Dear Editor
Platinum compounds, such as cisplatin, carboplatin, and oxaliplatin, are widely used as anti-cancer chemotherapeutic drugs in in vitro cell culture, animal preclinical and clinic studies [1,2]. Unlike other organic compounds which are routinely dissolved in organic solvents such as dimethyl sulfoxide (DMSO) for in vitro assay, platinum compounds are recommended to be dissolved in water-based solvents, especially for its clinic usage: 0.9% NaCl for cisplatin and 5% glucose (dextrose) for carboplatin and oxaliplatin [3]. However, platinum compounds dissolved in organic solvents, are still being used for in vitro studies in many laboratories. We repeatedly experience the loss or attenuation of cell killing effect of cisplatin when stored for a certain period of time in DMSO. Storage of cisplatin/DMSO in small aliquots at −20°C conditions also did not preserve the potency of cisplatin. Although stability of platinum compounds in infusion solutions (0.9% NaCl for cisplatin and 5% glucose for carboplatin and oxaliplatin) has been widely investigated [4–7], there are no reports on in vitro potencies or stabilities of platinum compounds in different solvents.
We chose water-based solvents (0.9% NaCl for cisplatin and 5% glucose for carboplatin and oxaliplatin), dimethylformamide (DMF), and DMSO to determine solvents’ effects on the potencies of three platinum compounds: cisplatin, carboplatin and oxaliplatin. Cisplatin and carboplatin were obtained from Sigma (St. Louis, MO), and oxaliplatin was purchased from LC Labs (Woburn, MA). Each platinum compound was dissolved in water-based solvents, DMF, and DMSO as follows: cisplatin, 5 mM in 0.9% NaCl, 25 mM in DMF, and 25 mM in DMSO; carboplatin, 5 mM in 5% glucose, 10 mM in DMF, and 25 mM in DMSO; oxaliplatin, 5 mM in 5% glucose, 10 mM in DMF, and 25 mM in DMSO. The newly prepared stock solutions were stored at 4°C in the refrigerator and further diluted in culture media to the desired concentration immediately before use. Human ovarian cancer cell lines (A2780) were purchased from Sigma and maintained as recommended [8]. For cell viability assays, A2780 cells were subcultured at a density of 2,000 cells/well in 96-well plates the day before treatment. Diluted platinum compounds were added to cells in triplicate for 72 hr and viable cells were measured by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay that is widely used for rapid determination of cell viability in the drug discovery field [9].
Since we are interested in the immediate potencies of the three platium compounds, each compound was freshly prepared in the three different solvents and was immediately added to the A2780 cells. EC50 values were calculated by CompuSyn software (ComboSyn Inc., Paramus, NJ). As shown in Table 1, all three platinum compounds dissolved in either DMF or DMSO showed slightly reduced potencies in A2780 cells compared to those in water-based solvents.
Table 1.
EC50 values of freshly prepared platinum compounds on proliferation of A2780 cells.
| Cells | Compound | H2O* | DMF | DMSO |
|---|---|---|---|---|
| A2780 | Cisplatin | 0.336 ± 0.069 | 0.497 ± 0.305 | 0.811 ± 0.131 |
| Carboplatin | 2.006 ± 0.342 | 14.248 ± 2.051 | 6.960 ± 3.072 | |
| Oxaliplatin | 0.132 ± 0.077 | 0.227 ± 0.096 | 0.223 ± 0.011 |
0.9% NaCl solution for cisplatin, 5% glucose solution for carboplatin and oxaliplatin.
