Abstract
The purpose of this cross-sectional, correlational study was to describe stomatitis-related pain in women with breast cancer undergoing autologous hematopoietic stem cell transplant. Hypotheses tested were that significant, positive relationships would exist between oral pain and stomatitis, state anxiety, depression, and alteration in swallowing. Stomatitis, sensory dimension of oral pain, and state anxiety were hypothesized to most accurately predict oral pain overall intensity. Thirty-two women were recruited at two East coast comprehensive cancer centers. Data were collected on BMT day +7 ± 24 hours using Painometer®, Oral Mucositis Index-20, Oral Assessment Guide, State-Trait Anxiety Inventory, and Beck Depression Inventory. Data analysis included descriptive statistics, correlations, and stepwise multiple regression. All participants had stomatitis; 47% had oral pain with a subset reporting continuous moderate to severe oral pain despite pain management algorithms. Significant, positive associations were seen between oral pain, stomatitis, and alteration in swallowing, and between oral pain with swallowing and alteration in swallowing. Oral pain was not significantly correlated with state anxiety and depression. Oral sensory and affective pain intensity most accurately predicted oral pain overall intensity. Future research needs to explore factors that affect perception and response to stomatitis-related oropharyngeal pain, and individual patient response to opioid treatment.
Introduction
Autologous hematopoietic stem cell transplantation (autoHSCT) is used frequently to treat lymphomas and leukemias, childhood brain tumors, multiple myeloma, neuroblastoma, and autoimmune diseases.1,2 AutoHSCT is also being evaluated through clinical trials for patients with ovarian and metastatic breast cancer.3 Although autoHSCT may extend progression-free survival time, patients often experience interrelated symptoms such as pain, fatigue, nausea, and psychological distress,4.5 and negative physiological sequelae including mucosal barrier injury. One such oral mucosal barrier injury is stomatitis.6,7,8
Stomatitis is an inflammation of the oral mucous membranes characterized by tissue erythema, edema, and atrophy, often progressing to ulceration.9 High-risk bone marrow transplantation (BMT) patients have reported stomatitis incidence rates of greater than 60%, and 78% for ulcerative stomatitis.10 A typical stomatitis time course begins on BMT day +3, lasts 9.5 days, and resolves by BMT day +12.7.11 Stomatitis-related oral pain may lead to dysphagia and related nutritional deficits, and difficulty speaking.12 Life-threatening sepsis may result from pathogen entry through damaged mucosa into the peripheral blood circulation.
Sonis13 described a five-phase pathogenic model of stomatitis - initiation, message generation, signaling and amplification, ulceration, and healing. Initiation of DNA damage occurs almost immediately after chemotherapy and radiation therapy administration, and is mediated through reactive oxygen species. Transcription factors activated include nuclear factor kappa B (NF-κB) that is capable of upregulating more than 200 genes including those coding for pro-inflammatory cytokines and adhesion molecules. This protein production leads to target cell injury, signaling, and amplification with the clinical outcome of mucosal injury. Bacteria colonizing oral ulcerations contain cell wall products that stimulate infiltrating macrophages to release additional cytokines. Stomatitis may be more severe in the presence of oral infection, in particular, herpes simplex virus.14 Healing, which is characterized by epithelial proliferation and differentiation, reestablishment of local microbial flora, and decrease in oral pain occurs in most patients by BMT day +15 in parallel with WBC recovery.
Pain is a complex, subjective experience with physiological, sensory, affective, behavioral, cognitive, and sociocultural dimensions.15 Stomatitis is the principal etiology of most pain reported during the three-week post-HSCT time period16 and is often described as the most unforgettable ordeal of HSCT.17 High incidence rates of oral pain up to 89% have been reported in BMT patients.18 The sensory intensity of this oral pain ranges from mild discomfort to severe pain requiring opioid use.19 Oral pain experienced by BMT patients has been described as increasing from BMT days +4 to +21, paralleling the trend for mucosal changes for 2 weeks post BMT.20 Conversely, patients have reported oral pain before, and limited or no pain after BMT.11 The affective dimension of pain in cancer populations is shown through positive associations between pain, anxiety, and depression.21,22 Elevated levels of depression have also been found in breast cancer patients.23 The behavioral dimension of oral pain includes altered communication patterns,24 and use of medications, sleep, and relaxation. Oral pain is experienced cognitively within the context of the already traumatic HSCT experience.21
Although evidence-based guidelines for prevention and treatment of cancer therapy-related stomatitis are available,25 the optimal treatments for stomatitis and related oral pain are not established. The scientific literature generally presents only the overall intensity dimension of stomatitis-related oral pain. Assessment of the multiple dimensions of this oropharyngeal pain experience is necessary to elucidate this symptom more comprehensively.
The purpose of this study was to describe multiple dimensions of the stomatitis-related acute oropharyngeal pain experience in women with breast cancer undergoing autoHSCT. Specific aims were the following:
To describe the clinical characteristics of stomatitis.
To describe multiple dimensions of oral pain with and without swallowing.
To describe oral care regimens and analgesics used by participants.
To examine the relationships between and among stomatitis severity, oral pain overall, sensory and affective dimensions intensity, state anxiety severity, and depression severity.
It was predicted that there would be significant positive relationships observed between oral pain and stomatitis severity, alteration in swallowing, state anxiety severity, and depression severity. Also hypothesized was that stomatitis severity, oral sensory pain intensity, and state anxiety severity would most accurately predict overall oral pain intensity.
Conceptual Framework
The relationships between and among the perception, evaluation, and response components of oral pain were explored within the Symptom Experience Dimension of the original Conceptual Model of Symptom Management (Figure 1).26 This model, which has been tested subsequently in research studies and expanded,27 views symptoms as subjective experiences reflecting biopsychosocial function, sensation, or cognitive changes.26 The three interrelated dimensions of the model are Symptom Experience, Symptom Management Strategies, and Symptom Management Outcomes. The Symptom Experience Dimension involves the interaction of the patient's perception of, evaluation of, and response to a symptom.26 Symptom perception refers to any perceived change in how a person usually feels or behaves as influenced by personal, environmental, and health illness variables. Symptom response includes feelings, thoughts, or behaviors related to actual or potential health problems. Symptom evaluation refers to self-judgments regarding severity, cause, potential treatment effects, and symptom effects, and includes symptom intensity, location, temporal nature, frequency, and associated pattern of disability. The goal of Symptom Management Strategies is to stop or delay a negative outcome using professional and self-care strategies through a dynamic patient-family-clinician partnership.26 The Symptom Outcomes Dimension evaluates symptom outcomes through ten multidimensional indicators with symptom status viewed as central.26
Figure 1.
