Abstract
BACKGROUND
Advanced maternal age is associated with reduced fertility and adverse pregnancy outcomes. This review details recent developments in our understanding of the biology and mechanisms underlying reproductive ageing in women and the implications for fertility and pregnancy.
METHODS
Sociological online libraries (IBSS, SocINDEX), PubMed and Google Scholar were searched for relevant demographic, epidemiological, clinical and biological studies, using key words and hierarchical MeSH terms. From this, we identified and focused on key topics where it was judged that there had been clinically relevant advances in the understanding of ovarian and uterine ageing with implications for improved diagnostics and novel interventions.
RESULTS
Mapping of the ovarian reserve, follicular dynamics and associated biomarkers, across the reproductive lifespan has recently been performed. This now allows an assessment of the effects of environmental, lifestyle and prenatal exposures on follicular dynamics and the identification of their impact during periods of germ cell vulnerability and may also facilitate early identification of individuals with shorter reproductive lifespans. If women choose to time their family based on their ovarian reserve this would redefine the meaning of family planning. Despite recent reports of the potential existence of stem cells which may be used to restore the primordial follicle and thereby the oocyte pool, therapeutic interventions in female reproductive ageing at present remain limited. Maternal ageing has detrimental effects on decidual and placental development, which may be related to repeated exposure to sex steroids and underlie the association of ageing with adverse perinatal outcomes.
CONCLUSIONS
Ageing has incontrovertible detrimental effects on the ovary and the uterus. Our enhanced understanding of ovarian ageing will facilitate early identification of individuals at greatest risk, and novel therapeutic interventions. Changes in both ovary and uterus are in addition to age-related co-morbidities, which together have synergistic effects on reducing the probability of a successful pregnancy outcome.
Keywords: ageing, fertility, infertility, ovarian reserve, anti-Müllerian hormone
Introduction
World and female reproductive demographics are changing dramatically. Within the current demographic transition, populations are moving from an initial state of high mortality and high fertility to a state of low mortality and low fertility. As recently reviewed, these changes have caused dramatic changes in the global population size, the rate of population growth, the working-age share and the age distribution (Bloom, 2011; Lee, 2011). Combinations of medical advances, public health initiatives and social changes have been responsible for this transformation. As a consequence of this, globally, adolescents and young adults aged 15–24 years currently outnumber those aged 60 years and above by 54% but the size of these two groups will equalize by 2025, after which those over age 60 years will outnumber adolescents and young adults (Bloom, 2011). For the foreseeable future we will therefore see a large number of women capable of reproduction, but with competing demands as educational attainment increases and their fiscal contribution to an ageing population is required. These factors underlie the progressive increase in age at childbirth.
Changes in fertility with female age have been widely described in historical populations where contraception was not practiced (Schmidt et al., 2012). Although the level of fertility varies, the age patterns of decline are quite similar; the fall is not large until after age 35 years (Olsen, 1990; van Balen et al., 1997; Dunson et al., 2002). A model that imposed a common age pattern but allowed the level of marital fertility to differ among populations demonstrated that compared with that of women age 20–24 years, fertility is reduced on average by 6% for women 25–29 years, by 14% for those 30–34 years and by 31% for women 35–39 years, with a much greater decline thereafter (Menken et al., 1986). These changes in natural fertility are mirrored by IVF success rates, which, while showing some variance by country, show an initial increase in success rates through the late teens to early 20s and then a general decline from the early 30s (Nelson and Lawlor, 2011; de Mouzon et al., 2012).
At a population level postponement of parenthood and an increased maternal age will have a relatively modest change in the mean number of children but a large increase in the proportion of couples suffering from infertility and seeking assisted reproduction techniques (ART) and a large increase in pregnancies in older mothers. In addition to the well-established age-related increase in the risk of miscarriage (Nybo Andersen et al., 2000; Cleary-Goldman et al., 2005), almost all other adverse fetal and maternal outcomes are also increased with advancing maternal age. Rates of placental abruption, placenta praevia, malpresentation, low-birthweight, large for gestational age, post-partum haemorrhage and preterm and post-term delivery are ∼2-fold higher in older mothers (Cnattingius et al., 1992; Aldous and Edmonson, 1993; Cleary-Goldman et al., 2005; Hoffman et al., 2007). The prevalence of pre-existing maternal medical conditions, including hypertension, obesity and diabetes also increases with age (Health, 2004), as do pregnancy-related maternal complications, such as pre-eclampsia and gestational diabetes (Solomon et al., 1997; Duckitt and Harrington, 2005). Consequently one quarter of pregnant women aged 45 years or over will have a chronic medical disease, with hypertension affecting 25% of 45–54-year-old women (Department of Health, 2004). Obesity affects ∼25% of women ages 35–54 years, with a further 33% overweight. Collectively this has resulted in many more women entering pregnancy with pre-existing morbidity or at increased risk of developing novel disease in response to the physiological demands of pregnancy. The clinical impact of this is substantial, as the risk of pregnancy-induced hypertension doubles with obesity and triples with morbid obesity (≥35 kg/m2), while the risk of pre-eclampsia increases 2-fold with each 5–7 kg/m2 increase in prepregnancy BMI (O'Brien et al., 2003b). The prevalence of gestational diabetes is also likely to increase, partly through its association with obesity (Chu et al., 2007) and also because of a redefining of the diagnostic criteria (International Association of Diabetes and Pregnancy Study Group, 2010), the latter alone increasing the prevalence almost 3-fold. Even using historical diagnostic criteria, in a cohort of healthy women aged over 50 years receiving oocyte donation, the risk of gestational diabetes was 20%, increasing to 40% in those over 55 years (Paulson et al., 2002).
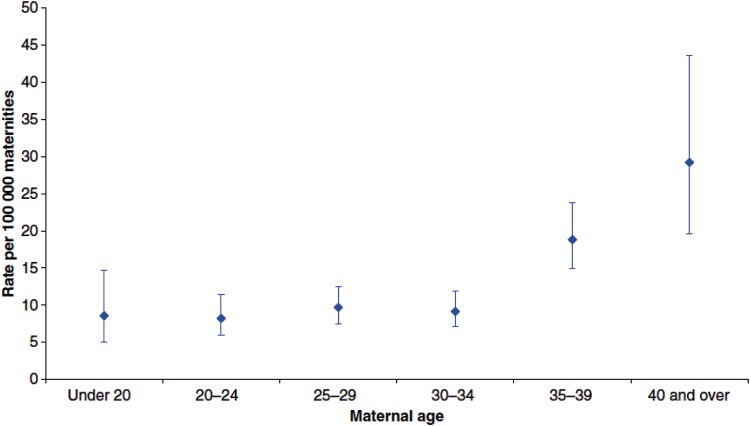
These medical co-morbidities can also all influence fetal health and are likely to compound the effect of age on the risk of pregnancy in an older mother. The extreme consequence of comorbidity or pregnancy-specific disorders is maternal mortality, which at present has continued to decline since systematic records began in 1952. The most recent triennial report from the UK reported 11.39 [95% confidence interval (CI) 10.09–12.86] deaths per 100 000 maternities from indirect and direct causes (Cantwell et al., 2011). Indirect deaths, primarily related to underlying medical or psychiatric causes, have however become more frequent with an incidence of 6.72 (95% CI 5.74–7.87) per 100 000 maternities when compared with direct deaths which had declined to 4.67 (95% CI 3.86–5.64) per 100 000 maternities. The impact of age is also striking, as overall the maternal death rate in the UK in women over 40 years is now three times higher than that in women aged <25 years (Figure 1). The objective of the present review is therefore to provide a discussion of recent findings in the biology and mechanisms underlying ovarian and uterine ageing in women, and their implications for fertility with particular focus on the establishment and decline in the finite pool of oocytes, and novel approaches questioning this concept.
Figure 1.
Maternal mortality rates by age group (years); UK: 2006–2008. Reproduced with permission from Cantwell et al. (2011).
Methods
Multiple strategies were used to identify relevant demographic, epidemiological, clinical and biological studies relevant to the broad topic of female reproductive ageing, without a date limit. We searched in sociological online libraries (IBSS, SocINDEX), PubMed and Google Scholar using the following key words and hierarchical MeSH terms: fertility, infertility, chromosome aberrations, reproduction, pregnancy, pregnancy complications, placenta, labour obstetric, assisted conception, maternal age, ovary, climacteric, stem cell. Additional journal articles were identified from the bibliography of studies included as well as textbooks and hand searches of other source materials including conference proceedings. Articles written in a language other than English without an available English translation were excluded from our review. From this, we identified and focused on key topics (listed in Table of Contents) where it was judged that there had been clinically relevant advances in the understanding of ovarian and uterine ageing with implications for improved diagnostics and novel interventions.
Ovarian ageing
Ovarian ageing, meaning the progressive loss of the primordial follicle pool, is strikingly different to the changes that occur in the other major organs in that the major functions of the ovary cease little more than half way through a woman's lifespan. This leads to a very long post-reproductive period in humans, and while a post-reproductive period of life is shared by a wide range of animals it appears to be only in women that it makes up such a large proportion of the overall lifespan (Hawkes et al., 1998). In humans, ovarian failure at menopause has been causally associated with increased risks for the development of a long list of significant health complications, including osteoporosis, cardiovascular disease, recurrent depression and cognitive dysfunction (Prior, 1998; Buckler, 2005; Frey et al., 2008). In addition, other less physically debilitating problems, such as heat intolerance and hot flushes, also negatively impact on the quality of life in peri- and post-menopausal women (Santoro, 2008). While the term premature ovarian failure has been historically widely used, it is increasingly replaced by ‘primary (or premature) ovarian insufficiency’ (POI; Welt, 2008; Nelson, 2009) to reflect the range of clinical presentations and aetiologies associated with this condition. A full review of this is outwith the scope of this review, and a European Society of Human Reproduction and Embryology guideline on this important subject is currently being developed.
The evolutionary advantage of a long post-menopausal life may be related to the very long period of dependence of the human infant and child on its mother, and indeed is not truly post-reproductive as it benefits the family and offspring. There remains debate as to whether post-menopausal longevity should be seen as an adaptive trait and indeed the longevity of grandmothers may be associated with fewer grandchildren (Madrigal and Melendez-Obando, 2008; Hawkes and Smith, 2009). It also appears that female fertility carries a cost in terms of lifespan, separate from any obstetric risks, which is linked to the ‘disposable soma’ theory, which proposes a trade-off between longevity and investment in reproduction. Genealogical analyses where apparently accurate and complete family data are available, suggest that age at first childbirth was lowest in women who died at a younger age and highest for women who died at the oldest ages. For older women, longevity was negatively correlated with the number of children and positively correlated with the age at first childbirth (Westendorp and Kirkwood, 1998).
Female reproductive potential differs qualitatively from that of men, in that our current understanding holds that the complete complement of primordial follicles is formed during fetal life (i.e. the true ovarian reserve) and that this is then progressively depleted until reproductive senescence, i.e. the menopause. This has been challenged recently, as discussed more fully below, but the concept of a finite pool of follicles remains a cornerstone of current understanding of ovarian function.
