Abstract
Isoflurane is a preferred anesthetic, due to its properties that allow a precise concentration to be delivered continually during in vivo experimentation. The major mechanism of action of isoflurane is modulation of the γ-amino butyric acid (GABAA) receptor-chloride channel, mediating inhibitory synaptic transmission. Animal studies have shown that isoflurane does not cause cell death, but it does inhibit cell growth and causes long-term hippocampal learning deficits. As there are no studies characterizing the effects of isoflurane on electrophysiological aspects of long-term potentiation (LTP) in the hippocampus, it is important to determine whether isoflurane alters the characteristic responses of hippocampal afferents to cornu ammonis region 3 (CA3). We investigated the effects of isoflurane on adult male rats during in vivo induction of LTP, using the mossy fiber pathway, the lateral perforant pathway, the medial perforant pathway, and the commissural CA3 (cCA3) to CA3, with intracranial administration of Ringer’s solution, naloxone, RS-aminoindan-1, 5-dicarboxylic acid (AIDA), or 3-[(R)-2-carboxypiperazin-4-yl]-propo-2-enyl-1-phosphonic acid (CPP). Then, we compared these responses to published electrophysiological data, using sodium pentobarbital as an anesthetic, under similar experimental conditions. Our results showed that LTP was exhibited in animals anesthetized with isoflurane under vehicle conditions. With the exception of AIDA in the lateral perforant pathway, the defining characteristics of the four pathways appeared to remain intact, except for the observation that LTP was markedly reduced in animals anesthetized with isoflurane compared to those anesthetized with sodium pentobarbital. The results suggest that isoflurane may affect amplitude through activation of GABAA receptors or mechanisms important to LTP in CA3 afferent fibers.
Keywords: in vivo electrophysiology, isoflurane, sodium pentobarbital
Introduction
The hippocampus is a structure within the limbic system that is known to play a fundamental role in learning and memory processes (Figure 1). Hippocampal long-term potentiation (LTP) is expressed as the persistent increase in the slope of the field excitatory postsynaptic potential (fEPSP) of an evoked response, which can be recorded from a single cell or a population of neurons.1 Another characteristic of LTP is an increase in neurotransmitter release.1–5 There are two forms of LTP: N-methyl-D-aspartic acid (NMDA) receptor-dependent and NMDA receptor-independent (or opioid-dependent). The hippocampal pathways that express NMDA receptor-dependent LTP are the medial perforant, commissural, Schaffer Collateral, and cornu ammonis region 3 associational/commissural pathways (cCA3). NMDA receptor-dependent LTP requires the activation of NMDA receptors by the neurotransmitter glutamate, where there is depolarization sufficient in the postsynaptic membrane to relieve a magnesium ion block, in order to allow the entry of calcium ions into the postsynaptic terminal.6,7 Once the magnesium block is removed and calcium ions enter the postsynaptic terminal, any number of calcium-sensitive second-messenger cascades can be activated, leading to LTP induction.
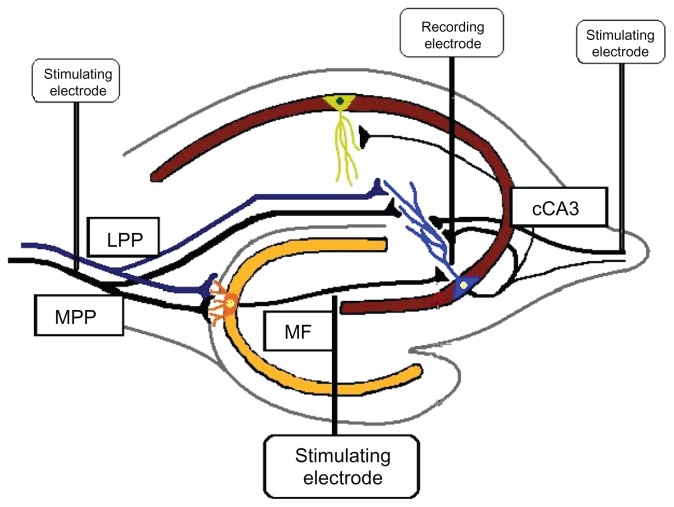
Figure 1.
Stimulating electrode placement.28
Notes: Stimulating electrodes were placed in the mossy fiber pathway, the lateral perforant pathway, the medial perforant pathway, or the associational/commissural pathway. A recording cannula was placed in the stratum lucidum of area CA3.
Abbreviations: LPP, lateral perforant pathway; MF, mossy fiber; MPP, medial perforant pathway; cCA3, commissural cornu ammonis region 3.
The hippocampal pathways that are NMDA receptor-independent are the lateral perforant pathway (LPP) and mossy fiber (MF) pathway to area CA3.8,9 Here, at least two mechanisms of opioid receptor-dependent LTP exist. LTP induction from the LPP to CA3, as well as MF to CA3, is dependent upon μ-opioid receptor activation, while induction in the LPP is dependent on δ-opioid receptor activation.7–11 When endogenous opioids are released in the hippocampus, a disinhibitory process occurs, resulting in the increase of excitatory postsynaptic potential (EPSP) amplitudes, by depressing tonic γ-amino butyric acid (GABAA) inhibition.12,13
Naloxone is an opioid receptor antagonist that blocks induction of opioid receptor-dependent LTP. RS-aminoindan-1, 5-dicarboxylic acid (AIDA) has also been shown to block opioid receptor-dependent LTP in the MF–CA3 pathway though its interaction as an antagonist of class I metabotropic glutamate receptors. The drug 3-[(R)-2-carboxypiperazin-4-yl]-propo-2-enyl-1-phosphonic acid (CPP) is a known NMDA receptor antagonist that is used to prevent LTP in NMDA receptor-dependent pathways. CPP is used in MF–LTP experiments to verify the dependence of this pathway on opioid receptor-dependent mechanisms, because it does not block induction of MF–LTP when applied to area CA3 prior to MF high-frequency stimulation (HFS).14,15
The purpose of this study was to determine whether isoflurane affected the physiological mechanisms that underlie in vivo LTP in the mossy fiber, medial and lateral perforant pathways to area CA3 and the commissural CA3 (cCA3) pathways, and whether there were interactions between isoflurane and LTP antagonists. We compared the data collected in this experiment to published LTP data collected previously from animals anesthetized with sodium pentobarbital in the laboratory of origin.
