Abstract
Although BRCA1 is the most prevalent genetic factor in breast cancer, the pathologic mechanism of tumorigenesis caused by its deficiency has not been elucidated. We have previously demonstrated that BRCA1 can modulate responses to xenobiotic stress by regulating expression of genes involved in metabolic activation, detoxification and antioxidant reactions. In this study, we examined whether BRCA1 deficiency is more vulnerable to xenobiotic stress by employing an in vitro cell model system. Benzo[a]pyrene (B[a]P), used as a xenobiotic insult, increased intracellular reactive oxygen species (ROS) levels in breast epithelial cells. Accumulation of ROS upon B[a]P exposure was significantly augmented by abrogation of BRCA1 compared to the control. Overexpression of Nrf2 in BRCA1 deficient cells reduced elevated ROS to the control levels. Bioactive food components such as sulforaphane (SFN) and resveratrol (RSV) significantly reduced B[a]P-induced ROS accumulation regardless of BRCA1 presence. In addition, these bioactive food components increased Nrf2 levels and Nrf2 transcriptional activity, which led to attenuation of B[a]P-induced DNA damages. Likewise, incubation with bioactive food components reduced B[a]P-mediated DNA damage in BRCA1 deficient cells. In conclusion, we demonstrated that the lack of BRCA1 renders cells more susceptible to ROS-induced DNA damage, which may eventually result in tumorigenesis, and that administration of Nrf2-activating bioactive food components can reduce those risks.
Keywords: BRCA1, Nrf2, oxidative stress, bioactive food components, carcinogenesis, chemoprevention
1. Introduction
Breast cancer is the most common female malignant cancer (Jemal et al., 2008). As a genetic factor, the breast cancer susceptibility gene (BRCA1) is known to be responsible for half of all inherited cases (Nathanson et al., 2001). The function of BRCA1 has been reported to be involved in tumor suppression (Deng and Brodie, 2000), DNA repair (Scully et al., 1997), cell cycle checkpoint control (Ruffner et al., 1999), and ubiquitination (Jensen et al., 1998). In addition, we have demonstrated that BRCA1 plays a crucial role in cellular responses to xenobiotic stress involved in both phase I and phase II systems (Bae et al., 2004; Kang et al., 2006, 2008a, 2008b, 2011a). BRCA1 is engaged in cellular responses to xenobiotic stress by up-regulation of Aryl hydrocarbon Receptor (AhR)/AhR nuclear translocator (ARNT)-driven transcription, which is the key feature of phase I (Kang et al., 2006). BRCA1 also stimulates antioxidant gene expression and modulates intracellular reactive oxygen species (ROS) levels by enhancing activity of the phase II transcription factor, nuclear factor (erythroid-derived 2)-like 2 (NFE2L2, Nrf2) (Bae et al., 2004).
Primary risk factors of breast cancer include exposure to environmental factors such as radiation, tobacco and xenoestrogen (Ibarluzea et al., 2004; Wolff et al., 1996). There are accumulating data that residual oxidative stresses from these xenobiotics promote tumorigenesis (Dunnick et al., 1995). For complete detoxification and excretion of xenobiotics, the cooperative processes of phase I and phase II enzymes are required (Xu et al., 2005). In this context, defects in both phase I and phase II systems resulting from a BRCA1 deficiency may hamper sufficient cytoprotection against those insults, which could result in DNA damage and tumorigenesis in the mammary gland.
Recently, applications of bioactive food components in cancer chemoprevention have been widely studied (reviewed in Surh, 2003). For example, sulforaphane (SFN), an isothiocyanate from cruciferous vegetables, has been shown to reduce the risk of developing many common cancers by altering the expression of various genes, including tumor suppressor genes (Cornblatt et al., 2007; Ho et al., 2009; Meeran et al., 2010; Pledgie-Tracy et al., 2007). Resveratrol (RSV) from grapes also showed anti-tumor activity by inducing apoptosis in various cancer models (Banerjee et al., 2002; Sareen et al., 2007; She et al., 2000). These two phytochemicals are strong antioxidants and induce the Nrf2-mediated phase II system (Fahey et al., 1997; Rubiolo et al., 2008). Hence, application of these bioactive food components could compensate for the weakness in the phase II system in a BRCA1 deficiency.