Establishment of the primordial follicle pool
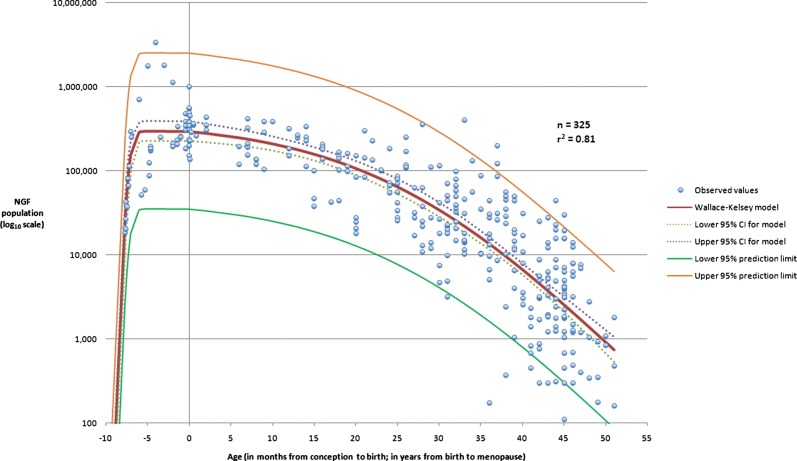
Ovarian development begins with colonization of the genital ridge from Week 5 of gestation onwards, and this is followed shortly afterwards by sex determination (Witschi, 1948). The primordial germ cells continue to proliferate for many weeks but some start to enter meiosis from 11 weeks gestation (Bendsen et al., 2006; Le Bouffant et al., 2010). In the mouse, the onset of meiosis occurs in a synchronized wave along the axis of the gonad from embryonic Day E13 (Bullejos and Koopman, 2004). In contrast, in the human fetal ovary the onset of meiosis occurs over an extended period of time such that when primordial follicles start to occur from ∼18 weeks gestation other germ cells still have a primordial phenotype and are still proliferating (Fulton et al., 2005). The human ovary thus contains a full range of developing germ cells arranged with the less differentiated cells undergoing mitosis around the edge of the ovary, with progressive stages of differentiation entry into meiosis and primordial follicle formation towards the centre of the organ (Anderson et al., 2007). Fetal life is therefore a critical time for establishing female reproductive potential and key factors involved in these processes are increasingly being identified in both mouse and human studies. These include growth factors involved in germ cell proliferation and survival (Coutts et al., 2008; Childs et al., 2010), and a number of germ cell-specific transcription factors that are essential for primordial follicle formation (Bayne et al., 2004; Pangas and Rajkovic, 2006). Meiosis is initiated by retinoic acid in the mouse, acting through its downstream target gene STRA8. The retinoic acid metabolizing enzyme CYP21B protects germ cells in the testes (Griswold et al., 2011). This model has been clearly elucidated in the mouse, and while the key aspects of this mechanism are likely to be conserved in the human there are also some differences, exemplified by the high level of CYP21B expression in the fetal ovary as well as the testis (Le Bouffant et al., 2010; Childs et al., 2011). There are few studies describing human ovarian development in later gestation: it is believed that primordial follicle formation is essentially complete before birth with any remaining oogonia (not within primordial follicles) being removed in the first years of life (Byskov et al., 2011). The limited data available, however, contribute to our understanding of the changes in primordial follicle number across the female lifespan (Wallace and Kelsey, 2010; Figure 2).
Figure 2.
Decline in primordial follicle pool with increasing age. The best model for the establishment of the NGF population after conception, and the subsequent decline until age at menopause as described by an asymmetric double Gaussian cumulative model with parameters = 5.56 (95% CI 5.38–5.74), =25.6 (95% CI 24.9–26.4), =52.7 (95% CI 51.1–54.2), =0.074 (95% CI 0.062–0.085) and =24.5 (95% CI 20.4–28.6). The figure shows the dataset (n = 325), the model, the 95% prediction limits of the model and the 95% CI for the model. The horizontal axis denotes age in months up to birth at age zero, and age in years from birth to 51 years. Reproduced with permission from Wallace and Kelsey (2010).
Fetal life is a period of germ cell vulnerability
Understanding development of the human ovary provides an opportunity for greater understanding of the regulation of adult ovarian lifespan. This is exemplified by the demonstration of expression of the transcription factor FOXL2 in germ cells of the developing human ovary from soon after sex determination (Duffin et al., 2009). Mutations in FOXL2 are a cause of syndromic POI, associated with blepharophimosis/ptosis/epicanthus inversus syndrome (Crisponi et al., 2001). Recently mutations in the homeobox gene NOBOX have been identified in a relatively large (6.2%) proportion of women with POI (Bouilly et al., 2011). NOBOX is one of several genes identified to be expressed specifically in the oocyte and is critical for primordial follicle formation and subsequent activation (Rajkovic et al., 2004). Relationships between these master regulators have been teased out using mouse knockout models (Pangas and Rajkovic, 2006; Matzuk and Lamb, 2008), but the growing body of relevant human data described above confirms that these key factors and their interrelationships are largely conserved.
The processes of ovarian development are potentially susceptible to external as well as internal influences. The influence of maternal smoking on the son's subsequent reproductive function has been recognized for some years (Vine et al., 1994; Ramlau-Hansen et al., 2007), and comparable female data are now emerging. In female fetuses, smoking may have a direct toxic effect on the primordial follicle, leading to premature exhaustion of the follicular germ pool. In animal models histological analysis of ovarian tissue from the exposed offspring mice demonstrates a markedly reduced number of primordial follicles (Vahakangas et al., 1985; Matikainen et al., 2002), with the combination of prepregnancy and lactational exposure to polycyclic hydrocarbons associated with a 70% reduction in primordial follicle number (Jurisicova et al., 2007). In humans maternal smoking has been associated with a reduction in human fetal ovarian germ and somatic cells (Lutterodt et al., 2009; Mamsen et al., 2010). Epidemiological studies examining the impact of maternal smoking on ovarian reserve of the offspring are consistent with a modest but significant decrease in adult fecundability following exposure to cigarette smoke in utero [adjusted fecundability odds ratio 0.96 (95% CI: 0.93, 0.99); Ye et al., 2010].
Exposure to environmental chemicals may also be of relevance. Bisphenol A (BPA) is a ubiquitous chemical found in plastics which can disrupt prophase of meiosis 1 (Susiarjo et al., 2007). The molecular basis for this has been elucidated in the nematode Caenorhabditis elegans showing that BPA disrupts the double-strand break repair processes (Allard and Colaiacovo, 2010). Intriguingly the genes involved in these repair processes are estrogen regulated, and BPA in this model has anti-estrogenic activity resulting in down-regulation of double-strand break repair gene expression in the germ line. Oocytes of the baboon fetal ovary are known to express estrogen receptor beta (Bocca et al., 2008) and expression in human fetal germ cells has recently been confirmed (Fowler et al., 2011). Estrogen depletion treatment using an aromatase inhibitor during primordial follicle formation in the baboon substantially reduced the number of primordial follicles that formed, with estrogen deprivation preventing breakdown of the germ cell nests that precede primordial follicle formation (Pepe et al., 2006). In the bovine fetal ovary estrogen production declines dramatically at follicle formation, and also regulates follicle growth activation (Yang and Fortune, 2008).
Androgen exposure during fetal life may also have a long-term impact on ovarian function. Intrauterine androgen treatment of sheep and non-human primates results in life-long ovarian dysfunction and a metabolic phenotype with considerable similarity to polycystic ovary syndrome (PCOS; Abbott et al., 2005). The relevance of this to the aetiology of PCOS is debated (Franks and Berga, 2012) but these models are of value for the investigation of this condition. Overall, these results suggest that the local steroid environment may be important in both follicle formation and chromatin integrity during fetal life.
In summary, emerging data demonstrate that genetic mutations in key genes and external factors, such as smoking and environmental chemicals, impact on the establishment of the primordial follicle pool during fetal life, with implications for subsequent reproductive function in adult women.
The regulation of initiation of follicle growth
Post-natal ovarian function is characterized by continuous follicle activation with subsequent follicle growth through the pre-antral and antral stages until follicle depletion at the menopause. Thus, the ability to regulate therapeutically this key aspect of ovarian function would have far-reaching consequences. The antral stages are clinically detectable because of their well-established hormonal products (the inhibins and estradiol) and they can also be assessed by ultrasound but our understanding of the early stages of human folliculogenesis is limited. Growth to early antral stages occurs before, as well as after, puberty (Peters et al., 1978) although later antral stages and of course ovulation do not occur until after maturation of the hypothalamo–pituitary axis at puberty with consequent increases in gonadotrophin secretion. The key issue for determination of reproductive lifespan is therefore the regulation of activation of primordial follicle growth. This appears to be a result of a balance between growth activating and inhibitory factors (Figure 3). Both groups of factors include those produced by the oocyte itself and by the surrounding somatic cells. These have been reviewed elsewhere (Zuccotti et al., 2011) but a clear overall understanding of their interaction remains elusive at present. A central pathway, however, is the phosphatidylinositol-3′-kinase (PI3K–AKT) signalling pathway within the oocyte. The phosphatase and tensin homolog deleted on chromosome 10 (PTEN) acts as a negative regulator of this pathway and suppresses initiation of follicle development (Reddy et al., 2008). The transcription factor FOXO3 is a downstream effector of this pathway and acts to inhibit follicle recruitment (Castrillon et al., 2003). An additional component of this pathway (S6K1-RPS6) is dependent on the mammalian target of rapamycin complex 1 (mTORC1), a serine/threonine kinase that regulates cell growth and proliferation in response to growth factors and nutrients and also regulates primordial follicle activation (Reddy et al., 2009). Oocyte PTEN therefore suppresses, whereas mTORC promotes, follicle activation.
Figure 3.
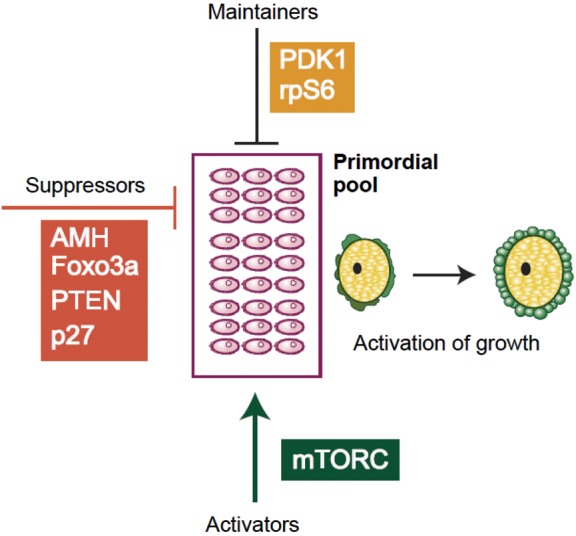
Mechanisms underlying activation of quiescent follicles. A summary of some of the components of the PI3K pathway that have been implicated in maintaining health of the primordial pool (black bar) and either suppressing (orange bar) or activating (green arrow) the initiation of growth. These details have been gathered through knockout mouse models (see Reddy et al., 2010 for review).
The role of the mTOR pathway is intriguing as it is also involved in oogenesis in Drosophila melanogaster. Rapamycin is an inhibitor of the TOR pathway and results in midstage loss of oogenesis in drosophila, similar to the effect of nutrient deprivation (Thomson et al., 2010). Treatment of early human growing follicles with rapamycin also results in oocyte loss (McLaughlin et al., 2011), postulated to result from destruction of the oocyte by adjacent somatic cells, as is believed to be the case in drosophila (Thomson and Johnson, 2010).
Oocyte quality and age
A key aspect of reproductive ageing is the decrease in oocyte quality as well as quantity. The impact of this is most strikingly demonstrated by the restoration of pregnancy rates to those seen in young women when older women use egg donation in IVF (Sauer et al., 1992; Templeton et al., 1996; Nelson and Lawlor, 2011). Successful conception is also more likely to result in miscarriage, the rate of which increases dramatically with increasing age (Nybo Andersen et al., 2000; Schmidt et al., 2012). This is largely associated with chromosomal aneuploidy in the embryo (the incidence of Trisomy 21 at age 25 years is 1 in 1500, and 1 in 16 at age 40 years), but it is likely that other biochemical processes in the oocyte may be relevant. One aspect of this may be changes in mitochondrial function with age (Bentov et al., 2011; Kujjo and Perez, 2011). All mitochondria are maternal in origin, and thus can only arise from mitochondria associated with the primordial germ cell and will remain with that cell throughout oogenesis. Ageing is associated with an increase in mitochondrial aggregation in oocytes and a reduction in cytoplasmic ATP concentrations in metaphase 2 oocytes (Tarin et al., 2001). It has been postulated that inheritance of dysfunctional aged maternal mitochondria could increase the risk of chromosomal abnormalities and indeed a range of metabolic disorders, and may account for the finding that advanced grandmaternal age is associated with an increase risk of trisomy 21 (Aagesen et al., 1984; Bentov et al., 2011).