Methods
All experimental procedures were approved in advance by the Institutional Animal Care and Use Committee at the University of Texas at San Antonio, and they are in accordance with National Institute of Health (NIH) guidelines. Adult male Sprague Dawley™ rats (275–299 g; Harlan Laboratories, Houston, TX) were anesthetized with isoflurane (Henry Schein, Melville, NY) and placed in a stereotaxic frame. A stimulating electrode was placed in the MF (anterior–posterior [AP]: 3.5 mm; medial–lateral [ML]: −2.0; dorsal–ventral [DV]: 3.0–3.2 mm) pathway, the LPP (AP: 8.1 mm; ML: 5.0 mm; DV 2.8–3.1 mm), the MPP (AP: 8.0 mm; ML: 4.4 mm; DV: 2.8–3.1 mm), or the cCA3 pathway (AP: −3.5 mm; ML: 3.5 mm; DV: 3.3–3.5 mm). A recording cannula-electrode was placed above the CA3 pyramidal layer of the dorsal hippocampus (AP: −2.9 mm; ML: 2.2 mm; DV: 3.1–3.3 mm below dura). For the MF pathway, we delivered stimulation at 0.05 Hz until antidromic spikes (2–3 ms to peak) were observed in the granule cells. We then evoked orthodromic responses by delivering stimulation through the dentate electrode and recording from the electrode placed in CA3. We adjusted the stimulating electrode until a characteristic MF excitatory postsynaptic potential (EPSP) was observed and easily elicited with low (10–50 μA) current intensity and an onset at ~2.5 ms and a peak at ~8–10 ms. For the medial and lateral perforant pathways, it is possible, when recording in the CA3 region, to distinguish field potentials elicited from the LPP versus the MPP. Due to the proximal location of MPP synapse to the CA3 cell body compared to the LPP, stimulation of the MPP produces larger evoked field potentials, with a faster rise time, than stimulation of the LPP. Therefore, when lowering the stimulating electrode, it is possible to see the transition in the slope and time to peak of the evoked response, as the electrode is moved dorsally between these two pathways in the angular bundle. The onset of the MPP EPSP is ~2 ms, with a peak at ~3–5 ms. The onset of the LPP EPSP is ~3 ms, with a peak af ~5–7 ms. The cCA3 pathway has an EPSP onset and peak latencies of 5–7 ms and 12–15 ms, respectively. Acute extracellular responses were evoked by direct stimulation of the MF, LPP, MPP, or cCA3 bundle, by Teflon-coated twisted tungsten wire (Small Parts Inc, Miramar, FL). Each electrode was connected to a Grass Stimulus Isolation Unit (Grass Technologies, West Warwick, RI) to deliver stimulation at 10–50 μA for 0.2 ms. Afferent CA3 responses were recorded, amplified, filtered at 0.1 Hz to 10 kHz, and stored for off-line analysis. The EPSP magnitude was measured by using the initial slope of the field; EPSP was measured 2–3 ms after response onset. After the collection of baseline CA3 afferent responses for ≥20 min at 0.05 Hz, animals received Ringer’s solution, naloxone (μ-opioid receptor antagonist, 10 nmol intracerebrally (ic); (RBI; Sigma-Aldrich, St Louis, MO), AIDA (mGluR1 receptor antagonist, 37.5 nmol ic; Sigma-Aldrich), or CPP (NMDA-R antagonist, 3 nmol ic; Sigma-Aldrich), depending on the pathway’s dependence on NMDA receptor activation or opioid receptor activation for LTP induction at 0.2 μL/min (1 μL total). Fifteen minutes after drug infusion by the cannula/recording electrode, LTP was induced by two 100-Hz, 1-second trains delivered at an intensity sufficient to evoke 50% of the maximal response of the CA3 afferent pathway examined (Figure 2).
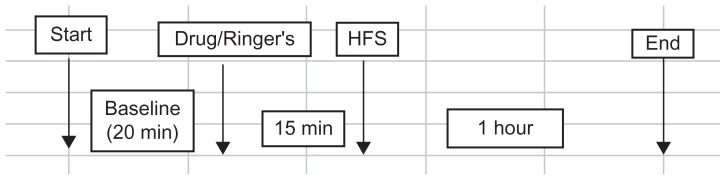
Figure 2.
Experimental paradigm for electrophysiological study of long-term potentiation of afferents to area CA3 in animals anesthetized with isoflurane.
Abbreviations: CA3, cornu ammonis region 3; HFS, high-frequency stimulation.