In this study, we employed a new assay system using Benzo[a]pyrene (B[a]P) as a carcinogenic insult in BRCA1 knockdown cells to demonstrate oxidative stress induction and genomic damage in BRCA1 defective cells. In addition, we monitored the efficacy of several bioactive food components, which can activate Nrf2 upon a B[a]P insult.
2. Experimental Procedures
2.1 Cell culture and reagents
The MCF10A cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in Dulbecco’s Modified Eagles’ Medium (DMEM)-F12 containing 5 % horse serum, 10 μg/ml insulin, 10 ng/ml epidermal growth factor and 0.5 μg/ml hydrocortisone. Benzo[a]pyrene (B[a]P), resveratrol (RSV; 3,5,4′-trihydroxy-trans-stilbene), and indole-3-carbinol (I3C: 1H-indol-3-ylmethanol) were purchased from Sigma (St. Louis, MO). Sulforaphane (SFN: 1-isothiocyanato-4-methylsulfinylbutane) and oltipraz (OTP: 5-2-pyrazinyl-4-methyl-1,2-dithiol-3-thione) were purchased from LKT Laboratories, Inc. (St. Paul, MN).
2.2 Transfection of DNA and siRNA
Control (non-targeting scrambled) and BRCA1 siRNAs were purchased from Dharmacon, Inc. (Lafayette, CO) and their sequences are as follows: Control-siRNA 5′-GAC GAG CGG CAC GUG CAC A -3′, BRCA1-siRNA 5′-GAA GGA GCT TCC ATC ATT C-3′. Transfection of BRCA1-siRNA and Flag-Nrf2 or UGT1A1 overexpressing vectors were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
2.3 ROS measurement
Measurements of ROS were performed by using CM-H2DCFDA (2 ′,7 ′-dichlorofluorescin diacetate), lucigenin (10-Methyl-9-[10-methylacridin-10-ium-9-yl]-acridin-10-ium dinitrate), and DAF-FM diacetate (4-Amino-5-methylamino-2′,7′-difluorofluorescein). CM-H2DCFDA (5μM), lucigenin (10 μM), or DAF-FM diacetate (5 μM) were added to cells incubated with B[a]P with or without pretreatment with siRNA, plasmid, or bioactive food components. After a 30 min incubation, cells were washed, then fluorescence was measured using Ultra 384 fluorometer (Tecan, Switzerland) at 495/535 nm for detection of CM-H2DCFDA and DAF-FM diacetate. A luminometer (Victor 2, Perkin Elmer, Walthan, MA) was used for detection of luminescence of lucigenin-treated cells every 1 min for 10 min. Data Analyses were performed at Genomics & Epigenomics Shared Resource, Lombardi Comprehensive Cancer Center of Georgetown University Medical Center.
2.4 Western blotting
Standard Western blotting was performed to measure the expression levels of Flag-Nrf2 and its downstream genes after knockdown of BRCA1. Anti-Flag M2 antibody (Sigma), anti-BRCA1 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-β-actin (Sigma) antibody, anti-GCLC antibody (Novus, Littleton, CO), and anti-UGT1A1 antibody (BD Gentest, San Jose, CA) were used for primary antibodies. Anti-mouse and anti-rabbit IgG–peroxidase antibodies (Sigma) were used for secondary antibody and ECL solution (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used for detection.
2.5 Reporter gene assay
MCF10A cells pretreated with control or BRCA1 specific siRNA for 72 hrs were transfected with NQO1-ARE-Luc vector (Bae et al., 2004) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Bioactive food components were co-incubated during DNA transfection for 24 hrs. Luciferase activity was determined according to the manufacturer’s instructions (Promega, Madison, WI). Luminescence of the total lysate in reporter lysis buffer was measured using a Luminometer (Victor 2). After normalization by β-galactosidase activity, luminescence readings were converted into fold increase measurements as compared to controls (control-siRNA and no bioactive food components).
2.6 Measurement of DNA damage
For determination of DNA damage by B[a]P, OxyDNA assay kit (8-oxo-guanine assay, Calbiochem, Philadelphia, PA) was applied. After pre-incubation with bioactive food components or chemopreventive agents for 6 hrs, cells were further incubated with 5 μM of B[a]P. Then cells were fixed and permeabilized, and FITC-labeled protein conjugate, which binds to the 8-oxoguanine moiety of oxidized DNA, was added for 1 hr. Fluorescence was counted for detection of FITC at 495/535 nm.
