Synopsis
Worldwide, breastfeeding saves the lives of infants and reduces their disease burden. Breastfeeding also reduces the disease burden for mothers. This article examines who chooses to breastfeed and for how long in the American context. It also reviews the latest evidence about the consequences of breastfeeding for the health of both the infant and mother. The results of this review provide support for current national and international recommendations that support breastfeeding.
Keywords: breastfeeding, lactation, postpartum weight retention, obesity, maternal health, infant health
Introduction
“Breastfeeding saves lives” and “Breast is best!” are well-known slogans for physicians and women. Putting the newborn to the breast to nurse is now considered “normative” in the United States with 75% of women doing so (1). Unfortunately, breastfeeding as a way to continue to feed infants is not yet normative. Women do not choose to breastfeed as long as recommended by health experts (2) and the government (3), which may result in a missed opportunity for improving infant health and, at the same time, maternal health. The evidence for this possibility is reviewed here.
This article considers some of the determinants of the duration and exclusivity of breastfeeding and its consequences because some of these determinants, such a socioeconomic status and maternal obesity, continue to influence the infant’s later health. This article considers the determinants and confounding factors that may be acting at the time the baby would be breastfed for the first time and during the breastfeeding period. The literature covered predominantly refers to feeding the term infant at the breast, with minimal or no use of pumped milk. Feeding the preterm infant and the use of pumped milk are covered elsewhere in this document [editor insert locations].
DETERMINANTS OF BREASTFEEDING DURATION AND EXCLUSIVITY
Breast milk is recommended as the infant’s sole source of nutrition for the first 6 months of life. It is recommended that complementary foods be added to the infant’s diet at 6 months of age and that breastfeeding continue up to two years of age and beyond (2). Although American women met the Healthy People 2010 goal for 75% of new mothers to initiate breastfeeding (Table 1), duration and exclusivity of breastfeeding remain below national goals. Determinants of breastfeeding duration and exclusivity can be grouped into five broad categories: a) demographic variables, b) biological factors, c) attitudinal characteristics, d) hospital practices, and e) social variables.
Table 1.
|
|
||||
|---|---|---|---|---|
| 20103 | 2010 Target | 20114 | 2020 Target | |
|
|
||||
| Any breastfeeding | ||||
| Ever | 75 | 75 | 74.6 | 81.9 |
| At 6 months | 43 | 50 | 44.3 | 60.6 |
| At 1 year | 22.4 | 25 | 23.8 | 34.1 |
| Exclusive breastfeeding | ||||
| To 3 months | 33 | 40 | 35 | 46.2 |
| To 6 months | 13.3 | 17 | 14.8 | 25.5 |
US Department of Health and Human Services. Healthy People 2010 midcourse review. Washington, DC: US Department of Health and Human Services; 2005. [cited Aug 14 2012]; Available from: http://www.heaIthypeople.gov/2010/data/rnidcourse/pdf/fa16.pdf
US Department of Health and Human Services. Healthy People 2020. Washington D.C.; [cited Aug 8, 2012]; Available from: www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=26.
Centers for Disease Control and Prevention. Breastfeeding Report Card — United States 2010. Atlanta, GA; 2010 [cited Aug 8, 2012]; Available from: http://www.cdc.gov/breastfeeding/pdf/BreastfeedingReportCard2010.pdf
Centers for Disease Control and Prevention. Breastfeeding Report Card — United States 2011. Atlanta, GA; 2011 [cited Aug 8, 2012]; Available from: www.cdc.gov/breastfeeding/pdf/2011breastfeedingreportcard.pdf
Demographic factors
The demographic determinants of breastfeeding duration are the subject of a large literature and it is widely acknowledged that women who are older, better educated and of higher income breastfeed longer (4–6). Black women less likely to breastfeed than non-black women (5) (Table 2). Degree of acculturation also has an impact on breastfeeding; every year of US residency reduces the odds of breastfeeding to any extent by 4% and breastfeeding to 6 months by 3% (7). Duration of breastfeeding among participants in the Special Supplemental Nutrition Program for Women, Infants and Children (WIC) lags behind that of non-participants, including those who are WIC-eligible but do not participate (8). Despite WIC’s aim to promote breastfeeding, the distribution of free formula undermines the program’s message.
Table 2.
Breastfeeding rates by race/ethnicity1
|
|
|||
|---|---|---|---|
| Ever breastfed (%) | BFat 3 mo (%) | BF at 6 mo (%) | |
|
|
|||
| Hispanic | 77.3 | 57 | 38.8 |
| Non-Hispanic white | 74.9 | 53.7 | 40.2 |
| Non-Hispanic black | 51.4 | 34.5 | 23.4 |
Abbreviations: BF, breastfeeding
Adapted from: Li R, Darling N, Maurice E, Barker L, Grummer-Strawn LM. Breastfeeding rates in the United States by characteristics of the child, mother, or family: the 2002 National Immunization Survey. Pediatrics. 2005;115(1):31–7.
Biological factors
A negative relationship between maternal obesity postpartum and breastfeeding duration was first reported in 1992 (9). Since then, the focus has been on maternal obesity at the time of conception, which is negatively associated with both the likelihood of successful initiation of breastfeeding and its duration (10, 11), though one study showed no association among black women (12). A recent systematic review summarized the potential reasons for the association between maternal obesity and breastfeeding as anatomical, medical, sociocultural and psychological (11) (Table 3).
Table 3.
Potential reasons why obese women breastfeed for shorter durations1
| Anatomical | Delayed lactogenesis Practical difficulties with latch and positioning |
| Medical | Complications of diabetes or polycystic ovary syndrome causing delayed lactogenesis or low milk supply |
| Socio-cultural | Obese women are more likely to be of lower SES, which is itself a determinant of reduced breastfeeding duration |
| Psychological | Increased body image dissatisfaction and this increased concern about their bodies makes them less likely to breastfeed |
Adapted from: Amir L, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy and Childbirth. 2007;7(1):9. PMCID: 1937008.
Maternal smoking during pregnancy is strongly negatively associated with breastfeeding duration (13) . A “dose-response” effect has been shown with the heaviest smokers having the least likelihood of establishing exclusive breastfeeding (4) . Mothers who smoke have significantly decreased milk production compared with non-smokers (14); this association may also be related to a decreased motivation to breastfeed among smokers (13).