Evoked CA3 afferent responses were collected for an additional 60 minutes in all groups (n = 4, MF–CPP; n = 4, MF–naloxone; n = 4, MF–AIDA; n = 4, MF–Ringer’s; n = 4, LPP–CPP; n = 4, LPP–naloxone; n = 4, LPP–AIDA; n = 4, LPP–Ringer’s; n = 4, MPP–CPP; n = 4, MPP–Ringer’s; n = 4, cCA3–CPP; n = 4, cCA3–Ringer’s; Table 1). LTP was measured in each group at 1 hour by obtaining the percent change in the field EPSP slope calculated for the last 5 minutes of baseline and the last 5 minutes of the 1-hour collection period. One hour after delivery of high-frequency stimulation, all the animals were euthanized by decapitation, while still under anesthesia. The magnitude of evoked responses following delivery of the conditioning trains was measured by the average amplitude of the maximal responses occurring between 20 and 60 minutes post-tetanus. The percentage change in the amplitude of the evoked response relative to the average amplitude observed in the 10-minute period following drug application was calculated. The data were subjected to a two-way analysis of variance (ANOVA) to determine significance in time and between control and antagonist groups. Data was considered significant if the P-value was ≤0.05. To ensure that appropriate electrode placement coordinates were used throughout the study, placements were verified histologically in only 60% of the subjects, due to obstacles that arose using the cryostat for tissue slicing.
Table 1.
Drug administration in CA3 afferent pathways for isoflurance-treated animals
| Area | CPP (3 nmol) | Naloxone (10 nmol) | AIDA (37.5 nmol) | Ringer’s |
|---|---|---|---|---|
| MF | 4 | 4 | 4 | 4 |
| LPP | 4 | 4 | 4 | 4 |
| MPP | 4 | 4 | ||
| cCA3 | 4 | 4 |
Note: Intracranial administration of Ringer’s solution and inhibitors to the afferent CA3 pathways during isoflurane treatment will determine any effects of the anesthetic on known molecular mechanisms of these pathways.
Abbreviations: MF, mossy fiber; LPP, lateral perforant pathway; MPP, medial perforant pathway; cCA3, commissural cornu ammonis region 3; CPP, 3-[(R)-2-carboxypiperazin-4-yl]-propo-2-enyl-1-phosphonic acid; AIDA, RS-aminoindan-1, 5-dicarboxylic acid.
Results
Effect of isoflurane on MF–CA3 responses
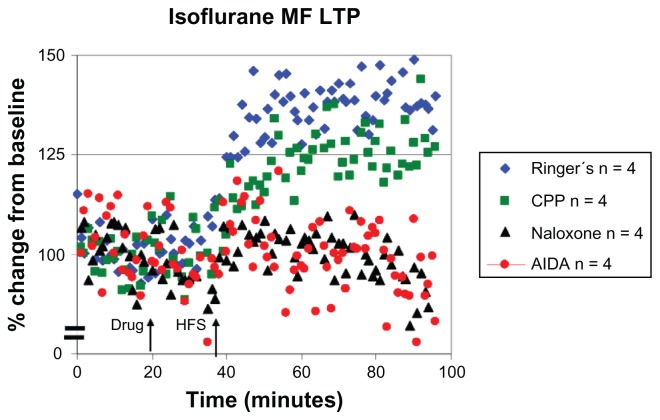
The isoflurane-treated rats exhibited MF–LTP under Ringer’s (+43% ± 20%) and CPP (29% ± 6%) conditions, while the amplitude of long-term potentiation was attenuated in both control groups when compared to potentiated responses measured under sodium pentobarbital anesthesia (mean difference in MF response compared to sodium pentobarbital during last 10 minutes of HFS is ~72% for Ringer’s, and ~94% LTP for CPP; see Figure 3 and Table 2). It also was noted that under sodium pentobarbital conditions, LTP amplitude in the MF pathway was higher in the CPP group than the Ringer’s group (Ringer’s = 115%; CPP = 123%), while the opposite was found in animals under isoflurane conditions (Ringer’s = +43% ± 20%; CPP = +29% ± 6%).
Figure 3.
Characteristic LTP responses are observed in the MF pathway under isoflurane anesthesia. A plot of normalized field excitatory postsynaptic potential (fEPSP) slope magnitude of mossy fiber-CA3 responses over time using current intensities eliciting 50% of the maximal response and the percent change in amplitude (mean ± SEM).
Notes: Blue diamonds: MF LTP is induced in the presence of Ringer’s solution in isoflurane (+43% ± 20%, n = 4) anesthetized animals. Green squares: MF LTP is induced in the presence of 3-[(R)-2-carboxypiperazin-4-yl]-propo-2-enyl-1-phosphonic acid (CPP; 3 nmol ic) with isoflurane (+29% ± 6% LTP, n = 4). Black triangles: LTP induction is blocked in the presence of naloxone (10 nmol ic) with isoflurane (−7% ± 20%, n = 4). Red circles: LTP induction is blocked in the presence of the class 1 metabotropic glutamate receptor (mGluR) antagonist AIDA (37.5 nmol ic) in isoflurane (−5% ± 18%, n = 4) anesthetized animals.
Abbreviations: MF, mossy fiber; LTP, long-term potentiation; HFS, high-frequency stimulation; CA3, cornu ammonis region 3; SEM, standard error of the mean; AIDA, RS-aminoindan-1, 5-dicarboxylic acid.
Table 2.
| LTP | ||
|---|---|---|
|
|
||
| Isoflurane | Sodium pentobarbital | |
| MF | ||
| Ringer’s | +43% ± 20% | 115% |
| CPP | +29% ± 6% | 123% |
| Naloxone | −7% ± 20% | Blocked |
| AIDA | −5% ± 18% | Blocked |
| LPP | ||
| Ringer’s | +22% ± 11% | 131% |
| CPP | +34% ± 17% | 146% |
| Naloxone | +9% ± 23% | Blocked |
| AIDA | −11% ± 15% | Blocked |
| MPP | ||
| Ringer’s | +66% ± 45% | 132% |
| CPP | +3% ± 15% | Blocked |
| cCA3 | ||
| Ringer’s | +74% ± 54% | 78% |
| CPP | +1% ± 12% | Blocked |
Note: Amplitude of long-term potentiation (LTP) is decreased under isoflurane anesthesia when compared to sodium pentobarbital anesthesia in the MF, LPP, MPP, and cCA3 pathways.
