Abstract
Background: Recent studies suggested that gamma-glutamyl transferase (GGT) and C-reactive protein (CRP) are good markers of metabolic abnormalities. We assessed the link between GGT, CRP and common metabolic abnormalities, as well their link to related diseases, such as cancer and cardiovascular disease (CVD). Methods: We selected 333,313 subjects with baseline measurements of triglycerides (TG), total cholesterol (TC), glucose, GGT and CRP in the Swedish AMORIS study. Baseline measurement of BMI was available for 63,900 persons and 77,944 had baseline measurements of HDL. Pearson correlation coefficients between CRP, GGT, and metabolic components (TG, HDL, BMI and TC) were calculated. To investigate the combined effect of GGT and CRP we created a score ranging from 0 to 6 and used Cox proportional hazard models to evaluate its association with CVD and cancer. Results: 21,216 individuals developed cancer and 47,939 CVD. GGT and TG had the strongest correlation (r=0.22). An increased risk of cancer was identified with elevated levels of GGT or CRP or both markers (GGT-CRP score ≥3); the greatest risk of cancer was found when GGT-CRP score = 6 (HR: 1.40 (95%CI: 1.31-1.48) and 1.60 (1.47-1.76) compared to GGT-CRP score = 0, respectively). Conclusion: While GGT and CRP have been shown to be associated with metabolic abnormalities previously, their association to the components investigated in this study was limited. Results did demonstrate that these markers were predictive of associated diseases, such as cancer.
Keywords: GGT, CRP, metabolic abnormalities, cardiovascular disease, cancer
Introduction
Recent research has linked metabolic abnormalities amongst other diseases with elevated levels of C-reactive protein (CRP) and gamma-glutamyl transferase (GGT) [1-5], however, some studies cite increased risk of a multitude of diseases in individuals with GGT in a normal range [6]. Evidence in large studies does appear limited. Aside from their link with the metabolic abnormalities, these biomarkers have been directly associated to increased risk of associated co-morbidities, such as cardiovascular disease (CVD) and cancer [7,8].
C-reactive protein is known for its sensitivity in response to inflammation and its prediction of risk for coronary diseases [9,10]. GGT is a protein found in many tissues, but has a particular affinity as a marker of liver dysfunction, making it invaluable as a diagnostic marker [11]. While traditionally interpreted as in indication of liver disease, new theories suggest that elevated GGT is reflective of atherogenesis and oxidative stress, making it a good marker for coronary diseases and also risk of stroke [11-14].
Additionally, a number of studies [15-18] have already cited a link between GGT and CRP and cancer, another disease with increased risk seen in individuals with abnormal metabolic profiles. This is thought to be biologically plausible given the strength of evidence of oxidative stress [19] and inflammation [20] seen across multiple cancer types.
The goal of this study is to broaden the existing evidence base for GGT and CRP as alternative markers of metabolic abnormalities through the use of a large European-based study population. While these previous studies do present interesting data, due to missing data on hypertension and BMI we will focus on dyslipidemia and hyperglycaemia. We assessed whether the direct associations between elevated levels of these biomarkers and dyslipidemia and hypertriglyceridemia were observed in a Swedish population with the use of a large, well-defined database [15-18].
Research design and methods
Study population and data collection
The Swedish AMORIS database has been described in detail elsewhere [21-23]. Briefly, this database is based on the linkage of the Central Automation Laboratory (CALAB) database to several Swedish national registries such as the National Cancer Register, the Cause of Death Register, the consecutive Swedish Censuses during 1970-1990, and the National Register of Emigration by using the Swedish 10-digit personal identity number to provide information on socio-economic status (SES), vital status, cancer diagnosis, and emigration. The CALAB database includes data from 351,487 male and 338,101 female healthy individuals having clinical laboratory testing as part of a general health check-up or outpatients referred for laboratory testing. This study complied with the Declaration of Helsinki, and the ethics review board of the Karolinska Institute approved the study.
We selected a sub-cohort of all persons age 20 years or older, whose levels of triglycerides (mmol/L), total cholesterol (mmol/L), glucose (mmol/L), GGT, ALT and CRP were measured at baseline (n=333,313). All subjects were free of cancer at time of entry and none were previously diagnosed with cancer or had a history of cardiovascular disease (CVD) included, a total of 63,852 had an additional. All subjects with a cancer diagnosis or CVD event < 1 year follow-up were excluded from the study. Of those baseline measurement of body-mass index (BMI, kg/m2) and a total of 78,006 persons had baseline information of HDL (mmol/L). Follow-up time was defined for each individual as the time from blood analyses until date of event (CVD or cancer diagnosis), emigration, death, or study closing date (31st of December 2002), whichever occurred first (Figure 1).
Figure 1.