Insufficient milk supply is consistently reported as a reason for early weaning (5, 6). Although up to 50% of women report that they perceive their milk supply to be insufficient, only about 5% of women suffer from a physiologically insufficient supply (5, 6). In response to the perception of having an insufficient milk supply, many women supplement breastfeeding with infant formula. This reduces demand for breast milk and decreases maternal supply, compounding the problem. This biological factor has a strong psychological component as low maternal self-efficacy for breastfeeding is associated with perceptions of insufficient milk supply (6).
Attitudinal characteristics
High maternal self-efficacy is associated with prolonged breastfeeding (4, 6) . A woman’s confidence in her breastfeeding ability is positively influenced by her exposure to breastfeeding and her personal breastfeeding experience (15). In addition, maternal attitudes toward breastfeeding have an impact on duration. Those who perceive breastfeeding to be healthier, easier and more convenient breastfeed longer than those who perceive that breastfeeding is restrictive, inconvenient and uncomfortable (4).
It is not surprising that intended duration of breastfeeding is associated with actual duration of breastfeeding (4, 6). This information is useful for clinicians because it has been suggested that “among women who intend to breastfeed, simply asking how long they plan to do so is an efficient method of identifying prenatally who is at risk for short breastfeeding duration” (16).
Hospital practices
Hospital practices shown to improve breastfeeding duration and exclusivity include early breastfeeding initiation, infant rooming-in and providing breast milk only (4, 17). These practices are included in the “10 steps” of the Baby Friendly Hospital Initiative (BFHI). Hospital participation in the BFHI increases rates of breastfeeding initiation, duration and exclusivity (18), but fewer than 5% of babies in the US are delivered in hospitals with BFHI certification (1).
Clinicians may also directly influence maternal breastfeeding behavior. In a prospective cohort study, researchers (19) found that mothers whose pediatricians recommended formula supplementation were significantly more likely to discontinue exclusive breastfeeding by 12 weeks. Moreover, clinicians can also potentially improve women’s breastfeeding behavior by making them aware of current national or international goals for breastfeeding duration as suggested in a recent report (20)[.
Social variables
Maternal employment negatively affects breastfeeding behavior (5). Returning to full-time work outside the home is associated with reduced duration of breastfeeding (21), whereas length of maternity leave is positively associated with duration of breastfeeding (4). Many women use breast pumps as a coping strategy for combing breastfeeding and employment (22). Breast milk expression is discussed in more detail elsewhere [editor provide location].
The impact of professional and lay support on breastfeeding outcomes was assessed in a 2007 Cochrane meta-analysis (23). All forms of lay and professional support increased the duration of any breastfeeding (23). However, lay support and combinations of lay and professional support were more effective for continuation of exclusive breastfeeding than professional support alone (23). The authors suggested that interventions/strategies to improve breastfeeding behaviors based on face-to-face support may be more effective than support via telephone contact.
Support from significant others also contributes to breastfeeding success (5, 6). Breastfeeding continuation is associated with the father’s knowledge, attitude and support (5) and also the support of the maternal grandmother (6). Fathers who receive breastfeeding information from professionals are more likely to promote and support their partner’s breastfeeding efforts (4).
It is important for clinicians to promote breastfeeding duration and exclusivity to avoid placing infants at risk of the poor health outcomes that result from being fed infant formula instead of breast milk. To optimize breastfeeding behavior, we must consider which of the determinants discussed are modifiable, when, and by whom. Attitudes, social variables, and health care practices represent a potential target for support and intervention. Evidence-based interventions to support breastfeeding are discussed in detail in [editor insert location].
PROTECTIVE EFFECTS FOR INFANTS
How Breast Milk Confers Its Benefits
Breast milk has evolved to provide the best nutrition, immune protection, and regulation of growth, development, and metabolism for the human infant (24). Breast milk is critical in compensating for developmental delays in immune function in the neonate, and responsible for reducing permeability of the intestine to prepare it for extrauterine life (25).
The predominant antibody in breast milk, secretory IgA (sIgA), confers its immunoprotection by inhibiting the adherence to or penetration of the gastrointestinal (GI) tract by pathogens and by phagocytosis or cytotoxicity of pathogens (26). sIgA is higher in colostrum than mature milk, is present in a form resistant to digestion, and provides key temporal and ubiquitous immunoprotection (27, 28). Additional, acquired secretory antibodies, such as IgM and IgG, depend on prior maternal exposure to pathogens, and provide the infant with environment-specific immunoprotection (27).
The favorable gut microbiome that results from breastfeeding protects the infant from pathogenic bacteria and has also been associated with reduced asthma and reduced obesity rates in children (29). This microbiome is a function of the interaction between human milk’s microbiota, such as Bifidobacteria and Lactobacilli, and the oligosaccharides which serve as fuel for these bacteria; these components resist digestion and have important antimicrobial activity (27, 30). The healthy microbiome promotes integrity of the intestinal barrier and competitively inhibits pathogen binding, thereby preventing inflammatory responses (25, 27). Additionally, the gut microbiota contribute to regulation of the expression of genes that affect fat metabolism and deposition (31).
Microbiota of the healthy GI tract are one of many examples of the functional efficiency of breast milk as they provide both immunoprotection and nutrients by synthesizing several essential micronutrients, namely vitamins B12, B6, folate, and vitamin K (31). Lactoferrin is another key example of functional efficiency as it aids in iron absorption, provides a significant proportion of digested amino acids, and provides immunoprotection by promoting epithelial growth and restricting bacterial access to iron (27, 28). Digested milk fat globules yield monoglycerides and medium- and long-chain fatty acids with additive antimicrobial properties (32), and undigested milk fat globules function as vehicles for small proportions of sIgA (33).
Finally, breast milk contains hormones, neuropeptides and growth factors that may affect growth, development, and self-regulation of food intake, contributing to the differences observed between breastfed and formula-fed infants (34). Leptin suppresses appetite, and infant serum leptin is positively correlated with maternal concentrations. Ghrelin, which stimulates appetite, is found in higher concentrations in foremilk than in hindmilk (35). This concentration difference may also contribute to the better self-regulation of intake in breastfed infants compared to formula-fed infants, and is thus a potential explanation for increased bottle-emptying behavior that is observed among bottle-fed infants (36).
Breastfeeding and Infant Health Outcomes
It is well-known that breastfeeding saves and improves the quality of lives even in relatively clean, industrialized contexts. In an analysis of data from the 2005 National Immunization Survey, researchers calculated that if 90% of infants were exclusively breastfed for 6 months, 911 deaths would be prevented (37). In an earlier analysis of the costs of formula-feeding, other investigators (38) found that, compared to 1,000 infants exclusively breastfed for 3 months, 1,000 infants never breastfed required 2,033 more office visits, 212 more days in the hospital, and 609 more prescriptions in the first year.