2.7 Statistical analysis
All experiments were performed more than three times. We used ANOVA analysis and Tukey’s multiple comparison procedure to adjust for p values. The test is performed at 5% significance level. * or # means the difference is significant after adjusting for multiple comparison.
3. Results
3.1 BRCA1 prevents ROS accumulation in normal breast epithelial cell
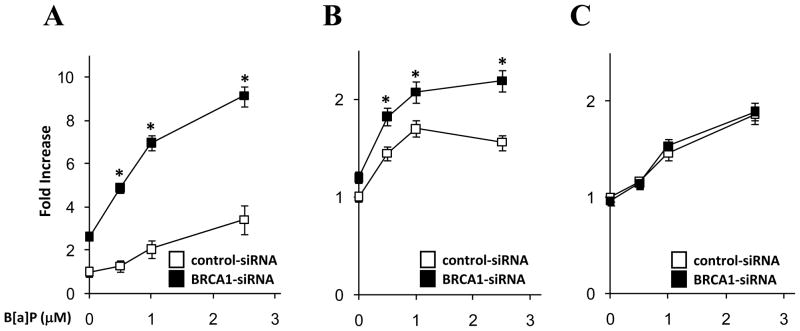
Previously we observed that B[a]P-mediated DNA adduct formation is negatively correlated with expression level of BRCA1 in MCF10A cells (Kang et al., 2011a). This observation implicates that loss of BRCA1 may cause impairs in modulation of intracellular ROS level. To address whether lack of BRCA1 increases B[a]P mediated ROS generation in a normal breast epithelial cell line, we treated MCF10A cells with B[a]P for 24 hrs after knockdown of BRCA1. Measurements of ROS were performed by using either CM-H2DCFDA or lucigenin. CM-H2DCFDA is a chemical fluorescent dye to detect hydrogen peroxide, hydroxyl radical, peroxyl radical, and peroxynitrite anion, while lucigenin is used to detect hydrogen peroxide and superoxide anion. Although ROS levels in both control and BRCA1-siRNA treated MCF10A were elevated in a dose-dependent manner with B[a]P, ROS accumulation in BRCA1 deficient cells were significantly accelerated compared to control cells (Fig. 1A and B). Next, we compared nitric oxide (NO) levels by measuring fluorescence after addition of DAF-FM diacetate. Incubation of B[a]P increased NO levels in a dose-dependent manner (Fig. 1C), but accumulation of NO was not dependent on the presence of BRCA1. Thus, these data implicate that BRCA1 plays a crucial role only in the regulation of ROS levels upon B[a]P-induced oxidative stress.
Figure 1.
ROS accumulation by B[a]P in BRCA1 defective cells. MCF10A cells pretreated with control or BRCA1 specific siRNA for 72 hrs were further incubated with B[a]P for 24 hrs. Then cellular ROS and NO levels were measured using (A) CM-H2DCFDA, (B) Lucigenin, and (C) DAF-FM diacetate. * indicates p < 0.05 (control siRNA vs. BRCA1 siRNA treated cells).
3.2 Overexpression of Nrf2 alleviates oxidative stress in the absence of BRCA1
Failure of ROS elimination in BRCA1 deficient cells is probably caused by the defect in phase II induction. As reported earlier, knockdown of BRCA1 resulted in down-regulation of several phase II enzymes, as well as Nrf2 (Bae et al., 2004). Thus, we hypothesized that significantly increased ROS accumulation in BRCA1 deficient cells is attributed to a weak activation of Nrf2. To test our hypothesis, we increased Nrf2 expression levels by transient DNA transfection after knockdown of BRCA1. Overexpression of Nrf2 significantly reduced the ROS levels in both BRCA1 proficient and deficient MCF10A cells (Fig. 2A and B). Consistent with the observation in Fig. 1C, knockdown of BRCA1 did not affect NO accumulation (Fig. 2C). Overexpression of Nrf2, however, significantly reduced NO levels in both BRCA1 proficient and deficient cells (Fig. 2C). Therefore, Nrf2 activity or expression level confers increased cytoprotection against oxidative stresses in BRCA1 deficient cells. Western blot analysis confirmed the expression levels of BRCA1 and Nrf2 after BRCA1 knockdown and Nrf2 overexpression (Fig. 2D).
Figure 2.
