Abstract
Recently, the biosynthesis of an unusual membrane phospholipid, N-acylphosphatidylethanolamine (NAPE), was found to increase in elicitor-treated tobacco (Nicotiana tabacum L.) cells (K.D. Chapman, A. Conyers-Hackson, R.A. Moreau, S. Tripathy [1995] Physiol Plant 95: 120–126). Here we report that before induction of NAPE biosynthesis, N-acylethanolamine (NAE) is released from NAPE in cultured tobacco cells 10 min after treatment with the fungal elicitor xylanase. In radiolabeling experiments [14C]NAE (labeled on the ethanolamine carbons) increased approximately 6-fold in the culture medium, whereas [14C]NAPE associated with cells decreased approximately 5-fold. Two predominant NAE molecular species, N-lauroylethanolamine and N-myristoylethanolamine, were specifically identified by gas chromatography-mass spectrometry in lipids extracted from culture medium, and both increased in concentration after elicitor treatment. NAEs were found to accumulate extracellularly only. A microsomal phospholipase D activity was discovered that formed NAE from NAPE; its activity in vitro was stimulated about 20-fold by mastoparan, suggesting that NAPE hydrolysis is highly regulated, perhaps by G-proteins. Furthermore, an NAE amidohydrolase activity that catalyzed the hydrolysis of NAE in vitro was detected in homogenates of tobacco cells. Collectively, these results characterize structurally a new class of plant lipids and identify the enzymatic machinery involved in its formation and inactivation in elicitor-treated tobacco cells. Recent evidence indicating a signaling role for NAPE metabolism in mammalian cells (H.H.O. Schmid, P.C. Schmid, V. Natarajan [1996] Chem Phys Lipids 80: 133–142) raises the possibility that a similar mechanism may operate in plant cells.
NAPE is a widespread, albeit minor, membrane phospholipid in animal and plant tissues (Schmid et al., 1990; Chapman and Moore, 1993). Its unusual structural features (a third fatty acid moiety linked to the amino head group of PE) impart stabilizing properties to membrane bilayers (Domingo et al., 1994; LaFrance et al., 1997). NAPE and its hydrolysis products, NAEs, are known to accumulate in vertebrate tissues under pathological conditions (for review, see Schmid et al., 1990). Recently, there has been renewed interest in NAEs because of the contention that anandamide (N-arachidonylethanolamine) is an endogenous ligand for the cannabinoid receptor in mammalian brain (Devane et al., 1992; Fontana et al., 1995; Schmid et al., 1996). The likely route for NAE formation in neural and nonneural tissues, although the matter of some debate, is via the signal-mediated hydrolysis of NAPE (DiMarzo et al., 1994; Schmid et al., 1996; Sugiura, et al., 1996).
In plants little is known regarding the catabolism of NAPE. In cottonseed microsomes NAPE was metabolized to NAE or NAlysoPE by PLD- or PLA-type activities, respectively (Chapman et al., 1995b). However, the metabolic fate of NAPE in vivo and the factors that regulate NAPE hydrolysis remain largely unknown. We previously noted that the biosynthesis of NAPE was increased in elicitor-treated cell suspensions of tobacco (Nicotiana tabacum L.). Here we extend our investigations with this model system to examine NAPE catabolism by plant cells in vivo. NAE was released from NAPE, and it accumulated extracellularly. We identified by GC-MS these tobacco NAEs as N-lauroylethanolamine and N-myristoylethanolamine. These NAEs were increased in elicitor-treated cell suspensions. Furthermore, we detected the enzymatic machinery capable of the release and the degradation of NAEs in tobacco cells. To our knowledge this represents the first identification of the NAE molecular species in plant cells. It is tempting to speculate that NAPE hydrolysis in elicitor-treated plant cells may be involved in a signaling pathway analogous to that found in mammalian cells.