Abbreviations: MF, mossy fiber; CPP, 3-[(R)-2-carboxypiperazin-4-yl]-propo-2-enyl-1-phosphonic acid; AIDA, RS-aminoindan-1, 5-dicarboxylic acid; LPP, lateral perforant pathway; MPP, 1-methyl-4-phenylpyridine; cCA3, commissural cornu ammonis region 3.
LTP was blocked in animals under isoflurane when mGluR1 antagonist AIDA (−5% ± 18%) and opioid receptor antagonist naloxone (−7% ± 20%) were injected into the stratum lucidum, which was also seen under sodium pentobarbital conditions.
Effect of isoflurane on LPP–CA3 responses
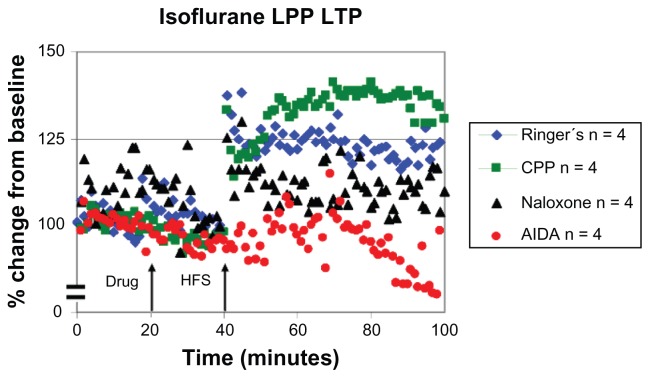
Isoflurane-treated animals exhibited LTP under Ringer’s (+22% ± 11%) and CPP (+34% ± 17%) conditions, while the amplitude of LTP was attenuated in both control groups when compared to responses measured under sodium pentobarbital anesthesia (mean difference in LPP response to sodium pentobarbital conditions during last 10 minutes of HFS is ~109% for Ringer’s and ~112% LTP for CPP; Figure 4; Table 2). It was also noted that under sodium pentobarbital conditions, LTP amplitude in the LPP was higher for the Ringer’s group than for the CPP group.
Figure 4.
Characteristic LTP responses are observed in the LPP under isoflurane anesthesia. A plot of normalized field excitatory postsynaptic potential (fEPSP) slope magnitude of lateral perforant path-CA3 responses over time using current intensities eliciting 50% of the maximal response and the percent change in amplitude (mean ± SEM).
Notes: Blue diamonds: LPP LTP is induced in the presence of Ringer’s solution in isoflurane (+22% ± 11%, n = 4) anesthetized animals. Green squares: LPP LTP is induced in the presence of 3-[(R)-2-carboxypiperazin-4-yl]-propo-2-enyl-1-phosphonic acid (CPP; 3 nmol ic) with isoflurane (+34% ± 17%, n = 4). Black triangles: LTP induction is blocked in the presence of naloxone (10 nmol ic) with isoflurane (+9% ± 23%, n = 4). Red circles: LTP induction is blocked in the presence of the class 1 mGluR antagonist AIDA (37.5 nmol ic) in isoflurane (−11% ± 15%, n = 4) anesthetized animals.
Abbreviations: LPP, lateral perforant pathway; LTP, long-term potentiation; HFS, high frequency stimulation; CA3, cornu ammonis region 3; SEM, standard error of the mean; AIDA, RS-aminoindan-1, 5-dicarboxylic acid.
LTP was blocked in animals under isoflurane when the opioid receptor antagonist naloxone (10 nmol) was injected into the stratum lucidum (+9% ± 23%), which was also seen under sodium pentobarbital conditions. Statistical analysis concluded that the fEPSP was affected by the combination of drug (F [1, 12] = 7.31, P < 0.05) and time (F [1, 12] = 5.08, P < 0.05). Under Ringer’s conditions, LPP potentiation increased from the 20-minute post-drug to the 60-minute post-HFS times, but in the naloxone condition, the fEPSP decreased from the 20-minute post-drug time point to the 60-minute post-HFS time point. When mGluR1R antagonist AIDA was applied to area CA3 before HFS, statistical analysis indicated that there was a significant drug effect (−11% ± 15%; F [1, 12] = 9.748, P < 0.05) as well as a statistically significant time by drug interaction (F [1, 12] = 5.886, P < 0.05). Therefore, it can be stated that under the anesthetic isoflurane, characteristic lateral perforant path LTP responses were observed when compared to those of sodium pentobarbital, although the amplitude of LTP was attenuated.