A recent report has provided a striking demonstration that calorie restriction, known to have a range of beneficial non-reproductive effects in non-human primates (Colman et al., 2009), may result in a reduction in age-associated oocyte aneuploidy (Selesniemi et al., 2011). Adult mice that were maintained with a 40% calorie restriction showed a similar aneuploidy rate in metaphase II oocytes to that in oocytes from younger animals, as well as a restoration of oocyte yield following gonadotrophin simulation to that of younger animals. Intriguingly, the number of non-atretic follicles per ovary was also increased by calorie restriction compared with age-matched animals, and this applied to primordial, primary and pre-antral follicles, although in all cases the number of follicles was lower than in young animals. Calorie restriction prevented spindle and chromosome alignment defects in oocytes, and also prevented age-associated mitochondrial aggregation (Selesniemi et al., 2011). While a 40% reduction is a severe calorie restriction, the results are striking and the mechanisms involved require further elaboration. In the converse situation oocytes of overfed animals also show mitochondrial abnormalities with a reduced oocyte mitochondrial membrane potential (Wu et al., 2010) and increased cumulus and granulosa cell apoptosis. Expression of the endoplasmic reticulum stress gene ATF4 was increased in mice fed on a high-fat diet, and was also increased in human granulosa cells from obese women (Wu et al., 2010). It may therefore be that biochemical changes during oocyte ageing have similarities to those caused by metabolic abnormalities, and the importance of the mTOR nutrient sensing pathway in the follicle has been mentioned above. This indicates that the adverse effects of ageing and of metabolic disturbance, for example as caused by obesity [itself associated with increased risk of miscarriage (Boots and Stephenson, 2011)], will be particularly detrimental in combination, with clear clinical implications. Additionally, the effects of maternal peri-conceptual nutrition on the subsequent health of the offspring are increasingly recognized (Fleming et al., 2012) but outwith the scope of this review.
Genetic and lifestyle determinants of reproductive ageing
Unlike the marked secular trends in age of menarche, the age of menopause is very similar both geographically and over time. This implies a strong genetic component (estimated as contributing to >50% of interindividual variability), which is reinforced by the association between ages of menopause of women and their daughters as well as in twin studies (Torgerson et al., 1997b; Sneider et al., 1998; de Bruin et al., 2001; van Asselt et al., 2004; Murabito et al., 2005). Recently large-scale genome wide association (GWA) studies have identified novel genetic loci associated with age at menopause (He et al., 2009; Stolk et al., 2009). Single nucleotide polymorphism analysis of 278 genes in 24 000 women from the Nurses Health Study has confirmed some of these associations, highlighting steroid hormone metabolism and biosynthesis pathways, but also identifying the importance of a group of genes associated with POI (including the auto-immune regulator AIRE, FMRI, FOXL2 and the BMP antagonist NOG) with age at natural menopause (He et al., 2010).
The expression of many of these genes identified in GWA studies during fetal ovarian development highlights the importance of that period in determining reproductive lifespan and the value of understanding the mechanisms involved in the action of single-gene defects causing POI. A substantial number of these have been identified, in themselves only being identified in very few women with POI (Matzuk and Lamb, 2008). However, the above candidate gene association study (He et al., 2010) demonstrates the importance of variations in these genes as contributors to the normal as opposed to pathologically early menopause. One example of a gene whose variation can cause a range of ovarian phenotypes, from POI to milder ovarian dysfunction, is the FMR1 gene, mutations in which cause fragile X syndrome. This is the most common cause of mental retardation in boys and is caused by an expansion of the number of CGG repeats to more than 200 in the 5′ untranslated region of the gene. Premutations (i.e. between 55 and 200 repeats) can also cause POI and the number of repeats can expand over generations. There is debate as to the significance of smaller repeat numbers (Bennett et al., 2010). A recent analysis has suggested that women with a reduced ovarian reserve show a shift towards an increased number of CGG repeats in FMR1 compared with women with a normal ovarian reserve, potentially indicating subtle effects on ovarian function across the spectrum of genetic variation (Karimov et al., 2011). The relatively high prevalence of mutations in the NOBOX gene in women with POI has been mentioned above; it is likely that data will emerge identifying genetic variations in a much larger proportion of women with POI than is currently recognized (Murray et al., 2011) with light consequently shed on the regulation of follicle formation, activation and early, gonadotrophin-independent, follicle growth.
Lifestyle and environmental factors by comparison appear to have only limited effects on age of menopause. A comprehensive review of a very large number of postulated factors calculated that the total contribution to age of menopause may be as low as 3% (Kok et al., 2005). A recent meta-analysis suggests that smoking is associated with a decreased age of menopause of 0.90 years (95% CI 1.58–0.21; Sun et al., 2011). In contrast, alcohol consumption is associated with a later age of menopause (Torgerson et al., 1997a) and it is possible that this impacts on the relationship between ovarian function and cardiovascular risk. The polycyclic aromatic hydrocarbon 7, 12-dimethyl benz[a]anthracene (DMBA) is an environmental carcinogen found in cigarette smoke as well as other products of combustion. Treatment of mouse ovaries with DMBA-induced widespread primordial follicle activation and pre-antral follicle atresia both in vitro and in vivo (Sobinoff et al., 2011). Widespread follicular activation also results in premature ovarian failure in other mouse models, for example the anti-Müllerian hormone (AMH), PTEN and FOXO3a knockout models (Durlinger et al., 1999; Castrillon et al., 2003; Reddy et al., 2008). Increased activation of primordial follicles has also been postulated to contribute to the POI resulting from chemotherapy owing to loss of inhibition from larger sizes of growing follicles, themselves directly targeted by chemotherapy (Meirow et al., 2010). Biochemical analysis of the effects of DMBA reveals increased AKT1 phosphorylation, mTOR activation and decreased FOXO3a expression (Sobinoff et al., 2011). These results indicate that exposure to this environmental toxin may contribute to ovarian ageing through increased follicular activation acting through pathways that physiologically regulate this process, as described above (Figure 3). As well as providing a potential mechanism for the effect of cigarette smoking on advancing the age at menopause, it is possible that this result is relevant to targeting these pathways therapeutically, either positively or negatively.
Assessment of ovarian reserve and changing follicle dynamics from birth to the menopause
Our understanding of the ovarian reserve and early follicle dynamics has been hindered by the absence of a good biomarker. Estradiol and inhibin A are largely produced by the dominant and pre-ovulatory follicle and thus largely reflect ovulatory activity. Inhibin B is produced by smaller ovarian follicles and is thus a step closer towards being a useful marker of early follicular activity but its serum concentration still shows substantial changes across the menstrual cycle (Illingworth et al., 1996), reflecting its association with gonadotrophin-dependent follicular activity as a key regulator of FSH secretion. It is, however, becoming increasingly clear that AMH is a useful although imperfect marker of pre- and early antral follicle growth. AMH is produced by the granulosa cells of follicles from the time at which follicle growth is first initiated. AMH expression continues until follicles reach ∼8 mm diameter and expression is very low in larger antral follicles (Weenen et al., 2004; Andersen et al., 2010). The relative contribution of the different follicle sizes to AMH concentrations in serum is incompletely described, as this will reflect the balance between the large number of small follicles and the smaller number of larger follicles and probably also the increasing blood supply to follicles as they grow. Early studies demonstrated that AMH concentrations fall with age in women (de Vet et al., 2002) and led to rapid development of the concept that AMH may be useful to predict reproductive lifespan and time to menopause (van Rooij et al., 2002). AMH concentrations are relatively, although not completely, stable during the menstrual cycle (La Marca et al., 2004; van Disseldorp et al., 2010), substantially increasing its utility as a marker as well as also reflecting its origin in smaller follicles. Histological data have confirmed that in humans as well as animal species the number of small growing follicles is related to the number of non-growing follicles (NGFs; Gougeon et al., 1994), supporting the interpretation that AMH concentrations may in fact reflect the primordial follicle pool size; indeed this has been directly demonstrated in both mice and humans (Kevenaar et al., 2006; Hansen et al., 2011). In the Hansen et al. (2011) study AMH concentrations showed a significant correlation with the histologically determined primordial follicle pool size. AMH concentrations are not completely gonadotrophin-independent and indeed are suppressed during prolonged pharmacological gonadotrophin suppression (Anderson et al., 2006) and in pregnancy (Nelson et al., 2010) but it appears that there is a physiologically valid and clinically useful relationship between serum AMH concentrations and the ovarian reserve, both ‘true’ (the number of NGFs) and ‘functional’ (the number of potentially recruitable small growing follicles).
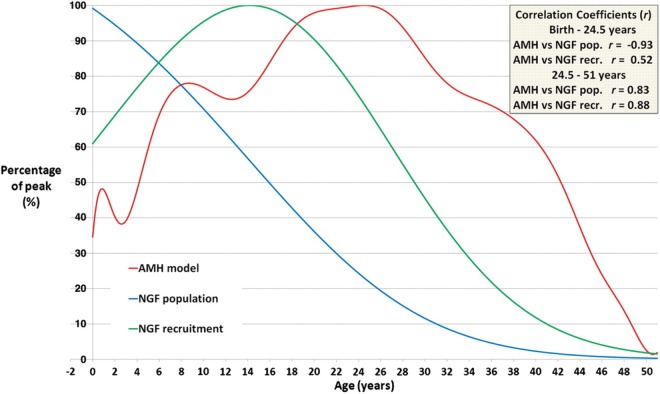
Data from very large cohorts of infertile women have now defined the decline in AMH during the second half of the reproductive lifespan (Nelson et al., 2011b, c; Seifer et al., 2011), with more limited data for childhood and adolescence (Hagen et al., 2010, 2012). A recent analysis has compiled AMH concentrations throughout life from conception to the menopause in healthy girls and women (Figure 4; Kelsey et al., 2011b). While these data are cross–sectional, this analysis reveals a number of important features which illustrate important and previously incompletely or unidentified aspects of human ovarian function. Specifically, AMH shows a clear rise in the first months of life, supporting the existence of a neonatal minipuberty in girls, similar to that well recognized in boys. This has recently been confirmed directly (Kuiri-Hanninen et al., 2011). AMH concentrations then progressively rise through childhood in girls reaching a peak in the mid-20s. This rise appears to start before puberty and continue beyond with, intriguingly, an inflection during adolescence, thus there appears not to be a clear increase at the time of other endocrinological manifestations of puberty. A decrease in AMH at early puberty has recently been demonstrated directly in a longitudinal study (Hagen et al., 2012). These data suggest increasing follicular activity from late childhood right through to some 10 years following menarche, and indicate the existence of a prolonged period of ovarian maturation.
Figure 4.
Comparison of serum AMH concentrations with NGF population and with NGF recruitment. The red line is the log-unadjusted validated AMH model (Kelsey et al., 2012), peaking at 24.5 years. The blue line denotes the decline in NGF population (Wallace and Kelsey, 2010), with peak population at 18–22 weeks gestation. The green line denotes the numbers of NGFs recruited towards maturation population (Wallace and Kelsey, 2010), with peak numbers lost at age 14.2 years on average. Each quantity has been normalized so that the peak occurs at 100%. Correlation coefficients (r) are given for AMH concentrations against the other two curves for birth to 24.5 years and for 24.5–51 years. Reproduced with permission from Kelsey et al. (2012).
