Abstract
Background
Heart failure (HF), a debilitating disease in a growing number of adults, exerts structural and neurohormonal changes in both cardiac and skeletal muscles. However, these alterations and their affected molecular pathways remain uncharacterized. Disease progression is known to transform skeletal muscle fiber composition by unknown mechanisms. In addition, perturbation of specific hormonal pathways, including those involving skeletal muscle insulin-like growth factor-1 (IGF-1) and insulin-like growth factor-binding protein-5 (IGFB-5) appears to occur, likely affecting muscle metabolism and regeneration. We hypothesized that changes in IGF-1 and IGFB-5 mRNA levels correlate with the transformation of single–skeletal muscle fiber myosin heavy chain isoforms early in disease progression, making these molecules valuable markers of skeletal muscle changes in heart failure.
Materials and methods
To investigate these molecules during “early” events in HF patients, we obtained skeletal muscle biopsies from New York Heart Association (NYHA) Class II HF patients and controls for molecular analyses of single fibers, and we also quantified isometric strength and muscle size.
Results
There were more (P < 0.05) single muscle fibers coexpressing two or more myosin heavy chains in the HF patients (30% ± 7%) compared to the control subjects (13% ± 2%). IGF-1 and IGFBP-5 expression was fivefold and 15-fold lower in patients with in HF compared to control subjects (P < 0.05), respectively. Strikingly, there was a correlation in IGF-1 expression and muscle cross-sectional area (P < 0.05) resulting in a decrease in whole-muscle quality (P < 0.05) in the HF patients, despite no significant decrease in isometric strength or whole-muscle size.
Conclusion
These data indicate that molecular alterations in myosin heavy chain isoforms, IGF-1, and IGFB-5 levels precede the gross morphological and functional deficits that have previously been associated with HF, and may be used as a predictor of functional outcome in patients.
Keywords: muscle quality, hybrid fibers, IGF1 and IGFBP-5
Background
Chronic heart failure is a major disease in older adults. In 2004 there were approximately 5.2 million Americans over the age of 20 years who suffered from heart failure, and of these, approximately 550,000 were new cases. It is estimated that these staggering statistics will result in the direct and indirect cost of heart failure reaching $33.2 billion in 2007.1 Clearly, a greater understanding of the molecular pathways involved, as well as the direct and peripheral structural changes that are known to occur in both cardiac and skeletal muscle during disease, would be useful for diagnosis and potential therapeutic intervention.
Chronic heart failure is characterized by compromised ventricular systolic or diastolic function or both and is frequently classified by an ejection fraction of <40%. The disease results in reduced exercise tolerance, stimulation of the sympathetic nervous system, and the resilience of neurohormonal activation (which turns from adaptive to maladaptive and leads to the progression of chronic heart failure). Specific neurohormones that are frequently involved include noradrenalin, atrial natriuretic peptide, and several hormones in the renin–angiotensin–aldosterone system (RAS).2 The progression of this disease continues to increase the activation of the RAS resulting in an upregulation of angiotensin II. In turn, angiotensin II downregulates both circulating and skeletal muscle insulin-like growth factor-1 (IGF-1),3 an anabolic hormone with multiple physiological effects.
Importantly, IGF-1 and its binding proteins (including insulin-like growth factor-binding protein-5 [IGFBP-5]) exert acute anabolic effects on metabolism as well as mediating myoblast proliferation, differentiation, and apoptosis.4–7 IGF-1 also is known to play a role in compensatory hypertrophy.8 Intriguingly, IGF-1 levels are perturbed (ie, downregulated) during the normal aging process9 but also pathologically in heart failure models10 and hemodialysis patients.11 Alterations of RAS hormones and IGF-1 levels, (together with reduced skeletal muscle blood perfusion as a consequence of increased sympathetic nervous system activity) alter the aerobic and anaerobic enzymatic activity of skeletal musculature. Chronically, this can transform the composition of skeletal muscle fibers,12–16 thus giving rise to highly fatigable muscle that manifests in exercise intolerance and weakness. These functions may make IGF-1 and its binding proteins good markers of skeletal muscle molecular changes and useful for understanding the peripheral effects of heart failure.
Chronic heart failure also causes a transformation of the skeletal muscle myosin heavy chain (MHC) isoforms from MHC I (slow-twitch, oxidative fibers) to MHC IIa and IIb (x) (fast-twitch, glycolytic fibers).17–22 This is in striking contrast with the “normal” aging process, in which skeletal muscles tend to shift toward the slow-twitch fibers23 or express hybrid type I/IIa fibers.24 For heart failure patients, shifting toward the fast, glycolytic fibers is expected to result in decreased muscle endurance and rapid fatigue upon physical stress. Indeed, studies have determined that cardiac cachexia (skeletal muscle wasting) results from moderate and severe left ventricular dysfunction: dramatically, MHC I, IIa, and IIx fibers are smaller in size compared with control fibers. The MHC isoform transformation, along with the cardiac cachexia, can lead to skeletal muscle reliance on glycolytic metabolism. This reliance contributes to an earlier onset of muscle fatigue and as a result, a decrease in functional work capacity and independent daily living.