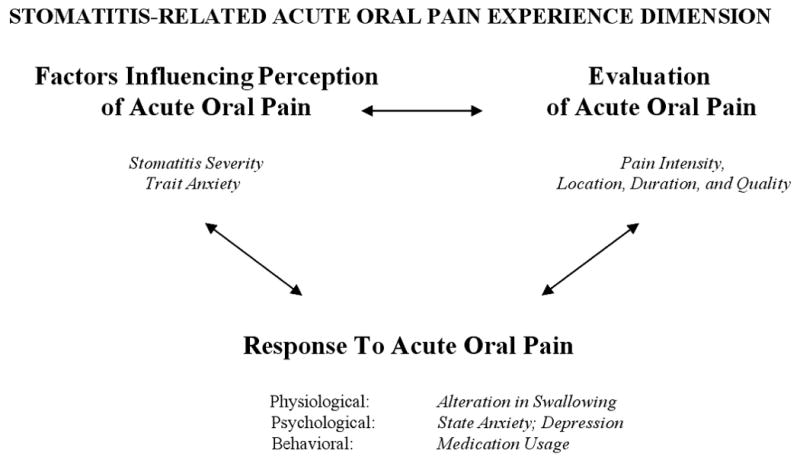
Relationships Among Research Variables Viewed Within the Symptom Experience Dimension of the Conceptual Model of Symptom Management
Methods
Design and Subjects
A descriptive, correlational, cross-sectional design was used to examine research variables and hypothesized relationships. Eligibility criteria were: a) diagnosis of stage II, III, or IV breast cancer; b) scheduled to receive conditioning chemotherapy and autoHSCT; c) age ≥18; and d) ability to read and comprehend English. The study was conducted at two urban, East coast, National Cancer Institute-designated comprehensive cancer centers with established HSCT programs. A convenience sample of 35 participants was recruited. Data were not collected for 3 participants on BMT day +7 due to critical illness, cancelled HSCT, or treatment site change. The resultant 32 participants were evenly divided between the two clinical sites.
Instruments
Instruments used were the Oral Assessment Guide,28 the Oral Mucositis Index-20,29 the Painometer,30 the State-Trait Anxiety Inventory,31 the Beck Depression Inventory,32 and patient and clinical data forms.
Oral Assessment Guide (OAG)
The OAG28 guided assessment of clinical and functional characteristics of oral cavity changes in eight categories - voice, swallowing, lips, tongue, saliva, mucous membranes, gingiva, and teeth/dentures - each rated on three levels of descriptors: normal findings = 1; mild alterations = 2; and definitely compromised = 3. The overall OAG score is the sum of all subscale scores (range = 8 to 24). Content-related and construct validity, clinical utility, and high trained nurse-nurse interrater reliability (r = .912) have been reported.28 The OAG has been used frequently to describe stomatitis incidence and severity in BMT patients.19 The OAG took about 5 minutes to complete.
Oral Mucositis Index-20 (OMI-20)
The OMI-20, which is a downsized version of the OMI,20 was used to assess stomatitis characteristics29. The OMI-20 rates atrophy of the dorsal tongue, erythema of the labial mucosa (upper and lower), buccal mucosa (right and left), and tongue (dorsal, lateral, and ventral), and edema for the lateral tongue from 0 = “normal” to 3 = “severe”. Pseudomembrane/ulceration size for these same areas is measured from 0 = “no ulceration/pseudomembrane” to 3 = ≥2cm2. The total possible score range is 0 to 60. Cronbach coefficient alphas have ranged from .90 to .96 for OMI-20 total, .92 to .95 for erythema, and .71 to .90 for ulceration.29 Interrater reliability coefficients ≥.80 were obtained for 17 of the 20 items. Test-retest reliability, construct-related, and criterion-related evidence were demonstrated. The OMI-20 performed well in the HSCT population with trained investigators using grading rules.29 The Oral Mucositis Index Template© was developed by G. D. Searle & Co (Skokie, Illinois) as a visual aid for scoring erythema severity and ulcer/pseudomembrane size (cm2). Grading rules were used to standardize oral assessment ratings (McGuire DB, personal communication, 1999).
Painometer (POM)
Multiple dimensions of oral pain were measured using the POM.30 The hand-held POM uses a vertical 10 cm visual analogue scale (VAS) to rate overall pain intensity, and 14 sensory and 11 affective pain word descriptors (WDS).30 The VAS is anchored with the words “no pain”= 0 and “worst possible pain” = 10. The weighted scores assigned to the selected words are summed to give sensory (range = 0 to 48) and affective (range = 0 to 37) pain intensity scores. The total pain intensity score for the WDS is the sum of sensory + affective scores (range = 0 to 85). High correlations were found between initial and repeat VAS ratings (r = .88, p < .001). Test-retest reliability of the WDS ranged from .68 (p < .001) to .73 (p < .001) in rheumatoid arthritis.30 Concurrent validity of the WDS was supported by correlations between the POM-WDS and the McGill Pain Questionnaire (r = 0.69, p < .001) and VAS (r = .85, p < .001).30 Construct validity evidence for the POM was shown by significant decrease in pain scores for the WDS (t = 5.53, p < .001), and VAS (t = 6.18, p < .001) after the patients had received pain medication.30 The POM Assessment Sheet captured oral pain intensity, worst intensity within the last 24 hours, sensory and affective word descriptors, length and duration of present episode, and locations.
State-Trait Anxiety Inventory (STAI)
The STAI measured anxiety through self-report state and trait anxiety scales.31 Each scale consists of 20 statements that are rated by respondents for how they feel in general (trait) and at present (state) using a Likert-type scale anchored from 1 = “not at all” to 4 = “very much so”. The total score is the sum of all responses ranging from a minimum score of 20 to 39 (low anxiety), 40 to 59 (moderate anxiety), to a maximum of 60 to 80 (high anxiety). Spielberger, Gorsuch, and Lushene32 reported test-retest reliability coefficients of 0.86 to 0.92 for the trait subscale and coefficients of 0.83 to 0.92 for the state subscale.33 Construct validity has been demonstrated.32
Beck Depression Inventory (BDI)
The BDI measured depression through participant self-report for how he/she has been feeling for the past week including today using a Likert-type scale rating each item 0 = “no symptom” to 3 = “severe or persistent symptom presence”.34 Depression severity values are categorized as scores of 1 to 9 = minimal, 10 to 16 = mild, 17 to 29 = moderate; and 30 to 63 = severe.35 The total possible score of 1 to 63 is obtained by summing the 21 responses. Concurrent validity for the BDI has ranged between 0.61 and 0.66.36 Construct validity was shown through correlation with the MMPI-D subscale (0.75).33 Internal reliability consistency was shown by a Cronbach coefficient alpha of 0.84 in a sample of autologous BMT patients.5 Test-retest reliability in nonpsychiatric patients ranged from 0.60 to 0.90.37 The BDI has been used in a wide variety of populations. patient Data Form (PDF) and Clinical Data Form (CDF) The PDF recorded sociodemographic, cancer treatment data, and risk factors for stomatitis. The CDF recorded laboratory data, and chemotherapy, analgesic, antifungal, and antibiotic agents' administration.