Our knowledge of human ovarian function and dynamics during puberty and adolescence is very limited owing to the lack of availability of relevant histological material and the inability of previous endocrinological analyses to reflect early follicle growth. Relating these changes in AMH to the number of NGFs in the ovary across life (Wallace and Kelsey, 2010) and data derived from that analysis giving the rate of loss of follicles from the non-growing pool illustrate that the rise in AMH during adolescence occurs in the face of a decline in NGF number but increasing NGF recruitment which peaks at an average age of 14 years (Kelsey et al., 2011a). Following this peak and the peak in AMH at age 24 both NGF recruitment and AMH fall in parallel (Figure 4). Disturbances in this relationship may occur in pathological states. Thus, for example girls with type 1 diabetes have higher AMH concentrations in childhood (Codner et al., 2011), which may reflect a larger primordial follicle pool but perhaps is more likely to reflect an increased number of small growing follicles. Conversely, in adult life AMH is reduced in women with type 1 diabetes (Soto et al., 2009), suggesting that there is already depletion of the primordial pool, which would be consistent with their observed increased risk of early age of menopause (Dorman et al., 2001). These data also illustrate the wide range in AMH concentrations between individual women at any given age. This is perhaps only to be expected given the similarly wide variation in the primordial follicle pool (Faddy et al., 1992; Wallace and Kelsey, 2010) and indeed age of menopause. While menopause occurs on average at age 51 years, it varies from 40 to 60 years, i.e. the reproductive lifespan of some women is 50% longer than others and, recognizing that fertility declines a decade before the menopause (Broekmans et al., 2009), the duration of fertile life in some women will be twice that in others. The ability to identify this in young women through measurement of AMH or other as yet unidentified biomarkers is unsubstantiated at present but is likely to have significant social and medical consequences (Baird and Steiner, 2012).
Many of the above studies have also used measurements of antral follicle count (AFC), with largely similar results to measurement of AMH. The relative merits and demerits of AMH and AFC assessment have been widely debated (Jayaprakasan et al., 2010; Nelson et al., 2011a; Rosen et al., 2012) and, as with AMH, large cross-sectional studies of the decline in AFC with age are now available (Almog et al., 2011; La Marca et al., 2011). Accurate interpretation of the ovarian reserve at any given age is therefore feasible and has been widely utilized for response prediction in ART (Broer et al., 2011a). We anticipate that by combining biomarkers, including age, the most accurate estimate of the underlying primordial follicle number will be achieved.
Predicting the reproductive lifespan
The later stages of ovarian ageing are characterized by increasing menstrual cycle variability followed by an increasing likelihood of missed cycles and ultimately amenorrhoea. The menopausal transition has been extensively reviewed clinically and endocrinologically with a standardized classification proposed as the ‘stages of reproductive ageing’ (STRAW; Soules et al., 2001). This classification includes five stages prior to the final menstrual period and two stages thereafter, accompanied by a progressive decrease in primordial follicle number (Hansen et al., 2012). Although initially based on menstrual characteristics and follicular phase FSH concentration, the most recent revision incorporates AMH, inhibin B and AFC as additional supportive criteria (Harlow et al., 2012). These studies all highlight the monotropic rise in FSH with ovarian ageing, a phenomenon first recognized in the mid-1970s. The rise in FSH largely reflects a decrease in inhibin B production by small antral follicles in the early follicular phase. A very large body of work has characterized these endocrinological changes in later reproductive life (Hale and Burger, 2009) but the ability of FSH and inhibin B to accurately determine the early stages is limited. The potential value of AMH as a marker of female reproductive ageing is likely to increase, particularly with the development of an international standard and harmonization of the assays (Nelson and La Marca, 2011).
Data are now becoming available from prospective studies on the use of AMH as a predictor of the menopause. In a study of 147 women aged over 40 years over a period of 6 years, initial AMH showed good prediction of not reaching the menopause (Tehrani et al., 2009). This supported previous studies (van Rooij et al., 2004) with the van Rooij study suggesting that inhibin B improved the predictive value of AMH. Comparable results were obtained in a study of 50 women assessed annually over 6 years with final menstrual period determined in all of them (Sowers et al., 2008). Baseline AMH was associated with time to, and age at, the final menstrual period, as was the rate of decline of inhibin B, which was less predictive. It is likely that improvements in the sensitivity of currently available AMH assays may improve the accuracy of AMH assessment in the years preceding the menopause, as currently AMH becomes undetectable ∼5 years before the final menstrual period (Sowers et al., 2008). A more recent study analysed AMH changes over a more prolonged period of 11 years in younger women (aged 21–46 years) and found that age-adjusted AMH was highly predictive for the timing of the menopause (Broer et al., 2011b). However, only 48 women had reached the menopause at the time of the second analysis. A larger study has now clearly confirmed that AMH is predictive of time to the menopause, although with wide CIs (Freeman et al., 2012). Importantly, the Freeman study also demonstrates that age and AMH are independent predictors. This indicates that there are mechanisms of ageing (presumably related to the rate of follicle activation) that are independent of the growing follicle pool, in addition to the links between them, clearly demonstrated in detailed histologically based studies (Gougeon et al., 1994).
The clinical implications of women being able to determine their reproductive lifespan by a blood test available to all health care professionals are potentially substantial. Certainly if women choose to time their family based on their ovarian reserve this would redefine the meaning of family planning.
Avoiding the age-related decline in oocyte quality
Given the inexorable decline in female fertility with age, what steps can be taken to mitigate against it? In essence, two very different therapeutic approaches have been promoted to address this. The first involves assessment of embryo quality in IVF cycles of older women to screen for aneuploidy, the second to try to preserve oocytes at a young age. Both of these approaches aim to circumvent rather than prevent the problem, in the absence of, for example, pharmacological ways of influencing chromosome segregation during meiosis. Alternative approaches, such as the administration of growth hormone and dehydroepiandrosterone, have been proposed in the context of IVF to increase the number of oocytes obtained, especially in older women and ‘poor responders’. Evidence as to their efficacy is unclear (Duffy et al., 2010; Sunkara et al., 2012), and this approach is not of relevance to reproductive ageing in general.
Screening for aneuploidy involves the biopsy of one or more blastomeres, with karyotypic analysis historically performed by fluorescence in situ hybridization (FISH). Unfortunately RCTs of this approach showed no evidence of a benefit in live birth rates (Staessen et al., 2004; Mastenbroek et al., 2007, 2011). Some of the technical aspects of those studies received adverse comment but subsequent RCTs gave similar results and the technology has largely moved on with increasing use of comparative genomic hybridization (CGH) analysis (Harper and Sengupta, 2011). Data using array CGH have recently supported the suggestion that most human aneuploidy arises from premature chromatid separation rather than non-dysjunction (Gabriel et al., 2011), a model first proposed as a key component of human reproductive ageing 20 years ago (Angell, 1991, 1994). Greater understanding of the biology of the cohesins that hold chromatids together is shedding light on this key aspect of oocyte ageing (Lister et al., 2010).
Despite previous adverse experiences of testing embryos at the cleavage stage, testing of blastomeres and polar bodies has been developed in an attempt to reduce the impact of mosaicism (Delhanty et al., 1993; van Echten-Arends et al., 2011). Analysis of the first polar body only allows partial identification of the maternal contribution, with at least as many errors arising during meiosis II as in meiosis I (Fragouli et al., 2011). Failure to analyse the second polar body could lead to the non-detection of 50% of oocyte-derived chromosomal errors. Furthermore, polar body biopsy will not identify post-zygotic errors. Consequently when combined with the issue of mosaicism, the inherent option of being able to sample more tissue at blastocyst biopsy (as many as 5–10 cells) would appear to make this a more logical approach.
From a practical perspective, a blastocyst-based strategy requires sufficient high-quality embryos to reach the blastocyst stage in the first place, thereby limiting the applicability to older women with an already reduced ovarian reserve. Furthermore, vitrification of the biopsied blastocysts while awaiting the report of diagnostic testing is required, however, alternative faster non-array-based diagnostic approaches are being developed (Treff et al., 2012). Given the lessons learned from results of RCTs on the initial use of FISH in older women, RCTs using array CGH and alternative technologies are eagerly awaited, with initial pilot studies confirming the feasibility of this approach (Geraedts et al., 2011; Magli et al., 2011).
Freezing of oocytes to avoid the decline in fertility related to age alone is essentially a social rather than a medical application. This is in contrast to women with a disease-related low ovarian reserve, for example secondary to chemotherapy. The increasing use of oocyte vitrification has advantages, with improved post-thaw viability, fertilization and clinical pregnancy rates when compared with slow freezing (Smith et al., 2010; Cobo and Diaz, 2011). It is likely that the number of women seeking oocyte storage will increase, particularly if early identification of women at risk of POI or reduced fecundability by screening with AMH is feasible (Steiner et al., 2011; Baird and Steiner, 2012). However, the financial costs and limited success rate likely from the restricted number of oocytes obtained from a single stimulation cycle mean that this approach will appeal and be available to only very few.
Another potential target to avoid age-related decline in fertility is the primordial pool of follicles. Given that the bulk of the human ovarian follicle reserve is made up of primordial follicles, this population is an ideal choice for in vitro growth to obtain fertilizable oocytes for potential use in ART and fertility preservation programmes (Telfer et al., 2008; Telfer and McLaughlin, 2011). The capacity of immature mammalian oocytes to develop fully in vitro has already been demonstrated in rodents with the birth of pups from in vitro grown murine primordial follicles (Eppig and O'Brien, 1996; O'Brien et al., 2003a), however, this has yet to be successfully repeated using immature human follicles. While the definition of a complete in vitro system has yet to be achieved, a great deal of basic scientific progress has been made using systems designed to support the partial growth of human follicles, with several developmental milestones accomplished, including follicle activation, follicle differentiation and oocyte maturation using fresh and cryopreserved human tissue (Smitz et al., 2010; Telfer and McLaughlin, 2011). An alternative strategy would be to regulate the rate of initiation of follicle growth, and thus the preservation of the primordial pool in vivo. This will inevitably require a much greater understanding of the molecular mechanisms underlying follicular activation (Figure 3) but is potentially a target for pharmacological intervention.
The effects of uterine ageing
In addition to the changes discussed above in oocyte quality, the ability to achieve and maintain a pregnancy will also be dependent on the uterus. This is starkly exemplified by the association of increasing maternal age and stillbirth (Huang et al., 2008; Flenady et al., 2011). This clear association remains even after controlling for co-morbidities and potential confounders, including fetal chromosomal abnormalities, multiple pregnancy, obesity, pre-eclampsia, insulin-dependent diabetes and multiple pregnancy (Fretts et al., 1995). Consequently the risk of stillbirths in mothers over the age of 40 years is approximately twice as high as that of younger mothers, however, the absolute risk would be <10 per 1000 births in most industrialized countries.
The biology underlying this association is largely undefined but potentially relates to impaired decidual and placental development and embryo interaction with the uterus. By 12 months of age, mice demonstrate a 15% reduction in the number of implantation sites during early pregnancy and a 50% reduction in fertility at term (Holinka et al., 1979). Even when blastocysts were transferred from young donors to older hosts there was greater implantation failure (Talbert and Krohn, 1966; Gosden, 1979). Older mice show an impairment of artificially induced decidual response, which in young rodents closely resembles the normal decidual response during implantation (Shapiro and Talbert, 1974; Holinka and Finch, 1977). Although this impaired decidual response may be in part be related to reduced progesterone secretion in older mice (Holinka et al., 1979), a uterine contribution is also likely. Even when progesterone is provided exogenously, the wet weight of the uterus and uterine glycogen, alkaline phosphatase and DNA content are all ∼25% lower in older mice (Holinka and Finch, 1977; Finch and Holinka, 1982). Microscopic changes have also been demonstrated in response to various experimental conditions, with unilateral hyperaemia inducing vascular development and growth of the myometrium and stroma in young hamsters (3–5 months) but not older animals (13–15 months) (Sorger and Soderwall, 1981). In response to deciduogenic stimuli, older rats exhibit more lysosomes and fewer luminal microvilli in the epithelial cells (Craig, 1981). It would appear that decidual development is partly impaired by prolonged exposure to hormonal stimulation, as in aged mice those that had undergone ovariectomy at 2 months had a greater decidual response compared with controls, albeit that the controls did not have sham operations (Goodrick and Nelson, 1989). A purely age effect is, however, still present as although young (4 months) and aged (10 months) ovariectomized mice, demonstrated equivalent uterine growth in response to estrogen and progesterone, the decidual response to a standardized stimulus was reduced in the older animals (Holinka and Finch, 1977). In rodents endometrial estrogen and progesterone receptor expression have also been observed to decline with age, with concomitant increases in collagen and fibrosis (Blaha and Leavitt, 1974; Ohta, 1987).