In this investigation, we aimed to examine the alterations in the structural composition of single muscle fiber MHC proteins in the skeletal muscles of heart failure patients and to determine whether these correlate with IGF-1 and IGFBP-5 mRNA expression, as well as whole-muscle strength, size, and quality. We were particularly interested in specifically analyzing these molecular events in New York Heart Association (NYHA) Class II chronic heart failure patients compared with healthy control subjects. This class was chosen for two main reasons: (1) to eliminate the complexity and variability in the skeletal muscle molecular alterations and whole-muscle functions that have been observed in previous studies involving NYHA Class II, III, IV, and V patients taken altogether; and (2) to focus on potential “early” events emerging in Class II patients that may serve as markers for skeletal muscle impairment.
For these studies, we evaluated single muscle fibers from each patient and a control muscle, a more accurate and powerful method of determining the expression profiles of MHC isoforms compared with whole-muscle homogenization experiments. We also used real-time polymerase chain reaction analysis (PCR) to quantify IGF-1 and IGFBP-5 transcript levels present in the muscle fibers. Our strategy enabled us to directly compare the MHC isoform profile with IGF-1 and IGF-5 mRNA expression in the single muscle fibers. Importantly, we also examined how these specific expression profiles translated into whole-muscle strength, size, and, ultimately, quality of the knee extensors in response to chronic heart failure. We hypothesized the following: (1) single muscle fiber MHC isoforms will demonstrate an increased composition of hybrid isoforms and a shift toward a faster fiber in the chronic heart failure group compared with the healthy control group; (2) IGF-1 and IGFBP-5 gene expression will be significantly lower in the chronic heart failure group compared to the healthy control group; and (3) functionally, whole-muscle strength and size will be decreased in the chronic heart failure group compared with the healthy control group, which will lead to a reduction in whole-muscle quality.
Material and methods
Subjects
Ten subjects were recruited to serve as subjects in this investigation (n = 5 heart failure and n = 5 control). The subjects in both the heart failure and control groups ranged in age from 46–78 years (with means of 61.8 ± 3.4 years and 55.0 ± 2.9 years for the heart failure and control groups, respectively). The heart failure volunteers had their disease state assessed by a board-certified cardiologist in cardiovascular diseases (NYHA Class II), and the control subjects were examined and screened by a board-certified family practice physician. Written informed consent was obtained from each participant. Our sample size was larger in many instances than what has been reported previously in studies of specific subclasses of heart failure.22,25 We felt that this was extremely important to control for when looking at physical activity levels and attempting to correlate whole-muscle function with cellular changes that were occurring in the skeletal musculature.
The inclusion criteria for the heart failure subjects in this study were: medically stable heart failure in NYHA Class II for at least 3 months; an echocardiographically determined ejection fraction at rest of less than 40% (average ejection fraction for the heart failure group was 28.6% ± 2.2%); and on angiotensin-converting enzyme (ACE) inhibitor therapy for at least 3 months. This last was selected in a continued effort to control for external influences that may affect some of the variables that we measured. Ace inhibitor therapy has not been controlled for in other similar studies.22,25 Though all of the heart failure subjects in a recent study by Toth et al26 were on ACE inhibitor therapy, 22% of the subjects in their investigation had diabetes mellitus, which could have a profound impact on skeletal muscle protein and gene expression. To this end, the exclusion criteria for all volunteers (heart failure and controls) were: obesity (BMI greater than 30 kg/m2); angina pectoris; cigarette smokers; persons taking nonsteroidal anti-inflammatory drugs, corticosteroids, or anticoagulants chronically; females taking estrogen during the time of the study or within the previous 12 months; and comorbidity, such as intermittent claudication, diabetes mellitus, severe chronic obstructive pulmonary disease, or any other disorder limiting physical performance other than heart failure.
Physical activity assessment
Assessment of physical activity was determined with the Yale Physical Activity Survey27 in both the heart failure and control groups. This is an interviewer-administered questionnaire, which was used to determine whether the physical activity levels of the two groups was similar in order to be able to eliminate physical activity as a secondary component that could have influenced skeletal muscle measurements (whole body and cellular).
Muscle biopsy
Percutaneous needle muscle biopsies of the vastus lateralis were obtained in each subject for single muscle fiber and mRNA analysis. A 5.0 mm biopsy needle (DePuy Inc, Warsaw, IN) was used to obtain the muscle sample. Muscle sections were placed in a skinning solution28 in preparation for single muscle fiber analysis or placed in RNAlater® (Life Technologies Inc, Carlsbad, CA) and stored at −20°C until the mRNA IGF-1 and IGFBP-5 analysis was performed.
Myosin stoichiometry
A muscle fiber bundle was transferred to a dissection dish containing a relaxing solution at 4°C.29 An individual fiber was then dissected under a microscope from the bundle and then transferred with a small glass capillary tube to a 1.5 mL Ependorff tube and solubilized in 80 μL of 1% sodium dodecyl sulfate (SDS) sample buffer. The tubes were stored at −80°C until SDS–polyacrylamide gel electrophoresis (SDS–PAGE) analysis was performed for the determination of the MHC isoform composition. For this analysis, 208 fibers from each of the muscle samples were obtained.
