Abstract
Objectives
Fibrin makes up the structural basis of an occlusive arterial thrombus and variability in fibrin phenotype relates to cardiovascular risk. The aims of the current study from the EU consortium EuroCLOT were to 1) determine the heritability of fibrin phenotypes and 2) identify QTLs associated with fibrin phenotypes.
Methods
447 dizygotic (DZ) and 460 monozygotic (MZ) pairs of healthy UK Caucasian female twins and 199 DZ twin pairs from Denmark were studied. D-dimer, an indicator of fibrin turnover, was measured by ELISA and measures of clot formation, morphology and lysis were determined by turbidimetric assays. Heritability estimates and genome-wide linkage analysis were performed.
Results
Estimates of heritability for d-dimer and turbidometric variables were in the range 17 - 46%, with highest levels for maximal absorbance which provides an estimate of clot density. Genome-wide linkage analysis revealed 6 significant regions with LOD>3 on 5 chromosomes (5, 6, 9, 16 and 17).
Conclusions
The results indicate a significant genetic contribution to variability in fibrin phenotypes and highlight regions in the human genome which warrant further investigation in relation to ischaemic cardiovascular disorders and their therapy.
Keywords: linkage, quantitative trait loci, twin, cardiovascular disease, thrombosis
Introduction
The EuroCLOT consortium is an EU-funded multi-centre collaborative study established to identify QTLs associated with fibrin clot phenotypes to shed light on the pathogenic mechanisms of ischaemic cardiovascular disease (stroke and myocardial infarction). In both myocardial infarction and ischaemic stroke, acute thrombosis is the key final step leading to tissue damage in the territory served by the vessel. Increased levels of haemostatic factors, including fibrinogen1 and tissue plasminogen activator (tPA)2 predict development of cardiovascular disorders and outcome.
Activation of the coagulation cascade results in thrombin generation and cleavage of fibrinogen to form fibrin monomers, which polymerise and are cross-linked by thrombin-activated factor XIII, to form the structural scaffold for thrombus formation. Fibrinolysis of fibrin clots is influenced by various factors including the fibrin binding characteristics of tPA/plasminogen, local concentrations of fibrinolysis inhibitors and the structure of the clot. For example, studies in vitro have shown that dense clots composed of thinner fibres lyse more slowly than less dense clots formed from thicker fibres3, 4. Alterations in clot structure/function, including increased clot density and decreased clot lysis times, have been observed in subjects with arterial and venous thrombosis5, 6 although the association with clinical outcome in healthy volunteers is unclear at present. We have recently shown that genetic factors contribute to variance in turbidimetric measures of clot structure/function7 and numerous studies have demonstrated genetic influences on proteins involved in coagulation and fibrinolysis. Furthermore, genetic factors have been estimated to account for approximately 60% of the risk of thrombosis8. Consequently the identification of genetic loci influencing clot structure/function may further our understanding of the underlying factors predisposing to occlusive vascular diseases.
The aims of the current study from EuroCLOT were to 1) determine the heritability of fibrin phenotypes and 2) identify QTLs associated with fibrin phenotypes in healthy twin volunteers from the UK and Denmark.
Materials and Methods
Subject recruitment and sample handling
The Twins UK Adult Twin Registry9 and the Danish Twin Registries study on endophenotypes10 provided the subjects for this study (please see http://atvb.ahajournals.org.). Venous blood was drawn at the participating centres following a standard protocol for venepuncture and sample processing for citrated plasma as previously reported11. EDTA-anticoagulated whole blood samples were also obtained for DNA extraction. Citrated plasma aliquots, for analysis of D-dimer and turbidimetric fibrin phenotypes, were snap frozen in liquid nitrogen then stored at −80°C. Samples were batched up and transported on dry ice to the University of Leeds for phenotyping, (see below). Genotyping for linkage analysis was performed by Gemini-Sequenom, Cambridge (UK samples) and by Genotyping Centre Helsinki, as part of GenomEUtwin (Denmark samples)12. Genotyping and phenotyping raw data were sent to King’s College London for collation and analysis.
Laboratory methods
Fibrin Phenotypes
D-dimer levels were determined according to the manufacturers’ instructions using TintElize(r) D-dimer ELISA kit, (Biopool, Umea, Sweden). Inter- and intraassay CVs were 5.6 and 3.3% respectively.