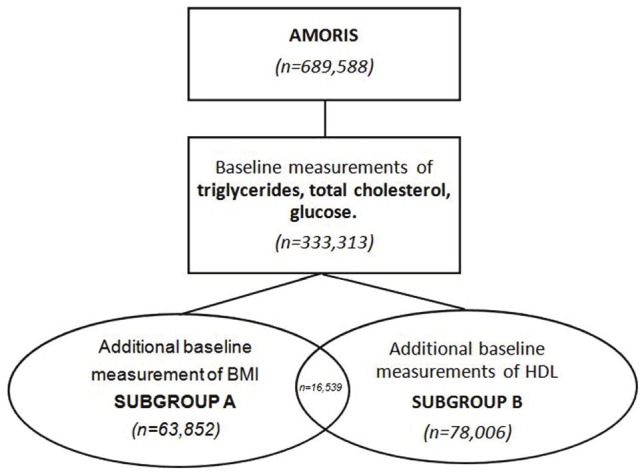
Study population and subgroup selection.
The CALAB database also contained information on age and fasting status. All other information was obtained from the above mentioned national registries. Diagnosis of cardiovascular disease (ICD-10: I00-I99) and lung disease (ICD-10: J00-J99) was taken from the National Patient Register and cancer diagnosis from the National Cancer Registry (ICD-7: 180). Socio-economic status (SES) is based on occupational groups and classifies gainfully employed subjects into manual workers and non-manual employees, below designated blue-collar and white-collar workers [24].
The quantitative determination of GGT was performed with an enzymatic colorimetric test using L-γ-glutamyl-3-carboxy-4-nitroanilide as donor substrate at a temperature of 37°C, which is the reference method recommended by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) [25]. ALT was measured with an enzymatic UV-test according to IFCC, including incubation with pyridoxal phosphate. The coefficient of variation was ≤6.0% for both GGT and ALT. The quantitative determination of CRP was done with an established turbidimetric assay (reagents from Orion Diagnostics, Finland) using fully automated multichannel analyzers (an AutoChemist-PRISMA, New Clinicon, Stockholm, Sweden, 1985-1992) and DAX 96, Technicon Instruments Corporation, Tarrytown, NY, USA, 1993-1996). High sensitive CRP was not available at any time of the period of blood sampling collection [26]. Total cholesterol and TG were measured enzymatically and levels were standardised according to the World Health Organisation International Federation of Clinical Chemists protocols (IFCC) [22,23]. Similarly, ALT was measured with an enzymatic UV-test according to IFCC, including incubation with pyridoxal phosphate. Glucose was measured enzymatically with a glucose-oxidase/peroxidase method. The concentration of HDL were calculated and the validation procedures have been reported [21]. All methods were fully automated with automatic calibration and performed at one accredited laboratory [23].
Statistical analysis
First, we calculated the Pearson correlation coefficients between components of the metabolic profile (glucose, TG, HDL, BMI, and TC) and GGT, CRP, as well as ALT to test for associations between the components included in our analysis. The latter one is a marker of hepatocellular damage [27] and was used to identify whether the association between GGT and components of the metabolic profile was driven by liver dysfunction. In the next step, we assessed whether GGT and CRP are predictive for risk of cardiovascular disease (CVD) and cancer, as is the case in those with abnormal metabolic profiles [7,28,29]. In order to investigate the combined effect of GGT and CRP, we created the ‘GGT-CRP score’. This score was devised based on levels of both GGT and CRP. The levels of GGT were based on tertiles, resulting in the assignment of a value of 1, 2, or 3 depending on what tertile the individual’s GGT level was. The levels of CRP were also divided into three groups using the clinical cut-off of 10 mg/L and the median amongst those with a CRP level > 10 mg/L. These cut-offs were chosen because CRP was not measured using a high-sensitive measurement. This also enabled the assignment of a value of 1, 2, or 3 depending on the individual’s CRP level. The GGT-CRP score was then the sum of the level value for GGT and CRP (Table 1).
Table 1.
Composition of GGT-CRP score based on levels of GGT and CRP
| GGT levels (U/L) | GGT value | CRP levels (mg/L) | CRP value | GGT-CRP score |
|---|---|---|---|---|
| <15 | 1 | <10 | 1 | 2 |
| <15 | 1 | 10-19 | 2 | 3 |
| 15-24 | 2 | <10 | 1 | 3 |
| 15-24 | 2 | 10-19 | 2 | 4 |
| 15-24 | 2 | ≥19 | 3 | 5 |
| ≥24 | 3 | 10-19 | 2 | 5 |
| ≥24 | 3 | ≥19 | 3 | 6 |
We evaluated the association between the GGT-CRP score and risk of CVD as well as with cancer using multivariate Cox proportional hazard models. A test for trend was conducted by using assignment to categories of the GGT-CRP score as an ordinal scale. All models took into account age, sex, SES, fasting status, history of circulatory disease (ICD-10: I00-I99), history of lung disease (ICD-10: J00-J99), and ALT (U/L).