The MHC analysis was performed on a 3.5% stacking gel and a 5% separating gel at 4°C (Hoefer SE 600 series; Hoefer® Inc, San Francisco, CA) for approximately 12 hours. Following the completion of the SDS–PAGE, the gels were removed from the glass slabs and transferred into an alcohol acid fixing solution for approximately 30 minutes. The gels were then removed from the alcohol acid fixing solution and transferred into a gluteraldehyde cross-linking solution for approximately 30 to 60 minutes. The gels were then subjected to a series of three rinses with distilled water (every 30 minutes). The methodology for the silver staining and developing of the SDS–PAGE gels has been described in detail previously.28
After the completion of the electrophoresis, the single muscle fiber types were determined from the MHCs. The MHC isoforms were identified according to the migration rates and compared with molecular weight standards for each specific fiber analyzed. This allowed the data for all measured parameters to be subclassified into the appropriate fiber-type group for proper interpretation of the results (Figure 1).
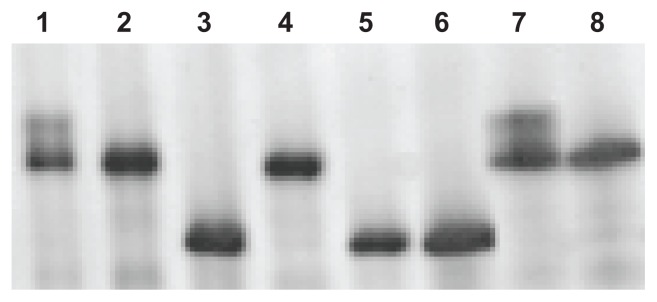
Figure 1.
Representative MHC single muscle fiber silver-stained SDS–PAGE from a heart failure patient.
Notes: Lanes 1 and 7 represent a hybrid isoform that coexpresses both MHC IIx and MHC IIa; lanes 2, 4, and 8 represent MHC IIa; and lanes 3, 5, and 6 represent MHC I isoforms.
Abbreviations: MHC, myosin heavy chain; SDS–PAGE, sodium dodecyl sulfate– polyacrylamide gel electrophoresis.
Real-time PCR
RNA was isolated from a portion of the vastus lateralis biopsy using the RNeasy® Kit (Qiagen Inc, Venlo, The Netherlands). Random primers were utilized to produce cDNA following instructions from the First Strand cDNA Synthesis Kit (GE Healthcare, Little Calfont, UK). Gene expression of human IGF-1 (Forward 5′ ATGTCCTCCTCGCATCTC3′, Reverse 5′ CAC GAACTGAAGAGCATCC3′-), and IGF binding protein-5 (IGFBP-5) (Forward 5′ TGAAGCAGTGAAGAAGGAC3′, Reverse 5′GAAGCCTCCATGTGTCTG3′) was measured with the iCycler (Bio-Rad Laboratories Inc, Hercules, CA) utilizing SYBR green chemistry (iQ SYBR green supermix; Bio-Rad Laboratories Inc). Cycling conditions included two-step amplification, with a denaturing step at 95°C and a combination anneal/extension at 53°C for 40 cycles, and incorporated a melt-curve analysis to ensure specificity of the product. Expression of these two target genes was made relative to β-actin for each sample using the ΔΔCt method:
where HF = heart failure and CON = control.
Muscular strength
Muscle function evaluations were performed on each subject. Two familiarization sessions were done on separate occasions before any data were collected for analysis. The average coefficient of variation for the whole-muscle strength testing was 2.2%. This testing was used to evaluate functional capacity of the knee extensors. Prior to any strength evaluations, the volunteers performed a warm-up consisting of 10 minutes of stationary cycling at a low resistance and slow speed as well as stretching of the muscle groups involved in the strength measurements. Using a Biodex dynamometer (Biodex Medical Systems Inc, Shirley, NY), volunteers performed the following measurements:
Isometric strength
This test involved applying a maximal force in a fixed, nonmoving position. The knee angle was fixed at 60° (with neutral representing a 90° knee position). Subjects were required to apply as much force as possible for 5 seconds at the test angle. Two trials were performed with values recorded from the better trial.
Isokinetic strength
This test was employed at three different velocities 60, 180, and 300°/second. Three maximal repetitions were performed at each velocity (to ensure a maximal effort) with 90 seconds rest between each velocity. The lower leg moved at a given velocity with the subject applying as much force as possible within the range of motion of the knee joint. The values of the best repetition were recorded.
Muscle size
Whole-muscle cross-sectional area (CSA) of the right thigh was determined with computed tomography (CT). The circumference of the anatomical midpoint of the femur was used as the point of CT measurement. An initial lower-body scout scan of the right leg was taken to determine the length of the femur from the greater trochanter to the lateral epicondyle, at an angle of 0°. Scan width was set at 5 mm and an exposure time of 3 seconds to enhance resolution quality. The image and its associated scale was printed on a standard imaging transparency and transferred to a computer using a flatbed scanner. The determination of the thigh CSA was made using Scion Image software (v 4.0; National Institute of Health, Bethesda, MD).