The high throughput turbidimetric assay of clot structure using customised software was performed as described elsewhere7. The following variables were analysed: LagC, (which represents the time at which sufficient protofibrils have formed to enable lateral aggregation, was taken as the time point at which an exponential increase in absorbance occurred), MaxAbsC (a measure of clot density reflected by the absorbance at which 3 consecutive readings were identical corrected for the LagC absorbance), Lys50t0 (calculated as the time from initiation of clot formation to the time at which a 50% fall in absorbance from MaxAbsL occurred) and AUC (area under the curve, reflecting the balance between coagulation and fibrinolysis) The inter-assay CVs for LagC, MaxAbsC, Lys50t0 and AUC were 8%, 4%, 7.0% and 16%, respectively.
Genotyping
Standard methods were used for linkage genotyping (http://atvb.ahajournals.org.).
Statistical analysis
Heritability Estimates
For each phenotype (D-dimer, LagC, MaxAbsC, Lys50t0 and AUC) data were transformed using a box cox transformation to make their distributions approximately normal. Observations that were more than 3 standard deviations away from the mean were considered outliers and excluded from the analyses 13. Modelling for heritability assumes phenotypic variance to be due to three latent factors namely additive polygenic effects (A), common environment (C) and specific individual effects and measurement error (E). Please see http://atvb.ahajournals.org.
Linkage Analysis
Genotype and phenotype data were available on 447 UK DZ pairs and 199 DZ Danish pairs. Phenotype data (D-dimer, LagC, MaxAbsC, Lys50t0 and AUC) were analyzed using R13. Data were transformed to optimize closeness to normality using a Box-Cox transformation using the box.cox.power function in the car package14, 15, to reduce the type I errors while preserving power for phenotypes having skewed distribution16. For details of joint linkage analysis please see http://atvb.ahajournals.org.
Results
Initially 2422 UK and 429 Danish twins were recruited to the study. Following genotyping and phenotyping and the removal of outliers as described above, complete data were available on 1814 UK female twins (447 DZ pairs and 460 MZ pairs), and 199 unselected Danish DZ twin pairs (Table 1).
Table 1.
Characteristics of UK and Danish twins and their phenotypes
| Population | UK twins | Denmark twins |
|---|---|---|
| Sample size | 1814 | 398 |
| % females | 100 | 50.3 |
| No MZ pairs | 447 | 0 |
| No DZ pairs | 460 | 199 |
| Age/mean (range) yr | 57 (48 – 64) | 36 (29 – 49) |
| Ddimer/median (IQ range)ng/ml |
77 (56 – 112) | 64 (48 – 87) |
| Lys50t0/median (IQ range) | 1716 (1531 – 1975) | 1377 (1270 – 1542) |
| AUC/median (IQ range) | 366 (250 – 533) | 302 (224 – 415) |
| Lagc/median (IQ range) | 343 (297 – 394) | 282 (250 -321) |
| MaxAbsc/median (IQ range) | 0.41 (0.35 – 0.47) | 0.31 (0.25 – 0.37) |
MZ represents monozygotic, DZ dizygotic, yr years, IQ interquartile
Phenotypes are defined in the Methods
Heritability Estimates
Narrow sense heritability (proportion of phenotypic variance attributable to additive genetic factors) requires data from both MZ and DZ twins so the UK twin sample was used. Examination of the ACE model and its sub-models revealed, in each phenotype, that the ACE model provided the best explanation of the observed data (data not shown). Heritability estimates obtained from the ACE model were in the range 17-46% (Table 2). These results indicate a significant contribution of additive genetic factors to variance in fibrin clot structure and function as measured by the 5 phenotypes (Table 2, column A).
Table 2.