Procedures
The investigator received training in oral assessment techniques and use of the OMI and the OAG. OMI training included calibrations and interrater reliability procedures using Kodachrome slides showing oral mucosal changes and corresponding scoring. Interrater reliability of ≥ .90 for the OMI and the OAG was obtained between the investigator and the dental consultant prior to data collection, and during the study. The Institutional Review Boards of the Georgetown University Hospital, Washington, DC, and the Johns Hopkins Hospital, Baltimore, MD, approved the study. The investigator obtained written informed consent from all participants during a regularly scheduled clinic visit pre-HSCT. Sociodemographic data were collected immediately after informed consent was obtained. Clinical data were collected on BMT day +7 ± 24 hours in recognition of potential patient morbidity, and were also abstracted from the medical record. The investigator performed all oral assessments and collected all data in the participant's room on the BMT unit as follows: POM; BDI; STAI; OAG; and OMI-20. The study otoscope (Welch Allyn, Skaneatles Falls, New York) provided an adequate intense white light illumination of the oral cavity. Infection control procedures required use of the BMT unit flashlight for one participant. All data were maintained confidentially in a locked file cabinet located in the clinical research office space, and were entered into a password-protected computer using Microsoft Access version 97SR238, and were analyzed using STATISTICA 6.039 computer software.
Statistical Analysis
Descriptive statistics provided a demographic and clinical profile of participants. The conservative separate variance t test was used to test for a statistically significant difference between the two clinical sites for research variables at the .05 level. Partial and semi-partial correlations were used to remove the effect of analgesic, anxiolytic, and antidepressant medications on the relationships observed between oral pain and stomatitis severity, alteration in swallowing, state anxiety severity, and depression severity. Although all eligible patients were recruited during the planned study time period, the pool of potential participants was much lower than predicted. Therefore the obtained power of a partial correlation was 45% (β = 55%) based on N = 31 and the preceding alpha, directionality, and effect size.40
Stepwise regression yielded the most accurate predictors of overall oral pain intensity. The variables were entered in the regression equation in order of significance levels using STATISTICA 6.0.39 The use of medications was not partialled out. However, the potential confounding effect of medications was considered in the interpretation of the regression results by estimating the percentage of variance accounted for by the medications. The obtained power was 36% (β = 64%) based on N = 31 and the same preceding regression characteristics and 58% (β = 42%) for one predictor.38 Following the step analysis and the order of the entering predictors determination, increments of change in the proportion of variance accounted for as each variable was added to the regression (R2 change) were tested for significance. The final regression equation with the beta weights for the predictors, the multiple correlation coefficient, the proportion of the explained variance, and the standard deviation for the predictor variables to predict overall intensity of oral pain was measured by the standard of estimate based on the regression equation containing only statistically significant predictors.
Results
Subject Characteristics
Sociodemographic profiles of the participants were similar between the two clinical sites, and were representative of breast cancer autoHSCT populations seen previously at the sites. The sample was 100% female, with a mean age of 49 years (range = 32 to 64 years), and primarily Caucasian (75%) (Table 1). All 32 participants had received previous cancer surgery and adjuvant chemotherapy. Breast cancer stage, past treatment for breast cancer, source of stem cells, and autoHSCT protocol are presented in Table 2. The t test computed between the two clinical sites for all research variables was not statistically significant at the .05 level.
Table 1. Sociodemographic Characteristics of the Sample (N=32).
| Age | Mean (SD) | 49.4 (7.3) | |
|---|---|---|---|
| N | % | ||
| Gender | |||
| Female | 32 | 100 | |
| Ethnicity | |||
| Caucasian | 24 | 75.0 | |
| African-American | 4 | 12.5 | |
| Hispanic | 1 | 3.1 | |
| Asian | 1 | 3.1 | |
| Native American | 2 | 6.3 | |
| Marital Status | |||
| Married | 21 | 65.6 | |
| Single | 5 | 15.6 | |
| Divorced | 6 | 18.8 | |
| Education | |||
| High School | 8 | 25.0 | |
| Some College | 11 | 34.4 | |
| College Graduate | 8 | 25.0 | |
| Graduate Degree | 5 | 15.6 | |
| Occupationa | |||
| Professional | 18 | 75.0 | |
| Non-Professional | 6 | 25.0 | |
| Work Status | |||
| Full Time | 12 | 37.5 | |
| Part Time | 4 | 12.5 | |
| Retired | 3 | 9.4 | |
| Unemployed | 5 | 15.6 | |
| Leave of Absence | 8 | 25.0 | |
n = 24 for Professional and Non-Professional excluding retired and unemployed categories.
Table 2. Breast Cancer Stage, Past Treatment for Breast Cancer, Source of Stem Cells, and Auto HSCT Protocol.