With respect to placental development in older mothers, many studies fail to control for associated maternal characteristics. It does, however, appear that maternal age is positively associated with placental weight, even after adjusting for birthweight, parity, smoking, pre-eclampsia and diabetes (Haavaldsen et al., 2011). Whether this reflects a biological compensatory mechanism for placental dysfunction is unclear. In the small mechanistic studies which have analysed indices of placental structure, healthy older women who had a normal pregnancy outcome exhibited reduced levels of apoptosis and increased levels of trophoblast proliferation compared with younger women (Rahima and Bruce, 1987; Yamada et al., 2001). In rats, placental weight was also increased by 40–70% in the older animals (9–12 months) when compared with the young rats (3–5 months). There was no difference in birthweight, suggesting placental adaptation, with compensatory hypertrophy of the placenta required to maintain fetal growth in the face of less favourable maternal physiology. Collectively these studies suggest that decidualization and placentation may be adversely affected by maternal age, however, given that dysfunctional implantation and placental development underlies almost all adverse obstetric outcomes, high-quality translational studies harnessing the power of appropriate animal models and human basic science and clinical studies on the effect of maternal age are required.
The donor oocyte model provides an invaluable paradigm to assess the role of age-related changes in the uterus on reproductive potential. Case reports of successful pregnancy and subsequent delivery in a 70-year-old recipient of donated oocytes anecdotally demonstrate that the uterus can support pregnancy far beyond the age at which it is normally required to do so. Initial studies from donor oocyte programmes revealed similar rates of implantation, pregnancy, miscarriage and delivery rates among donor oocyte recipients of different ages (Serhal and Craft, 1989; Navot et al., 1991; Sauer et al., 1992). However, it has subsequently become clear that oocyte recipients have an almost 2-fold increased risk of the major perinatal complications of preterm birth and low-birthweight when compared with women using their own oocytes after adjusting for other maternal confounders (Nelson and Lawlor, 2011), in keeping with the above-described impaired decidual and placental development.
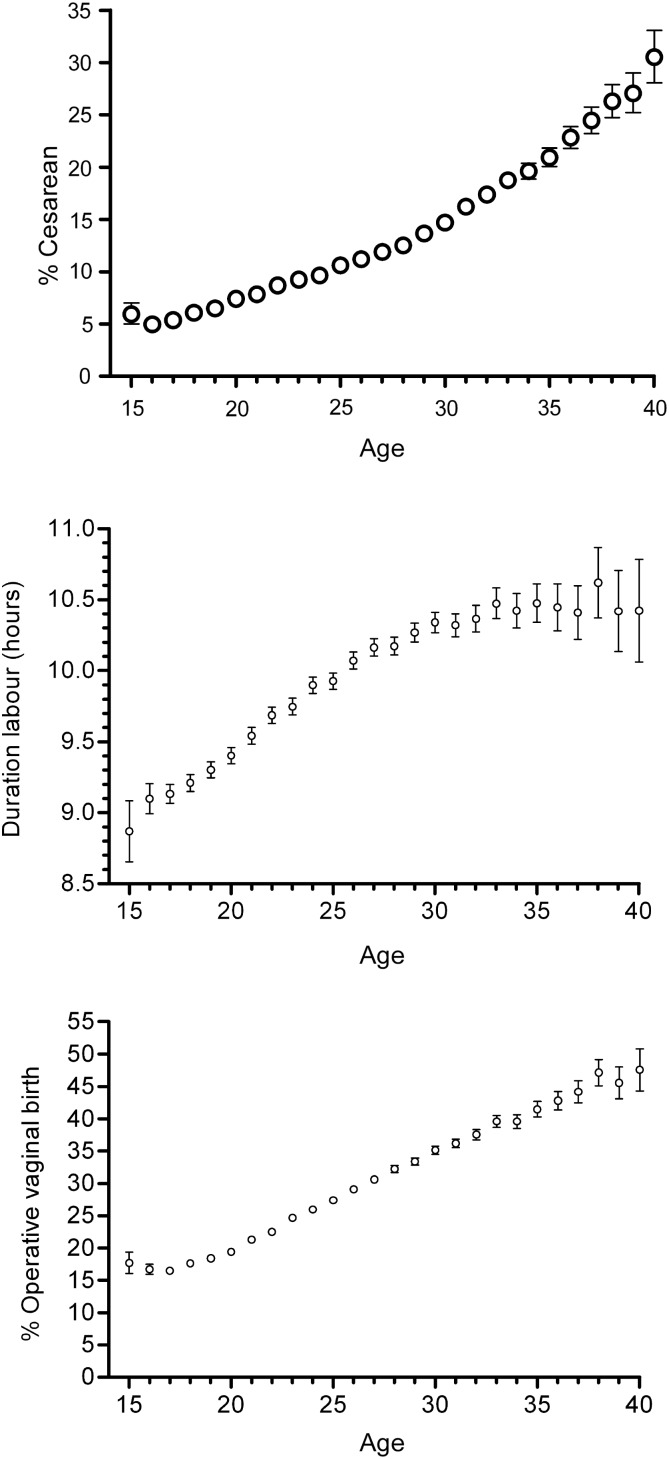
The older mother is also more likely to experience intrapartum complications, with a linear relationship between maternal age and the length of labour, the risk of emergency Caesarean delivery and operative delivery (Figure 5; Smith et al., 2008), suggesting that myometrial function is impaired by advanced maternal age. With increasing maternal age there appears to be failure of autophagy within the myometrium, with older women having an increased number of cytoplasmic lipofuscin inclusions in the myometrial smooth muscle cells, and ultrastructural changes including dissociation of myofilaments, mitochondrial destruction and abnormal endoplasmic reticulum structure (Gosden et al., 1978; Drampian et al., 1983). Myometrial functional assessment has demonstrated a negative association of maternal age and spontaneous activity, and also an increased risk of multiphasic contractions which are associated with dysfunctional labour in vivo (Smith et al., 2008). The basis for these structural and functional changes in the myometrium is unclear but, intriguingly, later menarche is protective and has been shown to be associated with a reduced risk of operative delivery (Smith, 2009).
Figure 5.
Maternal age and the risk of Caesarean delivery, duration of spontaneous labour and operative vaginal delivery. Top panel: proportion of women delivered by emergency intrapartum Caesarean section in relation to the age (years) of the mother (n = 583 847). The bars are binomial 95% CIs. Middle panel: mean duration of spontaneous labour in relation to maternal age (n = 409,703). The bars are 95% CIs of the mean. Bottom panel: proportion of nulliparous women who required operative vaginal delivery in relation to maternal age among the 518 787 women delivered by a means other than emergency Caesarean section. The bars are binomial 95% CIs. Reproduced with permission from Smith et al. (2008).
These observations raise the possibility that it is the repeated exposure of myometrium and endometrium to the rise and fall of sex steroids across the menstrual cycle that is detrimental (Smith et al., 2008). Historically, prior to the advent of contraception and alternatives to breastfeeding, the number of menstrual cycles would have been substantially less. Women would have conceived in adolescence, then breastfed exclusively and conceived again shortly after stopping breastfeeding, with a minimum number of menstrual cycles. In contrast, assuming menarche at 13 years, age at first birth at 29 years (the current mean age in UK) and a regular menstrual cycle, the uterus will now be exposed to 192 cycles of estrogen and progesterone prior to that first birth. With the use of the combined oral contraceptive pill, the pattern would differ but the repeated number of exposures would be similar. Average steroid exposure would perhaps even be higher, given the pharmacological doses necessary for contraceptive efficacy. We would therefore suggest the hypothesis that repeated and prolonged exposure to sex steroids induces both endometrial and myometrial uterine damage and partly underlies the age-related association with adverse outcomes.
Repeated exposures to hormonal fluctuations are known to increase the risk of disease in other hormone-sensitive tissues, such as the breast. Breast cancer risk is associated with early age of menarche, earlier development of regular ovulatory menstrual cycles, increased age of first birth, nulliparity or fewer children, not breast feeding, late age at menopause and exposure to combined hormone-replacement treatment (reviewed in Parsa and Parsa, 2009). Women with reduced exposure to menstrual cycle fluctuations, as seen with use of progestogen contraceptives, might therefore be expected to have a reduced risk of age-related pregnancy complications, when compared with women not using hormonal contraception or exposed to the combined oral contraceptive pill.
The future: making eggs
The dogma, as outlined above, that female mammals are born with all of the oocytes they will ever possess is based on a paper from Sir Solomon Zuckerman published in 1951 (Zuckerman, 1951). Zuckerman failed to find any experimental evidence that was inconsistent with an earlier hypothesis (Waldeyer, 1870) that germ cell production in female mammals ceases prior to birth [reviewed by (Zuckerman, 1971)]. This influential paper by Zuckerman formed the cornerstone of our understanding and subsequent interpretation of experimental and clinical observations relating to ovarian development, function and failure for the next 50 years (Tilly and Telfer, 2009). The consequences of this are extremely significant, not only in the context of a loss of fertile potential but also in the broader picture of the diverse spectrum of age-related health problems that emerge in post-menopausal women linked to failure of their ovaries (Prior, 1998; Buckler, 2005). If it were possible to repopulate adult ovaries with new oocytes and follicles, the female biological clock would no longer be an unreachable target to consider for clinical intervention.
In recent years there have been some exciting and controversial developments in female reproductive biology relating to a body of evidence that ovarian follicles may be formed during adult life by a rare population of putative germline stem cells (Johnson et al., 2004; Tilly and Telfer, 2009). Publications in support of oocyte renewal during adulthood have ranged from morphometry-based studies highlighting the mathematical improbability of a non-renewable oocyte pool being established at birth in rodents (Johnson et al., 2004; Kerr et al., 2006) to studies suggesting that oocyte regeneration can occur following a pathological insult that initially depletes the resting follicle pool (Johnson et al., 2005a; Borovskaya et al., 2006). These studies reopened the debate on neo-oogenesis resulting in the publication of many critiques (Telfer et al., 2005; Johnson et al., 2005b). However, the isolation and identification of oocyte-producing germline stem cells, also called oogonial stem cells (OSC), as unequivocal proof of their existence in ovaries of adult mammals in general, and humans in particular, remained elusive. A breakthrough in identifying such a cell was made in 2009 when putative germ line stem cells were isolated from adult mouse ovaries (Zou et al., 2009) and a more recent study (White et al., 2012) has now shown that a rare population of germ line stem cells can be extracted from adult human ovaries. This new study provides compelling evidence for the existence of a population of cells within adult human and mouse ovaries that can be multiplied in vitro and are capable of forming oocyte-like structures based upon morphology and expression of oocyte-specific biomarkers (e.g. DDX4, KIT and LHX8; White et al., 2012). Most importantly, following a period of differentiation these cells from mouse ovaries were capable of being fertilized and forming embryos (White et al., 2012). Unfortunately because of regulatory restrictions, fertilization of these cells from human ovaries has not yet been attempted.
This new work represents a major breakthrough by identifying cells with apparent germ line potential in both mouse and human ovary. However, many challenges lie ahead and many questions require answers before a practical and convincing demonstration of developmental potential of the oocyte-like structures derived from human cells is obtained. Clearly these ‘oocyte-like’ cells derived from the progenitor population in vitro require the somatic cell support of paracrine and junctional communication to form follicles and develop into functional oocytes. Combining these ‘oocyte-like’ cells with more conventional human ovarian culture models (Telfer et al., 2008) may facilitate follicle formation and growth, and enable detailed analysis and testing of any resulting oocytes to be carried out (Rodrigues et al., 2008; Telfer and Albertini, 2012). If these oocytes proved to be normal, this would indeed widen options for fertility preservation and treatment of ovarian ageing.