Estimates for additive genetics effects (A), common environment (C) and specific individual effects and experimental error (E) and 95% confidence intervals for UK twins
| Phenotype | A | C | E |
|---|---|---|---|
| D-dimer | 25% (6% - 44%) | 18% (3% - 33%) | 57% (50% - 64%) |
| Lys50t0 | 23% (11% – 35 %) | 47% (37%- 55%) | 30% (27% – 35%) |
| AUC | 28% (15% - 40%) | 41% (30% - 51%) | 31% (28% - 37%) |
| Lagc | 17% (8% - 25%) | 62% (55% - 69%) | 21% (18% - 25%) |
| MaxAbsc | 46% (30% - 62%) | 15% (2% - 28%) | 39% (34% - 45%) |
Estimates shown are derived from the ACE model which was found to be the best fitting model for each phenotype
Linkage Analysis
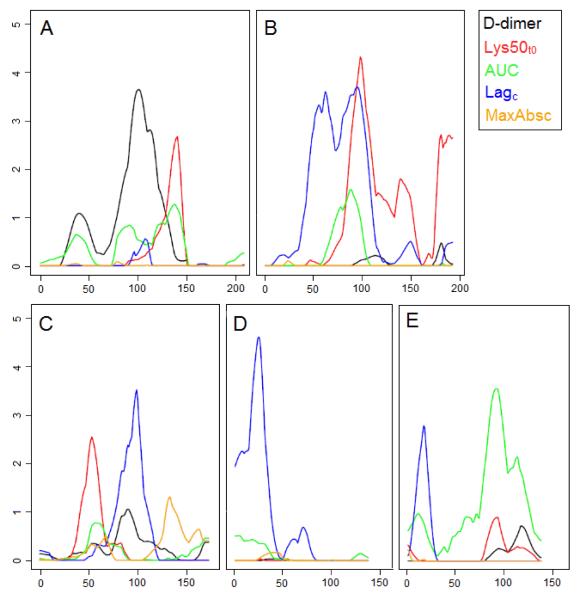
Genome-wide linkage analysis using the combined datasets identified evidence of linkage at a number of regions (Table 3). Regions with LOD>3 included Lagc on chromosome 6p22.2-q16.1 with peak LOD score 3.6 (Figure 1 panel B) at marker D6S426; on chromosome 9q22.1-q22.33 with maximum LOD=3.53 at marker D9S287 (Figure 1 panel C); chromosome 16p13.2-p13.13 with peak LOD score 4.57 (Figure 1 panel D) at marker D16S404. The first of these peaks, on chromosome 6, also showed linkage for Lys50t0 with a partially overlapping peak with LOD 4.2 at marker D6S462 (Figures 1 panel B). Evidence of linkage was also observed for AUC on chromosome 17q22-q24.3 with peak LOD score 3.51 (Figure 1) at marker D17S944 and for d-dimer on chromosome 5q14.1-q21.3 with LOD=3.51. Suggestive linkage17 was observed for Lagc on chromosomes 1, 3 and 17; for Lys50t0 on chromosomes 4, 5, 6, 9, 12 and 17; and for D-dimer on chromosome 14 (Table 3).
Table 3.
Details of the linkage peaks having LOD>2 for the DZ twins from UK and Denmark
| Chromosome | Trait | Flanking Markers* | Peak-LOD |
|---|---|---|---|
| 1p13.2-1q21.3 | Lagc | WIAF_3798 - WIAF_3272 | 2.27 |
| 3p26.3-3p25.3 | Lagc | D3S4559 - D3S1263 | 2.17 |
| 4p16.1-4p15.1 | Lys50t0 | D4S2935 - WIAF_3923 | 2.01 |
| 5q14.1-q21.3 | d-dimer | WIAF_1721 - WIAF_3198 | 3.51 |
| 5q23.1-5q31.3 | Lys50t0 | WIAF_3210 - WIAF_2528 | 2.57 |
| 6p22.2-q16.1 | Lagc | D6S1691 - WIAF_577 | 3.60 |
| 6q14.1-16.1 | Lys50t0 | D6S460 - WIAF_577 | 4.20 |
| 6q26-q-ter | Lys50t0 | D6S1599 - q-ter | 2.63 |
| 9q22.1-q22.33 | Lagc | WIAF_2079 - D9S1690 | 3.53 |
| 9p21.2-9p13.1 | Lys50t0 | D9S171 - WIAF_975 | 2.55 |
| 12q24.32-q-ter | Lys50t0 | D12S1679 - q-ter | 2.45 |
| 14q24.1- 14q32.13 |
D-dimer | WIAF_2677 - WIAF_1602 | 2.62 |
| 15q13.3-q21.3 | MaxAbsC | WIAF_1283 - D15S117 | 2.18 |
| 16p13.2-p13.13 | Lagc | D16S423 - D16S3075 | 4.57 |
| 17p13.3- 17p13.1 |
Lagc | D17S831 - D17S945 | 2.76 |
| 17q22-q24.3 | AUC | D17S787 - D17S949 | 3.51 |
1cM confidence interval
Figure 1.
Linkage plots for the UK and Danish twins over 5 phenotypes
Plot A represents chromosome 5, plot B chromosome 6, plot C chromosome 9, plot D chromosome 16 and Plot E chromosome 17. X axis represents distance along chromosome in cM, Y axis LOD score.