| Variables | Category | Johns Hopkins Oncology Center (n=16) | Lombardi Cancer Center (n=16) | Total (N=32) | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Breast Cancer | |||||||
| Stagea | Stage II | 8 | 57.1 | 7 | 43.8 | 15 | 50.0 |
| Stage III | 6 | 42.9 | 4 | 25.0 | 10 | 33.3 | |
| Stage IV | 0 | 0.0 | 5 | 31.3 | 5 | 16.7 | |
| Past Treatment | |||||||
| Surgery+Chemotherapy | 14 | 87.5 | 12 | 75.0 | 26 | 81.3 | |
| Surgery+Chemotherapy+RT | 1 | 6.25 | 2 | 12.5 | 3 | 9.4 | |
| Surgery+CTb +RTc +HTd | 0 | 0.0 | 1 | 6.3 | 1 | 3.1 | |
| SXe +CT+HT | 1 | 6.3 | 1 | 6.3 | 2 | 6.3 | |
| Surgery Type | |||||||
| Lumpectomy | 1 | 6.3 | 0 | 0.0 | 1 | 3.1 | |
| Lumpectomy+ALND f | 3 | 18.8 | 7 | 43.8 | 10 | 31.3 | |
| MRMg | 3 | 18.8 | 0 | 0.0 | 3 | 9.4 | |
| MRM+ALND | 3 | 18.8 | 2 | 12.5 | 5 | 15.6 | |
| MRM+Reconstruction | 2 | 12.5 | 4 | 25.0 | 6 | 18.7 | |
| Bilateral Mastectomy+Rh | 1 | 6.3 | 1 | 6.3 | 2 | 6.3 | |
| MRM+ALND+SMi | 0 | 0.0 | 1 | 6.3 | 1 | 3.1 | |
| MRM+ALND+R | 3 | 18.8 | 1 | 6.3 | 4 | 12.5 | |
| Chemotherapy | |||||||
| Adriamycin+Cytoxan+Taxol | 10 | 62.5 | 11 | 68.7 | 21 | 65.6 | |
| Adriamycin+Cytoxan | 3 | 18.8 | 0 | 0.0 | 3 | 9.4 | |
| NeoTaxolj +Adria+Cytoxan | 0 | 0.0 | 3 | 18.7 | 3 | 9.4 | |
| Cytoxan+Adria+ 5-FU k | 1 | 6.3 | 0 | 0.0 | 1 | 3.1 | |
| Adriamycin+Taxotere | 1 | 6.3 | 0 | 0.0 | 1 | 3.1 | |
| Cytoxan+MTXl +5-FU+ Taxotere+Adriamycin | 0 | 0.0 | 1 | 6.3 | 1 | 3.1 | |
| Adriamycin+Cytoxan+ 5-FU+Taxol | 0 | 0.0 | 1 | 6.3 | 1 | 3.1 | |
| Adriamycin+Taxol+5-FU | 1 | 6.3 | 0 | 0.0 | 1 | 3.1 | |
| Hormonal Agent Stem Cell Source | Tamoxifen | 1 | 6.3 | 2 | 12.5 | 3 | 9.4 |
| Bone Marrow (BM) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Peripheral Blood | 12 | 75.0 | 16 | 100.0 | 28 | 87.5 | |
| BM and Peripheral Blood | 4 | 25.0 | 0 | 0.0 | 4 | 12.5 | |
| HSCT Protocol | |||||||
| JH9819 | 14 | 87.5 | 0 | 0.0 | 14 | 43.7 | |
| JH9534 | 2 | 12.5 | 0 | 0.0 | 2 | 12.5 | |
| 97-311 | 0 | 0.0 | 14 | 87.5 | 14 | 43.7 | |
| LCCBMT | 0 | 0.0 | 2 | 12.5 | 2 | 12.5 | |
n=30;
CT=chemotherapy;
RT=radiation therapy;
HT=hormonal therapy;
SX=surgery;
ALND=axillary lymph node dissection;
MRM=modified radical mastectomy;
R=reconstruction;
SM=simple mastectomy;
Neo=neoadjuvant;
5-FU=5-fluoruracil;
MTX=methotrexate.
JH9819=cyclophosphamide 1.5g/m2/day, thiotepa 125mg/m2/day, carboplatin 200mg/m2/day via continuous infusion (CI) BMT day -8 to -4, cyclosporine A 2.5mg/kg/day/IV BMT day 0 to+28, gamma interferon 0.025mcg/m2 SQ QOD BMT day +7 to +28; JH9534=cyclophosphamide 1.5g/m2/day and thiotepa 200mg/m2/day via CI BMT day -8 to -4, cyclosporine A 2.5mg/kg/day/IV BMT day +7 to BMT day +28, gamma interferon 0.025mcg/m2 SQ QOD BMT day +7 to +28, and IL-2 beginning at 104 U/m2/day SQ; 97-311: STAMP V-cyclophosphamide 1500mg/m2/day, thiotepa 125mg/m2/day, carboplatin 200mg/m2/day CI BMT day -7 to -4, IL-2 5 days on, 2 days/weekly, recombinant HGCSF 5mcg/kg SQ QD BMT day +5 until AGC >5,000/mm3; LCCBMT: Stamp V.
Technical Adequacy of Instruments
Coefficient alphas were relatively high for the State Anxiety Scale and the Trait Anxiety Scale (.92), the BDI (.91), and the OMI (.83), and low for the OAG (.58).
Clinical Characteristics of Stomatitis
All participants had some degree of stomatitis as assessed by the OAG, and 31 participants (97%) had some severity of stomatitis as measured by the OMI (mean = 5.8). Stomatitis patterns (OMI) are presented in Table 3. The OAG mean score was 13.0 (SD = 2.4). Over half (59%) of all participants reported a normal voice, and 6 (19%) stated that it was difficult or painful to talk (Table 4). Thirteen (41%) participants reported some alteration in swallow. Dry cracked lips were evident in 20 (63%) participants. Only 8 (25%) participants had thick saliva. Reddened mucous membranes were seen in 24 (75%) participants, and gingiva was edematous in 12 (38%) participants.
Table 3. Anatomic Areas Affected by Stomatitis and Oral Mucositis Index Scores (N=32).
| Patterns of Stomatitis | Normal | Mild | Moderate | Severe | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| ERYTHEMA | ||||||||
| Labial Mucosa, Lower | 21 | 65.6 | 6 | 18.8 | 4 | 12.5 | 1 | 3.1 |
| Labial Mucosa, Upper | 25 | 78.1 | 5 | 15.6 | 2 | 6.3 | 0 | 0.0 |
| Buccal Mucosa, Right | 12 | 37.5 | 19 | 59.4 | 1 | 3.1 | 0 | 0.0 |
| Buccal Mucosa, Left | 12 | 37.5 | 19 | 59.4 | 0 | 0.0 | 1 | 3.1 |
| Tongue, Dorsal | 27 | 84.4 | 4 | 12.5 | 1 | 3.1 | 0 | 0.0 |
| Tongue, Lateral | 28 | 87.5 | 2 | 6.3 | 2 | 6.3 | 0 | 0.0 |
| Tongue, Ventral | 28 | 87.5 | 1 | 3.1 | 3 | 9.4 | 0 | 0.0 |
| Floor of Mouth | 17 | 53.1 | 13 | 40.7 | 1 | 3.1 | 1 | 3.1 |
| Soft Palatea | 9 | 30.0 | 18 | 60.0 | 2 | 6.7 | 1 | 3.3 |
| ATROPHY:Tongue Dorsal | 20 | 62.5 | 11 | 34.4 | 1 | 3.1 | 0 | 0.0 |
| EDEMA: Tongue, Lateral | 15 | 46.9 | 15 | 46.9 | 1 | 3.1 | 1 | 3.1 |
| ULCER/PSEUDOMEMBRANE | No Ul/P | >0cm2 but <1cm2 | ≥ 1cm2 but <2cm2 | ≥ 2cm2 | ||||
| Labial Mucosa, Lower | 28 | 87.5 | 4 | 12.5 | 0 | 0.0 | 0 | 0.0 |
| Labial Mucosa, Upper | 30 | 93.8 | 2 | 6.2 | 0 | 0.0 | 0 | 0.0 |
| Buccal Mucosa, Right | 30 | 93.8 | 2 | 6.3 | 0 | 0.0 | 0 | 0.0 |
| Buccal Mucosa, Left | 25 | 78.1 | 7 | 21.9 | 0 | 0.0 | 0 | 0.0 |
| Tongue, Dorsal | 32 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Tongue, Lateral | 30 | 93.8 | 1 | 3.1 | 1 | 3.1 | 0 | 0.0 |
| Tongue, Ventral | 32 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Floor of Mouth | 31 | 96.9 | 1 | 3.1 | 0 | 0.0 | 0 | 0.0 |
| Soft Palatea | 28 | 93.3 | 2 | 6.7 | 0 | 0.0 | 0 | 0.0 |
N = 30
Table 4. Oral Assessment Guide Scores.