Effect of isoflurane on MPP–CA3 responses
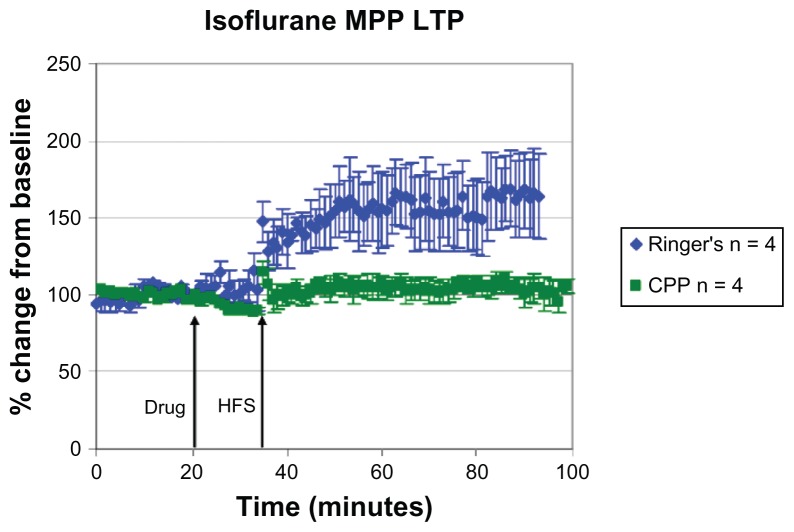
The isoflurane-treated animals exhibited LTP under Ringer’s solution (+66% ± 45%), while the amplitude of LTP was attenuated in the control group when compared to the response measured under sodium pentobarbital anesthesia (mean difference in MPP response during last 10 minutes of HFS is ~66% for Ringer’s; Figure 5; Table 2). LTP was blocked in animals under isoflurane and sodium pentobarbital when CPP was infused before HFS (+3% ± 15%), while a significant main effect for time (frequency [F] [1, 12] = 6.39, P < 0.05) and drug (F [1, 12] = 6.67, P < 0.05) was noted under isoflurane and CPP conditions. The results for the MPP pathway seem to agree with data showing that isoflurane depresses NMDA receptor-dependent LTP.16
Figure 5.
Characteristic LTP responses are observed in the MPP under isoflurane anesthesia. A plot of normalized field excitatory postsynaptic potential (fEPSP) slope magnitude of medial perforant path-CA3 responses over time using current intensities eliciting 50% of the maximal response and the percent change in amplitude (mean ± SEM).
Notes: Blue diamonds: MPP LTP is induced in the presence of Ringer’s solution in isoflurane (+66% ± 45%, n = 4) anesthetized animals. Green squares: LTP induction is blocked in the presence of 3-[(R)-2-carboxypiperazin-4-yl]-propo-2-enyl-1-phosphonic acid (CPP; 3 nmol ic; +3% ± 15%, n = 4).
Abbreviations: MPP, 1-methyl-4-phenylpyridine; LTP, long-term potentiation; HFS, high frequency stimulation; CA3, cornu ammonis region 3; SEM, standard error of the mean.
Effect of isoflurane on cCA3 responses
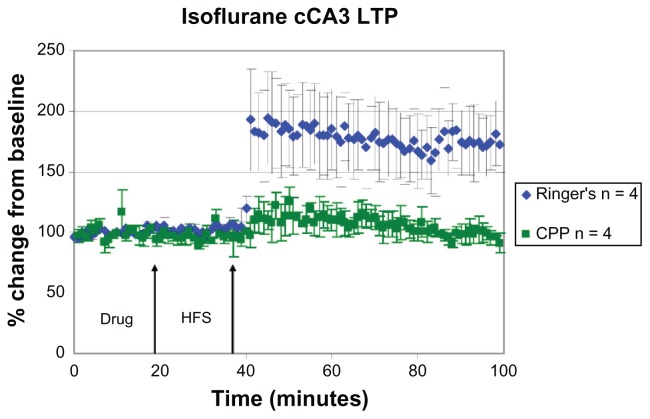
LTP was induced in animals treated with isoflurane, under Ringer’s solution (+74% ± 54%), while LTP was blocked when CPP was applied before HFS. These results were similar to those of sodium pentobarbital. Unlike the MF, LPP, and MPP, the amplitude of LTP did not show as large of a difference under control conditions when compared to sodium pentobarbital groups (~74% in the isoflurane group and ~78% in the sodium pentobarbital group; Figure 6; Table 2). Under CPP conditions in the cCA3 pathway, statistical analysis indicated an interaction approaching significance between drug and time (+1% ± 12%; F [1, 12] = 4.571, P = 0.054).
Figure 6.
Characteristic LTP responses are observed in the cCA3 pathway under isoflurane anesthesia. A plot of normalized field excitatory postsynaptic potential (fEPSP) slope magnitude of cCA3 responses over time using current intensities eliciting 50% of the maximal response and the percent change in amplitude (mean ± SEM).
Notes: Blue diamonds: cCA3 LTP is induced in the presence of Ringer’s solution in isoflurane (+74% ± 54%, n = 4) anesthetized animals. Green squares: LTP induction is blocked in the presence of 3-[(R)-2-carboxypiperazin-4-yl]-propo-2-enyl-1-phosphonic acid (CPP; 3 nmol ic, n = 4).
Abbreviations: cCA3, commissural cornu ammonis region 3; LTP, long-term potentiation; HFS, high frequency stimulation; SEM, standard error of the mean.
Discussion
Isoflurane effects on hippocampal mossy fiber and lateral perforant path LTP responses are likely mediated by glutamatergic transmission
When measuring the slope of MF LTP induction, it is characteristic to see a gradual increase in the slope over time, following high-frequency stimulation, which was observed in animals under both analgesic conditions. As the MF pathway depends on the activation of opioid receptors for LTP induction, the response of the MF pathway to NMDA receptor antagonist CPP was comparable to animals under sodium pentobarbital (123%, n = 3) anesthesia. Even though the MF pathway is capable of LTP induction and maintenance under isoflurane anesthesia, the difference in the amplitude of LTP in isoflurane-treated animals from sodium pentobarbital-treated animals was ~72% for the Ringer’s group and ~94% for the CPP group. When considering the possible mechanisms involved in MF–CA3 LTP, it is possible that isoflurane could affect mechanisms related to GABAB or glutamatergic transmission to decrease the amplitude of LTP in isoflurane-treated animals. After determining the time course of inhibition of GABA receptors following isoflurane treatment, Pearce et al suggested that volatile anesthetics, such as isoflurane, enhance GABAB inhibition instead of GABAA.17 As GABAB receptors are located both pre- and postsynaptically, it was suggested that activation of these receptors increases K+ conductance, while it may increase Ca2+ channel conductance and action potential duration, which will decrease cell excitability and reduce Ca2+ dependent release of neurotransmitter, resulting in a decrease in synaptic plasticity, explaining why an altered ionic flux was also suggested to be responsible for changes in synaptic potentiation.