Discussion
Over the last 20 years there has been considerable progress in understanding the contribution of abnormalities in haemostasis to the pathogenesis of vascular disease. Alterations in individual components of the coagulation and fibrinolytic pathways have been related to both cardio- and cerebrovascular disease in case control and prospective studies1, 2, 18. Evidence indicates genetic contributions to most aspects of these pathways19, 20 including a substantial genetic component to the structure and function of fibrin21,22. Taken together, these observations support the view that genetic factors contribute ultimately to vascular risk.
The heritability study shows varying degrees of genetic influence on fibrin phenotypes, with the strongest effect on MaxAbsc, a measure of clot density, at 46%. The heritabilities obtained are similar to those we have previously described in twin21 and family7 studies. These results confirm that the intermediate fibrin-related phenotypes are heritable and that a genome-wide approach offers a tractable strategy for the elucidation of underlying genetic factors. Multipoint linkage analysis of fibrin phenotypes revealed 6 chromosomal regions with significant linkage peaks (LOD scores >3.0) and a further 9 regions of suggestive linkage (LOD scores 2.0 – 3.0). The peaks having significant linkage did not harbour any obvious known structural genes for coagulation or fibrinolytic factors that might influence fibrin phenotypes.
The development of occlusive thrombotic disease is dependent on the formation of a cross-linked fibrin mesh which in the arterial system supports incorporation of platelets and platelet particles. The mechanisms involved in regulation of fibrin structure/function are complex and involve coagulation and fibrinolytic proteins, other plasma components and regulatory factors. Fibrin formation itself occurs following activation of the coagulation system with consequent thrombin generation, fibrinogen cleavage and activation of coagulation FXIII, all of which contribute to the final fibrin phenotype. That fibrin formed from plasma ex-vivo differs markedly from that formed by recombinant or purified fibrinogen23 suggests that additional plasma components contribute to the clot phenotype – mediated, perhaps, by diverse mechanisms. Preliminary data from the fibrin proteome indicate a range of immunoglobulins, inflammatory proteins and other plasma components associates with the fibrin matrix and could alter structure/function24, 25. Finally, regulatory factors influencing gene transcription, post-translational modification and protein degradation modify all processes leading to fibrin clot formation. For example, PAI-1 levels are related to a PAI-1 gene promoter polymorphism26, modulated by triglyceride levels27 and regulated by the transcriptional factor PPARγ28, 29 and components of the molecular clock30. Similarly, there are substantial data indicating that fibrinogen is regulated by complex genetic influences22.
The above should be borne in mind when interpreting the results. Although several highly significant linkage peaks were identified, there was little to suggest the peaks covered structural genes coding for coagulation or fibrinolytic proteins. There was, however, a suggestive linkage peak for Lys50t0 (6q26-q-ter) in proximity to genes encoding plasminogen and lipoprotein(a) - which have clearly defined roles in fibrinolysis31.
A variety of potentially important regulatory genes were identified. For the wide AUC 17q22-q24.3 peak a number of putative candidates were identified, including HLF (hepatic leukaemia factor) and GNA13 (guanine nucleotide binding protein α-13). HLF transactivates the genes encoding coagulation factors FVIII and FIX32 and GNA13 is involved in platelet signalling and deficiency in mice leads to decreased response to thrombin, thromboxane A2 and collagen and decreased thrombus formation under flow33. Furthermore, this region has been linked to clustering of metabolic syndrome components34. In addition, the 14q24.1-q32.13 D-dimer peak harbours a SERPIN cluster which includes SERPINA10 which inhibits Factors Xa and XIa and has been associated with thrombosis in a mouse model35. The 3p26.3-p25.3 peak for LagC contains SNPs associated with FVII and fibrinogen in the Framingham Heart Study36.
For several other linkage peaks putative candidate genes for thrombosis and/or cardiovascular disease were identified. The highest LOD scores were observed for Lagc and AUC (4.57 and 3.51) on chromosomes 16 and 17 respectively. The 16p13.2-p13.13 peak (Lagc) has been linked to type 1 diabetes (T1DM)37 and carotid artery calcified plaque in T2DM38. The 1p13.2-q21.3 peak includes 2 SNPs associated with LDL39. The 12q24.32-q-ter (Lys50t0) peak corresponds to a bivariate linkage locus for cholesterol and HDL40 and a locus for insulin resistance41 and contains a SNP associated with vWF36. The 6p22.2-q16.1 (LagC) peak is very wide and partially overlaps with the 6q14.1-q16.1 Lys50t0 peak. The former includes the lymphotoxin-α (LTA) gene - LTA SNPs have been associated with MI42. This region also corresponds to a QTL for LDL in a Samoan sample40 and 2 SNPs associated with vWF in the Framingham Heart Study36. Of particular note, the 9p21.2-p13.1 Lys50t0 peak corresponds with replicated loci for cardiovascular disease43 and T2DM44. Together, these results suggest mechanisms for linking genetic loci to cardiovascular risk through effects on fibrin structure/function.