Muscle quality
Voluntary maximal force production per unit of muscle mass (muscle quality) has been used to describe the relative contribution of muscle-mass components to the changes in strength throughout the aging process. Muscle quality, also termed specific tension, refers to strength per unit of muscle mass and may be a more accurate assessment of muscle function than strength alone. Muscle quality was determined by dividing the knee extensor maximal voluntary isometric contraction force produced by the thigh muscle cross-sectional area determined by computed tomography scanning.
Statistical analysis
Descriptive statistics (mean ± SE) were determined for all variables, and statistical significance was set at P < 0.05. All data were run using SPSS v.14 (SPSS Inc, Chicago, IL). A one-way analysis of variance (ANOVA) was used to determine significance between groups for MHC composition, IGF1, IGFBP5, whole-muscle strength, and whole-muscle CSA. Additionally, Pearson product-moment correlation coefficient was used to describe the linear relationship between whole-muscle CSA and mRNA expression of IGF-1.
Results
Physical activity assessment
There was no significant difference in the physical activity level between the heart failure (739.2 ± 69.3 kcal/day) and control (984 ± 52.4 kcal/day) groups, as determined by the Yale Physical Activity Survey (P = 0.12). This means that any significant findings obtained while comparing the heart failure and control groups cannot be a result of physical activity alone.
Myosin stoichiometry
Previous studies that examined alterations in skeletal muscle in chronic heart failure patients relied on whole-muscle sample preparations. Many of these studies reported no significant change in MHC isoform composition,30–32 most likely due to the poor resolution obtained when homogenizing whole-muscle preparations and utilizing densitometry to parse out the MHC isoform contributions. As a more accurate and sensitive approach, we used single muscle fiber analyses for these studies. MHC analysis was performed on 197.8 ± 2.1 single fibers from each sample (n = 1978 total fibers) (Table 1). There were no differences in MHC I isoform composition between the heart failure and control group (33% ± 7% and 45% ± 5%, respectively). Additionally, there were no differences between the groups in MHC IIa composition (33% ± 1% and 41% ± 3%, respectively). However, there were significantly more (P < 0.05) MHC isoforms coexpressing one or more pure MHC isoforms (hybrids) in the heart failure patients (30% ± 7%) compared with the control subjects (13% ± 2%). Additionally, a significant difference (P < 0.05) was found with the MHC IIa/IIx hybrid isoforms between the two groups (heart failure: 24% ± 6%; control: 9% ± 2%). These results demonstrate a molecular shift in the muscle of heart failure patients to a highly fatigable fiber type that may account for classical symptoms such as exercise intolerance.
Table 1.
Average of total fiber count out on approximately 200 fibers per subject that were analyzed for the MHC isoform distribution
| Group | MHC I% | MHC I/IIa% | MHC I/IIa/IIx% | MHC IIa% | MHC IIa/IIx% | MHC IIx% | MHC hybrids% |
|---|---|---|---|---|---|---|---|
| Heart failure | 66.4 ± 13.4 | 11.0 ± 3.1 | 1.4 ± 1.2 | 64.8 ± 2.1 | 46.2 ± 13.4* | 10.4 ± 4.3 | 58.6 ± 14.6* |
| Control | 87.6 ± 11.0 | 8.4 ± 2.1 | 0.4 ± 0.2 | 77.4 ± 5.3 | 17.8 ± 3.2 | 3.8 ± 1.9 | 26.6 ± 3.5 |
Notes: MHC hybrids represent a combination of MHCs expressing more than one isoform; all data are expressed as mean ± SD.
Statistically significant difference between groups (P < 0.05).
Abbreviations: MHC, myosin heavy chain; SD, standard deviation.
IGF-1 and IGFBP-5
A portion of the vastus lateralis biopsy was processed for RNA and used as the template for real-time PCR analysis of IGF-1 and IGFBP-5 transcript levels. The results indicated that IGF-1 mRNA expression was fivefold lower in patients with heart failure compared with the control subjects (P < 0.05). The reduction in local IGF-1 expression was accompanied by a 15-fold decrease in local IGFBP-5 mRNA expression in heart failure compared with control subjects (P < 0.05). In addition to IGF-1 and IGFBP-5, we used β-actin as a housekeeping gene to be sure that alterations in mRNA expression were not a result of universal changes of the heart failure condition (Figure 2). These decreases in local IGF-1 and IGFBP-5 could be an indication of the inability of heart failure skeletal muscle to undergo a compensatory hypertrophy. Additionally, reduction of these transcripts might imply that heart failure muscle is not capable of regeneration through upregulation of myoblast proliferation or differentiation.