| Variables | Category | N = 32 | |
|---|---|---|---|
| N | % | ||
| Voice | |||
| Normal | 19 | 59.4 | |
| Deeper or Raspy | 7 | 21.9 | |
| Difficult or Painful | 6 | 18.8 | |
| Ability to Swallow | |||
| Normal Swallowing | 19 | 59.4 | |
| Some Pain on Swallowing | 13 | 40.6 | |
| Condition of Lips | |||
| Smooth, Pink and Moist | 10 | 31.3 | |
| Dry or Cracked | 20 | 62.5 | |
| Ulcerated or Bleeding | 2 | 6.3 | |
| Condition of Tongue | |||
| Pink and Moist+Papillae | 7 | 21.9 | |
| Coated or Shiny | 24 | 75.0 | |
| Blistered or Cracked | 1 | 3.1 | |
| Character of Saliva | |||
| Watery | 24 | 75.0 | |
| Thick or Ropy | 8 | 25.0 | |
| Condition of Membranes | |||
| Pink and Moist | 3 | 9.4 | |
| Reddened or Coated | 24 | 75.0 | |
| Ulceration +/- Bleeding | 5 | 15.6 | |
| Condition of Gingiva | |||
| Pink, Stippled and Firm | 20 | 62.5 | |
| Edematous +/- Redness | 12 | 37.5 | |
| Cleanliness Teeth and Gums | |||
| Clean, No Debris | 15 | 48.9 | |
| Plaque/Debris Local | 10 | 31.3 | |
| Plaque/Debris General | 7 | 21.9 | |
Location, Intensity, Quality, and Duration of Oral Pain
Fifteen (47%) participants reported oral pain overall intensity mean VAS score = 1.3 (SD = 1.7; range = 0 to 5.4) (Table 5). Five (16%) participants had present oral pain overall intensity VAS scores > 3. The worst oral pain overall intensity mean VAS rating in the past 24 hours was 2.0 (SD = 2.7; range = 0 to 10); eight (25%) participants scored > 3. The sensory + affective pain mean score was 5.9 (SD = 9.3; range = 0 to 46). The most frequently chosen oral pain location was the throat (73.3%). Pain was also reported in the upper lips (20.0%), lower lips (26.6%), inside right cheek (46.6%), inside left cheek (46.6%), and tongue (73.3%). The most frequently chosen sensory words to describe oral pain were sore (66.7%), aching and dull (46.7%), and burning (26.7%). The most frequently chosen affective words used to describe oral pain were annoying (80.0%), nagging (53.3%), and troublesome (83.3%). Continuous oral pain was reported by 12 (80%) participants, and periodic oral pain by 4 (26.6%) participants.
Table 5. Stomatitis-Related Acute Oral Pain with and without Swallowing Intensity Mean Ratings (N=32).
| Variable | Mean (SD) Median (Range) |
|---|---|
| Oral Pain without Swallowing | |
| Pain Intensity | 1.3 (1.7) |
| (POM-VAS) | 0.0 (0-5.4) |
| Pain | 2.8 (3.6) |
| Sensory Dimension | 0.5 (0-10) |
| Pain | 3.0 (6.8) |
| Affective Dimension | 0.0 (0-37) |
| Pain | 5.9 (9.3) |
| Sensory + Affective Dimension | 1.0 (0-46) |
| Oral Pain With Swallowing | |
| Pain Intensity | 1.8 (2.8) |
| (POM-VAS) | 0.0 (0-10) |
| Pain | 4.6 (9.1) |
| Sensory Dimension | 1.0 (0-47) |
| Pain | 3.3 (7.1) |
| Affective Dimension | 0.0 (0-37) |
| Pain | 8.0 (16.0) |
| Sensory + Affective Dimension | 1.0 (0-84) |
Location, Intensity, Quality, and Duration of Oral Pain with Swallowing
Fifteen (47%) participants reported oral pain overall intensity with swallowing mean VAS score = 1.8 (range = 0 to 10) (Table 5). Seven (22%) participants reported oral pain with swallowing overall intensity mean VAS scores > 3. Sixteen (50%) participants reported a mean VAS score of 2.4 (range = 0 to 10) for worst intensity of oral pain with swallowing within the last 24 hours; nine (28%) participants scored > 3. The mean sensory + affective pain rating was 8.0 (range = 0 to 84). Sensory words chosen most frequently to describe the oral pain with swallowing were sore (86.7%), burning (26.5%), aching (26.7%), and pressing (26.7%). Affective words chosen most frequently to describe oral pain with swallowing were annoying (73.3%), nagging and miserable (33.3%), and troublesome (26.7%). Twelve participants (80.0%) had continuous pain and 4 (26.7%) participants had periodic pain with swallowing.
Oral Care Regimens
The two most frequently used mouth rinses prescribed by BMT unit practice were non-alcohol mouthwash with saline (n = 16), and sodium bicarbonate (n = 15). Non-alcohol mouthwash with saline was used at least 3 times daily during hospitalization by 14 participants, and 15 participants used this mouthwash at least once on data collection day. Sodium bicarbonate solution was used at least once per day during hospitalization with 12 participants using this solution more than 3 times daily. Twelve participants used sodium bicarbonate solution on data collection day. Self-care was practiced with frequent use of lip lubricants (91%), ice chips (19%) for dry mouth, and hard candy or gum (13%) to stimulate saliva production. All but one participant used a toothbrush, mainly soft bristles, and toothpaste daily.