The associations between breastfeeding behaviors and infant health outcomes are the subject of a large literature that, despite limitations, establishes breastfeeding as the “gold standard” against which alternative feeds should be evaluated. Most evidence is observational because of the ethical difficulties in randomizing individuals to breastfeeding or formula-feeding. Only one large-scale experimental trial exists in a developed country: the Promotion Of Breastfeeding Intervention Trial (PROBIT) in Belarus, in which hospitals were randomized to promotion of breastfeeding or standard care (39). As a result, the intervention and control arms of the trial comprise infants from hospitals with increased breastfeeding rates compared to infants at hospitals with baseline breastfeeding rates, and illustrate the benefits of improving breastfeeding behaviors. Because associations between breastfeeding behaviors and infant health outcomes are confounded by socioeconomic and psychosocial factors, this experimental design offers the best available evidence of causal relationships between breastfeeding and health outcomes. Moreover, among PROBIT participants, breastfeeding was nearly universal in both the intervention and control arms and illness rates were low, reducing the investigators’ power to detect a benefit of breastfeeding. Nonetheless, between-group differences were observed, and for these outcomes, a clear causal relationship can be inferred—particularly as biological evidence supports these effects and suggests mechanisms, as discussed elsewhere in this volume [editor provide location]. The evidence from PROBIT is supplemented by many systematic reviews and meta-analyses (summarized in Table 4 with associated effect measures) that, while subject to the same confounding factors, unequivocally support breastfeeding for optimal infant health.
Table 4.
Evidence supporting protective effects of breastfeeding on infant health.
| Health Outcome | Strongest Evidence | Source | Comparison Groups | ORa | Fold-Riskb |
|---|---|---|---|---|---|
| GI tract infection (0–12 mo) | Experimental (hospital BF promotion vs. standard care) | Kramer et al, 2001 1 | Intervention v. control (i.e baseline breastfeeding v. increased breastfeeding) | 0.6 | 1.67 |
|
|
|||||
| GI tract infection (3–6 mo) | Experimental (hospital BF promotion vs. standard care) | Kramer et al, 20032 | Exclusively BF at 3 mo and partially BF ≥ 6 mo v. exclusively BF ≥ 6 mo | 0.35 | 2.86 |
| Meta-analysis of cohorts | Chien et al, 20013 | Ever-BF v. never-BF | 0.36 | 2.78 | |
|
|
|||||
| Respiratory infection | Cohort | Duijts et al, 20104 | Exclusively BF at 4 mo and partially BF thereafter v. never-BF | URTI: 0.65 | URTI: 1.54 |
| LRTI: 0.50 | LRTI: 2.00 | ||||
| Exclusively BF ≥ 6 mo v. never-BF | URTI: 0.37 | URTI: 2.70 | |||
| LRTI: 0.33 | LRTI: 3.03 | ||||
|
|
|||||
| Hospitalization for respiratory infection | Meta-analysis | Bachrach et al, 20035 | Ever-BF v. never-BF | 0.26 | 3.85 |
|
|
|||||
| Otitis Media | Meta-analysis of cohorts | Ip et al, 20096 | Ever-BF v. never-BF | 0.77 | 1.30 |
| Exclusively BF ≥ 3 mo v. never-BF | 0.5 | 2.00 | |||
|
|
|||||
| Cognitive Development | Experimental (hospital BF promotion vs. standard care) | Kramer et al, 20087 | Intervention v. control (i.e. baseline breastfeeding v. increased breastfeeding) | +5.9 points on full-scale IQ | N/A |
|
|
|||||
| Sudden Infant Death Syndrome | Meta-analysis | Hauck et al, 20118 | Ever-BF v. never-BF | 0.55 | 1.82 |
| BF ≥ 2 mo v. never-BF | 0.38 | 2.63 | |||
| Exclusively BF any duration v. never-BF | 0.27 | 3.70 | |||
|
|
|||||
| Acute Lymphoblastic Leukemia | Meta-analysis | Ip et al, 20096 | BF > 6 mo v. never-BF | 0.81 | 1.23 |
|
|
|||||
| Obesity | Meta-analysis | Arenz et al, 20049 | Ever-BF v. never-BF | 0.79 | 1.27 |
| Meta-analysis | Owen at al, 2005 10 | Ever-BF v. never-BF | 0.87 | 1.15 | |
| BF duration 1–3 mo v. never-BF | 0.81 | 1.23 | |||
| Meta-analysis | Harder et al, 2005 11 | BF duration 4–6 mo v. never-BF | 0.76 | 1.32 | |
| BF duration 7–9 mo v. never-BF | 0.67 | 1.49 | |||
BF = breastfed, URTI = Upper Respiratory Tract Infection, LRTI = Lower Respiratory Tract Infection.
Odds Ratios as reported by original investigators, where the less-ideal behavior—i.e. the second comparison group listed—is used as the referent. The OR thus represents the benefit conferred by breastfeeding.
Fold-risk as recalculated by the authors, where the more-ideal breastfeeding behavior—i.e. the first comparison group listed—is used as the referent, reflecting the authors’ suggestion that breastfeeding be considered the normative standard. The fold-risk thus represents the increase in morbidity and mortality associated with formula-feeding.
Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–20.
Kramer MS, Guo T, Platt RW, et al. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am Journal Clin Nutr. 2003;78(2):291–5.
Chien P, Howie P. Breast milk and the risk of opportunistic infection in infancy in industrialized and non-industrialized settings. Advances in Nutritional Research. 2001;10:69–104.
Duijts L, Jaddoe VWV, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126(1):e18–e25.
Bachrach V, Schwarz E, Bachrach L. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med. 2003;157(3):237–43.
Ip S, Chung M, Raman G, et al. A summary of the agency for healthcare research and quality’s evidence report on breastfeeding in developed countries. Breastfeeding Medicine. 2009;4(1):s17–s30.
Kramer MS, Aboud F, Mironova E, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. 2008;65(5) :578–84.
Hauck FR, Thompson JMD, Tanabe KO, et al. Breastfeeding and reduced risk of Sudden Infant Death Syndrome: a meta-analysis. Pediatrics. 2011;128(1):103–10.
Arenz S, Ruckerl R, Koletzko B, et al. Breast-feeding and childhood obesity--a systematic review. International Journal of Obesity and Related Metabolic Disorders. 2004;28(10):1247–56.