MATERIALS AND METHODS
Cell Cultures, Elicitor Treatment, and Lipid Extractions
Tobacco (Nicotiana tabacum L. cv KY-14) cell suspensions were subcultured every 7 d (Chapman et al., 1995a); cell suspensions in log phase were treated with elicitor (xylanase, 1 μg/mL) as previously described (Chapman et al., 1995a). Control and experimental treatments were carried out on aliquots of the same population of cells. Culture supernatants were separated from cells by filtration. Cells were quick frozen in liquid N2, powdered in a mortar, and added to boiling 2-propanol in a ratio of 0.8:2 (grams fresh weight of cells:milliliters of 2-propanol). Culture supernatants were added directly to 2-propanol in the same ratio (v/v) without freezing. Lipids were extracted from samples with chloroform (Bligh and Dyer, 1959). For radiolabeling experiments in vivo, cells in log phase (3–4 d after subculture) were incubated for 4 h with [1,2-14C]ethanolamine (2 μCi; 3 μCi μmol−1, NEN) before treatment with elicitor.
Lipid Analyses
To assess radiolabeled ethanolamine-containing lipids, total lipids were subjected to TLC and radiometric scanning, as described previously (Chapman and Moore, 1993; Chapman et al., 1995a, 1995b). For structural characterization, NAE was separated from the total lipid extracts by a combination of Si gel cartridge chromatography and TLC (Chapman and Moore, 1993; Chapman et al., 1995b). Ethanolamine-containing lipids were identified on TLC plates by co-chromatography with authentic standards (Chapman and Moore, 1993; Chapman et al., 1995b; Sandoval et al., 1995). NAE-enriched fractions were recovered from Si gel plates in chloroform, O-acetylated (Fontana et al., 1995), and analyzed by GC-MS (model 5970 mass spectrometer equipped with a capillary interface to a model 5890 series II gas chromatograph, Hewlett-Packard). Derivatized samples in chloroform were chromatographed on a 30-m × 0.25-mm capillary column (Supelcowax 10, Supelco, Bellefonte, PA) with an oven temperature program of 100°C for 2 min, increased to 240°C at 10°C/min, and then held at 240°C for an additional 32 min. The injector temperature was 200°C and the inlet carrier gas (He) was 5 p.s.i. Synthetic NAEs were treated in the same manner, but with different TLC plates and glassware as a precaution to avoid contamination with these analytes. Synthetic NAE molecular species were kindly provided by Dr. Daniele Piomelli (The Neurosciences Institute, San Diego, CA) and their purity was verified by GC-MS.
Tobacco cell NAPE was purified by TLC and digested with Streptomyces chromofuscus PLD (Chapman and Moore, 1993). The NAEs derived from NAPE were derivatized and analyzed by GC-MS (as described above).
PLD and Amidohydrolase Assays
Tobacco cells (approximately 12 g fresh weight) were homogenized and microsomes isolated as previously described (Chapman et al., 1995a, 1995b). Radiolabeled NAPE was prepared fresh for each experiment from equimolar amounts of sn-1,2-dioleoylphosphatidyl[2-14C]ethanolamine (31.5 nmol; 31.8 nCi/nmol, Amersham) and palmitoyl chloride (Dawson et al., 1969) and purified by one-dimensional TLC (Chapman and Moore, 1993; Chapman et al., 1995b). Approximately 20,000 dpm were used per assay, and samples were sonicated briefly after the addition of the substrate (in 20 μL of diethyl ether). Reactions were carried out at 32°C in a final volume of 1 mL with shaking (120 rpm). Assays were buffered with potassium phosphate (100 mm, pH 6.0) and were started by the addition of the substrate, 14C-labeled NAPE. Assay reaction mixtures were incubated for 30 min and stopped by the addition of 2 mL of boiling 2-propanol. Lipids were extracted from the alcohol-water mixture into chloroform and separated by TLC (Chapman et al., 1995b). Released NAE was quantified by radiometric scanning (Bioscan System 200 Imaging Scanner, Bioscan, Washington, DC) and/or liquid-scintillation counting as described previously (Chapman et al., 1995b).
NAE amidohydrolase activity was measured by following the hydrolysis of [14C]NAE (release of water-soluble [14C]ethanolamine). [14C]NAE was prepared by enzymatic digestion of [14C]NAPE (prepared as described above) with S. chromofuscus PLD (Chapman and Moore, 1993). Approximately 5000 dpm of [14C]NAE (in a small volume of methanol) was added to aliquots of homogenates or microsomes and incubated for 30 min at 30°C. Lipids were extracted from enzyme reaction mixtures, and radioactivity in aqueous and organic fractions was quantified by liquid-scintillation counting. Enzyme activity was calculated based on the radiospecific activity of the original [14C]dioleoyl PE used for NAPE synthesis (as described above).
