Abstract
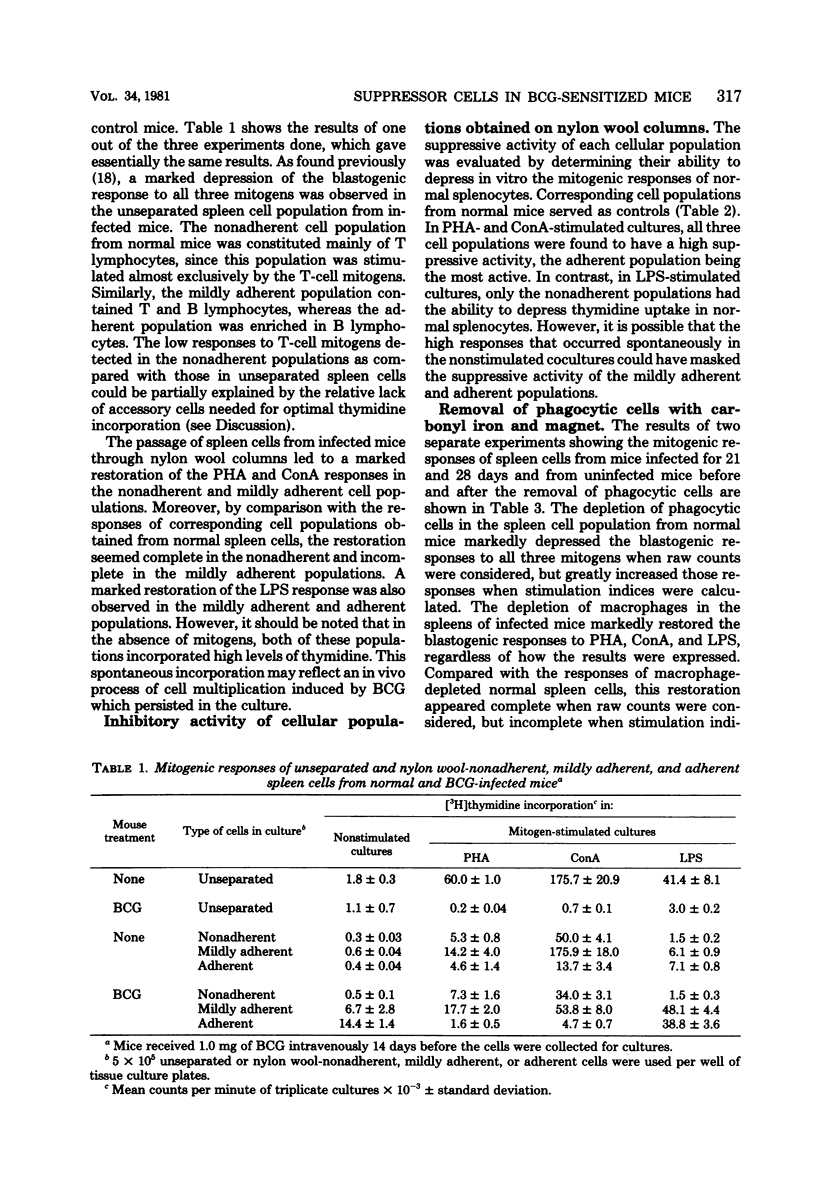
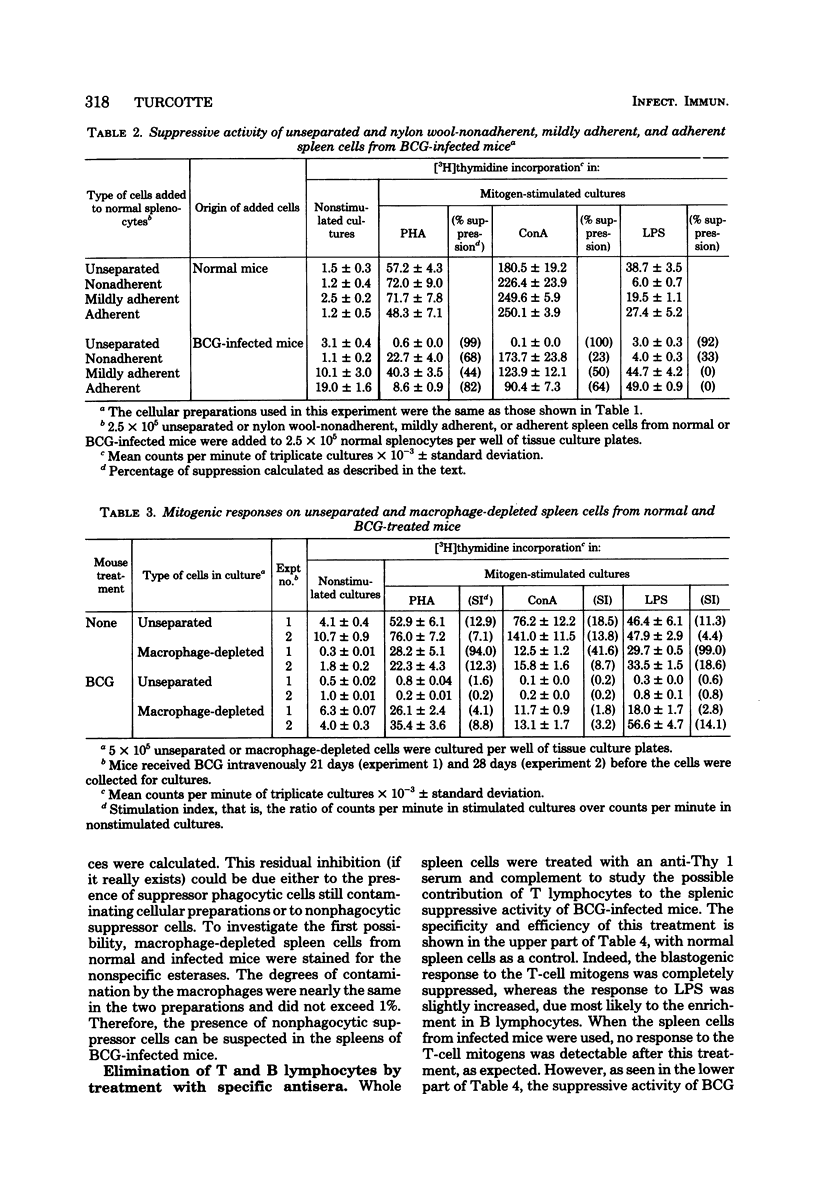
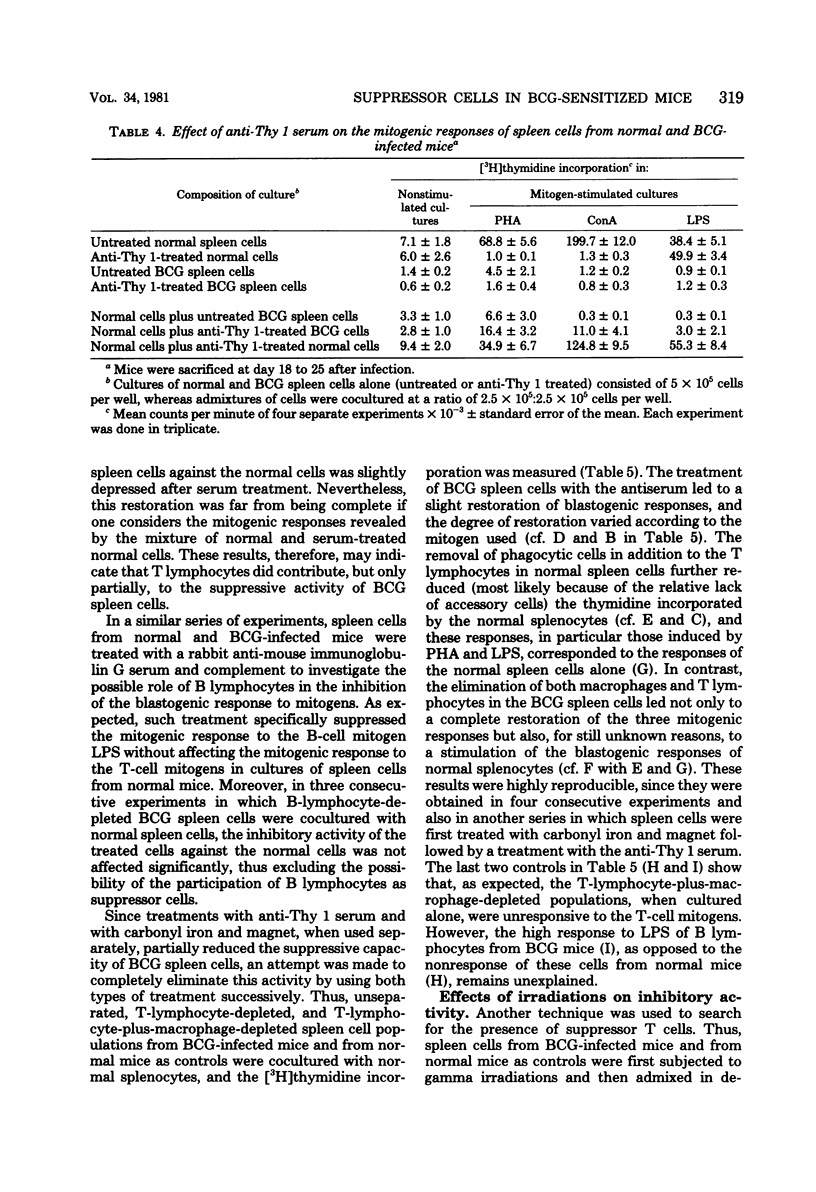
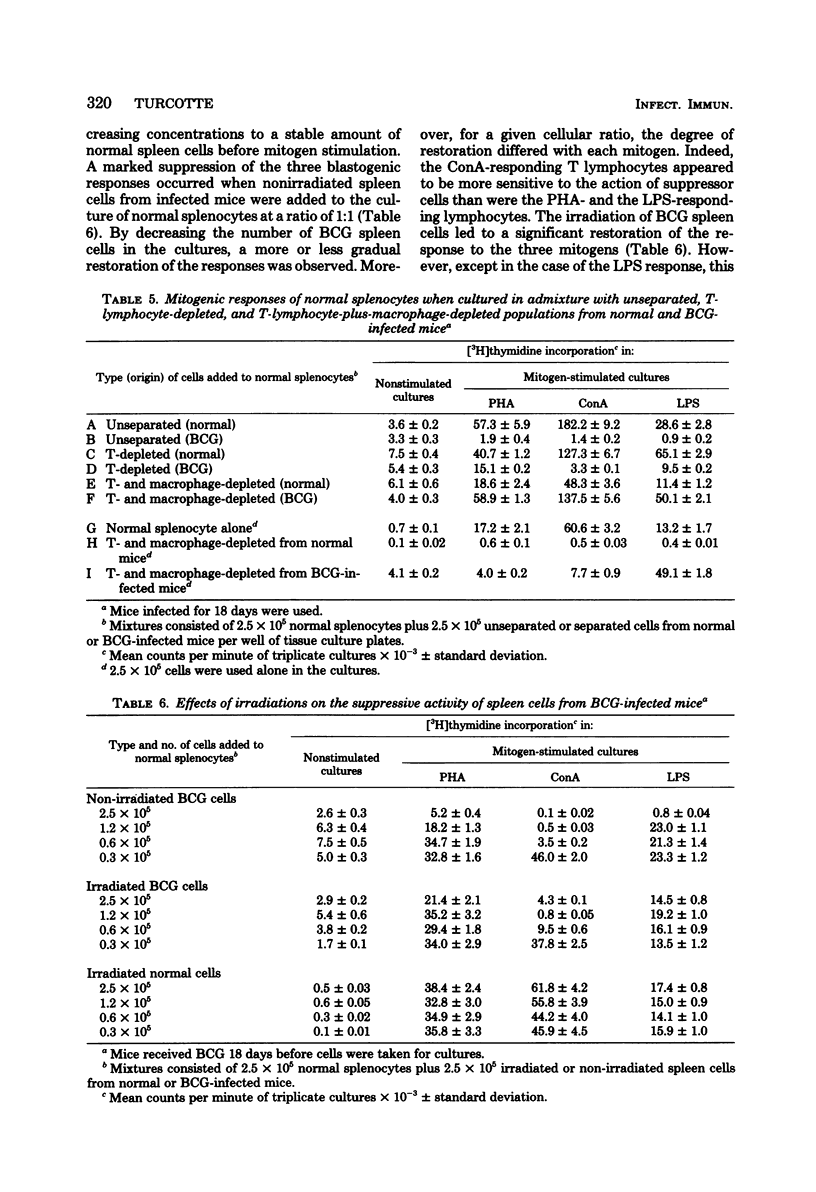
Spleen cells from female C57BL/6 mice infected intravenously with 1 mg (about 10(7) viable units) of bacillus Calmette-Guérin (BCG) were shown to suppress the blastogenic responses induced by the T-cell mitogens phytohemagglutinin and concanavalin A and by the B-cell mitogen lipopolysaccharide in spleen cells from normal syngeneic mice. By using various separation procedures or cellular treatments, evidence was found for two distinct populations of splenic suppressor cells. One population belonged to the monocyte-macrophage lineage on the basis of their adherence to plastic surfaces, their removal after treatment with carbonyl iron, and their resistance to gamma irradiation. The other population of suppressor cells belonged to the T lymphocytes due to their sensitivity to an anti-Thy 1 antiserum and complement