Harder T, Bergmann R, Kallischnigg G, et al. Duration of breastfeeding and risk of overweight: a meta-analysis. American Journal of Epidemiology. 2005;162(5):397–403.
Owen CG, Martin RM, Whincup PH, et al. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–77.
Infections and illnesses
Infants who are not breastfed, or who are breastfed for short periods or at low intensity, have a higher risk of infection and illness than those who are breastfed optimally. In the PROBIT trial, the standard-care group experienced more GI tract infections than the intervention group. These between-group differences were clear despite diminished power, as described above (39). In the U.S., where daycare is widespread and infection rates are higher than in Belarus (40), a greater effect would be expected. These findings are supported in the observational studies reviewed recently (41, 42), with breastfed infants 64% less likely to contract a GI infection.
PROBIT investigators were unable to confirm a similar protective effect of breastfeeding against respiratory ailments and otitis media with experimental data, but the unexpectedly high breastfeeding rates and low incidence of these infections may not have allowed adequate power to do so (39). Bachrach and colleagues (41, 43) found in their recent meta-analysis of studies from 1980–2001 that breastfed infants had a 72% lower risk of hospitalization for respiratory infections. In addition, investigators of a subsequent prospective cohort in the Netherlands found evidence to support a protective role for breastfeeding against GI and upper- and lower-respiratory tract infections. In the Netherlands cohort, only infants breastfed ≥6 months had lower risk for GI and respiratory-tract infections than non-breastfed controls (44). Moreover, the protective effects of breastfeeding persisted after cessation, although they diminished over time (41, 42).
In addition to sufficient breastfeeding duration, it is important to provide breast milk exclusively to reduce the risk of infection and illness as this behavior reduces the infant’s exposure to illness-causing agents. Among PROBIT infants who were exclusively breastfed for ≥3 months, those who continued to be exclusively breastfed were one-third less likely to have ≥1 GI infections in the first year than infants who were partially breastfed thereafter (45). The authors of a recent meta-analysis of cohorts from 1989–1997 found that, while infants ever breastfed were one-quarter less likely to contract otitis media as those never breastfed, infants exclusively breastfed for ≥3 months were half as likely (41). In a subsequent prospective cohort, infants exclusively breastfed for 4 months were at greater risk of contracting an upper-respiratory tract infection than those exclusively breastfed for a full 6 months (44).
Neurological outcomes
Breastfed and formula-fed babies differ in neurological outcomes, but this association is confounded by socioeconomic status, parental education, parental intelligence, and the home environment (41, 46). The experimental design of the PROBIT provides strong evidence of an effect independent of these confounders; at 6.5 y follow-up, children who were in the intervention arm had higher IQ scores and teacher ratings than those in the control arm (47). Although Der and colleagues (46) did not find support for the association between breastfeeding and cognitive outcomes in their recent prospective cohort and meta-analysis of prior studies through 2004, their sibling analysis in the cohort and the observational design of studies included in the meta-analysis may not have sufficiently controlled for confounding.
Sudden Infant Death Syndrome (SIDS)
Although SIDS deaths have declined substantially in 20 years (48), SIDS remains the leading cause of postneonatal death in the US (49). PROBIT was not statistically powered to detect differences in mortality, yet investigators found a non-significant trend in reduction of SIDS risk in the intervention group (provide percentage difference? p = 0.12) (39). The AAP recommends (48) breastfeeding to reduce SIDS risk further because, although this association is not well-understood, it has been recently shown to be independent of infant sleeping position (50). The authors of two recent meta-analyses found a protective effect of ever breastfeeding (41, 51). Hauck and colleagues analyzed studies conducted during 1966–2009 and found that, compared to formula-fed infants, those who were ever breastfed had a 45% reduction in SIDS risk, those breastfed ≥2 months had a 62% reduction, and those exclusively breastfed for any duration had a 73% reduction (51). The fact that breastfed infants are more easily aroused from sleep than formula-fed infants may explain these findings (52). This evidence and the AAP recommendation support the incorporation of breastfeeding promotion into the US SIDS-reduction campaign.
Asthma and atopic allergies
Although it is commonly thought that breastfeeding behavior is associated with risk of asthma and allergies, there was no difference in allergy risk between PROBIT groups (53). The authors of meta-analyses and reviews of observational evidence have been unable to clarify this association because of lack of power, inconsistent diagnostic criteria, and unresolved confounding. Authors of meta-analyses have found protective effects of breastfeeding, particularly when family history for allergic rhinitis (54), atopic allergies (55), and asthma (41) was present. However, there is some evidence from cohort studies that breastfed infants have increased risk of asthma (56) and similar or increased risk of allergy (56, 57).
Pediatric cancers
Despite a large literature, including recent meta-analyses (41, 58), evidence linking breastfeeding and risk of childhood cancers is limited. This results from limited exploration of certain cancers, small sample sizes, reliance on long-term recall, conflicting or null results, and between-study heterogeneity of findings. However, there is some evidence that breastfeeding may reduce risk of acute lymphoblastic leukemia, and duration of breastfeeding may be important, asstudies have reported that infants breastfed >6 months had a 24% (58) and 19% (41) reduction in risk of acute lymphoblastic leukemia compared to those not breastfed, while those breastfed ≤ 6 mo had a 12% reduction (58).
Childhood obesity
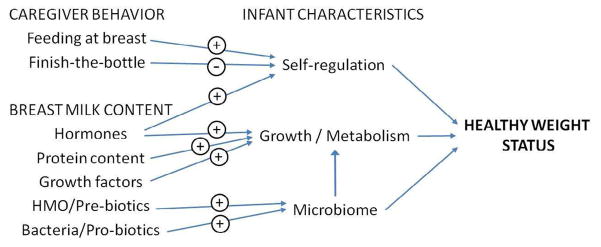
The two major mechanisms by which breastfeeding may protect against obesity in the child are through the components/composition of human milk and behaviors related to infant feeding (Figure 1). In addition to the effects of breast milk components and the microbiome described above, its lower protein concentration may help to protect the infant against later adiposity (59). Behaviors of the caregiver may also contribute to the higher obesity rates observed among formula-fed than breastfed infants. Caregivers who encourage bottle-fed infants to empty the bottle may override the infants’ internal satiety cues, which may result in poor infant self-regulation of intake. A study by Li et al. (36) supports this notion, as infants fed more often from a bottle (versus at the breast) were more likely to finish a bottle at a feeding.
Figure 1. Possible mechanisms through which breastfeeding promotes healthy infant weight status.