RESULTS
Radiolabeling experiments in vivo (ethanolamine-containing lipids were specifically radiolabeled with [1,2-14C]ethanolamine) demonstrated the occurrence of a xylanase-stimulated NAE release into the culture medium 10 min after treatment (Table I). This release appeared to be at the expense of NAPE, because radiolabeled cellular NAPE declined dramatically in elicitor-treated cells. There was little relative change in other ethanolamine-containing lipids. Replicate experiments, although varying in the efficiency of incorporation of radiolabel into lipids, consistently showed a release of NAE at the expense of NAPE when cells were treated with elicitor. The decrease in radiolabeled NAPE was not completely accounted for by the increase in radiolabeled NAE in the culture medium. We speculate that an amidohydrolase activity (see below) may be responsible for the subsequent metabolism of NAE.
Table I.
Release of 14C-labeled NAE from elicitor (xylanase)-treated tobacco cells (5 g fresh weight)
| Cell | Medium | Cell |
|---|---|---|
| dpm/flaska | ||
| Control (no xylanase) | ||
| Lipid class | ||
| NAE | 506 ± 54 | 649 ± 95 |
| NAPE | – | 8,651 ± 2,462 |
| PE | – | 154,396 ± 25,112 |
| Other (mostly phosphatidylcholine) | – | 15,921 ± 2,528 |
| Xylanase-treated (10 min) | ||
| Lipid class | ||
| NAE | 3104 ± 920 | 449 ± 131 |
| NAPE | – | 1,593 ± 382 |
| PE | – | 155,432 ± 56,207 |
| Other | – | 16,646 ± 1,852 |
Values represent the mean dpm and sd of four independent experiments.
Radiolabeled for 4 h with [1,2-14C]ethanolamine.
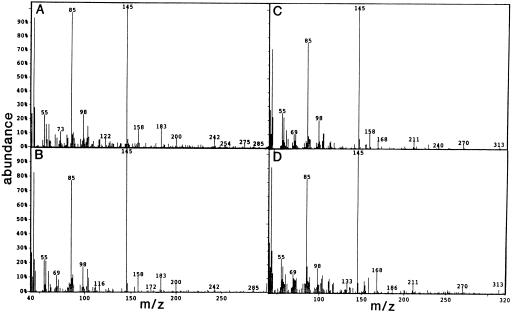
The NAEs that were specifically released into the tobacco cell culture medium after xylanase treatment were identified by GC-MS as N-lauroylethanolamine (NAE 12:0) and N-myristoylethanolamine (NAE 14:0) (Fig. 1). EIMS and retention times in GC of the O-acetylated derivatives of the endogenously released NAEs from tobacco cells were identical with authentic standards. Other types of NAEs (longer or unsaturated acyl chains) were not detected in tobacco cell suspensions. There was a measurable increase in both NAE molecular species 10 min after xylanase treatment, although not as pronounced as that inferred from radiolabeling experiments. In GC-MS experiments NAE 12:0 and NAE 14:0 in the culture medium were estimated to increase about 2-fold after elicitor treatment (from 5.6 to 10.0 and 3.6 to 8.4 ng/g fresh weight of cells, respectively). The apparent discrepancy between results from radiolabeling experiments and results from GC-MS experiments may be attributable to losses during processing/derivatization of samples for GC.
Figure 1.
EIMS of NAEs. O-Acetylated derivatives of putative tobacco NAE 12:0 (A), synthetic NAE 12:0 (B), synthetic NAE 14:0 (C), and putative tobacco NAE 14:0 (D) were analyzed by GC-MS. Retention times in GC and molecular ions [M]+ in EIMS of tobacco NAEs were identical to those of synthetic compounds (33.5 min and m/z 285 for NAE 12:0; 45.6 min and m/z 313 for NAE 14:0). Before GC-MS, tobacco NAEs were partially purified by TLC from total lipid extracts and derivatized according to Fontana et al. (1995). The synthetic NAEs were treated in the same manner, but with different TLC plates and glassware as a precaution to avoid contamination.