Reduction of ROS accumulation after Nrf2 overexpression. MCF10A cells pretreated with control or BRCA1 specific siRNA for 72 hrs were transfected with pCDNA or Flag-Nrf2 vectors for 24 hrs. Then indicated concentrations of B[a]P were further incubated for 24 hrs. The level of cellular ROS and NO were measured using (A) CM-H2DCFDA, (B) Lucigenin, and (C) DAF-FM diacetate. Open square, control-siRNA + pCDNA3; light gray square, control-siRNA + Flag-Nrf2; dark gray square, BRCA1-siRNA + pCDNA3; and black square, BRCA1-siRNA + Flag-Nrf2. (D) Expression levels of BRCA1 and Flag-Nrf2 after knockdown of BRCA1 and overexpression of Nrf2 were analyzed by Western blotting.
3.3 Bioactive food components trigger Nrf2 mediated transcription
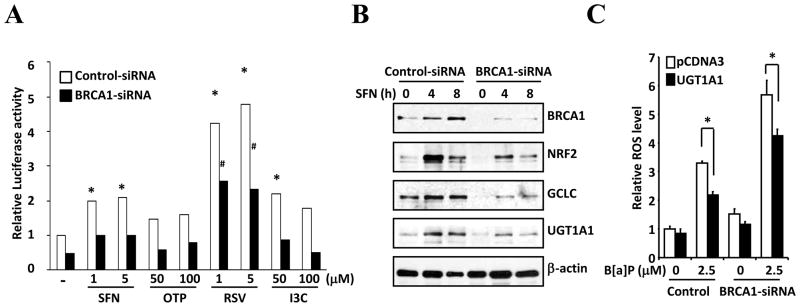
Previously we had observed that several bioactive food components increase Nrf2 activity (data not shown). Thus, we anticipated that the insufficient Nrf2 activity due to BRCA1 deficiency is alleviated by incubation of bioactive food components. For this purpose, we examined the effects of SFN, RSV, OTP and I3C on Nrf2 activation. Reporter gene assays using the NQO1-ARE-Luc vector revealed that all the agents increased transcriptional activity of Nrf2 regardless of BRCA1 presence (Fig. 3A). Especially, incubation of RSV significantly increased the activity up to 4.8 and 5.4 fold in the presence or absence of BRCA1, respectively. Interestingly, the inductions (fold increases) of Nrf2 transcriptional activity by these agents were not significantly different between control and BRCA1 specific siRNA treated cells. To further demonstrate that bioactive food components activate Nrf2 downstream genes, we analyzed the changes of expression levels of GCLC and UGT1A1, which are transcriptionally regulated by Nrf2. Incubation of SFN increased expression level of GCLC and UGT1A1 protein in both control and BRCA1-siRNA treated cells (Fig. 3B). As we expect from our prior study (Kang et al., 2011a), we found that overexpression of UGT1A1 significantly reduced B[a]P-induced ROS level (Fig. 3C). These data indicate that reduction of ROS level by bioactive food components is directly resulted from the overexpression of Nrf2 target genes.
Figure 3.
Nrf2 activation by bioactive food components. (A) Control or BRCA1 specific siRNA treated cells were transfected with the NQO1-ARE-Luc vector. Indicated concentrations of bioactive food components or chemopreventive agents were incubated for 24 hrs. Data show fold increases of luciferase activity compare to control. * indicates p < 0.05 (control vs. bioactive food components treated in control-siRNA treated cells), and # indicates p < 0.05 (control vs. bioactive food components treated in BRCA1-siRNA treated cells). (B) After treatment of 5 μM of SFN for indicated time, expression of Nrf2 target proteins were measured by Western blotting. (C) ROS levels were measured after knockdown of BRCA1 and overexpression of UGT1A1.