Medication Usage
Few subjects used topical anesthetics. Ulcerease (n = 3) was used 2-6 times/24 hours; viscous lidocaine (n = 2) was used as needed and 6 times/24 hours; and magic mouthwash (equal parts: Maalox, viscous lidocaine, nystatin suspension, benylin syrup) (n = 2) was used 2-4 times/24 hours. Table 6 presents acetaminophen and opioids used within their duration of action during data collection.
Table 6. Acetaminophen and Opioids Used Within Their Duration of Action During Data Collection (N=32).
| Total Dose Range | |
|---|---|
| Acetaminophen | 650 mg |
| Oxycodone PO | 5-10mg |
| Morphine Sulfate PO/IV | 2-40mg |
| Fentanyl Transdermal Patch | 25mcg/Hr =60mg* |
| Fentanyl Continuous Infusion | 50mcg/Hr =120mg* |
Morphine sulfate equivalent dose for 24 hours
Anxiety and Depression
All participants reported some degree of depression (mean = 10.2; SD = 9.3; range = 1 to 50). Depression severity ranged from minimal (61%), to mild (29%); two participants reporting moderate depression and one participant reporting severe depression. State anxiety scores (mean = 34.2; SD =11.5; range of 20 to 60) ranged from low (64%), to moderate (32%), to high (3%). Trait anxiety scores (mean = 33.2; SD = 10.6; range = 20 to 60) ranged from low (71%), to moderate (26%), to high (3%).
Intercorrelations Among Variables
Strength of the correlation coefficient was defined per Munro41 as 0.00 to 0.25 = little if any, 0.26 to 0.49 = low, 0.50 to 0.69 = moderate, 0.70 to 0.89 = high, and 0.90 to 1.00 = very high. The correlation coefficient, equal to its effect size, was interpreted per Cohen36 as small (r = .10), medium (r = .30), and large (r = .50). The critical value for the significance level of the Pearson product moment correlations based on a one-tailed test at the 0.05 level is .301.
Stomatitis-Related Oral Pain
Overall oral pain intensity was moderately correlated with stomatitis severity (OAG: r = .49, p < .01; OMI: r = .50, p < .01). Affective pain was highly correlated with overall oral pain intensity (r = .75, p < .001). Sensory pain was highly correlated with overall oral pain intensity (r = .75, p < .001).
Stomatitis-Related Oral Pain with Swallowing
Overall oral pain intensity was highly correlated with affective pain (r = .80, p < .001) and sensory pain (r = .79, p < .001) with swallowing. Affective pain with swallowing was highly correlated with sensory pain with swallowing (r = .83, p < .001). Sensory pain with swallowing was highly correlated with acute oral overall pain intensity with swallowing (r = .79, p < .001). Stomatitis severity (OMI) was correlated with alteration in both voice (r = .40, p < .05), and swallowing (r = .32, p < .05).
Anxiety and Depression
The correlations between overall oral pain intensity (VAS) and state anxiety (r = -.02) and trait anxiety (r = -.13) were extremely weak and did not reach significance. Depression was correlated with state anxiety (r = .42, p < .05), and moderately correlated with trait anxiety (r = .54, p < .01). Depression was not significantly correlated with overall oral pain intensity (VAS) (r = -.16). State and trait anxiety were highly correlated (r = .85, p < 001). Trait anxiety was also correlated with alteration in voice (r = .33, p < .05).
Hypotheses
There was a significant, positive relationship between oral pain and stomatitis severity (r = .499, p < .01) after controlling for the use of analgesics. The effect size of the coefficient was medium. These was a significant, positive relationship between oral pain and alteration in swallowing (r = .44, p < .05) after controlling for the use of analgesics. The effect size of the coefficient was medium. There was also a significant, positive relationship between alteration in swallowing and oral pain with swallowing (r = .66, p < .001) after controlling for the use of analgesics. The effect size of the coefficient was large.
The relationship between oral pain and state anxiety (r = .013, p = .947) was not statistically significant after controlling for the use of analgesics and anxiolytics. There was not a statistically significant, positive relationship between oral pain intensity and depression severity (r = -.105, p = .588) after controlling for the use of analgesics and antidepressants.
Each increment in the proportion of variance accounted for as each variable was added to the regression was tested for significance using the F-ratio (Table 7). This test for the criterion variable was used to develop the final regression equation. The final regression equation using standard (z) scores where OP = overall intensity of oral pain, SP = sensory pain, and AP = affective pain was: z'OP = .459zSP + .437zAP. The standard error of estimate for this equation was .927. Compared to the standard deviation of the criterion of 1.7, sensory pain and affective pain predict overall intensity of oral pain with fair to moderate accuracy.
Table 7. Summary of Stepwise Multiple Regression Analysis Predicting Overall Intensity of Stomatitis-Related Acute Oral Pain (N =31).
| Variable | R | R2 | R2 Change | Beta | F | p |
|---|---|---|---|---|---|---|
| Sensory Pain | .755 | .570 | - | .390 | 38.38 | <.001 |
| Affective Pain | .820 | .673 | .103 | .430 | 28.82 | <.01 |
| Stomatitis Severity | .831 | .691 | .018 | .140 | 20.11 | .225 |
| Depression | .838 | .702 | .012 | -.126 | 15.34 | .325 |
| State Anxiety | .839 | .704 | .002 | .045 | 11.88 | .721 |
This study had several limitations. The major limitation was the small sample size that significantly limited the statistical power of the study. In addition, the use of two clinical sites limits the findings to this sample. Cross-sectional data collection precluded potentially observing the participant's greatest stomatitis and oral pain variance. Although self-report questionnaires and grading rules for the OMI-20 were used, the potential for experimental bias exists.
Discussion
Utility and validity of the Symptom Experience Dimension of the Conceptual Model of Symptom Management24 to guide examination of perception, evaluation, and response components of the oral pain experience was demonstrated. Results of this study supported erythematous oral locations from previous reports.5,19 Interestingly, pseudomembrane/ulceration was observed less frequently than previously described, perhaps because a combined allogeneic and autoHSCT sample was not used. Prominent erythema observed on labial and buccal mucosa, floor of the mouth, and soft palate showed sensitivity of non-keratinized anatomical areas to chemotherapy effects,5,19 and supports the need for frequent, consistent inspection of these sites by nurses. Although low mean OMI and OAG scores were reported for stomatitis and alterations in voice and swallow (OAG), wide ranges indicated that some participants did experience moderate to severe stomatitis, and moderate alterations in swallowing and voice.
