [DOI] [PubMed] [Google Scholar]
- 51.Pickhardt PJ, Kim DH, Robbins JB. Flat (Nonpolypoid) Colorectal Lesions Identified at CT Colonography in a US Screening Population. Academic Radiology. 2010;17:784–90. doi: 10.1016/j.acra.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointestinal Endoscopy. 2001;54:62–6. doi: 10.1067/mge.2001.115729. [DOI] [PubMed] [Google Scholar]
- 53.Rubesin S, Saul S, Laufer I, Levine M. Carpet lesions of the colon. Radiographics. 1985;5:537–52. [Google Scholar]
- 54.Pickhardt PJ, Kim DH, Menias CO, Gopal DV, Arluk GM, Heise CP. Evaluation of submucosal lesions of the large intestine: part 1. Neoplasms Radiographics. 2007;27:1681–92. doi: 10.1148/rg.276075027. [DOI] [PubMed] [Google Scholar]
- 55.Pickhardt PJ, Kim DH, Menias CO, Gopal DV, Arluk GM, Heise CP. Evaluation of submucosal lesions of the large intestine: part 2. Nonneoplastic causes Radiographics. 2007;27:1693–703. doi: 10.1148/rg.276075028. [DOI] [PubMed] [Google Scholar]
- 56.Pickhardt PJ, Choi JR. Adenomatous polyp obscured by small-caliber rectal catheter at low-dose CT colonography: A rare diagnostic pitfall. American Journal of Roentgenology. 2005;184:1581–3. doi: 10.2214/ajr.184.5.01841581. [DOI] [PubMed] [Google Scholar]
- 57.Iafrate F, Rengo M, Ferrari R, Paolantonio P, Celestre M, Laghi A. Spectrum of normal findings, anatomic variants and pathology of ileocecal valve: CT colonography appearances and endoscopic correlation. Abdom Imaging. 2007;32:589–95. doi: 10.1007/s00261-007-9198-0. [DOI] [PubMed] [Google Scholar]
- 58.Prout TM, Taylor AJ, Pickhardt PJ. Inverted appendiceal stumps simulating large pedunculated polyps on screening CT colonography. American Journal of Roentgenology. 2006;186:535–8. doi: 10.2214/AJR.04.1791. [DOI] [PubMed] [Google Scholar]
- 59.Pickhardt PJ, Levy AD, Rohrmann CA, Kende AI. Primary neoplasms of the appendix: Radiologic spectrum of disease with pathologic correlation. Radiographics. 2003;23:645–62. doi: 10.1148/rg.233025134. [DOI] [PubMed] [Google Scholar]
- 60.Pickhardt PJ, Kim DH, Taylor AJ, Gopal DV, Weber SM, Heise CP. Extracolonic tumors of the gastrointestinal tract detected incidentally at screening CT colonography. Diseases of the Colon & Rectum. 2007;50:56–63. doi: 10.1007/s10350-006-0806-9. [DOI] [PubMed] [Google Scholar]