and to gamma irradiation. After separation on nylon wool columns, inhibitory activity was found in both the nonadherent and the adherent spleen cell populations. Both populations of suppressor cells were present in the spleens 14 days after BCG inoculation and persisted for at least 40 days after infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Moore V. L. Suppression of phytohemagglutinin and lipopolysaccharide responses in mouse spleen cells by Bacillus Calmette-Guerin. J Reticuloendothel Soc. 1979 Oct;26(4):349–356. [PubMed] [Google Scholar]
- Baird L. G., Kaplan A. M. Macrophage regulation of mitogen-induced blastogenesis. I. Demonstration of inhibitory cells in the spleens and peritoneal exudates of mice. Cell Immunol. 1977 Jan;28(1):22–35. doi: 10.1016/s0008-8749(77)80003-8. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Brown I. N., Sljivić V. S. Suppressed or enhanced antibody responses in vitro after BCG treatment of mice: importance of BCG viability. Immunology. 1979 Nov;38(3):481–488. [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doft B. H., Merchant B., Johannessen L., Chaparas S. D., Sher N. A. Contrasting effects of BCG on spleen and lymph node antibody responses in nude and normal mice. J Immunol. 1976 Nov;117(5 Pt 1):1638–1643. [PubMed] [Google Scholar]
- Farrar W. L., Elgert K. D. Inhibition of mitogen and immune blastogenesis by two distinct populations of supressor cells present in the spleens of fibrosarcoma-bearing mice: adoptive transfer of suppression. Int J Cancer. 1978 Aug 15;22(2):142–151. doi: 10.1002/ijc.2910220207. [DOI] [PubMed] [Google Scholar]
- Ito M., Ralph P., Moore M. A. Suppression of spleen natural killing activity induced by BCG. Clin Immunol Immunopathol. 1980 May;16(1):30–38. doi: 10.1016/0090-1229(80)90163-4. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Keller R. Major changes in lymphocyte proliferation evoked by activated macrophages. Cell Immunol. 1975 Jun;17(2):542–551. doi: 10.1016/s0008-8749(75)80058-x. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Orbach-Arbouys S., Poupon M. F. Active suppression of in vitro reactivity of spleen cells after BCG treatment. Immunology. 1978 Mar;34(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Pope B. L., Whitney R. B., Levy J. G. Two distinct populations of suppressor cells in the spleens of mice bearing methylcholanthrene-induced tumors. J Immunol. 1978 Jun;120(6):2033–2040. [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Turcotte R., Lafleur L., Labrèche M. Opposite effects of BCG on spleen and lymph node cells: lymphocyte proliferation and immunoglobulin synthesis. Infect Immun. 1978 Sep;21(3):696–704. doi: 10.1128/iai.21.3.696-704.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Studies on the regulation of lymphocyte reactivity by normal and activated macrophages. Cell Immunol. 1977 Apr;30(1):108–121. doi: 10.1016/0008-8749(77)90052-1. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