3.4 Bioactive food components attenuate ROS accumulation
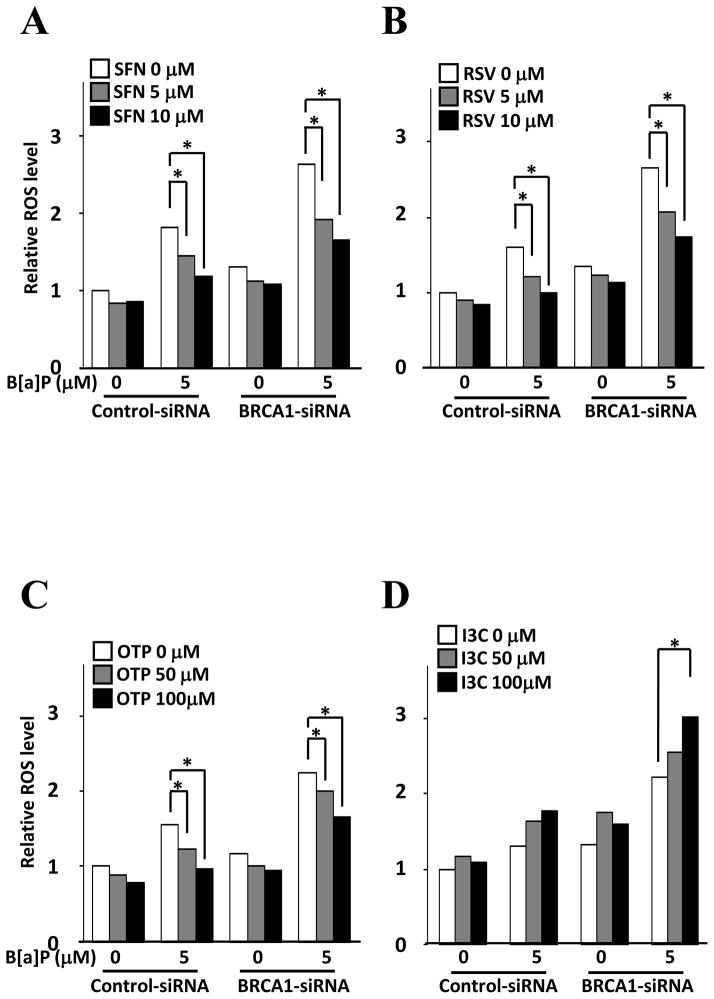
Since insufficient Nrf2 levels caused by BRCA1 deficiency renders cells more vulnerable to ROS accumulation, we investigated whether activation of Nrf2 by bioactive food components attenuates ROS accumulation in BRCA1 deficient cells. Pre-incubation of SFN significantly reduced B[a]P mediated ROS accumulation in both control and BRCA1 deficient cells (Fig. 4A). Incubation of RSV and OTP also significantly attenuated ROS accumulation in a dose-dependent manner (Fig. 4B and C). However, incubation of I3C augmented B[a]P mediated ROS accumulation (Fig. 4D), which was quite opposite from our expectation based on previous observation (Fig. 3A): I3C-mediated NQO1-ARE-Luc reporter activation.
Figure 4.
ROS accumulation after preincubation of bioactive food components. MCF10A cells pretreated with control or BRCA1 specific siRNA were incubated with indicated concentrations of SFN (A), RSV (B), OTP (C), and I3C (D) for 6 hrs then further incubated with B[a]P for 24 hrs. ROS accumulation was determined using CM-H2DCFDA.
3.5 Over-expression and activation of Nrf2 reduces DNA adduct formation
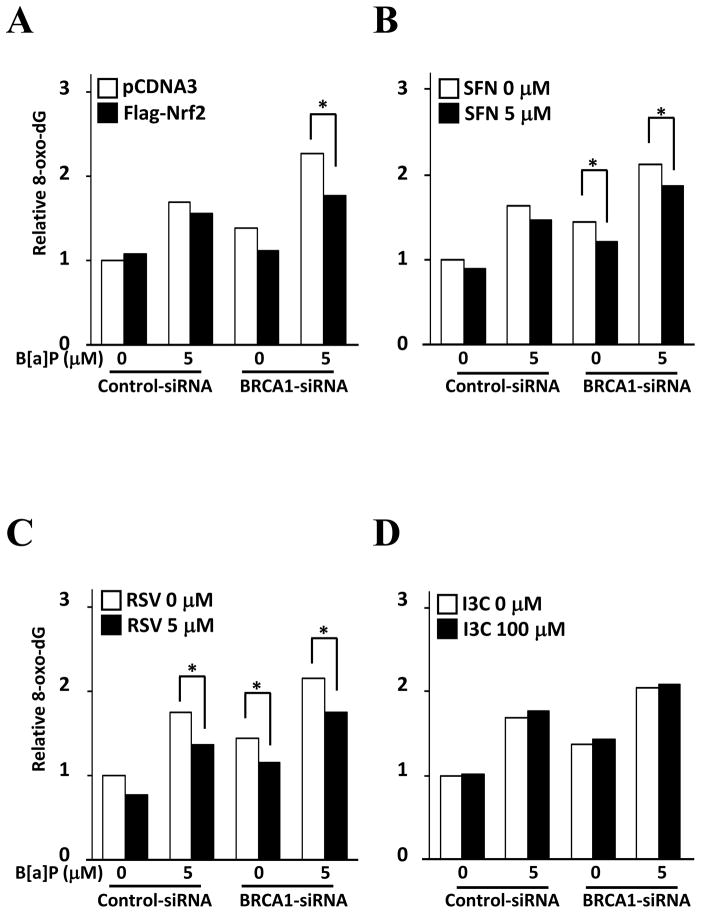
Accumulated ROS disturbs genomic DNA integrity through interaction of DNA with hydroxyl radicals. To assess whether BRCA1 deficiency increases the risk of oxidative stress-induced DNA damage, we measured these DNA damages using 8-oxodeoxyguanosine (8-oxo-dG), which is an oxidized derivative form of deoxyguanosine. Incubation of 5 μM B[a]P significantly increased 8-oxo-dG levels on genomic DNA extracted from BRCA1 deficient cells. In addition, overexpression of Nrf2 significantly reduced 8-oxo-dG level in BRCA1 deficient cell (Fig. 5A). Incubation of SFN and RSV also significantly reduced DNA damage in both BRCA1 deficient and proficient cells (Fig. 5B and C). However, incubation of I3C did not alter 8-oxo-dG level (Fig. 5D), which is consistent with data in Fig. 4D.
Figure 5.
Reduction of DNA damage by bioactive food components. Control or BRCA1 specific siRNA treated cells were transfected with Nrf2 expression vector for 24 hrs or preincubated with SFN, RSV, or I3C for 6 hrs, then incubated with B[a]P for 24 hrs. DNA damages by B[a]P were determined by measuring 8-oxo-guanine levels. Protection of DNA damage by (A) Nrf2 overexpression, (B) SFN, (C) RSV, and (D) I3C were compared to each control.