Represented here are caregiver behaviors and the contents of human milk have effects on the breastfed infant’s self-regulation of intake, growth and metabolism, and the intestinal microbiome, which in turn promote development of healthy weight. (+) beneficial effect; (−) detrimental effect; HMO=human milk oligosaccharides.
The association between breastfeeding and obesity is controversial. Data from the 6.5-year follow-up of PROBIT provide the only experimental evidence with which to determine whether or not formula-feeding instead of breastfeeding increases the risk of childhood obesity (60). No differences were observed between the intervention and standard-care groups in overweight or obesity. It is possible that the difference between some breastfeeding in the control versus more breastfeeding in the intervention groups was not large enough to observe an effect on child obesity as the majority of mothers in both groups initiated were still breastfeeding at 3 months postpartum. Additionally, the authors advise caution when generalizing these findings to contexts where the obesity epidemic is rampant as the proportions of children in PROBIT who were ≥ 85th (13%) or ≥ 95th (5%) were substantially lower than those in the US (33% and 18%, respectively) (61).
In meta-analyses of observational studies of breastfeeding and the risk of childhood obesity there were small, yet consistent reductions in obesity risk of 13% (62) and 22% (63) for breastfed compare to formula-fed infants. In another meta-analysis (64), a dose-response relationship was identified; there was a 4% reduction in obesity risk for each month of breastfeeding. In contrast, mean BMI was only minimally lower among breastfed compared to formula-fed individuals in a quantitative review of published and unpublished studies, which the authors attributed to confounding factors (65).
The importance of breastfeeding for growth may depend on the child’s existing adiposity. In one recent study, it appeared that breastfeeding resulted in a healthier BMI distribution overall (66) as fewer children were either underweight or obese.
Infants born to obese mothers are at high risk of developing obesity for several reasons. These infants may have inherited a genetic predisposition to obesity, are exposed to an obesogenic environment in utero, are likely to breastfeed for a shorter period than those normal-weight mothers, and the family food environment may also be obesogenic. Infants of heavier Danish mothers who were breastfed for longer periods gained 11% less in their first year of life than those who were breastfed for shorter periods (67). In a study of American infants, Li et al. (68) found that children of obese mothers who never breastfed had a 6-fold higher odds of becoming overweight compared to children of normal-weight mothers that breastfed for at least 4 months. Based on this evidence, mothers who are obese and children of obese mothers are a key group to target for breastfeeding assistance, and effective interventions are needed to help this population (69).
Cardiovascular and metabolic disease risk
Effects of breastfeeding on risk factors for cardiovascular and other metabolic diseases have also been examined in observational studies. In a meta-analysis of 7 studies, breastfeeding decreased the risk of type 2 diabetes by nearly 40% compared to formula-feeding (70). Fasting insulin values in later life were 3% lower among those who were breastfed, indicating an association with improved insulin-sensitivity. Breastfeeding may also decrease later risk of type 1 diabetes (41) and blood pressure in adulthood (71) although evidence for these outcomes are less conclusive because of potential problems of confounding and publication bias.
MATERNAL HEALTH OUTCOMES OF BREASTFEEDING
The advantages of breastfeeding for mothers are not as well studied as those for infants, but there is adequate evidence to state that women who breastfeed are likely to have improved health in the short-term, and are at lower risk of developing future diseases (72) (Box 1).
Box 1. Maternal Benefits of Breastfeeding.
| Breastfeeding may confer both immediate and long-term benefits to mothers, especially if recommendations for exclusivity and duration are met. Such benefits may strengthen motivation or commitment to breastfeeding. | |
Reasons to initiate breastfeeding:
|
Reasons to continue breastfeeding:
|
Immediate and Early Benefits to the Mother
Postpartum weight loss
Childbearing is associated with long-term weight gain (73), and postpartum weight retention has been associated with adverse outcomes in later pregnancies (74). Breastfeeding, conversely, is associated with postpartum weight loss (75, 76). In a large prospective cohort study, Baker et al. (76) showed that greater intensity (exclusivity) and duration of breastfeeding was associated with greater weight loss at 6 and 18 months postpartum in women of all BMI categories.
Bonding
Breastfeeding is often mentioned as a facilitator of mother-infant bonding (77), and bonding is reported by women as a reason for breastfeeding (78). Although potential hormonal and social mechanisms exist that may promote bonding, a systematic review by Jansen et al. (79) found that the empirical evidence is limited. Subsequently, evidence for a biologic link between breastfeeding and bonding is emerging as breastfeeding mothers had higher brain responses to their own infants’ cry and exhibited more sensitive behavior than formula-feeding mothers (80).
Lactational amennorrhea
Breastfeeding exclusively has the natural effect of suppressing ovulation, thereby acting as a natural birth control for up to 6 months (or as long as the woman is exclusively breastfeeding and her menses have not returned) (72). Lactation must be used with caution for family planning among women to do not breastfeed exclusively or only do so for a brief period.
Long-term Maternal Benefits of Breastfeeding
Diabetes, metabolic, and cardiovascular risk
Pregnancy is associated with changes in glucose and lipid metabolism that support the growing fetus; however, these changes can be deleterious to the mother’s health. Breastfeeding, on the other hand, is associated with favorable metabolic changes. The “Reset Hypothesis” (81) proposes that the favorable metabolic changes in lactation persist after weaning, resulting in the observed long-term decreases in chronic disease risk among women who have breastfed. All of the current evidence for this comes from observational studies, so confounding and selection bias cannot be ruled out.
Pregnancy is an insulin-resistant state, which results from the effects on the mother of placental hormones with anti-insulin effects. These metabolic changes can cause gestational diabetes, and may increase the risk of type 2 diabetes later in life. Conversely, during lactation, insulin-sensitivity improves and may have lasting effects (81) because a 4–12% reduction in the risk of type 2 diabetes was observed for every 12 months of lifetime lactation (82). Breastfeeding intensity may also be also important because a 50% higher risk of developing type 2 diabetes was observed among women who never exclusively breastfed compared to those who exclusively breastfed for 1–3 months (83).
Pregnancy is also a hyperlipidemic state, with increased concentrations of blood cholesterol and triglycerides; conversely, lactation promotes favorable effects on maternal blood lipids (81). Research has found that lactation is associated with lower risk of longer-term metabolic risk factors and cardiovascular disease (84, 85). Women who breastfed their children have been less likely to have developed hypertension, diabetes, hyperlipidemia, and cardiovascular disease when controlling for multiple important socio-demographic and lifestyle variables (84). Conversely, some studies have found no association of breastfeeding and disease risk (86). A systematic review is warranted to assess the totality of this growing literature.