As exciting as these new findings are, caution needs to be exercised in evaluating the immediate significance of the work to our understanding of the in vivo biology of ovarian function, as well as the ultimate relevance of this work to reproductive health in women. The successful purification and characterization of what appear to be bona fide OSCs from adult ovarian tissue in mice and humans, while important, does not immediately equate to proof that these cells serve a contributory role in determining the size of the post-natal follicle pool or the timing of ovarian failure under normal physiological conditions. Even if activity of the OSCs in vivo can be demonstrated and it is accepted they function to sustain the adult follicle pool by partially offsetting the high rate of follicle loss through atresia, it remains a fact that natural menopause happens approximately halfway or so through a woman's chronological lifespan. Nonetheless, these cells will remain an important target for future clinical applications. Studies such as these move us closer towards the application of stem cell-based regenerative medicine and the possibility that this may 1 day become a safe and effective strategy to control the timing of age-related ovarian failure and menopause when it might be clinically desirable to do so.
Conclusion
Ageing has incontrovertible effects on female reproductive function. This is most obvious for the ovary, and our understanding of the mechanisms by which oocyte quantity and quality decline with age is improving. It is also becoming clear that other reproductive organs, including the uterus, are detrimentally affected by age, and this may underlie the increased prevalence of late pregnancy complications in older women. These changes are in addition to age-related co-morbidities, which together may have synergistic effects on reducing the probability of a successful pregnancy outcome. At present, given the detrimental impact of ageing on reproductive outcomes, increasing public awareness and societal support for having a family at an earlier age are required. Although our understanding of ovarian ageing has improved dramatically and, in particular, the potential early identification of individuals at greatest risk may allow us to redefine the concept of family planning, therapeutic interventions at present remain limited. The potential existence of stem cells capable of being used to restore the primordial follicle, and thereby oocyte pool, raises the intriguing possibility of novel in vivo and in vitro strategies to reduce the inexorable decline in female reproductive potential.
Authors' roles
S.M.N., E.T. and R.A.A. all contributed substantially to the design of the review. The literature review was repeated by each author, with the data for their section used. S.M.N. and R.A.A. amalgamated the sections, with all authors editing and approving the final draft.
Funding
No external funding was either sought or obtained for this work, but the author's work in this field is supported by the Wellcome Trust WT092830/Z/10/Z (S.M.N.), UKRC G1001357 (S.M.N.) and the MRC G1100357 (R.A.A.) and G0901839 (E..E.T. and R.A.A.).
Conflict of interest
None declared.
References
- Aagesen L, Grinsted J, Mikkelsen M. Advanced grandmaternal age on the mother's side—a risk of giving rise to trisomy 21. Ann Hum Genet. 1984;48:297–301. doi: 10.1111/j.1469-1809.1984.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- Aldous MB, Edmonson MB. Maternal age at first childbirth and risk of low birth weight and preterm delivery in Washington State. J Am Med Assoc. 1993;270:2574–2577. [PubMed] [Google Scholar]
- Allard P, Colaiacovo MP. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc Natl Acad Sci USA. 2010;107:20405–20410. doi: 10.1073/pnas.1010386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog B, Shehata F, Shalom-Paz E, Tan SL, Tulandi T. Age-related normogram for antral follicle count: McGill reference guide. Fertil Steril. 2011;95:663–666. doi: 10.1016/j.fertnstert.2010.08.047. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–1287. doi: 10.1093/humrep/deq019. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Themmen APN, Al Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Human Reprod. 2006;21:2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PTK. Conserved and divergent patterns of gene expression in female and male germ cells during development of the human fetal gonad. BMC Dev Biol. 2007;7:136–145. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell RR. Predivision in human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum Genet. 1991;86:383–387. doi: 10.1007/BF00201839. [DOI] [PubMed] [Google Scholar]
- Angell RR. Aneuploidy in older women. Higher rates of aneuploidy in oocytes from older women. Hum Reprod. 1994;9:1199–1200. doi: 10.1093/oxfordjournals.humrep.a138675. [DOI] [PubMed] [Google Scholar]
- Baird DD, Steiner AZ. Anti-Müllerian hormone: a potential new tool in epidemiologic studies of female fecundability. Am J Epidemiol. 2012;175:245–249. doi: 10.1093/aje/kwr439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne RA, Martins Da Silva SJ, Anderson RA. Increased expression of the FIGLA transcription factor is associated with primordial follicle formation in the human fetal ovary. Mol Hum Reprod. 2004;10:373–381. doi: 10.1093/molehr/gah056. [DOI] [PubMed] [Google Scholar]
- Bendsen E, Byskov AG, Andersen CY, Westergaard LG. Number of germ cells and somatic cells in human fetal ovaries during the first weeks after sex differentiation. Hum Reprod. 2006;21:30–35. doi: 10.1093/humrep/dei280. [DOI] [PubMed] [Google Scholar]
- Bennett CE, Conway GS, Macpherson JN, Jacobs PA, Murray A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum Reprod. 2010;25:1335–1338. doi: 10.1093/humrep/deq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28:773–783. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha GC, Leavitt WW. Ovarian steroid dehydrogenase histochemistry and circulating progesterone in aged golden hamsters during the estrous cycle and pregnancy. Biol Reprod. 1974;11:153–161. doi: 10.1095/biolreprod11.2.153. [DOI] [PubMed] [Google Scholar]
- Bloom DE. 7 Billion and Counting. Science. 2011;333:562–569. doi: 10.1126/science.1209290. [DOI] [PubMed] [Google Scholar]
- Bocca SM, Billiar RB, Albrecht ED, Pepe GJ. Oocytes of baboon fetal primordial ovarian follicles express estrogen receptor beta mRNA. Endocrine. 2008;33:254–260. doi: 10.1007/s12020-008-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med. 2011;29:507–513. doi: 10.1055/s-0031-1293204. [DOI] [PubMed] [Google Scholar]
- Borovskaya TG, Gol'dberg VE, Pakhomova AV, Perova AV, Timina EA. Morphological and functional state of rat ovaries in the early and late periods after injection of vepesid. Bull Exp Biol Med. 2006;141:645–647. doi: 10.1007/s10517-006-0242-9. [DOI] [PubMed] [Google Scholar]
- Bouilly J, Bachelot A, Broutin I, Touraine P, Binart N. Novel NOBOX loss-of-function mutations account for 6.2% of cases in a large primary ovarian insufficiency cohort. Hum Mutat. 2011;32:1108–1113. doi: 10.1002/humu.21543. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJM. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011a;17:46–54. doi: 10.1093/humupd/dmq034. [DOI] [PubMed] [Google Scholar]
- Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, Laven JS, de Jong FH, Te Velde ER, Fauser BC, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011b;96:2532–2539. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- Buckler H. The menopause transition: endocrine changes and clinical symptoms. J Br Menopause Soc. 2005;11:61–65. doi: 10.1258/136218005775544525. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev. 2004;68:422–428. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Høyer PE, Yding Andersen C, Kristensen SG, Jespersen A, Møllgård K. No evidence for the presence of oogonia in the human ovary after their final clearance during the first two years of life. Hum Reprod. 2011;26:2129–2139. doi: 10.1093/humrep/der145. [DOI] [PubMed] [Google Scholar]
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, Harper A, Hulbert D, Lucas S, McClure J, et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Childs AJ, Kinnell HL, Collins CS, Hogg K, Bayne RA, Green SJ, McNeilly AS, Anderson RA. BMP signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells. 2010;28:1368–1378. doi: 10.1002/stem.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PT. Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS One. 2011;6:e20249. doi: 10.1371/journal.pone.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, Saade GR, Eddleman KA, Klugman S, Dugoff L, et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105:983–990. doi: 10.1097/01.AOG.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, Forman MR, Berendes HW, Isotalo L. Delayed childbearing and risk of adverse perinatal outcome. A population-based study. J Am Med Assoc. 1992;268:886–890. [PubMed] [Google Scholar]
- Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–285. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Codner E, Iniguez G, Hernandez IM, Lopez P, Rhumie HK, Villarroel C, Rey RA. Elevated anti-Mullerian hormone (AMH) and inhibin B levels in prepubertal girls with type 1 diabetes mellitus. Clin Endocrinol (Oxf) 2011;74:73–78. doi: 10.1111/j.1365-2265.2010.03887.x. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts SM, Childs AJ, Fulton N, Collins C, Bayne RA, McNeilly AS, Anderson RA. Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev Biol. 2008;314:189–199. doi: 10.1016/j.ydbio.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Craig SS. Effect of age upon uterine response to deciduagenic stimulus. Acta Anat (Basel) 1981;110:146–158. doi: 10.1159/000145424. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Bovenhuis H, van Noord PA, Pearson PL, van Arendonk JA, te Velde ER, Kuurman WW, Dorland M. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16:2014–2018. doi: 10.1093/humrep/16.9.2014. [DOI] [PubMed] [Google Scholar]
- de Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, Kupka M, Nygren KG, Andersen AN. Assisted reproductive technology in Europe, 2007: results generated from European registers by ESHRE. Hum Reprod. 2012;27:954–966. doi: 10.1093/humrep/des023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- Delhanty JDA, Griffin DK, Handyside AH, Harper J, Atkinson GHG, Pieters MHEC, Winston RML. Detection of aneuploidy and chromosomal mosaicism in human embryos during preimplantation sex determination by fluorescent in situ hybridisation, (FISH) Hum Mol Genet. 1993;2:1183–1185. doi: 10.1093/hmg/2.8.1183. [DOI] [PubMed] [Google Scholar]
- Department of Health. Health Survey for England 2003. London: Department of Health; 2004. http://www.dh.gov.uk/asset/Root/04/09.89/11/04098911.pdf . [Google Scholar]
- Dorman JS, Steenkiste AR, Foley TP, Strotmeyer ES, Burke JP, Kuller LH, Kwoh CK. Menopause in type 1 diabetic women. Diabetes. 2001;50:1857–1862. doi: 10.2337/diabetes.50.8.1857. [DOI] [PubMed] [Google Scholar]
- Drampian G, Okoev GG, Allaverdian AG. [Ultrastructural characteristics of the myometrium in the lower segment of the uterus in elderly primigravidas] Akush Ginekol (Mosk) 1983;8:18–21. [PubMed] [Google Scholar]
- Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. Br Med J. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin K, Bayne RA, Childs AJ, Collins C, Anderson RA. The forkhead transcription factor FOXL2 is expressed in somatic cells of the human ovary prior to follicle formation. Mol Hum Reprod. 2009;15:771–777. doi: 10.1093/molehr/gap065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JM, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database Syst Rev. 2010:CD000099. doi: 10.1002/14651858.CD000099.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17:1399–1403. doi: 10.1093/humrep/17.5.1399. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- Finch CE, Holinka CF. Aging and uterine growth during implantation in C57BL/6J mice. Exp Gerontol. 1982;17:235–241. doi: 10.1016/0531-5565(82)90030-4. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Velazquez MA, Eckert JJ, Lucas ES, Watkins AJ. Nutrition of females during the peri-conceptional period and effects on foetal programming and health of offspring. Anim Reprod Sci. 2012;130:193–197. doi: 10.1016/j.anireprosci.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, Coory M, Gordon A, Ellwood D, McIntyre HD, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Anderson RA, Saunders PT, Kinnell H, Mason JI, Evans DB, Bhattacharya S, Flannigan S, Franks S, Monteiro A, et al. Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J Clin Endocrinol Metab. 2011;96:1754–1762. doi: 10.1210/jc.2010-2618. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Alfarawati S, Goodall N-n, Sánchez-García JF, Colls P, Wells D. The cytogenetics of polar bodies: insights into female meiosis and the diagnosis of aneuploidy. Mol Hum Reprod. 2011;17:286–295. doi: 10.1093/molehr/gar024. [DOI] [PubMed] [Google Scholar]