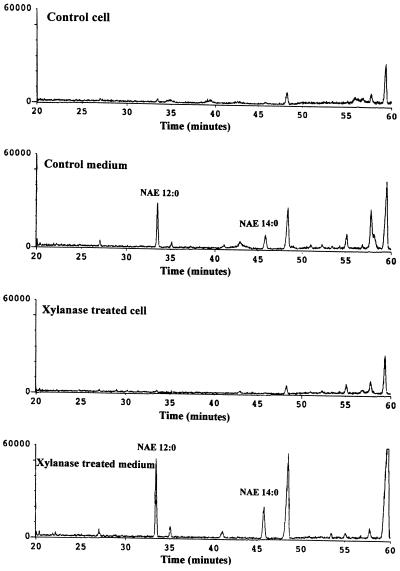
Single-ion chromatograms (m/z 145, major diagnostic ion characteristic of NAEs) for NAE-enriched samples from cells and culture medium of unelicited and elicitor-treated cells are compared in Figure 2. NAE 12:0 and NAE 14:0 (identified by their respective EIMS, Fig. 1) were detected in culture medium, but were barely detectable in lipids from cells. These data suggest that NAEs are released into the extracellular medium and do not accumulate intracellularly. The low levels of intracellular NAEs may be caused by the degradation of these molecules by an amidohydrolase activity (see below). In separate experiments we confirmed that lauric acid and myristic acid were present endogenously as the predominant N-acyl constituents of tobacco NAPE (not shown). Longer N-acyl chains, as previously identified in cottonseed (Chapman and Moore, 1993; Sandoval et al., 1995), were not detected in tobacco cells. This may be indicative of different physiological roles for NAPE metabolism in these different cell types or different developmental stages (germinated seeds versus cell suspensions).
Figure 2.
Single-ion chromatograms at m/z 145 of O-acetylated, NAE-enriched samples from unelicited (control) cells and medium and xylanase-treated cells and medium. Peaks identified by electron-impact MS as NAE 12:0 and NAE 14:0 are labeled. Other lipid molecules in the chromatograms did not show mass spectra characteristic of NAEs (Fontana et al., 1995; see text).
Two other abundant lipid species in these chromatograms, one at 48.5 min and one at 59.8 min, were present in the cell and medium samples, but appeared more abundant in the medium after elicitor treatment (similar to the results for NAEs). EIMS of these species (not shown) indicated that they were likely bis-O-acetylated derivatives of monoacylglycerols, palmitylglycerol and stearylglycerol, respectively. Diagnostic ions in both spectra of [M-175]+, representing the fatty acyl moiety, were evident (at m/z 239 for palmityl and at m/z 267 for stearyl). Also, the prominent ion in both spectra was at m/z 159, which would correspond to the derivatized glyceryl fragment ion. Although our experimental results do not address the origin of these lipids, their occurrence suggests the involvement of PLA (and phospholipase C, or PLD and phosphatidic acid phosphatase) action on membrane phospholipids.
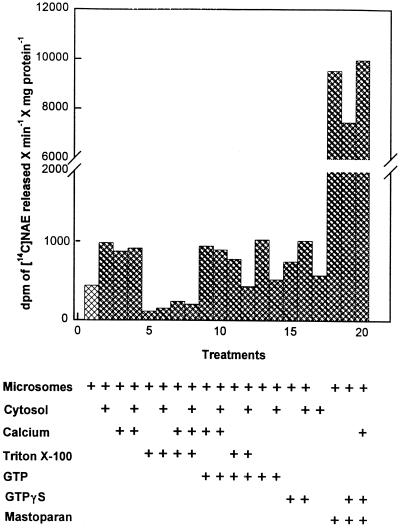
A microsomal enzyme activity from tobacco cells was identified that hydrolyzed NAPE to NAE in vitro (Fig. 3). The tobacco PLD-type activity was present in cytosolic fractions as well. NAE formation by tobacco microsomes was stimulated somewhat by Ca2+ and GTP-γ-S. However, the activity was inhibited by concentrations of Triton X-100 that are known to stimulate a similar PLD activity previously characterized in mammalian liver (Schmid et al., 1996). Most notably, the tobacco microsomal PLD activity toward exogenously supplied [14C]NAPE was increased about 20-fold in the presence of mastoparan, suggesting the possibility of G-protein-mediated regulation of NAPE hydrolysis. It should be pointed out that a direct stimulation of tobacco PLD activity by mastoparan, as opposed to involvement of an activated G-protein, cannot be ruled out. The xylanase protein preparation itself had no hydrolytic activity toward NAPE in vitro, and adding elicitor to the microsomes did not stimulate PLD activity above control levels (not shown).
Figure 3.