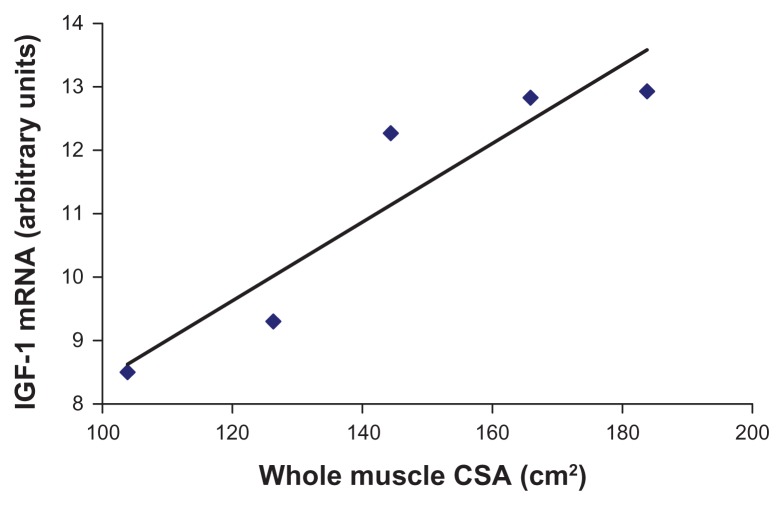
Figure 2.
Whole-muscle CSA and insulin-like growth factor-1 mRNA expression in heart failure patients.
Notes: Pearson product-moment correlation coefficient was used to describe the linear relationship. This relationship was highly correlated at r = 0.931 (P < 0.05).
Abbreviations: IGF-1, insulin-like growth factor-1; CSA, cross-sectional area.
Whole-muscle strength, size, and quality
After observing a decrease in IGF-1 and IGFBP-5, as well as an increase in myosin hybrid fibers in the chronic heart failure patients, we next wanted to quantify skeletal muscle function in both cohorts. Our hypothesis was that the heart failure group would exhibit decreased strength when compared with the controls. We found no significant difference (P = 0.15) in whole-muscle isometric strength (heart failure: 164.6 ± 25.6 nm; control: 193.3 ± 19.2 nm) or isokinetic strength at 60°/second, 180°/second, and 300°/second (Table 2). Additionally, whole-muscle CSA was not significantly different between the two groups (heart failure: 144.8 ± 14.1 mm2; control: 146.0 ± 13.1 mm2). These results were unexpected, as decreases in muscle strength and size frequently accompany alterations in MHC isoform and decrease in IGF-1. However, the muscle quality (torque/CSA) was significantly less in the heart failure patients (1.24 ± 0.1) compared with the control subjects (1.49 ± 0.1) (P < 0.05), meaning that for a given area of muscle, the heart failure patients produced less force. Muscle quality (also known as specific tension) refers to strength per unit of muscle mass and may be a better indicator of muscle function than strength alone. Tracy et al33 regards this phenomenon as alterations in neuromuscular input, which may be evidenced in the current study, as shifting motor unit input to the altered MHC-expressing skeletal muscle fibers. Additionally, whole-muscle CSA and IGF-1 mRNA expression was highly correlated (r = 0.931) in the chronic heart failure group (P < 0.05) (Figure 2), with no significant correlation detected in the control group. This correlation describes that 86% of the variability in whole-muscle CSA is accounted for by the variability in IGF-1 mRNA expression in these heart failure patients. This would imply that molecular changes can be found prior to whole-muscle changes with heart failure and that IGF-1 mRNA expression might be an early predictor of disruptions in muscle function.
Table 2.
Average whole-muscle strength measurements for both isometric and isokinetic maximal voluntary contractile (MVC) strength of the right knee extensors for both the heart failure and control groups
| Group | Isometric MVC 0°/sec |
Isokinetic MVC 60°/sec |
Isokinetic MVC 180°/sec |
Isokinetic MVC 300°/sec |
|---|---|---|---|---|
| Heart failure | 164.6 ± 25.6 nm | 161.7 ± 27.2 nm | 115.3 ± 21.0 nm | 97.3 ± 14.3 nm |
| Control | 193.3 ± 19.2 nm | 165.7 ± 17.6 nm | 118.7 ± 13.2 nm | 97.3 ± 7.6 nm |
Notes: All data are represented as means ± SE. There were no statistically significant differences in any of the variables between the groups.
Discussion
The purpose of this investigation was to examine the alterations of single skeletal muscle fiber MHC composition and IGF-1 and IGFBP-5 mRNA expression along with the functional muscle alterations that occur during chronic heart failure. The direct effects of NYHA Class II heart failure on single–muscle fiber MHC phenotype changes, on IGF-1 and IGFBP-5, and on whole-muscle strength and size of the knee extensors were studied to determine the potential relationship between cellular alterations and whole-muscle function. Specifically focusing on Class II heart failure patients enabled us to “focus in” on potential early events occurring during heart failure, and also eliminated the variability found in studying all classes of heart failure in general.
The primary findings of this study indicate that negative local cellular changes take place before whole-muscle strength and size changes are detectable in NYHA Class II heart failure patients. However, whole-muscle quality was significantly decreased in the heart failure patients compared with the control subjects (P < 0.05), and there was a significant relationship between whole-muscle CSA and mRNA expression of IGF-1 in the chronic heart failure patients. These data demonstrate that there are significant destructive alterations taking place at the cellular level in NYHA Class II heart failure patients that precede severe declines in muscle function. Each of the molecular alterations that we studied is described below.