LTP was blocked in animals under isoflurane when mGluR1 antagonist AIDA (−5% ± 18%) and opioid receptor antagonist naloxone (−7% ± 20%) were injected into the stratum lucidum, which was also seen under sodium pentobarbital conditions. When statistical analysis was performed, comparing the fEPSP amplitudes 20 minutes post-drug infusion and 60 minutes post-HFS, there was statistical significance, indicating that long-term depression (LTD) was induced when HFS followed the antagonist infusions (naloxone = F [1, 12] = 8.87 P < 0.05 ; AIDA = F [1, 12] = 5.73, P < 0.05). LTD induction following drug infusion of the MF pathway was also observed by Derrick and Martinez,18 and it was an indication that MF LTP still required activation of μ-opioid receptors. Because opioids reduce GABA release and the MFs are close to the soma where most GABAA receptors are found, drugs that have a larger effect on GABAA currents may alter MF LTP, whereas drugs that alter GABAB receptors may alter LTP at synapses more distal from the soma, such as cCA3 and the perforant pathways.
Thompson et al determined that the metabotropic glutamate class I receptor antagonist AIDA inhibited LTP induction in the MF pathway under sodium pentobarbital anesthesia in vivo.19 Twenty minutes after injection of AIDA to area CA3 under isoflurane conditions, HFS failed to induce LTP in the MF pathway. Based on the results of this experiment, it can be stated that under the anesthetic isoflurane, characteristic MF LTP responses were still observed when compared to those of sodium pentobarbital. Yet visual analysis of the amplitude of LTP in the MF pathway of control groups appeared to show attenuation. These experiments also showed that the difference in amplitude between the Ringer’s group and the CPP group under sodium pentobarbital conditions was similar in animals under isoflurane anesthesia for LTP in the lateral perforant pathways.
Isoflurane effects on the medial perforant and cCA3 pathways possibly mediated by reduction of presynaptic Ca2+ levels
When considering the mechanism of LTP in the MPP–CA3, as well as the requirements for LTP to occur in this system, it can be determined which mechanisms occurring in this pathway are affected by isoflurane treatment. The molecular basis for LTP in this pathway includes the activation of NMDA receptors. NMDA-associated channels allow passage of Na+, K+, and Ca2+ under two conditions: presynaptic release and postsynaptic depolarization causing the relief of a Mg2+-dependent channel blockade for LTP to occur.17 Nishikawa and MacIver have shown that isoflurane depresses NMDA-dependent LTP.16 Another study examining the effects on learning of isoflurane anesthesia has recently shown that acquisition of new memory and performance improvement is enhanced in already-learned spatial memory task from days to up to two weeks following two hours of isoflurane treatment when using the holeboard behavioral learning paradigm and in vitro electrophysiology studying the Schaffer collateral pathway.20 By western blot analysis, Rammes et al attributed improved memory performance to a specific upregulation of the NR2B subunit of the NMDA receptor in the hippocampus, which is dependent on age and learning task.20 In addition to these qualifications, the amount of time that the animal has been exposed to isoflurane should also be considered as a component in determining the cellular mechanisms affected by isoflurane exposure.
Winegar and MacIver showed that isoflurane appeared to depress CA1 synapses presynaptically at sites downstream from voltage gated Na+ channels, while fiber volleys did not exhibit depression, as had been reported for other brain regions in vitro.21 This paper also reported that isoflurane depressed EPSP amplitude by about 60% in the Schaffer collateral glutamate nerve terminals located at CA1. The depression of fEPSP amplitude caused by isoflurane coincides with data collected in this study at the MPP synapse at CA3, where there is a depression of about 66% of LTP amplitude under control conditions, compared to the LTP amplitude of animals treated with sodium pentobarbital anesthesia. Winegar and MacIver also suggest that a likely mechanism of isoflurane in its ability to attenuate fEPSP amplitude is by the reduction of presynaptic Ca2+ levels following nerve terminal depolarization and/or disruption of the vesicle release process.21 Similar findings were reported by Hemmings et al on the role of Ca2+ channel activation in the attenuation of LTP under isoflurane treatment.22 Hemmings et al determined, through quantitative laser-scanning fluorescence microscopy, that isoflurane reversibly inhibited action potential-evoked exocytosis over a range of concentrations, with little effect on vesicle pool size.22 He also determined that inhibition of exocytosis by isoflurane was not due to GABAA receptor modulation, but instead, a reduction in action potential initiation, conduction, or coupling to Ca2+ channel activation. He suggested that the effects of isoflurane on synaptic transmission are caused primarily by inhibition of synaptic vesicle exocytosis by an action potential at a site upstream of Ca2+ entry and exocytosis, possibly as a result of Na+ channel blockage and/or K+ channel activation. Additional supporting evidence by El Beheiry et al show that L-Type calcium blockade opposes anesthetic-induced depression of excitatory synaptic transmission in vivo.23 As the cCA3 pathway to CA3 LTP is conducted by very similar mechanisms of action, it would be expected that these findings would also support the following results.