4. Discussion
Although several genetic factors have been identified in breast cancer, the majority of the cases are attributed to environmental factors. Moreover extensive twin studies revealed that inherited factors accounted for only one fourth of breast cancer risk (Lichtenstein et al., 2000). On the other hand, environmental factors such as xenobiotics are the main risk factors in breast cancer. To study the impact of those factors on breast cancer risk, various xenobiotics have been investigated through epidemiologic studies (Ibarluzea et al., 2004). The polycyclic aromatic hydrocarbons (PAHs) ubiquitously present in the environment and are acknowledged as breast carcinogens. They increase ROS generation during intracellular metabolism and ROS has been reported to result in malignant transformation of cellular macromolecules (Vinothini et al., 2000). In the study of mammary gland carcinogenesis, DMBA (7,12-dimethylbenz[a]anthracene) is most commonly used among PAHs (Keshava et al., 2001).
To further investigate our previous observations that BRCA1 deficient cells are more susceptible to tumorigenesis due to insufficient clearance of oxidative stress, we employed a model system using MCF10A cells and B[a]P. MCF10A cells have the closest phenotype to normal mammary epithelial cells among currently available cell lines. It has normal BRCA1 function and exhibits normal responses to oxidative stress via Nrf2 activation in our previous studies (Kang et al., 2011a). As an oxidative stress or a DNA damaging insult, we used B[a]P, a polycyclic aromatic hydrocarbon (PAH). Previously, we have shown that B[a]P induces oxidative stress and DNA adduct formation in breast and pancreatic cells (Kang et al., 2011a; Kang et al., 2011b). This model exhibited that cellular accumulation of ROS and 8-oxo-dG DNA is manipulated in accordance with B[a]P treatment and gene expression levels. Using this system, we demonstrated the cooperative responses of BRCA1 and Nrf2 to oxidative stresses and revealed the efficacy of SFN and RSV on the prevention of oxidative stress/DNA damage in mammary epithelial cells. Thus, our system may be useful for further screening of bioactive food components or chemopreventive agents, which suppress oxidative stress-induced tumorigenesis in breast cancer.