Oral pain was described by subjects as a multidimensional experience. Although the significant positive relationship seen between oral pain intensity and stomatitis severity supports previous reports20, this oral pain is more complex than a direct correlation with the amount of tissue damage.11 Although low mean scores for oral pain were reported, the wide range of pain VAS scores indicated that some participants had moderate to severe oral pain intensity. Mild to moderate sensory oral pain intensity was reported. Mild affective oral pain intensity was reported. Clinically significant findings included 5 participants reporting oral pain intensity ratings > 3, 7 participants experiencing worst oral pain intensity ratings within the last 24 hours > 3, and twelve participants having continuous oral pain for 3 to 10 days. Continuous pain places patients at risk for fatigue, symptom distress, and unintended biological effects. Oral pain ratings > 3 reported by patients using either a fentanyl transdermal patch or receiving fentanyl via continuous infusion demonstrates the importance of frequent pain assessment to determine effectiveness of both opioid type and titration of opioid dose to this dynamic oral pain experience.
The wide range of pain VAS scores with swallowing indicated that some participants had moderate to severe oral pain intensity, mild to severe sensory oral pain intensity, and moderate affective oral pain intensity with swallowing. Clinically significant findings included pain with swallowing intensity ratings > 3 (n = 7), worst oral pain within last 24 hours ratings > 3 (n = 10); and continuous oral pain for 2 to 7 days (n = 12). Assessing pain on swallowing is critical to manage this important symptom that is related to alterations in nutritional intake often seen in the HSCT setting.
The findings that a subset of participants experienced uncontrolled oropharyngeal pain despite the use of clinical site specific pain management algorithms reinforces the need for more individualized pain assessments and interventions to manage pain and mucosal injury. Valid and reliable assessment tools are key components of stomatitis-related oropharyngeal pain assessment, and the OAG, OMI, and POM demonstrated clinical utility in this sample. The potential communication challenges related to altered voice from edematous and painful oral mucous membranes require the use of appropriate pain assessment tools such as the POM.
The lack of a significant positive relationship between oral pain and state anxiety and depression may be related to limited statistical power of the study, and to assessing anxiety in a sample that did not experience high morbidity pre-HSCT. Oral sensory and affective pain intensity most accurately predicted oral pain overall intensity, and participants were able to discriminate between these two dimensions of the oropharyngeal pain experience. Assessment of multiple dimensions of stomatitis-related oropharyngeal pain would yield a more comprehensive understanding of this pain experience than measuring only the pain overall intensity.
Although this study focused on patients with breast cancer receiving autoHSCT, the research design is appropriate to examine stomatitis-related oral pain in diverse oncology populations. Future research is needed to explore risk factors for and factors that affect perception and response to stomatitis-related oropharyngeal pain,42 individual patient response to opioid treatment, and effectiveness of pain management algorithms for multiple dimensions of this oropharyneal pain. This clinical research may also be enhanced though use of interview techniques to assess subjective tolerance of symptoms.43
Acknowledgments
The authors thank Dr. Raymond A. Dionne, Dr. Alexis Bakos, and Ms. Elise Herlihy for advice regarding manuscript preparation. They also thank the clinical staff of the hematopoietic stem cell transplantation units for assistance with study procedures, and the subjects who chose to join this study.
This work was supported by an American Cancer Society Doctoral Scholarship in Cancer Nursing (ACS, Atlanta, GA), and a Sigma Theta Tau, Nu Beta Chapter, Johns Hopkins University School of Nursing, Nursing Research Award for Outstanding Research Proposal.
Contributor Information
Jane M. Fall-Dickson, Email: dicksonj@mail.nih.gov, National Institute of Nursing Research, National Institutes of Health, CRC 2NE-1339, 10 Center Drive, Bethesda, MD 20892, Phone: (301) 451-1675; FAX: (301) 480-1413.
Victoria Mock, Center for Nursing Research, Johns Hopkins University School of Nursing.
Ronald A. Berk, Johns Hopkins University.
Patricia M. Grimm, Clinical Staff formerly, HopeWell Cancer Support.
Nancy Davidson, Johns Hopkins University School of Medicine.
Fannie Gaston-Johansson, International and Extramural Programs, Johns Hopkins University School of Nursing.
References
- 1.Boyiadzis M, Pavletic S. Haematopoietic stem cell transplantation: Indications, clinical developments and future directions. Expert Opin Pharmacother. 2004;5:97–108. doi: 10.1517/14656566.5.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall A, Daikeler T. Autologous hematopoietic stem cell transplantation for autoimmune diesases. Acta Haematol. 2005;114:239–247. doi: 10.1159/000088415. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar C, Basser R, Hetrich S, Lethaby A, Marjoribanks J. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with metastatic breast cancer. Cochrane Database Systematic Reviews. 2003;1:CD003142. doi: 10.1002/14651858.CD003142. [DOI] [PubMed] [Google Scholar]
- 4.Gaston-Johansson F, Fall-Dickson JM, Nanda J, et al. The effectiveness of the comprehensive coping strategy program on clinical outcomes in breast cancer autotransplantation patients. Can Nurs. 2000;23:277–285. doi: 10.1097/00002820-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Gaston-Johansson F, Franco T, Zimmerman L. Pain and psychological distress in patients undergoing autologous bone marrow transplantation. Oncol Nurs For. 2000;19:441–48. [PubMed] [Google Scholar]
- 6.Scully C, Sonis S, Diz PD. Oral mucositis. Oral Diseases. 2006;12:229–241. doi: 10.1111/j.1601-0825.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 7.Filicko J, Lazarus HM, Flomerberg N. Mucosal injury in patients undergoing hematopoietic progenitor cell transplantation: New approaches to prophylaxis and treatment. Bone Marrow Transplantation. 2003;31:1–10. doi: 10.1038/sj.bmt.1703776. [DOI] [PubMed] [Google Scholar]