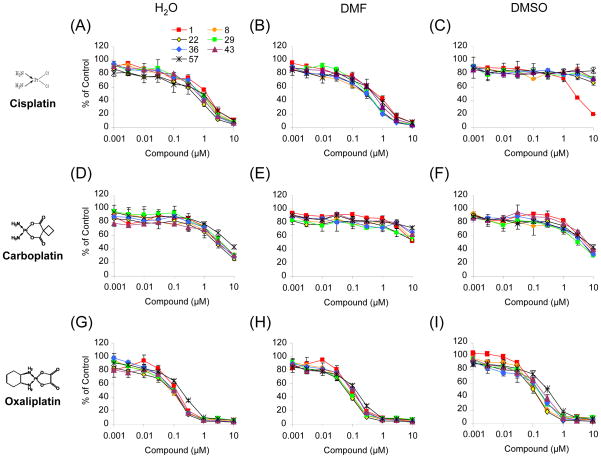
For long-term storage effects we performed MTT assays with the treatment of platinum compounds to A2780 cells at 8, 22, 29, 36, 43, and 57-days after preparation of stock solutions. Strikingly, cisplatin in DMSO nearly completely lost its potency to A2780 cells within 8-days after preparation (Fig. 1C). On the contrary, no significant loss of cell killing effects was observed with cisplatin in 0.9% NaCl or DMF for up to 57-days of storage at 4°C (Fig. 1A and C). Visible precipitates were observed in the 5 mM stock solution of cisplatin dissolved in 0.9% NaCl stored in the refrigerator, but these did not affect the potency of cisplatin. In case of carboplatin and oxaliplatin, the potency of each compound was maintained at least 43-days after preparation in 5% glucose solution with relatively higher potencies than those prepared in DMF or DMSO (Fig. 1D and G vs. Fig. 1E, F, H, and I).
Fig. 1. Cytotoxicity of cisplatin, carboplatin, and oxaliplatin after storage of stock solutions at 4°C.
A2780 cells were subcultured at 2,000 cells/well in 96-well plates the day before treatment. Cells were treated with indicated concentrations of platinum compounds at 1, 8, 22, 29, 36, 43, and 57-days after preparation of stock solutions. Viable cells were measured by MTT assay. Data are mean ± S.D. performed in triplicate.
These results suggest that platinum compounds prepared in water-based solvents and stored at 4°C can be used without significant loss of potency for up to 40-days after preparation.
Acknowledgments
This work was supported by Susan G. Komen for the Cure (FAS0703858) and by R31-10069 (WCU Program) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
References
- 1.Muggia F. Platinum compounds 30 years after the introduction of cisplatin: implications for the treatment of ovarian cancer. Gynecol Oncol. 2009;112:275–281. doi: 10.1016/j.ygyno.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Olszewski U, Hamilton G. A better platinum-based anticancer drug yet to come? 2010;10:293–301. doi: 10.2174/187152010791162306. [DOI] [PubMed] [Google Scholar]
- 3.Trissel LA, Zhang Y. Physical and chemical stability of palonosetron HCl with cisplatin, carboplatin, and oxaliplatin during simulated Y-site administration. J Oncol Pharm Pract. 2004;10:191–195. [Google Scholar]
- 4.Schuldes H, Bade S, Knobloch J, Jonas D. Loss of in vitro cytotoxicity of cisplatin after storage as stock solution in cell culture medium at various temperature. Cancer. 1997;79:1723–1728. [PubMed] [Google Scholar]
- 5.Cubells MP, Aixela JP, Bru VG. Pharm World Sci. 1993;15:34–36. the container’s material. [Google Scholar]
- 6.Gust R, Schnurr B. Investigations on the stability of carboplatin infusion solutions. Monatsh Chem. 1999;130:637–644. [Google Scholar]
- 7.Junker A, Roy S, Desroches MC, Moussay C, Berhoune M, Bellanger A, Fernandez C, Farinotti R. Stability of oxaliplatin solution. Ann Pharmacother. 2009;43:390–391. doi: 10.1345/aph.1L122. [DOI] [PubMed] [Google Scholar]
- 8.Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young RC, Ozols RF. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987;47:414–418. [PubMed] [Google Scholar]
- 9.Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov. 2011;10:221–237. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]