Recent epidemiological researches have consistently shown that increased dietary intake of fruits or vegetables is strongly associated with reduced risk of developing chronic diseases and cancers (Meeran et al., 2010; Patil et al., 2009). In this study, we demonstrated that bioactive food components such as SFN and RSV significantly decrease ROS accumulation and DNA damage in BRCA1 deficient mammary epithelial cells. Since incubation with SFN, RSV, or OTP increase Nrf2 activity, these effects might be derived from activation of Nrf2 and expression of phase II proteins. On the other hand, I3C failed to exhibit a chemopreventive effect against B[a]P by protecting from either ROS or DNA damage, although our reporter assay revealed that I3C also induces Nrf2 transcriptional activity. These results are consistent with the N-diethylnitrosamine administered rat model in which diet feeding of I3C significantly induced upregulation of Nrf2 downstream proteins, but oxidative stress and DNA damages were significantly increased (Shimamoto et al., 2011). The researchers attributed this phenomenon to “redox imbalance”, i.e., ROS production from I3C exceeds the capacity of the Nrf2 gene batteries. Taken together, these data demonstrate that we should be careful in choosing anti-oxidant dietary factors or their derivatives.
We have demonstrated that BRCA1 can modulate phase I and II systems upon xenobiotic stresses. BRCA1 is recruited to the promoter of CYP1A1 and CYP1B1 upon xenobiotic exposure, which results in activation of phase I. The possibility of direct interaction between BRCA1 and xenobiotic response elements (XREs) of the promoters of those genes are very low. Instead, we demonstrated the physical interaction between AD1 domain of BRCA1 and AhR (Kang et al., 2008a) and between N-terminal of BRCA1 and ARNT (Kang et al., 2006). Since Nrf2 contains XRE on its promoter regions and its transcripts are induced via XRE after TCDD treatment (Miao et al., 2005), BRCA1 may utilize the very same mechanism to regulate Nrf2 transcript via AhR-ARNT-XRE pathway. Our previous (Kang et al., 2011) and current studies demonstrated that BRCA1 positively regulates Nrf2 at transcriptional level.
From the novel finding that abrogation of BRCA1 reduces expression of anti-oxidant genes to the latest report that detoxification processes of UGTs on xenobiotic stresses are affected by BRCA1 deficiency (Kang et al., 2011a), we have raised a new hypothesis that BRCA1 plays a pivotal role in cellular defense systems against xenobiotic stresses, which eventually deteriorate DNA integrity. In this context, our results that BRCA1 deficiency renders breast cells more susceptible to oxidative stress and DNA damage is consistent with our hypothesis. Moreover, manipulation of Nrf2 levels also influenced the stress levels and complemented the BRCA1 deficiency. Thus, activation of Nrf2 can protect or delay BRCA1 deficient mediated tumorigenesis.
Currently, there are neither therapeutic options nor preventive agents, which specifically target BRCA1 mutant patients. Our findings that BRCA1 deficiency is more susceptible to xenobiotic stress and that bioactive food components reduce this stress may be a significant clue to the direction of breast cancer prevention efforts. Further experiments using animal models may clearly demonstrate the efficacy of bioactive food components.
Acknowledgments
Dr. Bae has been supported by the Susan G. Komen for the Cure (FAS0703858) and by grants R31-10069 (WCU program) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology. We appreciate BioMedText, Inc./Dr. Rashmi Nemade for helpful discussions and editing.