Reproductive cancers
A decrease in risk for reproductive cancers has been observed among women who have breastfed, possibly reduce their reduced lifetime exposure to hormones such as estrogen. According to a 2002 meta-analysis, women with breast cancer were less likely to have breastfed, and they had a shorter average lifetime duration of breastfeeding did women who had not developed this disease (87). Furthermore the risk of breast cancer decreased by 4.3% for each year of breastfeeding, which indicates that longer breastfeeding may increase protection against breast cancer. In another meta-analysis, there was a 28% lower risk of developing ovarian cancer among women who breastfed for at least 12 months compared to women who never breastfed (41).
Together, the evidence of effects of breastfeeding on maternal health suggest that breastfeeding protects the mother from many short- and long-term health problems, and that breastfeeding exclusively and for longer durations result in the most optimal maternal health.
CONCLUSIONS
As the overview presented here makes clear, there is persuasive evidence available to support recommendations by the health authorities (2, 3, 88) and to support national goals for breastfeeding duration. These recommendations and goals treat breastfeeding as the optimal way to feed infants during their first year of life, along with the timely addition of complementary foods. Moreover, there is a growing body of evidence that supports breastfeeding as a way to improve a woman’s health after pregnancy as it may help her to return to a normal metabolic profile and to lose the weight she gained during pregnancy—among other benefits. Indeed “breast is best!” for mothers as well as their babies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christine M. Dieterich, Email: cmd76@cornell.edu, Division of Nutritional Sciences, Cornell University, Ithaca, NY 14850; tel: 415-609-5438.
Julia P. Felice, Email: jpf99@cornell.edu, Division of Nutritional Sciences, Cornell University, Ithaca, NY 14850; tel: 617-797-8618.
Elizabeth O’Sullivan, Email: eo238@cornell.edu, Division of Nutritional Sciences, Cornell University, Ithaca, NY 14850; tel: 607-379-5624.
Kathleen M. Rasmussen, Email: kathleen.rasmussen@cornell.edu, Division of Nutritional Sciences, Cornell University, Ithaca, NY 14850; tel: 607-255-2290.
References
- 1.Centers for Disease Control and Prevention. Breastfeeding Report Card –– United States 2011. Atlanta, GA: 2011. [cited Aug 8, 2012]; Available from: www.cdc.gov/breastfeeding/pdf/2011breastfeedingreportcard.pdf. [Google Scholar]
- 2.Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Healthy People 2020. Washington D.C: [cited Aug 8, 2012]; Available from: www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=26. [Google Scholar]
- 4.Dennis CL. Breastfeeding initiation and duration: a 1990–2000 literature review. J Obstet Gynecol Neonatal Nurs. 2002;31(1):12–32. doi: 10.1111/j.1552-6909.2002.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 5.Thulier D, Mercer J. Variables associated with breastfeeding duration. J Obstet Gynecol Neonatal Nurs. 2009;38(3):259–68. doi: 10.1111/j.1552-6909.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 6.Meedya S, Fahy K, Kable A. Factors that positively influence breastfeeding duration to 6 months: a literature review. Women and Birth. 2010;23(4):135–45. doi: 10.1016/j.wombi.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Gibson-Davis CM, Brooks-Gunn J. Couples’ immigration status and ethnicity as determinants of breastfeeding. Am J Public Health. 2006;96(4):641–6. doi: 10.2105/AJPH.2005.064840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R, Darling N, Maurice E, et al. Breastfeeding rates in the United States by characteristics of the child, mother, or family: the 2002 National Immunization Survey. Pediatrics. 2005;115(1):31–7. doi: 10.1542/peds.2004-0481. [DOI] [PubMed] [Google Scholar]
- 9.Rutishauser IH, Carlin JB. Body mass index and duration of breast feeding: a survival analysis during the first six months of life. Journal of Epidemiology and Community Health. 1992;46(6):559–65. doi: 10.1136/jech.46.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annual Review of Nutrition. 2007;27(1):103–21. doi: 10.1146/annurev.nutr.27.061406.093738. [DOI] [PubMed] [Google Scholar]
- 11.Amir LH, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy and Childbirth. 2007;7(1):9. doi: 10.1186/1471-2393-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kugyelka JG, Rasmussen KM, Frongillo EA. Maternal obesity is negatively associated with breastfeeding success among Hispanic but not Black women. Journal of Nutrition. 2004;134(7):1746–53. doi: 10.1093/jn/134.7.1746. [DOI] [PubMed] [Google Scholar]
- 13.Donath SM, Amir LH. The relationship between maternal smoking and breastfeeding duration after adjustment for maternal infant feeding intention. Acta Paediatrica. 2004;93(11):1514–8. doi: 10.1080/08035250410022125. [DOI] [PubMed] [Google Scholar]
- 14.Hopkinson JM, Schanler RJ, Fraley JK, et al. Milk production by mothers of premature infants: influence of cigarette smoking. Pediatrics. 1992;90(6):934–8. [PubMed] [Google Scholar]
- 15.Blyth R, Creedy D, Dennis C, et al. Effect of maternal confidence on breastfeeding duration: an application of breastfeeding self-efficacy theory. Birth. 2002;29(4):278–84. doi: 10.1046/j.1523-536x.2002.00202.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Campo P, Faden RR, Gielen AC, et al. Prenatal factors associated with breastfeeding duration: recommendations for prenatal interventions. Birth. 1992;19(4):195–201. doi: 10.1111/j.1523-536x.1992.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 17.Murray EK, Ricketts S, Dellaport J. Hospital practices that increase breastfeeding duration: results from a population-based study. Birth. 2007;34(3):202–11. doi: 10.1111/j.1523-536X.2007.00172.x. [DOI] [PubMed] [Google Scholar]
- 18.Philipp BL, Merewood A. The Baby-Friendly way: the best breastfeeding start. Pediatr Clin N Am. 2004;51(3):761–83. doi: 10.1016/j.pcl.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Taveras EM, Li R, Grummer-Strawn L, et al. Opinions and practices of clinicians associated with continuation of exclusive breastfeeding. Pediatrics. 2004;113(4):283–90. doi: 10.1542/peds.113.4.e283. [DOI] [PubMed] [Google Scholar]