To verify the known association between perturbed metabolic profiles and risk of CVD and cancer in our own study population, we repeated the above multivariate Cox proportional hazards models in the two subgroups with BMI and HDL measurements. We first assessed the components of the metabolic profile available in our study by indicating how many of the following components were above the NCEP cut-offs: glucose (>6.11 mmol/L), TG (>1.71 mmol/L), and TC (>6.50 mmol/L) [30]. In those individuals with additional baseline measurements of BMI we also used obesity (BMI >25 kg/m2) as a component we investigated. In the subgroup with additional baseline measurements of HDL, we assessed the presence of a perturbed metabolic profile by using measurements of glucose, TG, and HDL (<1.03 mmol/L) instead of TC [30]. In addition, we evaluated the additivity of GGT and CRP using a synergy index.
We then considered the probability of reverse causation by conducting a sensitivity analysis in which all men with follow-up < 3 years were excluded [31].
A test for additivity was conducted to determine whether the simultaneous influence of the metabolic variables under consideration was greater than their individual effects on the outcome.
Finally, we used the Pearson correlation coefficient to test whether there was a close association between the GGT/CRP score and the components of the metabolic profile available in our study and then ran tests for concordance to determine whether the score was an accurate predictor of the risk of developing CVD and cancer [32]. All analyses were conducted with Statistical Analysis Systems (SAS) release 9.2 (SAS Institute, Cary, NC).
Results
A total of 21,216 individuals (6.37%) developed cancer, whereas 47,939 individuals (14.38%) developed CVD. The majority of the study population (83.99%) were gainfully employed as the data was collected through routine health checkups across companies. A description of the demographics is shown in Table 2.
Table 2.
Descriptive characteristics by number of abnormal metabolic components
| ≤ 2 Abnormal Metabolic Components (N= 329036) | >2 Abnormal Metabolic Components (N= 4277) | |
|---|---|---|
|
|
||
| N (%) | N (%) | |
| Age (years) Mean (SD) | 45.14 (13.98) | 55.67 (11.46) |
| Gender | ||
| Men | 156272 (42.14) | 1541 (35.00) |
| Women | 172625 (57.86) | 2862 (65.00) |
| Socioeconomic Status | ||
| White collar | 126646 (38.52) | 1547 (35.14) |
| Blue collar | 149738 (46.03) | 2001 (45.45) |
| Not gainfully employed/ Missing | 52513 (7.73) | 855 (9.71) |
| Fasting status | ||
| Fasting | 173916 (51.27) | 2077 (47.17) |
| Non-fasting | 79268 (25.51) | 1359 (30.87) |
| Missing | 75713 (23.21) | 967 (21.96) |
| History of Circulatory disease (ICD10: I00-I99) | ||
| Yes | 21007 (8.20) | 768 (17.44) |
| No | 307890 (91.80) | 3635 (82.56) |
| History of Lung Disease (ICD10: J00-J99) | ||
| Yes | 23245 (6.97) | 314 (7.13) |
| No | 305652 (93.03) | 4089 (92.87) |
| Follow-up time (years) Mean (SD) | 10.46 (3.38) | 9.15 (3.63) |
| CRP (mg/L) | ||
| <10 | 280114 (84.79) | 3575 (81.19) |
| 10-15 | 35575 (10.88) | 498 (11.31) |
| 15-25 | 6273 (2.26) | 192 (4.36) |
| 25-50 | 4299 (1.31) | 89 (2.02) |
| >50 | 2636 (0.77) | 49 (1.11) |
| GGT (U/L) | ||
| Mean (SD) | 28.53 (45.66) | 71.37 (135.27) |
| Normal (<18) | 158745 (40.42) | 494 (14.17) |
| Normal high (18-36) | 114317 (39.60) | 1465 (42.01) |
| Elevated (36-72) | 39951 (18.58) | 1370 (39.29) |
| Highly elevated (>72) | 2576 (1.39) | 158 (4.53) |
| ALT (U/L) | ||
| Mean | 0.46 (0.63) | 0.72 (0.70) |
| <50 | 301450 (88.18) | 3247 (73.75) |
| ≥ 50 | 27447 (11.82) | 1156 (26.25) |
| Glucose (mmol/L) | ||
| Mean (SD) | 5.02 (1.31) | 8.68 (3.61) |
| <6.11 | 310357 (93.15) | 0 (0.00) |
| ≥6.11 | 18540 (6.85) | 4403 (100.00) |
| Triglycerides (mmol/L) | ||
| Mean (SD) | 1.35 (1.02) | 3.71 (2.95) |
| <1.71 | 261987 (78.60) | 0 (0.00) |
| ≥1.71 | 66910 (21.40) | 4403 (100.00) |
| Total Cholesterol (mmol/L) | ||
| Mean (SD) | 5.56 (1.14) | 7.44 (1.02) |
| <6.50 | 265582 (79.68) | 0 (0.00) |
| ≥6.50 | 63315 (20.32) | 4403 (100.00) |
| HDL (mmol/L)* | ||
| Mean | 1.51 (0.41) | 1.12 (0.40) |