- Franks S, Berga SL. Does PCOS have developmental origins? Fertil Steril. 2012;97:2–6. doi: 10.1016/j.fertnstert.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretts RC, Schmittdiel J, McLean FH, Usher RH, Goldman MB. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333:953–957. doi: 10.1056/NEJM199510123331501. [DOI] [PubMed] [Google Scholar]
- Fulton N, Martins da Silva SJ, Bayne RAL, Anderson RA. Germ cell proliferation and apoptosis in the developing human ovary. J Clin Endocrinol Metab. 2005;90:4664–4670. doi: 10.1210/jc.2005-0219. [DOI] [PubMed] [Google Scholar]
- Gabriel AS, Thornhill AR, Ottolini CS, Gordon A, Brown AP, Taylor J, Bennett K, Handyside A, Griffin DK. Array comparative genomic hybridisation on first polar bodies suggests that non-disjunction is not the predominant mechanism leading to aneuploidy in humans. J Med Genet. 2011;48:433–437. doi: 10.1136/jmg.2010.088070. [DOI] [PubMed] [Google Scholar]
- Geraedts J, Montag M, Magli MC, Repping S, Handyside A, Staessen C, Harper J, Schmutzler A, Collins J, Goossens V, et al. Polar body array CGH for prediction of the status of the corresponding oocyte. Part I: clinical results. Hum Reprod. 2011;26:3173–3180. doi: 10.1093/humrep/der294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick GJ, Nelson JF. The decidual cell response in aging C57BL/6J mice is potentiated by long-term ovariectomy and chronic food restriction. J Gerontol. 1989;44:B67–B71. doi: 10.1093/geronj/44.3.b67. [DOI] [PubMed] [Google Scholar]
- Gosden RG. Effects of age and parity on the breeding potential of mice with one or two ovaries. J Reprod Fertil. 1979;57:477–487. doi: 10.1530/jrf.0.0570477. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Hawkins HK, Gosden CA. Autofluorescent particles of human uterine muscle cells. Am J Pathol. 1978;91:155–174. [PMC free article] [PubMed] [Google Scholar]
- Gougeon A, Echochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: the case for retinoic acid. Biol Reprod. 2011;14:35–37. doi: 10.1095/biolreprod.111.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavaldsen C, Samuelsen SO, Eskild A. The association of maternal age with placental weight: a population-based study of 536,954 pregnancies. BJOG. 2011;118:1470–1476. doi: 10.1111/j.1471-0528.2011.03053.x. [DOI] [PubMed] [Google Scholar]
- Hagen CP, Aksglaede L, Sorensen K, Main KM, Boas M, Cleemann L, Holm K, Gravholt CH, Andersson AM, Pedersen AT, et al. Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 turner syndrome patients. J Clin Endocrinol Metab. 2010;95:5003–5010. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- Hagen CP, Aksglaede L, Sørensen K, Mouritsen A, Andersson A-M, Petersen JH, Main KM, Juul A. Individual serum levels of anti-Müllerian hormone in healthy girls persist through childhood and adolescence: a longitudinal cohort study. Hum Reprod. 2012;27:861–866. doi: 10.1093/humrep/der435. [DOI] [PubMed] [Google Scholar]
- Hale GE, Burger HG. Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23:7–23. doi: 10.1016/j.bpobgyn.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Craig LB, Zavy MT, Klein NA, Soules MR. Ovarian primordial and nongrowing follicle counts according to the Stages of Reproductive Aging Workshop (STRAW) staging system. Menopause. 2012;19:164–171. doi: 10.1097/gme.0b013e31823b0b2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. Executive summary of the stages of reproductive aging workshop +10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JC, Sengupta SB. Preimplantation genetic diagnosis: State of the ART 2011. Hum Genet. 2011;131:175–186. doi: 10.1007/s00439-011-1056-z. [DOI] [PubMed] [Google Scholar]
- Hawkes K, Smith KR. Brief communication: evaluating grandmother effects. Am J Phys Anthropol. 2009;140:173–176. doi: 10.1002/ajpa.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, O'Connell JF, Jones NG, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc Natl Acad Sci USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009;41:724–728. doi: 10.1038/ng.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Kraft P, Chasman DI, Buring JE, Chen C, Hankinson SE, Pare G, Chanock S, Ridker PM, Hunter DJ. A large-scale candidate gene association study of age at menarche and age at natural menopause. Hum Genet. 2010;128:515–527. doi: 10.1007/s00439-010-0878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MC, Jeffers S, Carter J, Duthely L, Cotter A, Gonzalez-Quintero VH. Pregnancy at or beyond age 40 years is associated with an increased risk of fetal death and other adverse outcomes. Am J Obstet Gynecol. 2007;196:e11–e13. doi: 10.1016/j.ajog.2006.10.862. [DOI] [PubMed] [Google Scholar]
- Holinka CF, Finch CE. Age-related changes in the decidual response of the C57BL/6J mouse uterus. Biol Reprod. 1977;16:385–393. doi: 10.1095/biolreprod16.3.385. [DOI] [PubMed] [Google Scholar]
- Holinka CF, Tseng Y-C, Finch CE. Reproductive ageing in C57BL/6J mice: plasma progesterone, viable embryos and resorption frequency throughout pregnancy. Biol Reprod. 1979;20:1201–1211. doi: 10.1095/biolreprod20.5.1201. [DOI] [PubMed] [Google Scholar]
- Huang L, Sauve R, Birkett N, Fergusson D, van Walraven C. Maternal age and risk of stillbirth: a systematic review. Can Med Assoc J. 2008;178:165–172. doi: 10.1503/cmaj.070150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth PJ, Groome NP, Byrd W, Rainey WE, McNeilly AS, Mather JP, Bremner WJ. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab. 1996;81:1321–1325. doi: 10.1210/jcem.81.4.8636325. [DOI] [PubMed] [Google Scholar]
- International Association of Diabetes and Pregnancy Study Group. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93:855–864. doi: 10.1016/j.fertnstert.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005a;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Johnson J, Skaznik-Wikiel M, Lee HJ, Niikura Y, Tilly JC, Tilly JL. Setting the record straight on data supporting postnatal oogenesis in female mammals. Cell Cycle. 2005b;4:1471–1477. doi: 10.4161/cc.4.11.2186. [DOI] [PubMed] [Google Scholar]
- Jurisicova A, Taniuchi A, Li H, Shang Y, Antenos M, Detmar J, Xu J, Matikainen T, Benito Hernandez A, Nunez G, et al. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J Clin Invest. 2007;117:3971–3978. doi: 10.1172/JCI28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimov CB, Moragianni VA, Cronister A, Srouji S, Petrozza J, Racowsky C, Ginsburg E, Thornton KL, Welt CK. Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Hum Reprod. 2011;26:2077–2083. doi: 10.1093/humrep/der168. [DOI] [PubMed] [Google Scholar]
- Kelsey TW, Anderson RA, Wright P, Nelson SM, Wallace WH. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18:79–87. doi: 10.1093/molehr/gar059. [DOI] [PubMed] [Google Scholar]
- Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-Mullerian hormone from conception to menopause. PLoS One. 2011b;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147:3228–3234. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- Kok HS, van Asselt KM, van der Schouw YT, Peeters PH, Wijmenga C. Genetic studies to identify genes underlying menopausal age. Hum Reprod Update. 2005;11:483–493. doi: 10.1093/humupd/dmi024. [DOI] [PubMed] [Google Scholar]
- Kuiri-Hanninen T, Kallio S, Seuri R, Tyrvainen E, Liakka A, Tapanainen J, Sankilampi U, Dunkel L. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J Clin Endocrinol Metab. 2011;96:3432–3439. doi: 10.1210/jc.2011-1502. [DOI] [PubMed] [Google Scholar]
- Kujjo LL, Perez GI. Ceramide and mitochondrial function in ageing oocytes: joggling a new hypothesis and old players. Reproduction. 2011;143:1–10. doi: 10.1530/REP-11-0350. [DOI] [PubMed] [Google Scholar]
- La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P, Volpe A. Anti-Mullerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004;19:2738–2741. doi: 10.1093/humrep/deh508. [DOI] [PubMed] [Google Scholar]
- La Marca A, Spada E, Sighinolfi G, Argento C, Tirelli A, Giulini S, Milani S, Volpe A. Age-specific nomogram for the decline in antral follicle count throughout the reproductive period. Fertil Steril. 2011;95:684–688. doi: 10.1016/j.fertnstert.2010.07.1069. [DOI] [PubMed] [Google Scholar]
- Le Bouffant R, Guerquin MJ, Duquenne C, Frydman N, Coffigny H, Rouiller-Fabre V, Frydman R, Habert R, Livera G. Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum Reprod. 2010;25:2579–2590. doi: 10.1093/humrep/deq195. [DOI] [PubMed] [Google Scholar]
- Lee R. The Outlook for Population Growth. Science. 2011;333:569–573. doi: 10.1126/science.1208859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–1521. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Lutterodt MC, Sorensen KP, Larsen KB, Skouby SO, Andersen CY, Byskov AG. The number of oogonia and somatic cells in the human female embryo and fetus in relation to whether or not exposed to maternal cigarette smoking. Hum Reprod. 2009;24:2558–2566. doi: 10.1093/humrep/dep226. [DOI] [PubMed] [Google Scholar]
- Madrigal L, Melendez-Obando M. Grandmothers’ longevity negatively affects daughters’ fertility. Am J Phys Anthropol. 2008;136:223–229. doi: 10.1002/ajpa.20798. [DOI] [PubMed] [Google Scholar]
- Magli MC, Montag M, Köster M, Muzi L, Geraedts J, Collins J, Goossens V, Handyside AH, Harper J, Repping S, et al. Polar body array CGH for prediction of the status of the corresponding oocyte. Part II: technical aspects. Hum Reprod. 2011;26:3181–3185. doi: 10.1093/humrep/der295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamsen LS, Lutterodt MC, Andersen EW, Skouby SO, Sorensen KP, Andersen CY, Byskov AG. Cigarette smoking during early pregnancy reduces the number of embryonic germ and somatic cells. Hum Reprod. 2010;25:2755–2761. doi: 10.1093/humrep/deq215. [DOI] [PubMed] [Google Scholar]
- Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NE, Arts EG, de Vries JW, Bossuyt PM, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17:454–466. doi: 10.1093/humupd/dmr003. [DOI] [PubMed] [Google Scholar]
- Matikainen TM, Moriyama T, Morita Y, Perez GI, Korsmeyer SJ, Sherr DH, Tilly JL. Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology. 2002;143:615–620. doi: 10.1210/endo.143.2.8624. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M, Patrizio P, Kayisli U, Luk J, Thomson TC, Anderson RA, Telfer EE, Johnson J. mTOR kinase inhibition results in oocyte loss characterized by empty follicles in human ovarian cortical strips cultured in vitro. Fertil Steril. 2011;96:1154–1159. doi: 10.1016/j.fertnstert.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Meirow D, Biederman H, Anderson RA, Wallace WHB. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389–1394. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- Murabito JM, Yang Q, Fox C, Wilson PW, Cupples LA. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90:3427–3430. doi: 10.1210/jc.2005-0181. [DOI] [PubMed] [Google Scholar]
- Murray A, Bennett CE, Perry JR, Weedon MN, Jacobs PA, Morris DH, Orr N, Schoemaker MJ, Jones M, Ashworth A, et al. Common genetic variants are significant risk factors for early menopause: results from the Breakthrough Generations Study. Hum Mol Genet. 2011;20:186–192. doi: 10.1093/hmg/ddq417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PLoS Med. 2011;8:e1000386. doi: 10.1371/journal.pmed.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Stewart F, Fleming R, Freeman DJ. Longitudinal assessment of antimullerian hormone during pregnancy-relationship with maternal adiposity, insulin, and adiponectin. Fertil Steril. 2010;93:1356–1358. doi: 10.1016/j.fertnstert.2009.07.1676. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Anderson RA, Broekmans FJ, Raine-Fenning N, Fleming R, La Marca A. Anti-Müllerian hormone: clairvoyance or crystal clear? Hum Reprod. 2011a;27:631–636. doi: 10.1093/humrep/der446. [DOI] [PubMed] [Google Scholar]