- 20.Wen LM, Simpson JM, Rissel C, et al. Awareness of breastfeeding recommendations and duration of breastfeeding: findings from the Healthy Beginnings Trial. Breastfeeding Medicine. 2012;7:223–9. doi: 10.1089/bfm.2011.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan AS, Zhou W, Arensberg MB. The effect of employment status on breastfeeding in the United States. Women’s Health Issues. 2006;16(5):243–51. doi: 10.1016/j.whi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Fein SB, Mandal B, Roe BE. Success of strategies for combining employment and breastfeeding. Pediatrics. 2008;122(Suppl 2):s56–s62. doi: 10.1542/peds.2008-1315g. [DOI] [PubMed] [Google Scholar]
- 23.Britton C, McCormick FM, Renfrew MJ, et al. Support for breastfeeding mothers. Cochrane Database of Systematic Reviews. 2007;(1) doi: 10.1002/14651858.CD001141.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Goldman AS. Evolution of immune functions of the mammary gland and protection of the infant. Breastfeeding Medicine. 2012;7(3):132–42. doi: 10.1089/bfm.2012.0025. [DOI] [PubMed] [Google Scholar]
- 25.Goldman AS. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. The Journal of Nutrition. 2000;130(Suppl 2):426s–31s. doi: 10.1093/jn/130.2.426S. [DOI] [PubMed] [Google Scholar]
- 26.Brandtzaeg P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine. 2003;21(24):3382–8. doi: 10.1016/s0264-410x(03)00338-4. [DOI] [PubMed] [Google Scholar]
- 27.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatric Research. 2007;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 28.Chirico G, Marzollo R, Cortinovis S, et al. Antiinfective properties of human milk. Journal of Nutrition. 2008;138(9):1801s–6s. doi: 10.1093/jn/138.9.1801S. [DOI] [PubMed] [Google Scholar]
- 29.Isolauri E. Development of healthy gut microbiota early in life. Journal of Paediatrics and Child Health. 2012;48(Suppl 3):1–6. doi: 10.1111/j.1440-1754.2012.02489.x. [DOI] [PubMed] [Google Scholar]
- 30.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annual Review of Nutrition. 2005;25(1):37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 31.Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isaacs CE. Antimicrobial lipids in milk. In: Thormar H, editor. Lipids and Essential Oils as Antimicrobial Agents. Chichester, West Sussex: Wiley; 2011. pp. 81–97. [Google Scholar]
- 33.Schroten H, Bosch M, Nobis-Bosch R, et al. Anti-infectious properties of the human milk fat globule. In: Newburg D, editor. Bioactive Components of Human Milk. New York: Plenum Publishers; 2001. pp. 189–92. [DOI] [PubMed] [Google Scholar]
- 34.Savino F, Liguori SA. Update on breast milk hormones: leptin, ghrelin and adiponectin. Clinical Nutrition. 2008;27(1):42–7. doi: 10.1016/j.clnu.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Karatas Z, Durmus Aydogdu S, Dinleyici EC, et al. Breastmilk ghrelin, leptin, and fat levels changing foremilk to hindmilk: Is that important for self-control of feeding? European Journal of Pediatrics. 2011;170(10):1273–80. doi: 10.1007/s00431-011-1438-1. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Grummer-Strawn LM, Fein SB. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics. 2010;125(6):e1386–e93. doi: 10.1542/peds.2009-2549. [DOI] [PubMed] [Google Scholar]
- 37.Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics. 2010;125(5):e1048–e56. doi: 10.1542/peds.2009-1616. [DOI] [PubMed] [Google Scholar]
- 38.Ball TM, Wright AL. Health care costs of formula-feeding in the first year of life. Pediatrics. 1999;103(4):870–6. [PubMed] [Google Scholar]
- 39.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–20. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 40.Singleton RJ, Holman RC, Folkema AM, et al. Trends in lower respiratory tract infection hospitalizations among American Indian/Alaska Native children and the general US child population. J Pediatr. 2012;161(2):296–302. e2. doi: 10.1016/j.jpeds.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Ip S, Chung M, Raman G, et al. A summary of the agency for healthcare research and quality’s evidence report on breastfeeding in developed countries. Breastfeeding Medicine. 2009;4(1):S17–S30. doi: 10.1089/bfm.2009.0050. [DOI] [PubMed] [Google Scholar]
- 42.Chien PF, Howie PW. Breast milk and the risk of opportunistic infection in infancy in industrialized and non-industrialized settings. Advances in Nutritional Research. 2001;10:69–104. doi: 10.1007/978-1-4615-0661-4_4. [DOI] [PubMed] [Google Scholar]
- 43.Bachrach VR, Schwarz E, Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med. 2003;157(3):237–43. doi: 10.1001/archpedi.157.3.237. [DOI] [PubMed] [Google Scholar]
- 44.Duijts L, Jaddoe VWV, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126(1):e18–25. doi: 10.1542/peds.2008-3256. [DOI] [PubMed] [Google Scholar]
- 45.Kramer MS, Guo T, Platt RW, et al. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am J Clin Nutr. 2003;78(2):291–5. doi: 10.1093/ajcn/78.2.291. [DOI] [PubMed] [Google Scholar]
- 46.Der G, David BG, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333(7575):945. doi: 10.1136/bmj.38978.699583.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kramer MS, Aboud F, Mironova E, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. 2008;65(5):578–84. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- 48.Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128(5):e1341–e67. doi: 10.1542/peds.2011-2285. [DOI] [PubMed] [Google Scholar]
- 49.Heron M. National Vital Statistics Reports. 5. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. Deaths: leading causes for 2004. [PubMed] [Google Scholar]
- 50.Vennemann MM, Brinkmann B, Yucesan K, et al. Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics. 2009;123(3):e406–e10. doi: 10.1542/peds.2008-2145. [DOI] [PubMed] [Google Scholar]
- 51.Hauck FR, Tanabe KO, Thompson JMD, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128(1):103–10. doi: 10.1542/peds.2010-3000. [DOI] [PubMed] [Google Scholar]
- 52.Horne RS, Parslow PM, Harding R. Respiratory control and arousal in sleeping infants. Paediatric Respiratory Reviews. 2004;5(3):190–8. doi: 10.1016/j.prrv.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Kramer MS, Matush L, Vanilovich I, et al. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trial. BMJ. 2007;335(7624):815. doi: 10.1136/bmj.39304.464016.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mimouni Bloch A, Mimouni D, Mimouni M, et al. Does breastfeeding protect against allergic rhinitis during childhood? A meta-analysis of prospective studies. Acta Paediatrica. 2002;91(3):275–9. doi: 10.1080/08035250252833914. [DOI] [PubMed] [Google Scholar]