| <1.03 | 8147 (17.18) | 490 (40.53) |
| ≥1.03 | 68650 (82.82) | 719 (59.47) |
| Missing | 255294 | |
| Body Mass Index (kg/m2)** | ||
| Mean (SD) | 24.59 (3.77) | 28.11 (4.18) |
| <18.5 | 1062 (1.13) | 1 (0.13) |
| 18.5-25 | 37154 (49.15) | 156 (20.97) |
| 25-30 | 20271 (31.19) | 361 (48.52) |
| >30 | 4621 (10.53) | 226 (30.38) |
| Missing | 269448 | |
| CVD | ||
| Yes | 46333 (18.55) | 1606 (36.48) |
| No | 282564 (81.45) | 2797 (63.52) |
| Cancer | ||
| Yes | 20781 (7.22) | 435 (9.88) |
| No | 308116 (92.78) | 3968 (90.12) |
| Score (%) | ||
| GGT-CRP score = 2 | 98544 (22.82) | 246 (5.59) |
| GGT-CRP score = 3 | 101429 (27.91) | 601 (13.65) |
| GGT-CRP score = 4 | 108791 (41.58) | 2884 (65.50) |
| GGT-CRP score = 5 | 15907 (6.02) | 481 (10.92) |
| GGT-CRP score = 6 | 4424 (1.66) | 191 (4.34) |
Firstly, we assessed the association between GGT and CRP in relation to the components of the metabolic profile available in our study (results not shown). The strongest correlation observed was observed for GGT and TG (r=0.22) indicating little to no linear dependence between the variables included. Stratification by ALT levels had no effect on the relation between GGT or CRP and components of the metabolic profile except for GGT and BMI where the correlation was negated when ALT was ≥50 (r=0.01). There was also a reduction in the correlation between GGT and triglycerides when ALT was ≥50 (r=0.15).
Next we assessed how GGT and CRP (when combined) had an effect on risk of cancer and CVD, as both diseases have been linked to metabolic abnormalities. This index, independent of abnormal metabolic profiles, is associated with an increased risk of developing cancer and possibly, albeit weaker, also CVD. The greatest risk of CVD was found when GGT-CRP score = 6 (HR: 1.40 (95%CI: 1.31-1.48)), compared to GGT-CRP score = 0. A similar trend was found when studying the number of components of metabolic profile (as available in our study) in relation to CVD risk; risk increases with the presence of each additional component (e.g. HR for those with three abnormal metabolic profile components: 1.40 (95%CI: 1.31-1.48) (Table 3).
Table 3.
Hazard ratios (HR) and 95% Confidence Intervals (95%CI) for the risk of CVD by GGT-CRP score and number of abnormal metabolic components. All models were adjusted for gender, age, socioeconomic status, fasting status, history of circulatory disease, history of lung disease, and ALT
| N (%) | N (%) | HR (95%CI) | |
|---|---|---|---|
|
| |||
| With CVD N=47,939 | Without CVD N=285,361 | ||
| All | |||
|
| |||
| GGT-CRP score = 2 | 9089 (18.96) | 89701 (31.43) | 1.00 (Ref) |
| GGT-CRP score = 3 | 13335 (27.82) | 88695 (31.08) | 1.04 (1.01 - 1.07) |
| GGT-CRP score = 4 | 20208 (42.15) | 91467 (81.90) | 1.29 (1.26 - 1.32) |
| GGT-CRP score = 5 | 4047 (8.44) | 12143 (4.26) | 0.97 (0.94 - 1.01) |
| GGT-CRP score = 6 | 1260 (2.63) | 3355 (1.18) | 1.40 (1.31 - 1.48) |
| P-value for trend | <0.001 | ||
|
| |||
| Number of components of the MetS (triglycerides ≥1.71, total cholesterol ≥6.50, glucose ≥6.11) | |||
|
| |||
| 0 | 21874 (45.63) | 190528 (66.77) | 1.00 (Ref) |
| 1 | 15993 (33.24) | 68292 (23.93) | 1.28 (1.22-1.35) |
| 2 | 8526 (17.79) | 23744 (8.32) | 1.61 (1.52-1.71) |
| 3 | 1606 (3.35) | 2798 (0.98) | 2.19 (1.93-2.48) |
| P-value for trend | <0.001 | ||
|
| |||
| Number of components of the MetS with BMI* (triglycerides ≥1.71, total cholesterol ≥6.50, glucose ≥6.11, BMI ≥30) | |||
|
| |||
| 0 | 3671 (42.24) | 35170 (63.76) | 1.00 (Ref) |
| 1 | 2814 (32.38) | 13517 (24.50) | 1.29 (1.23- 1.36) |
| 2 | 1647 (18.95) | 5267 (9.55) | 1.58 (1.49- 1.67) |
| 3 | 472 (5.43) | 1068 (1.94) | 2.15 (1.95- 2.37) |
| 4 | 86 (0.99) | 140 (0.25) | 2.35 (1.90- 2.92) |
| P-value for trend | <0.001 | ||
|
| |||
| Number of components of the MetS (triglycerides ≥1.71, HDL <1.03**, glucose ≥6.11) | |||
|
| |||
| 0 | 7441 (58.64) | 48184 (58.64) | 1.00 (Ref) |
| 1 | 3029 (23.87) | 11321 (23.87) | 1.35 (1.25-1.47) |
| 2 | 1765 (13.91) | 1765 (13.91) | 1.52 (1.37-1.68) |
| 3 | 454 (3.58) | 454 (3.58) | 2.04 (1.80-2.32) |
| P-value for trend | <0.001 | ||
Measured in a subgroup of 63,852,
Measured in a subgroup of 78,006.