NAE formation from NAPE by tobacco microsomal PLD. Plus signs below the treatment indicate the addition of that component to the reaction mixture (microsomes, 0.04 mg of protein; cytosol, 0.025 mg of protein; calcium, 15 mm; Triton X-100, 2 mg/mL; GTP, 3 mm; GTP-γ-S, a nonhydrolyzable GTP analog, 25 μm; and mastoparan, 25 μm). Reactions were carried out at 30°C in a final volume of 1 mL with shaking (120 rpm). Assays were started by adding the substrate (14C-labeled NAPE) and stopped by adding 2-propanol. Released NAE was quantified by radiometric scanning (System 200 Imaging Scanner, Bioscan) (Chapman et al., 1995a) of TLC separations of lipids extracted from reaction mixtures. Values are the averages of duplicate samples (in all cases the range was less than 14%) and are representative of several replicate experiments.
Because only trace levels of NAEs were detected in cellular lipid extracts, we postulated that there was an intracellular NAE amidohydrolase activity like that found in some mammalian tissues (Desarnaud et al., 1995; Ueda et al., 1995) that could catalyze the hydrolysis of NAE. Such an activity was readily detected in tobacco cell homogenates and membranes (Table II), although the latter accounted for only a small proportion of the total activity. This hydrolytic activity released water-soluble radioactivity from [14C]NAE (radiolabeled on carbon 2 of the ethanolamine) and was inactivated by boiling the cell fractions. Consequently, we conclude that an amidohydrolase-type activity is present in tobacco cells and could be responsible for the rapid removal of free intracellular NAE.
Table II.
Enzymatic hydrolysis of N-palmitoyl[2-14C]ethanolamine by homogenates and membranes of tobacco cells
| Cell Fraction | Total Activity | Specific Activity |
|---|---|---|
| pmol h−1 | units mg−1 protein | |
| Homogenate (650g supernatant) | 1926 ± 465 | 66.3 ± 13.8 |
| Membranes (150,000g pellet, 60 min) | 121.0 ± 17.5 | 75.8 ± 6.9 |
Activity was quantified as the amount of water-soluble radioactive [2-14C]ethanolamine released after 30 min and is attributed to an amidohydrolase-type enzyme. Values represent the average enzyme activity and sd from three independent cell-fractionation experiments.
DISCUSSION
Recent studies indicated that NAPE biosynthesis was increased in elicitor-treated tobacco cells (Chapman et al., 1995a). Approximately 2 h were required for the maximum induction of NAPE biosynthesis, as judged by enzyme activity and lipid accumulation, suggesting that NAPE biosynthesis was not involved in the early membrane permeability-related events of pathogen perception. Instead, we hypothesized that the increase in NAPE biosynthesis might be required to replenish NAPE levels depleted by the signal-mediated hydrolysis of NAPE. The results presented here are consistent with such a hypothesis.
The tobacco-cell/fungal-elicitor model system has been used by a number of research groups to identify and characterize various components involved in plant defense responses. These include changes in ion flux across the plasma membrane (Bailey et al., 1992), changes in plasma membrane lipid metabolism (Moreau and Presig, 1993; Moreau et al., 1994; Chapman et al., 1995a), transient protein (Tyr) phosphorylation (Suzuki and Shinshi, 1995), induction of phytoalexin biosynthesis (Moreau and Presig, 1993), induction of ethylene biosynthesis (Anderson et al., 1993), induction of pathogenesis-related protein expression (Lotan and Fluhr, 1990), and induction of defense gene expression (Bailey et al., 1995; Suzuki et al., 1995). Recently, a receptor for the xylanase protein was identified in tobacco plasma membranes (Hanania and Avni, 1997), and a single gene trait has been linked to xylanase sensitivity (Bailey et al., 1993). Hence, our work on the regulation of NAPE metabolism in tobacco cells treated with xylanase may be relevant to signal-transduction pathways in plant defense responses. Additional work to characterize the biological effects of NAEs on plant cells will be necessary to understand the possible role of NAEs in plant cell signaling.