This study contained an excess of women, present for historical reasons. There are significant gender differences in vascular risk, mediated by hormonal influences and environmental exposure and it is possible that a study of men might produce different results. Another potential limitation is that the samples comprised twins. However, twins have been shown to be comparable to singletons for many common traits and diseases45,46 and same sex dizygotic twin pairs actually offer considerable advantages in linkage studies because intra-pair age and sex differences are eliminated, and the effect of the individual-specific random environment is reduced.
Several steps need to be taken in EuroCLOT to link the current findings to vascular outcomes. These include fine mapping, prospective and case control studies of cardiovascular disease and functional studies of the genes and their encoded proteins. Ultimately, this work will further understanding of the mechanisms underlying the pathogenesis of occlusive vascular disease and could potentially provide novel therapeutic targets for the amelioration of the effects of these common disorders.
Supplementary Material
Acknowledgements
The EuroCLOT consortium would like to acknowledge all the twin pairs who gave up their time to contribute to this study, and the staff of TwinsUK, King’s College London, and at the Danish Twin Registry in Odense.. We would also like to acknowledge the EU Framework 6 support for funding EuroCLOT and genotyping by Sequenom/Gemini genomics, Cambridge and GenomEUtwin for genotyping the Danish twins. TwinsUK is also supported by an NIHR Biomedical Resource Centre grant to Guy’s and St Thomas’ NHS Foundation Trust and KCL. May Boothby and Jessica Surr from The Division of Cardiovascular and Diabetes Research in Leeds, UK are thanked for their contributions to this programme of work.
Reference List
- (1).Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984 Aug 23;311(8):501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- (2).Ridker PM. Plasma Concentration of Endogenous Tissue Plasminogen Activator and the Occurrence of Future Cardiovascular Events. J Thromb Thrombolysis. 1994;1(1):35–40. doi: 10.1007/BF01061993. [DOI] [PubMed] [Google Scholar]
- (3).Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000 May;20(5):1354–1361. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- (4).Collet JP, Lesty C, Montalescot G, Weisel JW. Dynamic changes of fibrin architecture during fibrin formation and intrinsic fibrinolysis of fibrin-rich clots. J Biol Chem. 2003 Jun 13;278(24):21331–21335. doi: 10.1074/jbc.M212734200. [DOI] [PubMed] [Google Scholar]
- (5).Collet JP, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, Blanchet B, Dumaine R, Gianetti J, Payot L, Weisel JW, Montalescot G. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006 Nov;26(11):2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- (6).Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005 Feb 1;105(3):1102–1105. doi: 10.1182/blood-2004-08-3253. [DOI] [PubMed] [Google Scholar]
- (7).Carter AM, Cymbalista CM, Spector TD, Grant PJ. Heritability of Clot Formation, Morphology, and Lysis. The EuroCLOT Study. Arterioscler Thromb Vasc Biol. 2007 Oct 11;27(12):2783–2789. doi: 10.1161/ATVBAHA.107.153221. [DOI] [PubMed] [Google Scholar]
- (8).Souto JC, Almasy L, Borrell M, Blanco-Vaca F, Mateo J, Soria JM, Coll I, Felices R, Stone W, Fontcuberta J, Blangero J. Genetic susceptibility to thrombosis and its relationship to physiological risk factors: the GAIT study. Genetic Analysis of Idiopathic Thrombophilia. Am J Hum Genet. 2000 Dec;67(6):1452–1459. doi: 10.1086/316903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Spector TD, Williams FM. The UK Adult Twin Registry (TwinsUK) Twin Res Hum Genet. 2006 Dec;9(6):899–906. doi: 10.1375/183242706779462462. [DOI] [PubMed] [Google Scholar]