Given the known association between metabolic abnormalities and cancer, we then assessed the association between GGT-CRP and cancer (Table 4). A positive trend was identified between GGT-CRP and the risk of cancer with the greatest cancer risk when GGT-CRP score = 6 (HR: 1.60 (95%CI: 1.47-1.76)), compared to GGT-CRP score = 0. Again, an increasing number of abnormal metabolic components (as available in our study) was found to be positively associated with risk of cancer (e.g. for those with three abnormal components: 1.11 (95%CI: 1.00-1.22)) (Table 4).
Table 4.
Hazard ratios (HR) and 95% Confidence Intervals (95%CI) for the risk of cancer by GGT-CRP score and number of abnormal metabolic components. All models were adjusted for gend, age, socioeconomic status, fasting status, history of circulatory disease, history of lung disease, and ALT
| N (%) | N (%) | HR (95%CI) | |
|---|---|---|---|
|
| |||
| With Cancer N=21,216 | Without Cancer N=312,084 | ||
| All | |||
|
| |||
| GGT-CRP score = 2 | 4983 (23.49) | 93807 (30.06) | 1.00 (Ref) |
| GGT-CRP score = 3 | 6104 (28.77) | 95926 (30.74) | 1.01 (0.97 - 1.05) |
| GGT-CRP score = 4 | 7921 (37.34) | 103754 (33.25) | 1.12 (1.08 - 1.16) |
| GGT-CRP score = 5 | 1664 (7.84) | 14526 (4.65) | 1.23 (1.16 - 1.30) |
| GGT-CRP score = 6 | 544 (2.56) | 4071 (1.30) | 1.60 (1.47 - 1.76) |
| P-value for trend | <0.001 | ||
|
| |||
| Number of components of the MetS (triglycerides ≥1.71, total cholesterol ≥6.50, glucose ≥6.11) | |||
|
| |||
| 0 | 11607 (54.71) | 200795 (64.34) | 1.00 (Ref) |
| 1 | 6395 (30.14) | 77830 (24.94) | 0.98 (0.91- 1.05) |
| 2 | 2779 (13.10) | 29491 (9.45) | 0.94 (0.85- 1.04) |
| 3 | 435 (2.05) | 3968 (1.27) | 1.07 (0.84- 1.37) |
| P-value for trend | <0.370 | ||
|
| |||
| Number of components of the MetS with BMI* (triglycerides ≥1.71, total cholesterol ≥6.50, glucose ≥6.11, BMI ≥30) | |||
|
| |||
| 0 | 2049 (52.01) | 36792 (61.41) | 1.00 (Ref) |
| 1 | 1211 (30.74) | 15120 (25.24) | 1.00 (0.93- 1.07) |
| 2 | 514 (13.05) | 6400 (10.68) | 0.92 (0.83- 1.01) |
| 3 | 151 (3.83) | 1389 (2.32) | 1.22 (1.04- 1.45) |
| 4 | 15 (0.38) | 211 (0.35) | 0.83 (0.50- 1.38) |
| P-value for trend | 0.998 | ||
|
| |||
| Number of components of the MetS (triglycerides ≥1.71, HDL <1.03**, glucose ≥6.11) | |||
|
| |||
| 0 | 3489 (66.47) | 52136 (71.66) | 1.00 (Ref) |
| 1 | 1094 (20.84) | 13256 (18.22) | 1.09 (0.97-1.23) |
| 2 | 554 (10.55) | 6332 (8.70) | 0.83 (0.69-1.00) |
| 3 | 112 (2.13) | 1033 (1.42) | 0.94 (0.73-1.22) |
| P-value for trend | 0.760 | ||
Measured in a subgroup of 63,900,
Measured in a subgroup of 77,944.
A sensitivity analysis was carried out in order to identify the possibility of reverse causation. This was done by excluding those with follow-up of < three years; it did not alter the trends observed (results not shown).