Other workers have implicated PLD induction in plant pathogen perception or wounding (Ryu and Wang, 1996; Young et al., 1996; Wang, 1997). Young et al. (1996) reported changes in plasma membrane distribution of a rice PLD in response to bacterial pathogens. In other studies a mastoparan-stimulated PLD activity was reported in carnation petals and Chlamydomonas eugametos cells; however, the endogenous lipid substrate was not identified (Munnik et al., 1995). Our results are consistent with an emerging role for a highly regulated PLD activity(ies) (Causier and Milner, 1996; Ryu and Wang, 1996; Pappan et al., 1997; Wang, 1997) that is involved in signal transduction pathways in plants. Moreover, our studies identify at least one type of endogenous membrane lipid substrate for PLD and characterize for the first time to our knowledge the structure of the hydrolysis products, NAEs.
The NAEs identified here have shorter acyl chains than the biologically active neurotransmitters (anandamide; Devane et al., 1992) or the closely related sleep-inducing compounds (oleoylamide; Cravatt et al., 1995) found in mammalian brain. Nonetheless, there are many similarities in NAE metabolism evident from our results that are shared between plants and animals. First, the molecular origin of NAE appears to be a relatively minor membrane phospholipid (NAPE). Second, NAEs are released from NAPE in a signal-mediated fashion and accumulate extracellularly. A PLD appears to catalyze the formation of NAE and an amidohydrolase appears to be responsible for its intracellular degradation. Whereas a signaling role for these NAEs in plants has yet to be firmly established, the emerging role for NAE as a signaling molecule in mammalian tissues (Schmid et al., 1996) and the similarities in its metabolism suggest that this mechanism may be widespread in evolution.
ACKNOWLEDGMENTS
We thank Dr. Daniele Piomelli for providing the synthetic NAEs and also for insightful discussions regarding NAPE metabolism in mammalian neurons. We also thank Drs. James D. Anderson, Bryan Bailey, and James Jennings for helpful discussions relating to elicitor treatment of tobacco cells.
Abbreviations:
- EIMS
electron-impact mass spectrum(a)
- NAE
N-acylethanolamine
- NAPE
N-acylphosphatidylethanolamine
- PE
phosphatidylethanolamine
- PLA
phospholipase A
- PLD
phospholipase D
- X:Y
a fatty acyl group containing X carbon atoms and Y cis double bonds
Footnotes
This research was supported by grants from the U.S. Department of Agriculture-National Research Initiative Competitive Grants Program to K.D.C., agreement nos. 94-37304-1230 and 96-35304-3862.
LITERATURE CITED
- Anderson JD, Bailey BA, Taylor R, Sharon A, Avni A, Matoo AK, Fuchs Y. Fungal xylanase elicits ethylene biosynthesis and other defense responses in tobacco. In: Pech JC, Latche A, Balague C, editors. Cellular and Molecular Aspects of the Plant Hormone Ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 197–204. [Google Scholar]
- Bailey BA, Avni A, Anderson JD. The influence of ethylene and tissue age on the sensitivity of Xanthi tobacco leaves to a Trichoderma viride xylanase. Plant Cell Physiol. 1995;36:1669–1676. [Google Scholar]
- Bailey BA, Korcak RF, Anderson JD. Alterations in Nicotiana tabacum L. cv Xanthi cell membrane function following treatment with an ethylene biosynthesis-inducing endoxylanase. Plant Physiol. 1992;100:749–755. doi: 10.1104/pp.100.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey BA, Korcak RF, Anderson JD. Sensitivity to an ethylene biosynthesis-inducing endoxylanase in Nicotiana tabacum L. cv Xanthi is controlled by a single dominant gene. Plant Physiol. 1993;101:1081–1088. doi: 10.1104/pp.101.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WG. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Causier BE, Milner PA. G-protein regulated phospholipase D: another piece in the plant cell signalling jigsaw. Trends Plant Sci. 1996;1:168–170. [Google Scholar]
- Chapman KD, Conyers-Jackson A, Moreau RA, Tripathy S. Increased N-acylphosphatidylethanolamine biosynthesis in elicitor-treated tobacco cells. Physiol Plant. 1995a;95:120–126. [Google Scholar]
- Chapman KD, Lin I, Desouza AD. Metabolism of cottonseed microsomal N-acylphosphatidylethanolamine. Arch Biochem Biophys. 1995b;318:401–407. doi: 10.1006/abbi.1995.1246. [DOI] [PubMed] [Google Scholar]