Molecular alterations in MHC fiber types in heart failure patients
Modifications to crucial components of the contractile machinery, including MHC isoforms, play a significant role in muscle function. Several longitudinal studies have established that distinct MHC isoforms (I, IIa, IIx) have diverse contractile properties, and in fact, even within one single MHC isoform there can be variations of contractile phenotype and performance.34–36 These alterations have been demonstrated in athletes, younger and older adults, and with spaceflight. Therefore it is likely that the observed alterations in MHC isoform distribution result in decrements in whole-muscle function in heart failure patients.
Indeed, our approach of using single-fiber molecular analyses enabled us to determine that there is a significantly (P < 0.05) higher percentage of MHC hybrid isoforms in NYHA Class II heart failure patients (30% ± 7%) compared with the healthy controls (13% ± 2%). Of the 30% ± 7% of hybrid isoforms in the heart failure fibers, 24% ± 6% of these were MHC IIa/IIx, compared with only 9% ± 2% in the healthy controls (P < 0.05). These results indicate that the muscle fibers likely are in a state of transition and therefore not “purely” expressed as just MHCI, MHCIIa, or MHCIIx. Instead, it appears that the fibers from Class II heart failure patients are shifting towards a faster MHC isoform. These alterations in MHC composition have potentially noteworthy outcomes on the force–velocity and power–velocity relationships, unloaded shortening velocity, muscle-cell diameter, and calcium sensitivity of the whole muscles.34,35,36,37 We hypothesize that these changes in single muscle fiber function due to MHC isoform composition contribute to the negative alterations detected in skeletal muscle quality and function with chronic heart failure.
The specific alterations responsible for the MHC phenotype changes seen with chronic heart failure have not been elucidated. One possible contributing factor is the potential reduction in physical activity levels of heart failure patients compared with healthy individuals of similar age. Other studies have found that decrements in neuromuscular activity result in an alteration in the MHC isoform expression from slow to fast.38,39 However, in our study, there was no significant difference in physical activity levels measured via an interviewer-administered Yale Physical Activity Survey between the healthy control group and the NYHA Class II heart failure group. Although other investigations have examined MHC isoform distribution in heart failure patients,19,21,22 our study is the first to control for physical activity levels and for the disease state of the heart failure group. This indicates that physical activity is not the sole cause of the alterations in MHC isoform expression with heart failure. Instead, we speculate that this alteration is due, at least in part, to decrements in IGF-1 and IGFBP-5 expression in Class II heart failure patients.
IGF-1 and IGFBP-5 transcript levels are downregulated and correlate with MHC isoform shifting and a decrease in whole-muscle quality in Class II heart failure patients
Strikingly, real-time PCR quantification in the single skeletal muscle fiber analyses enabled us to determine that there was a 15-fold decrease in IGFBP-5 and a fivefold decrease in IGF-1 transcript levels in heart failure patients compared with controls. Our findings that IGF-1 mRNA expression was highly related to whole-muscle CSA (r = 0.931) in chronic heart failure patients (Figure 2) are in agreement with Hambrecht et al40 who also reported a significant correlation (r = 0.75) with IGF-1 mRNA expression and whole-muscle CSA in heart failure patients. 40 In our investigation, this relationship signifies that 86% of the variability in whole-muscle CSA is accounted for by the variability in IGF-1 mRNA expression in these heart failure patients. This same relationship was not found in the healthy control subjects, therefore demonstrating that the negative alterations of the heart failure disease process are at least partially explained by decrements in IGF-1: decrements in IGF-1 translate to alteration in MHC isoforms to exhibit more glycolytic properties and in turn, to the initiation of negative whole-muscle alterations, which are caused by these detected cellular changes.
Contrary to the study by Hambrecht et al,40 we did not find a significant decrease in whole-muscle CSA in the chronic heart failure patients compared with the healthy controls. These conflicting results are potentially due to the differences in the chronic heart failure disease state between the two studies. In the current investigation we limited our chronic heart failure group to those who had NYHA functional Class II disease, whereas the Hambrecht et al study included patients with NYHA functional Class II–IV disease. Similar to this study we also found no difference in peak isometric force, but we did find a significant decrease in whole-muscle quality between the groups (maximal voluntary isometric force/thigh CSA), which was not reported in Hambrecht et al.40 There appears to be agreement that IGFBP-5 modulates the effects of IGF-1 via regulation of the free IGF-1 concentration in muscle.41,42 The 15-fold decrease in IGFBP-5 in the heart failure patients compared with the healthy control subjects that was demonstrated in our current study underscores the dramatic impact of the heart failure disease process on IGF-1 (fivefold decrease) and, subsequently, cellular and whole-muscle functionality. Combining the data from our current study with data from previous studies13–22 investigating molecular changes that occur during heart failure, we propose a mechanistic model.