Isoflurane possibly affects multiple receptor sites and synaptic mechanisms at CA3 dendrites
Isoflurane is an inhaled anesthetic, and the effective brain concentration of isoflurane depends upon multiple pharmacokinetic factors that influence its uptake and distribution, including solubility (the relative affinity of an anesthetic for the blood compared to the air), anesthetic concentration in the inspired air, pulmonary ventilation, pulmonary blood flow, and the arteriovenous concentration gradient. Isoflurane binds to GABA receptors (specifically as a positive allosteric modulator that binds to a site on the GABAA receptor at the beta-alpha subunit interface), and inhibits conduction in activated potassium channels.24 The beta subunit of the GABAA receptor possesses sites for phosphorylation by cAMP-dependent protein kinase and by protein kinase C. The GABAA receptor complex is composed of five subunits, each with four transmembrane domains. The second membrane-spanning region of each subunit forms the wall of the Cl− channel.
When compared to the anesthetic sodium pentobarbital, both isoflurane and sodium pentobarbital bind to the molecular components of the GABAA receptor system, but very likely on different modulatory sites. Another distinction between the two drugs is that sodium pentobarbital is used for sedation and the treatment of seizures, while isoflurane reduces pain sensitivity and relaxes muscles. Because sodium pentobarbital is a barbiturate given intraperitoneally, its rate of action is slower, and it is necessary to administer booster shots to maintain a surgical level of anesthesia. In in vitro studies of the CA1 area of the hippocampus, it has been suggested that the presence of the GABAA receptor antagonist bicuculline causes the inhibitory actions of sodium pentobarbital to be completely antagonized, while those of isoflurane are only partially blocked.25 In reference to NMDA-mediated population spikes in vitro, isoflurane was shown to decrease NMDA population spikes, while sodium pentobarbital had no effect on the NMDA population spikes.25 In a paper by Nishikawa and MacIver, it was shown that isoflurane depresses both the fEPSPs mediated by non-NMDA and NMDA receptors, while having greater depressive effects on the latter in vitro.16 The depressive effects of isoflurane could indicate that a reduction in depolarization is the reason for the lower amplitudes that were observed in the LTP of the non-NMDA pathways.
Tachibana et al showed that low-concentration isoflurane anesthesia caused depression of the EPSP response with enhanced synaptic efficacy by increased paired pulse facilitation in the Schaffer collateral pathway in vivo.26 As the CA1 and CA3 pyramidal cell dendrites both have glutamatergic synapses, it appears plausible that CA1 and CA3 dendrites may show some similar mechanisms under the influence of isoflurane anesthesia. However, there are several differences in the experimental protocol that could account for variance in the results of this experimentation. The current acute electrophysiological experimentation examines synaptic connections to CA3, which differ in several aspects from CA1, and while we examined the effects of control and inhibitory drugs to the afferents of CA3, the animal was under a surgical dose of anesthesia.
In Tachibana et al’s paper, chronic implantation of electrodes to the Schaffer collateral and the mechanical ventilation of animals under low concentrations of isoflurane were performed, which introduces different possible mechanisms into the electrophysiological response.26 Data from this research and from Tachibana et al suggest that possible similarities in the mechanisms that appear to be affected in each pathway may exist; for example, depressed EPSP amplitude in the Schaffer collateral pathway following isoflurane anesthesia indicates depression of glutamatergic transmission, while a change in concentration of isoflurane might be shown to modulate synaptic efficacy. Tachibana also suggested that greater synaptic efficacy could indicate the depression of interneurons, which could also indicate that the concentration of isoflurane may determine whether or not interneuron activity at pyramidal cells is depressed or enhanced.26 There is also a lack of evidence showing how isoflurane possibly interacts with CPP, naloxone or AIDA. Future studies of drug interaction in this case could determine whether inhibition occurs between the pathways in question and their appropriate inhibitory drugs, indicating a change in the molecular mechanisms of LTP that may occur in each pathway.
Acknowledgments
We would like to acknowledge the Ewing Halsell Endowment, the Alfred P. Sloan Foundation, and the Texas Consortium in Behavioral Neuroscience (T32-MH065728).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shepherd G. The Synaptic Organization of the Brain. New York: Oxford University Press; 1994. [Google Scholar]
- 2.Lynch MA, Bliss TV. On the mechanism of enhanced release of [14C] glutamate in hippocampal long-term potentiation. Brain Res. 1986;369(1–2):405–408. doi: 10.1016/0006-8993(86)90561-5. [DOI] [PubMed] [Google Scholar]
- 3.Lynch MA, Voss KL. Arachidonic acid increases inositol phospholipid metabolism and glutamate release in synaptosomes prepared from hippocampal tissue. J Neurochem. 1990;55(1):215–221. doi: 10.1111/j.1471-4159.1990.tb08841.x. [DOI] [PubMed] [Google Scholar]
- 4.Martinez CO, Do VH, Martinez JL, Jr, Derrick BE. Associative long-term potentiation (LTP) among extrinsic afferents of the hippocampal CA3 region in vivo. Brain Res. 2002;940(1–2):86–94. doi: 10.1016/s0006-8993(02)02598-2. [DOI] [PubMed] [Google Scholar]