- (10).Benyamin B, Sorensen TI, Schousboe K, Fenger M, Visscher PM, Kyvik KO. Are there common genetic and environmental factors behind the endophenotypes associated with the metabolic syndrome? Diabetologia. 2007 Sep;50(9):1880–1888. doi: 10.1007/s00125-007-0758-1. [DOI] [PubMed] [Google Scholar]
- (11).Freeman MS, Mansfield MW, Barrett JH, Grant PJ. Genetic contribution to circulating levels of hemostatic factors in healthy families with effects of known genetic polymorphisms on heritability. Arterioscler Thromb Vasc Biol. 2002 Mar 1;22(3):506–510. doi: 10.1161/hq0302.104906. [DOI] [PubMed] [Google Scholar]
- (12).Wilson SG, Reed PW, Bansal A, Chiano M, Lindersson M, Langdown M, Prince RL, Thompson D, Thompson E, Bailey M, Kleyn PW, Sambrook P, Shi MM, Spector TD. Comparison of genome screens for two independent cohorts provides replication of suggestive linkage of bone mineral density to 3p21 and 1p36. Am J Hum Genet. 2003 Jan;72(1):144–155. doi: 10.1086/345819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ihaka R, Gentleman R. R: A language for data analysis and graphics. J J Comp Graph Stat. 1996;5:299–314. [Google Scholar]; Journal of Computational and Graphical Statistics. 1996;5(3):299–314. [Google Scholar]
- (14).Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society. 1964;26:211–252. [Google Scholar]
- (15).Cook RD, Weisberg S. Applied regression including computing and graphics. Wiley; New York: 1999. [Google Scholar]
- (16).Etzel CJ, Shete S, Beasley TM, Fernandez JR, Allison DB, Amos CI. Effect of Box-Cox transformation on power of Haseman-Elston and maximum-likelihood variance components tests to detect quantitative trait Loci. Hum Hered. 2003;55(2-3):108–116. doi: 10.1159/000072315. [DOI] [PubMed] [Google Scholar]
- (17).Morton NE. Significance levels in complex inheritance. Am J Hum Genet. 1998 Mar;62(3):690–697. doi: 10.1086/301741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kannel WB. Overview of hemostatic factors involved in atherosclerotic cardiovascular disease. Lipids. 2005 Dec;40(12):1215–1220. doi: 10.1007/s11745-005-1488-8. [DOI] [PubMed] [Google Scholar]
- (19).Ariens RA, de Lange M, Snieder H, Boothby M, Spector TD, Grant PJ. Activation markers of coagulation and fibrinolysis in twins: heritability of the prethrombotic state. Lancet. 2002 Feb 23;359(9307):667–671. doi: 10.1016/S0140-6736(02)07813-3. [DOI] [PubMed] [Google Scholar]
- (20).de Lange M, Snieder H, Ariens RA, Spector TD, Grant PJ. The genetics of haemostasis: a twin study. Lancet. 2001 Jan 13;357(9250):101–105. doi: 10.1016/S0140-6736(00)03541-8. [DOI] [PubMed] [Google Scholar]
- (21).Dunn EJ, Ariens RA, de LM, Snieder H, Turney JH, Spector TD, Grant PJ. Genetics of fibrin clot structure: a twin study. Blood. 2004 Mar 1;103(5):1735–1740. doi: 10.1182/blood-2003-07-2247. [DOI] [PubMed] [Google Scholar]
- (22).Carter AM, Standeven KF, Grant PJ. Common genetic determinants of coagulation and fibrinolysis. In: Rimoin DL, Connor JM, Pyeritz RE, Korf BR, editors. Emery & Rimoin’s Principles and Practice of Medical Genetics. Churchill Livingstone Elsevier; Philadelphia: 2006. pp. 1316–1328. [Google Scholar]
- (23).Carr ME. Fibrin formed in plasma is composed of fibers more massive than those formed from purified fibrinogen. Thromb Haemost. 1988 Jun 16;59(3):535–539. [PubMed] [Google Scholar]
- (24).Howes JM, Keen JN, Findlay JB, Carter AM. The application of proteomics technology to thrombosis research: the identification of potential therapeutic targets in cardiovascular disease. Diab Vasc Dis Res. 2008 Sep;5(3):205–212. doi: 10.3132/dvdr.2008.033. [DOI] [PubMed] [Google Scholar]