- 8.Logan RM, Stringer AM, Bowen JM, et al. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat Reviews. 2007;33:448–60. doi: 10.1016/j.ctrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Hyland S. Assessing the oral cavity. In: Stromborg F, Olsen SJ, editors. Instruments for clinical health-care research. London: Jones and Bartlett Publishers International; 1997. pp. 519–527. [Google Scholar]
- 10.Woo SB, Sonis ST, Monopoli MM, Sonis AL. A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer. 1993;72:1612–1217. doi: 10.1002/1097-0142(19930901)72:5<1612::aid-cncr2820720520>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.McGuire DB, Altomonte V, Peterson DE, Wingard JR, Jones RJ, Grochow LB. Patterns of mucositis and pain in patients receiving preparative chemotherapy. Oncol Nurs For. 1993;20:1493–1502. [PubMed] [Google Scholar]
- 12.Dose DM. The symptom experience of mucositis, stomatitis, and xerostomia. Sem Oncol Nurs. 1995;11:248–255. doi: 10.1016/s0749-2081(05)80005-1. [DOI] [PubMed] [Google Scholar]
- 13.Sonis ST. The pathobiology of mucositis. Nature Reviews Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 14.Woo SB, Lee SFK. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Path Oral Rad Oral Endo. 1997;83:239–243. doi: 10.1016/s1079-2104(97)90011-1. [DOI] [PubMed] [Google Scholar]
- 15.Coyle N, Foley KM. Alterations in comfort: Pain. In: Baird SB, Baird R, McCorkle R, Grant M, editors. Cancer nursing: A comprehensive textbook. Philadelphia: WB Saunders; 1991. pp. 782–805. [Google Scholar]
- 16.Syrjala KL, Chapko M. Evidence for a biophychosocial model of cancer treatment-pain. Pain. 1995;(61):69–79. doi: 10.1016/0304-3959(94)00153-6. [DOI] [PubMed] [Google Scholar]
- 17.Eisen D, Essell J, Broun ER. Oral cavity complications of bone marrow transplantation. Sem Cutaneous Med Surg. 1997;16:265–272. doi: 10.1016/s1085-5629(97)80015-6. [DOI] [PubMed] [Google Scholar]
- 18.Waterman MR, Wool A, Brown J. The incidence and severity of stomatitis and oral pain in bone marrow transplant patients. Oncol Nur For. 1993;20:325. [Google Scholar]
- 19.Schubert MM, Williams BE, Lloid ME, Chapko MK. Clinical scale for the rating of oral mucosal changes associated with bone marrow transplantation. Cancer. 1992;69:2469–2477. doi: 10.1002/1097-0142(19920515)69:10<2469::aid-cncr2820691015>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Kolbinson DA, Schubert MM, Flournoy N, Truelove E. Early oral changes following bone marrow transplantation. Oral Surg, Oral Med, Oral Path. 1988;66:130–138. doi: 10.1016/0030-4220(88)90080-1. [DOI] [PubMed] [Google Scholar]
- 21.Gaston-Johansson F, Fall-Dickson JM, Bakos AB, Kennedy MJ. Fatigue, pain, and depression in pre-autotransplant breast cancer patients. Can Prac. 1997;7:240–247. doi: 10.1046/j.1523-5394.1999.75008.x. [DOI] [PubMed] [Google Scholar]
- 22.Massie MJ, Holland J. The cancer patients with pain: Psychiatric complications and their management. J Pain Symptom Management. 1992;7:99–109. doi: 10.1016/0885-3924(92)90121-w. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel D, Sands S, Koopman C. Pain and depression in patients with cancer. Cancer. 1994;74:2570–2578. doi: 10.1002/1097-0142(19941101)74:9<2570::aid-cncr2820740927>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Shuster GF, Steeves RH, Onega L, Richardson B. Coping patterns among bone marrow transplant patients: A hermeneutical inquiry. Can Nurs. 1996;19:290–297. doi: 10.1097/00002820-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;54:2026–2046. doi: 10.1002/cncr.20163. [DOI] [PubMed] [Google Scholar]
- 26.Larson PJ, Carrieri-Kohlman V, Dodd MJ, et al. A model for symptom management. Image. J Nurs Scholar. 1994;26:272–276. [PubMed] [Google Scholar]
- 27.Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 28.Eilers J, Berger AM, Petersen MC. Development, testing, and application of the oral assessment guide. Oncol Nurs For. 1988;15:325–330. [PubMed] [Google Scholar]
- 29.McGuire DB, Peterson DE, Muller S, Owen DC, Slemmons M. The 20-item oral mucositis index: Reliability and validity in bone marrow and stem cell transplant patients. 5th National Conference on Cancer Nursing Research; February 10-13, 1999; Newport Beach Marriott, Newport Beach, CA. [DOI] [PubMed] [Google Scholar]
- 30.Gaston-Johansson F. Measurement of pain: The psychometric properties of the Pain-O-Meter, a simple, inexpensive pain assessment tool that could change health care practices. J Pain Sym Man. 1996;12:172–181. doi: 10.1016/0885-3924(96)00128-5. [DOI] [PubMed] [Google Scholar]
- 31.Spielberger CG. State-Trait Anxiety Inventory (Form Y) Palo Alto: Mind Garden; 1983. [Google Scholar]
- 32.Beck AT, Steer RA. Beck Depression Inventory Manual. San Antonio: The Psychological Corporation Harcourt Brace & Company; 1993. [Google Scholar]
- 33.Spielberger CG, Gorsuch F, Lushene R. STAI Manual for the S-T-A-I (“Self-evaluation questionnaire”) Palo Alto: Consulting Psychologist Press; 1971. [Google Scholar]
- 34.Weintrab F, Hagopian G. The effect of nursing consultation on anxiety, and side effects and self-care of patents receiving radiation therapy. Oncol Nurs For. 1990;17(supplement 3):31–38. [PubMed] [Google Scholar]
- 35.Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;46:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA. Beck Depression Inventory Manual. San Antonio: The Psychological Corporation, Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 37.Beck AT. Depression: Causes and treatment. Philadelphia: University of Philadelphia Press; 1987. [Google Scholar]
- 38.Halvorson M, Young M. Running Microsoft Office 2000, Professional. Redmond, WA: Microsoft; 1999. [Google Scholar]
- 39.STATISTICA 6.0. Statistica for Windows. Tulsa, OK: StatSoft Inc; 1999. [Google Scholar]
- 40.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 41.Munro BH. Statistical methods for health care research. 3rd. Philadelphia: JB Lippincott Publishers; 1997. p. 235. [Google Scholar]
- 42.Spross JA, McGuire DB, Schmitt R. Oncology Nursing Society position paper on cancer pain. Part II. Oncol Nurs For. 1990;17:751–760. [PubMed] [Google Scholar]
- 43.Dodd MJ, Facione NC, Dibble SL, McPhail L. Comparison of methods to determine the prevalence and nature of oral mucositis. Can Prac. 1996;4:312–317. [PubMed] [Google Scholar]