When testing for an additive effect between the GGT/CRP score and TC and TG, there were no differences in the risk of either cancer or CVD when compared to TC alone (e.g., HR for cancer 0.93 (0.90-0.96); and therefore no indication of an additive effect. Notably, the risk of an individual having a higher GGT or CRP did increase significantly by the number of abnormal metabolic components the individual had (e.g., 84% of individuals with five abnormal metabolic components had high levels of GGT (≥24mg/L).
The Pearson correlation coefficient (r=0.27) indicated that the GGT/CRP score was statistically significantly, albeit weak, associated with an abnormal metabolic profile (results not shown). The C-statistic for the association between the GGT/CRP score and CVD was also statistically significant (0.59 (95% 0.58-0.59)), as it was for cancer (0.54 (0.53-0.55)).
Discussion
The findings from the current study showed that GGT and CRP, independent of one another, are weak markers of an abnormal metabolic profile. However, when these two markers were combined, they proved to be a fairly strong predictor of cancer independent of an abnormal metabolic profile.
Studies which have examined the link between GGT and CRP in relation to the risk of abnormal metabolic profiles have generally found a positive association [22,33,34]. For example, one study found that elevated levels of GGT and high sensitivity CRP had a synergistic effect and were associated with the Metabolic Syndrome and insulin resistance [33].
Although an association was identified between GGT, CRP and abnormal levels of metabolic components in the current study, our findings suggest that these markers are not strong predictors as has been suggested in previous literature [2-5]. It is possible that previous studies saw associations because GGT and CRP are more strongly related to BMI and/or hypertension, two measurements for which we had limited or no information available.
In addition, further studies also found a significant association between GGT, CRP and risk of developing cancer and CRP [16,35,36]. The link between GGT and CRP in relation to risk of CVD and cancer have mainly been investigated in isolation, therefore making this study the first to examine GGT and CRP levels simultaneously in relation to CVD and cancer risk and thus explore the possibility of a synergistic effect.
When testing the additive effect of the GGT/CRP score and TC and TG there were no marked differences observed in risk of either CVD or cancer. This may indicate that these markers could impact the risk of these diseases via different mechanisms, working largely independently of one another. Another possibility is that an elevated GGT/CRP score is an intermediary between hypertension and/or BMI and CVD or cancer. This is plausible as CRP is a known marker of inflammation [10], thus in the presence of hypertension and/or obesity, we would expect levels to rise [37].
The increased likelihood of a higher GGT/CRP with a higher number of abnormal metabolic components observed in this study does suggest that GGT and/or CRP could be intermediaries between perturbed metabolic profiles and associated diseases. When combined with existing evidence for an association between hypertension/BMI and risk of both CVD and cancer [38,39], the results obtained here support a plausible argument that CRP could be an intermediary which, in turn, could potentially prove helpful in predicting higher risk individuals, subject to further research.
Given that there are only a small number of studies that have assessed the relation between GGT, CRP and the risk of developing an abnormal metabolic profile it is too early for any firm conclusions to be drawn. Although the findings in this study were statistically significant, it remains difficult to identify whether these commonly used biomarkers (GGT and CRP) are intermediates between developing an abnormal metabolic profile and then CVD or cancer, or whether they act as confounders. For example, due to an unhealthy lifestyle individuals may develop risk factors associated with abnormal metabolic profiles, which consequently may result in liver dysfunction and inflammation and thus elevated levels of GGT and CRP. This can eventually increase risk of developing associated diseases which include CVD and cancer.
Further research in this field is required to develop a better understanding of the mechanism behind the association between these commonly used biomarkers, perturbed metabolic profiles and risk of developing associated diseases, including CVD and cancer. In particular, multicentre studies are warranted amongst European populations to identify whether GGT and CRP are good predictors of abnormal metabolic profiles as well as associated diseases. This is particularly important given the high prevalence of obesity and the associated dyslipidemia across these regions [40,41].
The main strengths of this study was the large sample size consisting of individuals with prospective measurements of GGT, CRP, ALT, triglycerides, total cholesterol and glucose from the AMORIS database, all measured at the same clinical laboratory, and the longitudinal cohort study design. In addition, the database also provided complete follow-up for all subjects including detailed information on cancer diagnosis, emigration and time of death. The study population in AMORIS was selected by analysing blood samples from routine health check-ups in non-hospitalised persons. Nonetheless, the selection of a healthy cohort does not affect the internal validity in this study. A limitation to the current study is that there was no access to other commonly used inflammatory markers such as high sensitive-CRP (hs-CRP) and IL-6. Furthermore, hs-CRP did not exist at the time of data collection or analysis and thus it was not possible to specify CRP concentrations of <10mg/L. However, 10mg/L is found to be a reliable cut off as it has been widely used in general medicine prior to the introduction of hs-CRP assay [42]. Another limitation to this study was the lack of data on hypertension and BMI for the whole study cohort as well as the lack of data on potential confounding variables such as smoking status and alcohol consumption. However as a proxy for smoking status all models were adjusted for history of lung disease.