- (25).Rijken DC, Dirkx SP, Luider TM, Leebeek FW. Hepatocyte-derived fibrinogen-related protein-1 is associated with the fibrin matrix of a plasma clot. Biochem Biophys Res Commun. 2006 Nov 10;350(1):191–194. doi: 10.1016/j.bbrc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- (26).Mansfield MW, Stickland MH, Grant PJ. Environmental and genetic factors in relation to elevated circulating levels of plasminogen activator inhibitor-1 in Caucasian patients with non-insulin-dependent diabetes mellitus. Thromb Haemost. 1995 Sep;74(3):842–847. [PubMed] [Google Scholar]
- (27).Eriksson P, Nilsson L, Karpe F, Hamsten A. Very-low-density lipoprotein response element in the promoter region of the human plasminogen activator inhibitor-1 gene implicated in the impaired fibrinolysis of hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 1998 Jan;18(1):20–26. doi: 10.1161/01.atv.18.1.20. [DOI] [PubMed] [Google Scholar]
- (28).Chen JG, Li X, Huang HY, Liu HL, Liu DG, Song TJ, Ma CG, Ma D, Song HY, Tang QQ. Identification of a peroxisome proliferator responsive element (PPRE)-like cis-element in mouse plasminogen activator inhibitor-1 gene promoter. Biochem Biophys Res Commun. 2006 Sep 1;347(3):821–826. doi: 10.1016/j.bbrc.2006.06.170. [DOI] [PubMed] [Google Scholar]
- (29).Zirlik A, Leugers A, Lohrmann J, Ernst S, Sobel BE, Bode C, Nordt TK. Direct attenuation of plasminogen activator inhibitor type-1 expression in human adipose tissue by thiazolidinediones. Thromb Haemost. 2004 Apr;91(4):674–682. doi: 10.1160/TH03-06-0384. [DOI] [PubMed] [Google Scholar]
- (30).Ohkura N, Oishi K, Fukushima N, Kasamatsu M, Atsumi GI, Ishida N, Horie S, Matsuda J. Circadian clock molecules CLOCK and CRYs modulate fibrinolytic activity by regulating the PAI-1 gene expression. J Thromb Haemost. 2006 Nov;4(11):2478–2485. doi: 10.1111/j.1538-7836.2006.02210.x. [DOI] [PubMed] [Google Scholar]
- (31).Angles-Cano E, de la Pena DA, Loyau S. Inhibition of fibrinolysis by lipoprotein(a) Ann N Y Acad Sci. 2001;936:261–275. doi: 10.1111/j.1749-6632.2001.tb03514.x. [DOI] [PubMed] [Google Scholar]
- (32).Begbie M, Mueller C, Lillicrap D. Enhanced binding of HLF/DBP heterodimers represents one mechanism of PAR protein transactivation of the factor VIII and factor IX genes. DNA Cell Biol. 1999 Feb;18(2):165–173. doi: 10.1089/104454999315556. [DOI] [PubMed] [Google Scholar]
- (33).Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, Gawaz M, Offermanns S. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003 Nov;9(11):1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- (34).Day IN, Chen XH, Gaunt TR, King TH, Voropanov A, Ye S, Rodriguez S, Syddall HE, Sayer AA, Dennison EM, Tabassum F, Barker DJ, Cooper C, Phillips DI. Late life metabolic syndrome, early growth, and common polymorphism in the growth hormone and placental lactogen gene cluster. J Clin Endocrinol Metab. 2004 Nov;89(11):5569–5576. doi: 10.1210/jc.2004-0152. [DOI] [PubMed] [Google Scholar]
- (35).Zhang J, Tu Y, Lu L, Lasky N, Broze GJ., Jr Protein Z-dependent protease inhibitor deficiency produces a more severe murine phenotype than protein Z deficiency. Blood. 2008 May 15;111(10):4973–4978. doi: 10.1182/blood-2007-12-126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Yang Q, Kathiresan S, Lin JP, Tofler GH, O’Donnell CJ. Genome-wide association and linkage analyses of hemostatic factors and hematological phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S12. doi: 10.1186/1471-2350-8-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, Ionescu-Tirgoviste C, Simmonds MJ, Heward JM, Gough SC, Dunger DB, Wicker LS, Clayton DG. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007 Jul;39(7):857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Bowden DW, Lehtinen AB, Ziegler JT, Rudock ME, Xu J, Wagenknecht LE, Herrington DM, Rich SS, Freedman BI, Carr JJ, Langefeld CD. Genetic Epidemiology of Subclinical Cardiovascular Disease in the Diabetes Heart Study. Ann Hum Genet. 