- Chapman KD, Moore TS., Jr N-Acylphosphatidylethanol-amine synthesis in plants: occurrence, molecular composition and phospholipid origin. Arch Biochem Biophys. 1993;301:21–33. doi: 10.1006/abbi.1993.1110. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula N-B, Henriksen SJ, Boger DL, Lerner RA. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- Dawson RMC, Clarke N, Quarles RH. N-Acylphosphatidylethanolamine: a phospholipid that is rapidly metabolized during early germination of pea seeds. Biochem J. 1969;114:265–270. doi: 10.1042/bj1140265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes: identification and partial characterization. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson L, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DiMarzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid annadamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Domingo J, Mora M, Africa De Madariaga M. Role of headgroup structure in the phase behaviour of N-acylethanol-amine phospholipids: hydrogen bonding ability and headgroup size. Chem Phys Lipids. 1994;69:229–240. doi: 10.1016/0009-3084(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Fontana A, Di Marzo V, Cadas H, Piomelli D. Analysis of anadamide, an endogenous cannabinoid substance, and of other natural N-acylethanolamines. Prostaglandins Leukot Essent Fatty Acids. 1995;53:301–308. doi: 10.1016/0952-3278(95)90130-2. [DOI] [PubMed] [Google Scholar]
- Hanania U, Avni A. High-affinity binding site for ethylene-inducing xylanase elicitor on Nicotiana tabacum membranes. Plant J. 1997;12:113–120. [Google Scholar]
- LaFrance CP, Blochet JE, Pezolet M. N-Acylphosphatidylethanolamines: effect of the N-acyl chain length on its orientation. Biophys J. 1997;72:2559–2568. doi: 10.1016/S0006-3495(97)78899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T, Fluhr R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, uses a non-ethylene pathway for induction. Plant Physiol. 1990;93:811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau RA, Powell MJ, Whitaker BD, Bailey BA, Anderson JD. Xylanase treatment of plant cells induces glycosylation and fatty acylation of phytosterols. Physiol Plant. 1994;91:575–580. [Google Scholar]
- Moreau RA, Presig CL. Lipid changes in tobacco cell suspensions following treatment with cellulase elicitor. Physiol Plant. 1993;87:7–13. [Google Scholar]
- Munnik T, Arisz SA, de Vrije T, Musgrave A. G-protein activation of phospholipase signaling in plants. Plant Cell. 1995;7:2197–2210. doi: 10.1105/tpc.7.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappan K, Zheng S, Wang X. Identification and characterization of a novel plant phospholipase D that requires phosphoinositides and submicromolar calcium for activity in Arabidopsis. J Biol Chem. 1997;272:7055–7061. doi: 10.1074/jbc.272.11.7048. [DOI] [PubMed] [Google Scholar]
- Ryu SB, Wang X. Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor leaves. Biochim Biophys Acta. 1996;1303:243–250. doi: 10.1016/0005-2760(96)00096-3. [DOI] [PubMed] [Google Scholar]
- Sandoval J, Huang Z, Garrett D, Gage D, Chapman KD. N-Acylphosphatidylethanolamine in dry and imbibing cottonseeds: amounts, molecular species, and enzymatic synthesis. Plant Physiol. 1995;109:269–275. doi: 10.1104/pp.109.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid HHO, Schmid PC, Natarajan V. N-Acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Schmid HHO, Schmid PC, Natarajan V. The N-acylation-phosphodiesterase pathway and cell signalling. Chem Phys Lipids. 1996;80:133–142. doi: 10.1016/0009-3084(96)02554-6. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, Waku K. Enzymatic synthesis of anandamide, an endogenous cannabinoid receptor ligand, through N-acylphosphatidylethanolamine pathway in testis. Biochem Biophys Res Commun. 1996;218:113–117. doi: 10.1006/bbrc.1996.0020. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Fukada Y, Shinshi H. Studies on elicitor-signal transduction leading to differential expression of defense genes in cultured tobacco cells. Plant Cell Physiol. 1995;36:281–289. [Google Scholar]
- Suzuki K, Shinshi H. Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. Partial purification and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. J Biol Chem. 1995;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- Young SA, Wang X, Leach JE. Changes in the plasma membrane distribution of rice phospholipase D during resistant interactions with Xanthomonas oryzae pv oryzae. Plant Cell. 1996;8:1079–1090. doi: 10.1105/tpc.8.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Molecular analysis of phospholipase D. Trends Plant Sci. 1997;2:261–266. [Google Scholar]