Our current model demonstrates that chronic neurohormonal activation of the renin–angiotensin system decreases local skeletal muscle IGF-1 and its binding partner, IGFBP-5. Consequently, this confounds the MHC isoform shift that may result from alterations in peripheral blood flow alone, resulting in an extreme shift towards hybrid fast-twitch, glycolytic muscle fibers. These molecular alterations precede the gross morphological and functional deficits that have previously been associated with chronic heart failure and may be used as a predictor of functional outcome in patients.
Mechanistically, it is well known that IGF-1, acting through the IGF-1 receptor, has acute anabolic effects on metabolism in addition to longer term effects on cell replication and differentiation;4 and that IGF-1 has potent inhibitory effects on apoptosis.5,6 Two recent studies43,44 have shown an increased incidence of apoptosis in the skeletal muscle of heart failure patients. Therefore, a decline in local IGF-1 expression may intensify the apoptotic process in the skeletal muscle. Hambrecht et al reported a significant downregulation of local IGF-1 expression and an upregulation of IGF-1 receptor expression in skeletal muscle of heart failure patients despite normal IGF-1 serum concentrations,40 suggesting a possible feedback mechanism between local IGF-1 concentrations and receptor density. IGFBP-5 is expressed at high levels in skeletal muscle, is regulated by IGF-1, and may have IGF-1 receptor-independent effects that contribute to muscle function.42 In fact, reductions of IGFBP-5 mRNA levels have been reported in muscle-atrophy models.45 These studies underscore the critical roles of IGF-1 and IGFBP-5 on normal physiological functioning of human skeletal muscle.
Conclusion
Our data indicate that decrements in IGF-1 and IGFBP-5 levels in the skeletal muscles of Class II heart failure patients play critical roles in MHC isoform distribution, which switch to more glycolytic properties (as evidenced by a significant increase in hybrid isoforms [P < 0.05] and in particular the MHC IIa/IIx isoforms). These negative alterations may partly be explained by the strong correlation in mRNA IGF-1 expression and whole-muscle CSA (P < 0.05) and the decrease in whole-muscle quality (P < 0.05). Future directions for research include addressing the specific single muscle fiber physiological alterations (peak force, maximal shortening velocity, peak power) that are occurring with heart failure in conjunction with the IGF-1 system and other growth factor systems.
Figure 3.
The melt-curve analysis for IGF-1 and β-actin.
Note: The curve to the left represents IGF-I and the curve to the right represents the β-actin.
Abbreviation: IGF-1, insulin-like growth factor-1.
Acknowledgment
The authors would like to thank Abigail McElhinny, PhD for her critical review of the manuscript and helpful comments.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics: 2007 Update At-a-Glance. Dallas: American Heart Association; 2007. [Accessed October 10, 2012]. Available from: http://www.harcdata.org/UserFiles/File/AHA-heartandstrokestats.pdf. [Google Scholar]
- 2.Sigurdsson A, Amtorp O, Gundersen T, Nilsson B, Remes J, Swedberg K. Neurohormonal activation in patients with mild or moderately severe congestive heart failure and effects of ramipril. The Ramipril Trial Study Group. Br Heart J. 1994;72(5):422–427. doi: 10.1136/hrt.72.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115(2):451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 5.Butt AJ, Firth SM, Baxter RC. The IGF axis and programmed cell death. Immunol Cell Biol. 1999;77(3):256–262. doi: 10.1046/j.1440-1711.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 6.Resnicoff M, Baserga R. The role of the insulin-like growth factor I receptor in transformation and apoptosis. Ann N Y Acad Sci. 1998;842:76–81. doi: 10.1111/j.1749-6632.1998.tb09634.x. [DOI] [PubMed] [Google Scholar]
- 7.Musarò A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400(6744):581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 8.DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ. Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol. 1990;259(1 Pt 1):E89–E95. doi: 10.1152/ajpendo.1990.259.1.E89. [DOI] [PubMed] [Google Scholar]
- 9.Léger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11(1):163B–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- 10.Serose A, Prudhon B, Salmon A, Doyennette MA, Fiszman MY, Fromes Y. Administration of insulin-like growth factor-1 (IGF-1) improves both structure and function of delta-sarcoglycan deficient cardiac muscle in the hamster. Basic Res Cardiol. 2005;100(2):161–170. doi: 10.1007/s00395-004-0506-3. [DOI] [PubMed] [Google Scholar]
- 11.Kopple JD, Cohen AH, Wang H, et al. Effect of exercise on mRNA levels for growth factors in skeletal muscle of hemodialysis patients. J Ren Nutr. 2006;16(4):312–324. doi: 10.1053/j.jrn.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Drexler H. Effect of angiotensin-converting enzyme inhibitors on the peripheral circulation in heart failure. Am J Cardiol. 1992;70(10):50C–54C. doi: 10.1016/0002-9149(92)91358-b. [DOI] [PubMed] [Google Scholar]
- 13.Mancini DM, Coyle E, Coggan A, et al. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989;80(5):1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 14.Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85(4):1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 15.Schaufelberger M, Eriksson BO, Grimby G, Held P, Swedberg K. Skeletal muscle alterations in patients with chronic heart failure. Eur Heart J. 1997;18(6):971–980. doi: 10.1093/oxfordjournals.eurheartj.a015386. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80(4):769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 17.Delp MD, Duan C, Mattson JP, Musch TI. Changes in skeletal muscle biochemistry and histology relative to fiber type in rats with heart failure. J Appl Physiol. 1997;83(4):1291–1299. doi: 10.1152/jappl.1997.83.4.1291. [DOI] [PubMed] [Google Scholar]