Conclusion
In conclusion, while GGT and CRP have been shown to be associated with abnormal metabolic profiles previously, their association to the components investigated in this study was limited. That said the present study did demonstrate that these markers were predictive of the risk of diseases commonly associated with perturbed metabolic profiles. This area would benefit from intervention studies aimed to determine whether lifestyle changes could reduce GGT and CRP levels and subsequently the risk of abnormal metabolic profiles and associated diseases.
Acknowledgements
Funding was received from Cancer Research UK and the Swedish Cancer Society. This research was also supported by the Experimental Cancer Medicine Centre at King’s College London and also by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest statement
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide licence to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to i) publish, reproduce, distribute, display and store the Contribution, ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, iii) create any other derivative work(s) based on the Contribution, iv) to exploit all subsidiary rights in the Contribution, v) the inclusion of electronic links from the Contribution to third party material where-ever it may be located; and, vi) licence any third party to do any or all of the above.
References
- 1.Wannamethee SG, Lennon L, Shaper AG. The value of gamma-glutamyltransferase in cardiovascular risk prediction in men without diagnosed cardiovascular disease or diabetes. Atherosclerosis. 2008;201:168–175. doi: 10.1016/j.atherosclerosis.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan A, Jaiswal A, Tabassum R, Podder A, Ghosh S, Madhu SV, Mathur SK, Tandon N, Bharadwaj D. Elevated levels of C-reactive protein as a risk factor for Metabolic Syndrome in Indians. Atherosclerosis. 2012;220:275–281. doi: 10.1016/j.atherosclerosis.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Kang YH, Min HK, Son SM, Kim IJ, Kim YK. The association of serum gamma glutamyltransferase with components of the metabolic syndrome in the Korean adults. Diabetes Res Clin Pract. 2007;77:306–313. doi: 10.1016/j.diabres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Frohlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U. S. youth. Diabetes Care. 2005;28:878–881. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- 6.Lee DH, Buijsse B, Steffen L, Holtzman J, Luepker R, Jacobs DR Jr. Association between serum gamma-glutamyltransferase and cardiovascular mortality varies by age: the Minnesota Heart Survey. Eur J Cardiovasc Prev Rehabil. 2009;16:16–20. doi: 10.1097/HJR.0b013e32830aba5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 9.Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, Park JR, Kim SW. High sensitivity C-reactive protein as an independent risk factor for essential hypertension. Am J Hypertens. 2003;16:429–433. doi: 10.1016/s0895-7061(03)00566-1. [DOI] [PubMed] [Google Scholar]
- 10.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 11.Turgut O, Tandogan I. Gamma-glutamyltransferase to determine cardiovascular risk: shifting the paradigm forward. J Atheroscler Thromb. 2011;18:177–181. doi: 10.5551/jat.6189. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi N, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004;27:1427–1432. doi: 10.2337/diacare.27.6.1427. [DOI] [PubMed] [Google Scholar]
- 13.Cheung BM, Ong KL, Tso AW, Cherny SS, Sham PC, Lam TH, Lam KS. Gamma-glutamyl transferase level predicts the development of hypertension in Hong Kong Chinese. Clin Chim Acta. 2011;412:1326–1331. doi: 10.1016/j.cca.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto R, Kohara K, Tabara Y, Kusunoki T, Otsuka N, Miki T. Association between serum gamma-glutamyl transferase level and prehypertension among community-dwelling men. Tohoku J Exp Med. 2008;216:213–221. doi: 10.1620/tjem.216.213. [DOI] [PubMed] [Google Scholar]
- 15.Strasak AM, Rapp K, Brant LJ, Hilbe W, Gregory M, Oberaigner W, Ruttmann E, Concin H, Diem G, Pfeiffer KP, Ulmer H. Association of gamma-glutamyltransferase and risk of cancer incidence in men: a prospective study. Cancer Res. 2008;68:3970–3977. doi: 10.1158/0008-5472.CAN-07-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Hemelrijck M, Jassem W, Walldius G, Fentiman IS, Hammar N, Lambe M, Garmo H, Jungner I, Holmberg L. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study. Eur J Cancer. 2011;47:2033–2041. doi: 10.1016/j.ejca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Fentiman IS, Allen DS. gamma-Glutamyl transferase and breast cancer risk. Br J Cancer. 2010;103:90–93. doi: 10.1038/sj.bjc.6605719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strasak AM, Pfeiffer RM, Klenk J, Hilbe W, Oberaigner W, Gregory M, Concin H, Diem G, Pfeiffer KP, Ruttmann E, Ulmer H. Prospective study of the association of gamma-glutamyltransferase with cancer incidence in women. Int J Cancer. 2008;123:1902–1906. doi: 10.1002/ijc.23714. [DOI] [PubMed] [Google Scholar]