References
- Bae I, Fan S, Meng Q, Rih JK, Kim HJ, Kang HJ, Xu J, Goldberg ID, Jaiswal AK, Rosen EM. BRCA1 Induces Antioxidant Gene Expression and Resistance to Oxidative Stress. Cancer Res. 2004;64:7893–7909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- Deng CX, Brodie SG. Roles of BRCA1 and its interacting proteins. Bioessays. 2000;22:728–737. doi: 10.1002/1521-1878(200008)22:8<728::AID-BIES6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Elwell MR, Huff J, Barrett JC. Chemically induced mammary gland cancer in the National Toxicology Program’s carcinogenesis bioassay. Carcinogenesis. 1995;16:173–179. doi: 10.1093/carcin/16.2.173. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho E, Clarke J, Dashwood R. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J Nutr. 2009;139:2393–2396. doi: 10.3945/jn.109.113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarluzea JM, Fernández MF, Santa-Marina L, Olea-Serrano MF, Rivas AM, Aurrekoetxea JJ, Expósito J, Lorenzo M, Torné P, Villalobos M, Pedraza V, Sasco AJ, Olea N. Breast cancer risk and the combined effect of environmental estrogens. Cancer Causes Control. 2004;15:591–600. doi: 10.1023/B:CACO.0000036167.51236.86. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz DC, Wilkinson KD, Maul GG, Barlev N, Berger SL, Prendergast GC, Rauscher FJ., 3rd BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim HJ, Kim SK, Barouki R, Cho CH, Khanna KK, Rosen EM, Bae I. BRCA1 modulates xenobiotic stress-inducible gene expression by interacting with ARNT in human Breast Cancer Cells. J Biol Chem. 2006;281:14654–14662. doi: 10.1074/jbc.M601613200. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim HJ, Cho CH, Hu Y, Li R, Bae I. BRCA1 transcriptional activity is enhanced by interactions between its AD1 domain and AhR. Cancer Chemother Pharmacol. 2008a;62:965–975. doi: 10.1007/s00280-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kim HJ, Kwon SH, Kang BD, Eling TE, Lee SH, Bae I. BRCA1 modulates sensitivity to 5F-203 by regulating xenobiotic stress-inducible protein levels and EROD activity. Cancer Chemother Pharmacol. 2008b;62:689–697. doi: 10.1007/s00280-007-0657-7. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Hong YB, Kim HJ, Rodriguez OC, Nath RG, Tilli EM, Albanese C, Chung FL, Kwon SH, Bae I. Detoxification: a novel function of BRCA1 in tumor suppression? Toxicol Sci. 2011a;122:26–37. doi: 10.1093/toxsci/kfr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Hong YB, Kim HJ, Yi YW, Nath RG, Chang YS, Cho HC, Bae I. A novel in vitro pancreatic carcinogenesis model. Toxicol Lett. 2011b;202:15–22. doi: 10.1016/j.toxlet.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshava N, Mandava U, Kirma N, Tekmal RR. Acceleration of mammary neoplasia in aromatase transgenic mice by 7,12-dimethylbenz[a]anthracene. Cancer Lett. 2001;167:125–133. doi: 10.1016/s0304-3835(01)00478-5. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and Heritable Factors in the Causation of Cancer: Analyses of Cohorts of Twins From Sweden, Denmark and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5:e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- Nathanson KL, Wooster R, Weber BL, Nathanson KN. Breast cancer genetics: what we know and what we need. Nat Med. 2001;7:552–556. doi: 10.1038/87876. [DOI] [PubMed] [Google Scholar]
- Patil B, Jayaprakasha G, Chidambara Murthy K, Vikram A. Bioactive compounds: historical perspectives, opportunities, and challenges. J Agric Food Chem. 2009;57:8142–8160. doi: 10.1021/jf9000132. [DOI] [PubMed] [Google Scholar]
- Pledgie-Tracy A, Sobolewski M, Davidson N. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Ruffner H, Jiang W, Craig AG, Hunter T, Verma IM. BRCA1 is phosphorylated at serine 1497 in vivo at a cyclin-dependent kinase 2 phosphorylation site. Mol Cell Biol. 1999;19:4843–4854. doi: 10.1128/mcb.19.7.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, Darjatmoko SR, Albert DM, Polans AS. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol Pharmacol. 2007;72:1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston DM. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2000;61:1604–1610. [PubMed] [Google Scholar]
- Shimamoto K, Dewa Y, Ishii Y, Kemmochi S, Taniai E, Hayashi H, Imaoka M, Morita R, Kuwata K, Suzuki K, Shibutani M, Mitsumori K. Indole-3-carbinol enhances oxidative stress responses resulting in the induction of preneoplastic liver cell lesions in partially hepatectomized rats initiated with diethylnitrosamine. Toxicology. 2011;283:109–117. doi: 10.1016/j.tox.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Vinothini G, Nagini S. Correlation of xenobiotic-metabolizing enzymes, oxidative stress and NFkappaB signaling with histological grade and menopausal status in patients with adenocarcinoma of the breast. Clin Chim Acta. 2010;411:368–374. doi: 10.1016/j.cca.2009.11.034. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Collman GW, Barrett JC, Huff J. Breast cancer and environmental risk factors: epidemiological and experimental findings. Annu Rev Pharmacol Toxicol. 1996;36:573–596. doi: 10.1146/annurev.pa.36.040196.003041. [DOI] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]