2008 May 5;72(Pt 5):598–610. doi: 10.1111/j.1469-1809.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Sandhu MS, Waterworth DM, Debenham SL, Wheeler E, Papadakis K, Zhao JH, Song K, Yuan X, Johnson T, Ashford S, Inouye M, Luben R, Sims M, Hadley D, McArdle W, Barter P, Kesaniemi YA, Mahley RW, McPherson R, Grundy SM, Bingham SA, Khaw KT, Loos RJ, Waeber G, Barroso I, Strachan DP, Deloukas P, Vollenweider P, Wareham NJ, Mooser V. LDL-cholesterol concentrations: a genome-wide association study. Lancet. 2008 Feb 9;371(9611):483–491. doi: 10.1016/S0140-6736(08)60208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Aberg K, Dai F, Sun G, Keighley ED, Indugula SR, Bausserman L, Viali S, Tuitele J, Deka R, Weeks DE, McGarvey ST. A genome-wide linkage scan identifies multiple chromosomal regions influencing serum lipid levels in the population on the Samoan islands. J Lipid Res. 2008 Jul 1;49(10):2169–2178. doi: 10.1194/jlr.M800194-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Voruganti VS, Lopez-Alvarenga JC, Nath SD, Rainwater DL, Bauer R, Cole SA, Maccluer JW, Blangero J, Comuzzie AG. Genetics of variation in HOMA-IR and cardiovascular risk factors in Mexican-Americans. J Mol Med. 2008 Mar;86(3):303–311. doi: 10.1007/s00109-007-0273-3. [DOI] [PubMed] [Google Scholar]
- (42).Ozaki K, Ohnishi Y, Iida A, Sekine A, Yamada R, Tsunoda T, Sato H, Sato H, Hori M, Nakamura Y, Tanaka T. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat Genet. 2002 Dec;32(4):650–654. doi: 10.1038/ng1047. [DOI] [PubMed] [Google Scholar]
- (43).Schunkert H, Gotz A, Braund P, McGinnis R, Tregouet DA, Mangino M, Linsel-Nitschke P, Cambien F, Hengstenberg C, Stark K, Blankenberg S, Tiret L, Ducimetiere P, Keniry A, Ghori MJ, Schreiber S, El Mokhtari NE, Hall AS, Dixon RJ, Goodall AH, Liptau H, Pollard H, Schwarz DF, Hothorn LA, Wichmann HE, Konig IR, Fischer M, Meisinger C, Ouwehand W, Deloukas P, Thompson JR, Erdmann J, Ziegler A, Samani NJ. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation. 2008 Apr 1;117(13):1675–1684. doi: 10.1161/CIRCULATIONAHA.107.730614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Bostrom KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, Frayling TM, Freathy RM, Gianniny L, Grallert H, Grarup N, Groves CJ, Guiducci C, Hansen T, Herder C, Hitman GA, Hughes TE, Isomaa B, Jackson AU, Jorgensen T, Kong A, Kubalanza K, Kuruvilla FG, Kuusisto J, Langenberg C, Lango H, Lauritzen T, Li Y, Lindgren CM, Lyssenko V, Marvelle AF, Meisinger C, Midthjell K, Mohlke KL, Morken MA, Morris AD, Narisu N, Nilsson P, Owen KR, Palmer CN, Payne F, Perry JR, Pettersen E, Platou C, Prokopenko I, Qi L, Qin L, Rayner NW, Rees M, Roix JJ, Sandbaek A, Shields B, Sjogren M, Steinthorsdottir V, Stringham HM, Swift AJ, Thorleifsson G, Thorsteinsdottir U, Timpson NJ, Tuomi T, Tuomilehto J, Walker M, Watanabe RM, Weedon MN, Willer CJ, Illig T, Hveem K, Hu FB, Laakso M, Stefansson K, Pedersen O, Wareham NJ, Barroso I, Hattersley AT, Collins FS, Groop L, McCarthy MI, Boehnke M, Altshuler D. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008 May;40(5):638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Andrew T, Hart DJ, Snieder H, de Lange M, Spector TD, MacGregor AJ. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res. 2001 Dec;4(6):464–477. doi: 10.1375/1369052012803. [DOI] [PubMed] [Google Scholar]
- (46).Christensen K, Vaupel JW, Holm NV, Yashin AI. Mortality among twins after age 6: fetal origins hypothesis versus twin method. BMJ. 1995 Feb 18;310(6977):432–436. doi: 10.1136/bmj.310.6977.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.