- 18.Lang CC, Chomsky DB, Rayos G, Yeoh TK, Wilson JR. Skeletal muscle mass and exercise performance in stable ambulatory patients with heart failure. J Appl Physiol. 1997;82(1):257–261. doi: 10.1152/jappl.1997.82.1.257. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan MJ, Duscha BD, Klitgaard H, Kraus WE, Cobb FR, Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc. 1997;29(7):860–866. doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81(2):518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 21.Toth MJ, Matthews DE, Ades PA, et al. Skeletal muscle myofibrillar protein metabolism in heart failure: relationship to immune activation and functional capacity. Am J Physiol Endocrinol Metab. 2005;288(4):E685–E692. doi: 10.1152/ajpendo.00444.2004. [DOI] [PubMed] [Google Scholar]
- 22.Vescovo G, Serafini F, Facchin L, et al. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart. 1996;76(4):337–343. doi: 10.1136/hrt.76.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Short KR, Vittone JL, Bigelow ML, et al. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol. 2005;99(1):95–102. doi: 10.1152/japplphysiol.00129.2005. [DOI] [PubMed] [Google Scholar]
- 24.Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13(1):40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan MJ, Green HJ, Cobb FR. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity. Circulation. 1991;84(4):1597–1607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]
- 26.Toth MJ, Ades PA, Lewinter MM, Tracy RP, Tchernof A. Skeletal muscle myofibrillar mRNA expression in heart failure: relationship to local and circulating hormones. J Appl Physiol. 2006;100(1):35–41. doi: 10.1152/japplphysiol.00570.2005. [DOI] [PubMed] [Google Scholar]
- 27.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25(5):628–642. [PubMed] [Google Scholar]
- 28.Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol. 2000;88(2):627–633. doi: 10.1152/jappl.2000.88.2.627. [DOI] [PubMed] [Google Scholar]
- 29.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552(Pt 1):47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coirault C, Guellich A, Barbry T, Samuel JL, Riou B, Lecarpentier Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292(2):H1009–H1017. doi: 10.1152/ajpheart.00438.2006. [DOI] [PubMed] [Google Scholar]
- 31.Duscha BD, Kraus WE, Keteyian SJ, et al. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in Class II–III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33(7):1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 32.Toth MJ, Palmer BM, LeWinter MM. Effect of heart failure on skeletal muscle myofibrillar protein content, isoform expression and calcium sensitivity. Int J Cardiol. 2006;107(2):211–219. doi: 10.1016/j.ijcard.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Tracy BL, Ivey FM, Hurlbut D, et al. Muscle quality. II. Effects of strength training in 65- to 75-yr-old men and women. J Appl Physiol. 1999;86(1):195–201. doi: 10.1152/jappl.1999.86.1.195. [DOI] [PubMed] [Google Scholar]
- 34.Godard MP, Gallagher PM, Raue U, Trappe SW. Alterations in single muscle fiber calcium sensitivity with resistance training in older women. Pflugers Arch. 2002;444(3):419–425. doi: 10.1007/s00424-002-0821-1. [DOI] [PubMed] [Google Scholar]
- 35.Trappe S. Effects of spaceflight, simulated spaceflight and countermeasures on single muscle fiber physiology. J Gravit Physiol. 2002;9(1):P323–P326. [PubMed] [Google Scholar]
- 36.Trappe S, Harber M, Creer A, et al. Single muscle fiber adaptations with marathon training. J Appl Physiol. 2006;101(3):721–727. doi: 10.1152/japplphysiol.01595.2005. [DOI] [PubMed] [Google Scholar]
- 37.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol. 2004;557(Pt 2):501–513. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve. 2000;23(5):661–679. doi: 10.1002/(sici)1097-4598(200005)23:5<661::aid-mus3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Talmadge RJ, Roy RR, Edgerton VR. Persistence of hybrid fibers in rat soleus after spinal cord transection. Anat Rec. 1999;255(2):188–201. doi: 10.1002/(SICI)1097-0185(19990601)255:2<188::AID-AR9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Hambrecht R, Schulze PC, Gielen S, et al. Reduction of insulin-like growth factor-I expression in the skeletal muscle of noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2002;39(7):1175–1181. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- 41.Adams V, Nehrhoff B, Späte U, et al. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54(1):95–104. doi: 10.1016/s0008-6363(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 42.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278(6):E967–E976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 43.Adams V, Jiang H, Yu J, et al. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33(4):959–965. doi: 10.1016/s0735-1097(98)00626-3. [DOI] [PubMed] [Google Scholar]
- 44.Vescovo G, Volterrani M, Zennaro R, et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart. 2000;84(4):431–437. doi: 10.1136/heart.84.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lecker SH, Jagoe RT, Gilbert A, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18(1):39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]