- Nelson SM, La Marca A. The journey from the old to the new AMH assay: how to avoid getting lost in the values. Reprod Biomed Online. 2011;23:411–420. doi: 10.1016/j.rbmo.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Messow MC, McConnachie A, Wallace H, Kelsey T, Fleming R, Anderson RA, Leader B. External validation of nomogram for the decline in serum anti-Mullerian hormone in women: a population study of 15,834 infertility patients. Reprod Biomed Online. 2011b;23:204–206. doi: 10.1016/j.rbmo.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Messow MC, Wallace AM, Fleming R, McConnachie A. Nomogram for the decline in serum antimullerian hormone: a population study of 9,601 infertility patients. Fertil Steril. 2011c;95:736–741. doi: 10.1016/j.fertnstert.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. Br Med J. 2000;320:1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003a;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003b;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Ohta Y. Age-related decline in deciduogenic ability of the rat uterus. Biol Reprod. 1987;37:779–785. doi: 10.1095/biolreprod37.4.779. [DOI] [PubMed] [Google Scholar]
- Olsen J. Subfecundity according to the age of the mother and the father. Dan Med Bull. 1990;37:281–282. [PubMed] [Google Scholar]
- Pangas SA, Rajkovic A. Transcriptional regulation of early oogenesis: in search of masters. Hum Reprod Update. 2006;12:65–76. doi: 10.1093/humupd/dmi033. [DOI] [PubMed] [Google Scholar]
- Parsa P, Parsa B. Effects of reproductive factors on risk of breast cancer: a literature review. Asian Pac J Cancer Prev. 2009;10:545–550. [PubMed] [Google Scholar]
- Paulson RJ, Boostanfar R, Saadat P, Mor E, Tourgeman DE, Slater CC, Francis MM, Jain JK. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. J Am Med Assoc. 2002;288:2320–2323. doi: 10.1001/jama.288.18.2320. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Billiar RB, Albrecht ED. Regulation of baboon fetal ovarian folliculogenesis by estrogen. Mol Cell Endocrinol. 2006;247:41–46. doi: 10.1016/j.mce.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Peters H, Byskov AG, Grinsted J. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clin Endocrinol Metab. 1978;7:469–485. doi: 10.1016/s0300-595x(78)80005-x. [DOI] [PubMed] [Google Scholar]
- Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- Rahima A, Bruce NW. Fetal and placental growth in young, primiparous and old, multiparous rats. Exp Gerontol. 1987;22:257–261. doi: 10.1016/0531-5565(87)90004-0. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Storgaard L, Toft G, Olsen J, Bonde JP. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol. 2007;165:1372–1379. doi: 10.1093/aje/kwm032. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF. Oogenesis: Prospects and challenges for the future. J Cell Physiol. 2008;216:355–365. doi: 10.1002/jcp.21473. [DOI] [PubMed] [Google Scholar]
- Rosen MP, Johnstone E, McCulloch CE, Schuh-Huerta SM, Sternfeld B, Reijo-Pera RA, Cedars MI. A characterization of the relationship of ovarian reserve markers with age. Fertil Steril. 2012;97:238–243. doi: 10.1016/j.fertnstert.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer MV, Paulson RJ, Lobo RA. Reversing the natural decline in human fertility. An extended clinical trial of oocyte donation to women of advanced reproductive age. J Am Med Assoc. 1992;268:1275–1279. doi: 10.1001/jama.268.10.1275. [DOI] [PubMed] [Google Scholar]
- Santoro N. Symptoms of menopause: hot flushes. Clin Obstet Gynecol. 2008;51:539–548. doi: 10.1097/GRF.0b013e31818093f6. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Sobotka T, Bentzen JG, Nyboe Andersen A. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update. 2012;18:29–43. doi: 10.1093/humupd/dmr040. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747–750. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci USA. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhal PF, Craft IL. Oocyte donation in 61 patients. Lancet. 1989;1:1185–1187. doi: 10.1016/s0140-6736(89)92762-1. [DOI] [PubMed] [Google Scholar]
- Shapiro M, Talbert GB. The effect of maternal age on decidualization in the mouse. J Gerontol. 1974;29:145–148. doi: 10.1093/geronj/29.2.145. [DOI] [PubMed] [Google Scholar]
- Smith GC. Age at menarche and the risk of operative first delivery. BJOG. 2009;116:1613–1621. doi: 10.1111/j.1471-0528.2009.02340.x. [DOI] [PubMed] [Google Scholar]
- Smith GC, Cordeaux Y, White IR, Pasupathy D, Missfelder-Lobos H, Pell JP, Charnock-Jones DS, Fleming M. The effect of delaying childbirth on primary cesarean section rates. PLoS Med. 2008;5:e144. doi: 10.1371/journal.pmed.0050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, Alegretti JR, Motta EL. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94:2088–2095. doi: 10.1016/j.fertnstert.2009.12.065. [DOI] [PubMed] [Google Scholar]
- Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. JCEM. 1998;83:1875–1880. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA. Understanding the villain: DMBA-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol Sci. 2011;123:563–575. doi: 10.1093/toxsci/kfr195. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Stampfer MJ, Speizer FE, Spiegelman D, Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. J Am Med Assoc. 1997;278:1078–1083. [PubMed] [Google Scholar]
- Sorger T, Soderwall A. The aging uterus and the role of edema in endometrial function. Biol Reprod. 1981;24:1135–1144. [PubMed] [Google Scholar]
- Soto N, Iñiguez G, López P, Larenas G, Mujica V, Rey RA, Codner E. Anti-Müllerian hormone and inhibin B levels as markers of premature ovarian aging and transition to menopause in type 1 diabetes mellitus. Hum Reprod. 2009;24:2838–2844. doi: 10.1093/humrep/dep276. [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF., Jr Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–3483. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, Devroey P, Liebaers I, Van Steirteghem A. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19:2849–2858. doi: 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, Baird DD. Antimullerian hormone as a predictor of natural fecundability in women aged 30-42 years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk L, Zhai G, van Meurs JB, Verbiest MM, Visser JA, Estrada K, Rivadeneira F, Williams FM, Cherkas L, Deloukas P, et al. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat Genet. 2009;41:645–647. doi: 10.1038/ng.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Tan L, Yang F, Luo Y, Li X, Deng HW, Dvornyk V. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause. 2011;19:126–132. doi: 10.1097/gme.0b013e318224f9ac. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, Coomarasamy A, Arlt W, Bhattacharya S. Should androgen supplementation be used for poor ovarian response in IVF? Hum Reprod. 2012;27:637–640. doi: 10.1093/humrep/der464. [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert GB, Krohn PL. Effect of maternal age on viability of ova and uterine support of pregnancy in mice. J Reprod Fertil. 1966;11:399–406. doi: 10.1530/jrf.0.0110399. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Cano A. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod. 2001;65:141–150. doi: 10.1095/biolreprod65.1.141. [DOI] [PubMed] [Google Scholar]
- Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16:797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med. 2012;18:353–354. doi: 10.1038/nm.2699. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29:15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Gosden RG, Byskov AG, Spears N, Albertini D, Andersen CY, Anderson R, Braw-Tal R, Clarke H, Gougeon A, et al. On regenerating the ovary and generating controversy. Cell. 2005;122:821–822. doi: 10.1016/j.cell.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- Templeton A, Morris JK, Parslow W. Factors that affect outcome of in-vitro fertilisation treatment. Lancet. 1996;348:1402–1406. doi: 10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- Thomson TC, Johnson J. Inducible somatic oocyte destruction in response to rapamycin requires wild-type regulation of follicle cell epithelial polarity. Cell Death Differ. 2010;17:1717–1727. doi: 10.1038/cdd.2010.49. [DOI] [PubMed] [Google Scholar]
- Thomson TC, Fitzpatrick KE, Johnson J. Intrinsic and extrinsic mechanisms of oocyte loss. Mol Hum Reprod. 2010;16:916–927. doi: 10.1093/molehr/gaq066. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Telfer EE. Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol Hum Reprod. 2009;15:393–398. doi: 10.1093/molehr/gap036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson DJ, Thomas RE, Campbell MK, Reid DM. Alcohol consumption and age of maternal menopause are associated with menopause onset. Maturitas. 1997a;26:21–25. doi: 10.1016/s0378-5122(96)01075-4. [DOI] [PubMed] [Google Scholar]
- Torgerson DJ, Thomas RE, Reid DM. Mothers and daughters menopausal ages: is there a link? Eur J Obstet Gynecol Reprod Biol. 1997b;74:63–66. doi: 10.1016/s0301-2115(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT., Jr Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril. 2012;97:819–824. doi: 10.1016/j.fertnstert.2012.01.115. [DOI] [PubMed] [Google Scholar]
- Vahakangas K, Rajaniemi H, Pelkonen O. Ovarian toxicity of cigarette smoke exposure during pregnancy in mice. Toxicol Lett. 1985;25:75–80. doi: 10.1016/0378-4274(85)90103-1. [DOI] [PubMed] [Google Scholar]
- van Asselt KM, Kok HS, Pearson PL, Dubas JS, Peeters PH, Te Velde ER, van Noord PA. Heritability of menopausal age in mothers and daughters. Fertil Steril. 2004;82:1348–1351. doi: 10.1016/j.fertnstert.2004.04.047. [DOI] [PubMed] [Google Scholar]
- van Balen F, Verdurmen JE, Ketting E. Age, the desire to have a child and cumulative pregnancy rate. Hum Reprod. 1997;12:623–627. doi: 10.1093/humrep/12.3.623. [DOI] [PubMed] [Google Scholar]
- van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, Broekmans FJ. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod. 2010;25:221–227. doi: 10.1093/humrep/dep366. [DOI] [PubMed] [Google Scholar]
- van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, Korevaar JC, Heineman MJ, van der Veen F, Repping S. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update. 2011;17:620–627. doi: 10.1093/humupd/dmr014. [DOI] [PubMed] [Google Scholar]
- van Rooij IAJ, Broekmans FJM, te Velde ER, Fauser BCJM, Bancsi LF, Jong FH, Themmen APN. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, Themmen AP, te Velde ER. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- Vine MF, Margolin BH, Morrison HI, Hulka BS. Cigarette smoking and sperm density: a meta-analysis. Fertil Steril. 1994;61:35–43. [PubMed] [Google Scholar]
- Waldeyer W. Eierstock und Ei. Leipzig: Engelmann; 1870. [Google Scholar]
- Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BCJM, Themmen APN. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- Westendorp RG, Kirkwood TB. Human longevity at the cost of reproductive success. Nature. 1998;396:743–746. doi: 10.1038/25519. [DOI] [PubMed] [Google Scholar]
- White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschi E. Migration of the germ cells of human embryos from the yolksac to the primitive gonadal fold. Contrib Embryol. 1948;32:67–80. [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- Yamada Z, Kitagawa M, Takemura T, Hirokawa K. Effect of maternal age on incidences of apoptotic and proliferative cells in trophoblasts of full-term human placenta. Mol Hum Reprod. 2001;7:1179–1185. doi: 10.1093/molehr/7.12.1179. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during mid-gestation and is associated with meiotic arrest of oocytes. Biol Reprod. 2008;78:1153–1161. doi: 10.1095/biolreprod.107.066688. [DOI] [PubMed] [Google Scholar]
- Ye X, Skjaerven R, Basso O, Baird DD, Eggesbo M, Uicab LA, Haug K, Longnecker MP. In utero exposure to tobacco smoke and subsequent reduced fertility in females. Hum Reprod. 2010;25:2901–2906. doi: 10.1093/humrep/deq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Merico V, Cecconi S, Redi CA, Garagna S. What does it take to make a developmentally competent mammalian egg? Hum Reprod Update. 2011;17:525–540. doi: 10.1093/humupd/dmr009. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. The number of oocytes in the mature ovary. Rec Prog Horm Res. 1951;6:63–108. [Google Scholar]
- Zuckerman S. The Frontiers of Public and Private Science. New York: Taplinger; 1971. Beyond the Ivory Tower. [Google Scholar]