- 5.Schulz PE. Long-term potentiation involves increases in the probability of neurotransmitter release. Proc Natl Acad Sci U S A. 1997;94(11):5888–5893. doi: 10.1073/pnas.94.11.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez JL, Jr, Derrick BE. Long-term potentiation and learning. Annu Rev Psychol. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- 7.Martinez JL, Jr, Barea-Rodriguez E, Derrick BE. Neurobiology of Learning and Memory. San Diego: Academic Press; 1998. [Google Scholar]
- 8.Breindl A, Derrick BE, Rodriguez SB, Martinez JL., Jr Opioid receptor-dependent long-term potentiation at the lateral perforant path–CA3 synapse in rat hippocampus. Brain Res Bull. 1994;33(1):17–24. doi: 10.1016/0361-9230(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 9.Derrick BE, Weinberger SB, Martinez JL., Jr Opioid receptors are involved in an NMDA receptor-independent mechanism of LTP induction at hippocampal mossy fiber–CA3 synapses. Brain Res Bull. 1991;27(2):219–223. doi: 10.1016/0361-9230(91)90071-q. [DOI] [PubMed] [Google Scholar]
- 10.Jin W, Chavkin C. Mu opioids enhance mossy fiber synaptic transmission indirectly by reducing GABAB receptor activation. Brain Res. 1999;821(2):286–293. doi: 10.1016/s0006-8993(99)01089-6. [DOI] [PubMed] [Google Scholar]
- 11.Bramham CR, Sarvey JM. Endogenous activation of mu and delta-1 opioid receptors is required for long-term potentiation induction in the lateral perforant path: dependence on GABAergic inhibition. J Neurosci. 1996;16(24):8123–8131. doi: 10.1523/JNEUROSCI.16-24-08123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupica CR, Proctor WR, Dunwiddie TV. Dissociation of mu and delta opioid receptor-mediated reductions in evoked and spontaneous synaptic inhibition in the rat hippocampus in vitro. Brain Res. 1992;593(2):226–238. doi: 10.1016/0006-8993(92)91312-3. [DOI] [PubMed] [Google Scholar]
- 13.Madison DV, Nicoll RA. Enkephalin hyperpolarizes interneurons in the rat hippocampus. J Physiol. 1988;398:123–130. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson KJ, Orfila JE, Achanta P, Martinez JL., Jr Gene expression associated with in vivo induction of early-phase long-term potentiation (LTP) in the hippocampal mossy fiber–cornus ammonis (CA3) pathway. Cell Mol Biol. 2003;49(8):1281–1287. [PubMed] [Google Scholar]
- 15.Escobar ML, Barea-Rodriguez EJ, Derrick BE, Reyes JA, Martinez JL., Jr Opioid receptor modulation of mossy fiber synaptogenesis: independence from long-term potentiation. Brain Res Bull. 1997;751(2):330–335. doi: 10.1016/s0006-8993(96)01373-x. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa K, MacIver MB. Excitatory synaptic transmission mediated by MNDA receptors is more sensitive to isoflurane than are non-NMDA receptor-mediated responses. Anesthesiology. 2000;92(1):228–236. doi: 10.1097/00000542-200001000-00035. [DOI] [PubMed] [Google Scholar]
- 17.Pearce RA, Stringer JL, Lothman EW. Effect of volatile anesthetics on synaptic transmission in the rat hippocampus. Anesthesiology. 1989;71(4):591–598. doi: 10.1097/00000542-198910000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Derrick BE, Martinez JL., Jr Associative, bidirectional modifications at the hippocampal, mossy fibre–CA3 synapse. Nature. 1996;381(6581):429–434. doi: 10.1038/381429a0. [DOI] [PubMed] [Google Scholar]
- 19.Thompson KJ, Mata ML, Orfila JE, Barea-Rodriguez EJ, Martinez JL., Jr Metabotropic glutamate receptor antagonist AIDA blocks induction of mossy fiber-CA3 LTP in vivo. J Neurophysiol. 2005;93(5):2668–2673. doi: 10.1152/jn.00901.2004. [DOI] [PubMed] [Google Scholar]
- 20.Rammes G, Starker LK, Haseneder R, et al. Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009;56(3):626–636. doi: 10.1016/j.neuropharm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Winegar BD, MacIver MB. Isoflurane depresses hippocampal CA1 glutamate nerve terminals without inhibiting fiber volleys. BMC Neurosci. 2006;7:5. doi: 10.1186/1471-2202-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmings HC, Jr, Yan W, Westphalen RI, Ryan TA. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol. 2005;67(5):1591–1599. doi: 10.1124/mol.104.003210. [DOI] [PubMed] [Google Scholar]
- 23.El Beheiry H, Ouanounou A, Carlen PL. L-type calcium channel blockade modifies anesthetic actions on aged hippocampal neurons. Neuroscience. 2007;147(1):117–126. doi: 10.1016/j.neuroscience.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Li GD, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem. 2010;285(12):8615–8620. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bieda MC, Su H, MacIver MB. Anesthetics discriminate between tonic and phasic gamma-aminobutyric acid receptors on hippocampal CA1 neurons. Anesth Analg. 2009;108(2):484–490. doi: 10.1213/ane.0b013e3181904571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tachibana K, Takita K, Hashimoto T, Matsumoto M, Yoshioka M, Morimoto Y. Isoflurane bidirectionally modulates the paired-pulse responses in the rat hippocampal CA1 field in vivo. Anesth Analg. 2007;105(4):1006–1011. doi: 10.1213/01.ane.0000281433.73260.8d. [DOI] [PubMed] [Google Scholar]
- 27.Do VH, Martinez CO, Martinez JL, Jr, Derrick BE. Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J Neurophysiol. 2002;87(2):669–678. doi: 10.1152/jn.00938.2000. [DOI] [PubMed] [Google Scholar]
- 28.Orfila J. Necessity of Protein Synthesis in Long-Term Potentiation (LTP) in the CA3 Area of the Rat Hippocampus. San Antonio: University of Texas at San Antonio; 2008. [Google Scholar]