- 55.Gdalevich M, Mimouni D, David M, et al. Breast-feeding and the onset of atopic dermatitis in childhood: a systematic review and meta-analysis of prospective studies. Journal of the American Academy of Dermatology. 2001;45(4):520–7. doi: 10.1067/mjd.2001.114741. [DOI] [PubMed] [Google Scholar]
- 56.Sears M, Greene J, Willan A, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360(9337):901–7. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 57.Wegienka G, Ownby D, Havstad S, et al. Breastfeeding history and childhood allergic status in a prospective birth cohort. Annals of Allergy, Asthma & Immunology. 2006;97(1):78–83. doi: 10.1016/S1081-1206(10)61374-9. [DOI] [PubMed] [Google Scholar]
- 58.Kwan M, Buffler P, Abrams B, et al. Breastfeeding and the risk of childhood leukemia: a meta-analysis. Public Health Rep. 2004;119(6):521–35. doi: 10.1016/j.phr.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singhal A, Lanigan J. Breastfeeding, early growth and later obesity. Obesity Reviews. 2007;8(Suppl 1):51–4. doi: 10.1111/j.1467-789X.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 60.Kramer MS, Matush L, Vanilovich I, et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86(6):1717–21. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- 61.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among us children and adolescents, 1999–2010. JAMA. 2012;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Owen CG, Martin RM, Whincup PH, et al. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 63.Arenz S, Ruckerl R, Koletzko B, et al. Breast-feeding and childhood obesity--a systematic review. International Journal of Obesity and Related Metabolic Disorders. 2004;28(10):1247–56. doi: 10.1038/sj.ijo.0802758. [DOI] [PubMed] [Google Scholar]
- 64.Harder T, Bergmann R, Kallischnigg G, et al. Duration of breastfeeding and risk of overweight: a meta-analysis. American Journal of Epidemiology. 2005;162(5):397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 65.Owen CG, Martin RM, Whincup PH, et al. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;82(6):1298–307. doi: 10.1093/ajcn/82.6.1298. [DOI] [PubMed] [Google Scholar]
- 66.Beyerlein A, Von Kries R, Toschke AM. Breastfeeding and childhood obesity: Shift of the entire BMI distribution or only the upper parts? Obesity. 2008;16(12):2730–3. doi: 10.1038/oby.2008.432. [DOI] [PubMed] [Google Scholar]
- 67.Baker JL, Michaelsen KF, Rasmussen KM, et al. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80(6):1579–88. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 68.Li C, Kaur H, Choi WS, et al. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obesity Research. 2005;13(2):362–71. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- 69.Rasmussen KM, Dieterich CM, Zelek ST, et al. Interventions to increase the duration of breastfeeding in obese mothers: The Bassett Improving Breastfeeding Study. Breastfeeding Medicine. 2011;6(2):69–75. doi: 10.1089/bfm.2010.0014. [DOI] [PubMed] [Google Scholar]
- 70.Owen CG, Martin RM, Whincup PH, et al. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84(5):1043–54. doi: 10.1093/ajcn/84.5.1043. [DOI] [PubMed] [Google Scholar]
- 71.Owen CG, Whincup PH, Cook DG. Symposium II: Infant and childhood nutrition and disease: Breast-feeding and cardiovascular risk factors and outcomes in later life: Evidence from epidemiological studies. Proc Nutr Soc Proceedings of the Nutrition Society. 2011;70(4):478–84. doi: 10.1017/S0029665111000590. [DOI] [PubMed] [Google Scholar]
- 72.Godfrey JR, Lawrence RA. Toward optimal health: the maternal benefits of breastfeeding. Journal of Women’s Health. 2010;19(9):1597–602. doi: 10.1089/jwh.2010.2290. [DOI] [PubMed] [Google Scholar]
- 73.Wolfe WS, Sobal J, Olson CM, et al. Parity-associated weight gain and its modification by sociodemographic and behavioral factors: a prospective analysis in US women. International Journal of Obesity. 1997;21(9):802–10. doi: 10.1038/sj.ijo.0800478. [DOI] [PubMed] [Google Scholar]
- 74.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. The Lancet. 2006;368(9542):1164–70. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 75.Dewey KG, Heinig MJ, Nommsen LA. Maternal weight-loss patterns during prolonged lactation. The American Journal of Clinical Nutrition. 1993;58(2):162–6. doi: 10.1093/ajcn/58.2.162. [DOI] [PubMed] [Google Scholar]
- 76.Baker JL, Gamborg M, Heitmann BL, et al. Breastfeeding reduces postpartum weight retention. American Journal of Clinical Nutrition. 2008;88(6):1543–51. doi: 10.3945/ajcn.2008.26379. [DOI] [PubMed] [Google Scholar]
- 77.Leung AK, Sauve RS. Breast is best for babies. Journal of the National Medical Association. 2005;97(7):1010–9. [PMC free article] [PubMed] [Google Scholar]
- 78.Arora S, McJunkin C, Wehrer J, et al. Major factors influencing breastfeeding rates: mother’s perception of father’s attitude and milk supply. Pediatrics. 2000;106(5):e67. doi: 10.1542/peds.106.5.e67. [DOI] [PubMed] [Google Scholar]
- 79.Jansen J, de Weerth C, Riksen-Walraven JM. Breastfeeding and the mother-infant relationship - a review. Developmental Review. 2008;28(4):503–21. [Google Scholar]
- 80.Kim P, Feldman R, Mayes LC, et al. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry. 2011;52(8):907–15. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuebe AM, Rich-Edwards JW. The reset hypothesis: Lactation and maternal metabolism. American Journal of Perinatology. 2009;26(1):81–8. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294(20):2601–10. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 83.Schwarz EB, Brown JS, Creasman JM, et al. Lactation and maternal risk of type 2 diabetes: a population-based study. Am J Med. 2010;123(9):863–6. doi: 10.1016/j.amjmed.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5):974–82. doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Natland ST, Nilsen TIL, Midthjell K, et al. Lactation and cardiovascular risk factors in mothers in a population-based study: the HUNT-study. International Breastfeeding Journal. 2012;7(8) doi: 10.1186/1746-4358-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stuebe A, Kleinman K, Gillman MW, et al. Duration of lactation and maternal metabolism at 3 years postpartum. Journal of Women’s Health. 2010;19(5):941–50. doi: 10.1089/jwh.2009.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50,302 women with breast cancer and 96,973 women without the disease. The Lancet. 2002;360(9328):187–95. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 88.World Health Organization. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: WHO and UNICEF; 2003. [Google Scholar]