- 19.Pathak SK, Sharma RA, Steward WP, Mellon JK, Griffiths TR, Gescher AJ. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: targets for chemopreventive strategies. Eur J Cancer. 2005;41:61–70. doi: 10.1016/j.ejca.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo E, Krieg A, Weiner GJ, Fox BA, Coukos G, Wang E, Abraham RT, Carbone M, Lotze MT. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 22.Jungner I, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I in relation to serum cholesterol and triglycerides in 43,000 Swedish males and females. Int J Clin Lab Res. 1992;21:247–255. doi: 10.1007/BF02591655. [DOI] [PubMed] [Google Scholar]
- 23.Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I values in 147576 Swedish males and females, standardized according to the World Health Organization-International Federation of Clinical Chemistry First International Reference Materials. Clin Chem. 1998;44:1641–1649. [PubMed] [Google Scholar]
- 24.Central Bureau for Statistics: Statistics Sweden. Stockholm, Sweden: 2008. [Google Scholar]
- 25.Targher G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer - a narrative review. Clin Chem Lab Med. 2010;48:147–157. doi: 10.1515/CCLM.2010.031. [DOI] [PubMed] [Google Scholar]
- 26.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Inflammatory markers, lipoprotein components and risk of major cardiovascular events in 65,005 men and women in the Apolipoprotein MOrtality RISk study (AMORIS) Atherosclerosis. 2010 Nov;213:299–305. doi: 10.1016/j.atherosclerosis.2010.08.049. Epub 2010 Aug 19. [DOI] [PubMed] [Google Scholar]
- 27.Clark PKM, editor. Clinical Medicine. Saunders Ltd; 2005. [Google Scholar]
- 28.Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer. 2008;44:293–297. doi: 10.1016/j.ejca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 30.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 31.Ahn J, Lim U, Weinstein SJ, Schatzkin A, Hayes RB, Virtamo J, Albanes D. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2814–2821. doi: 10.1158/1055-9965.EPI-08-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolai N, Colecchia M, Biasoni D, Catanzaro M, Stagni S, Torelli T, Necchi A, Piva L, Milani A, Salvioni R. Concordance and prediction ability of original and reviewed vascular invasion and other prognostic parameters of clinical stage I nonseminomatous germ cell testicular tumors after retroperitoneal lymph node dissection. J Urol. 2011;186:1298–1302. doi: 10.1016/j.juro.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 33.Kawamoto R, Tabara Y, Kohara K, Miki T, Kusunoki T, Takayama S, Abe M, Katoh T, Ohtsuka N. High-sensitivity C-reactive protein and gamma-glutamyl transferase levels are synergistically associated with metabolic syndrome in community-dwelling persons. Cardiovasc Diabetol. 2010;9:87. doi: 10.1186/1475-2840-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andre P, Balkau B, Vol S, Charles MA, Eschwege E. Gamma-glutamyltransferase activity and development of the metabolic syndrome (International Diabetes Federation Definition) in middle-aged men and women: Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort. Diabetes Care. 2007;30:2355–2361. doi: 10.2337/dc07-0440. [DOI] [PubMed] [Google Scholar]
- 35.Van Hemelrijck M, Holmberg L, Garmo H, Hammar N, Walldius G, Binda E, Lambe M, Jungner I. Association between levels of C-reactive protein and leukocytes and cancer: three repeated measurements in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prev. 2011;20:428–437. doi: 10.1158/1055-9965.EPI-10-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Choe JW, Kim HK, Sung J. High-sensitivity C-reactive protein and cancer. J Epidemiol. 2011;21:161–168. doi: 10.2188/jea.JE20100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi KH, Kim HK, Kim JH, Choi ES, Shin JA, Lee SO, Chintharlapalli S, Safe S, Abdelrahim M, Kong G, Choi HS, Jung JY, Cho HT, Cho NP, Cho SD. The p38 MAPK pathway is critical for 5,5’-dibromodiindolylmethane-induced apoptosis to prevent oral squamous carcinoma cells. Eur J Cancer Prev. 2010;19:153–159. doi: 10.1097/CEJ.0b013e328333d088. [DOI] [PubMed] [Google Scholar]
- 38.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 39.Kannel WB. Hypertension: reflections on risks and prognostication. Med Clin North Am. 2009;93:541–558. doi: 10.1016/j.mcna.2009.02.006. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ervin R. Prevalence of Metabolic Syndrome Among Adults 20 Years of Age and Over, by Sex, Age, Race and Ethnicity, and Body Mass Index: United States, 2003-2006. Hyattsville, MD: National Center for Health Statistics; 2009. National Health Statistics Reports. [PubMed] [Google Scholar]
- 41.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–375. doi: 10.1016/j.ecl.2004.03.005. table of contents. [DOI] [PubMed] [Google Scholar]
- 42.Wilkins J, Gallimore JR, Moore EG, Pepys MB. Rapid automated high sensitivity enzyme immunoassay of C-reactive protein. Clin Chem. 1998;44:1358–1361. [PubMed] [Google Scholar]


