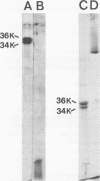
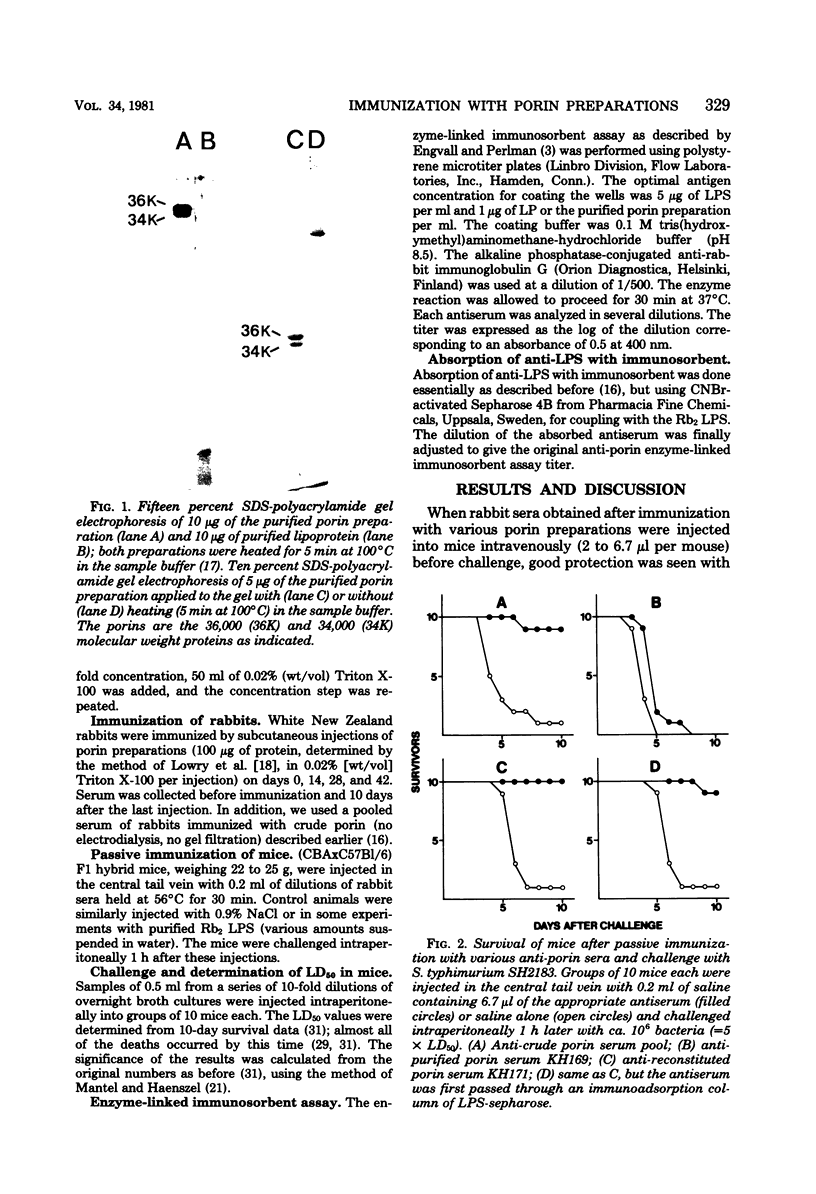
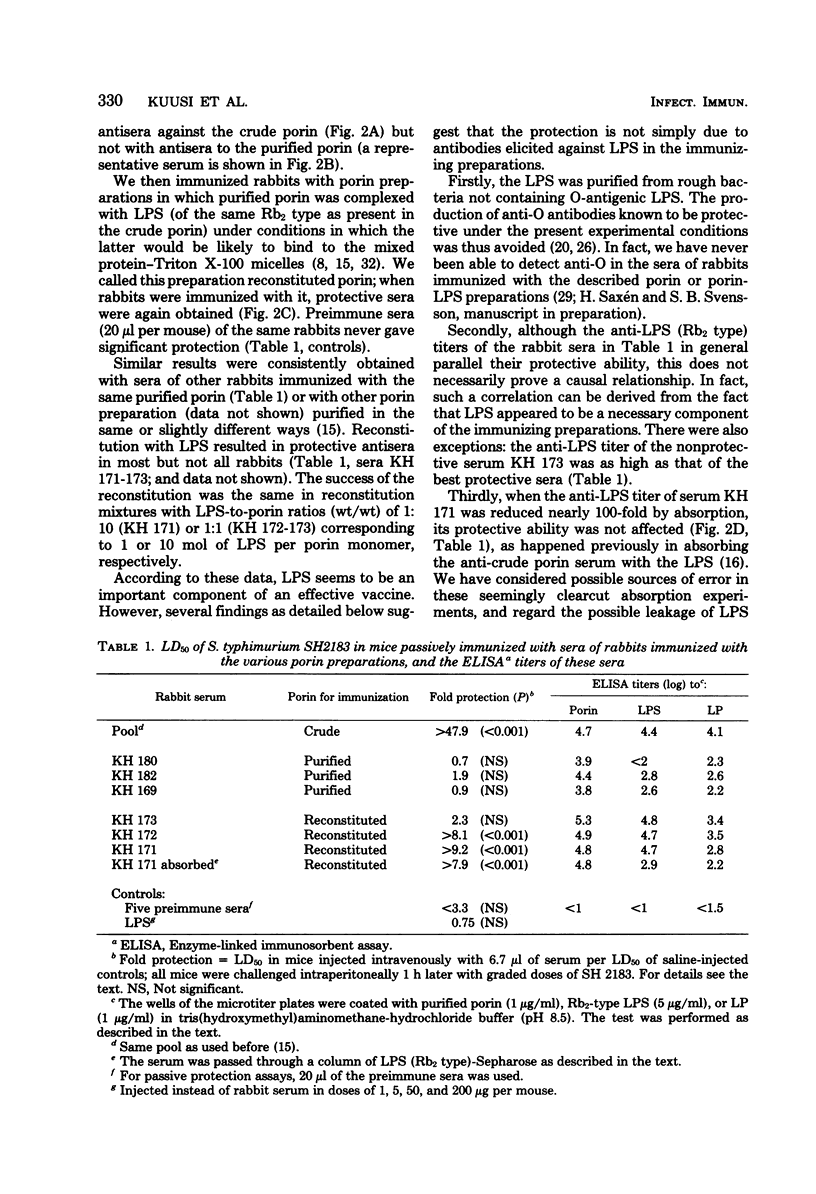
Abstract
A crude major outer membrane (porin) preparation, obtained from a rough strain of Salmonella typhimurium earlier shown to be protective both in active and passive immunization of mice against challenge with smooth S. typhimurium, was further purified. Removal of the main impurities, lipopolysaccharide (LPS) and lipoprotein, was accompanied by loss of protective capacity in passive immunization experiments. Reconstitution with rough LPS restored the protective capacity. Protection was, however, concluded not to be due to anti-LPS, because a large fraction of the anti-LPS antibodies could be removed from the protective rabbit antiserum with an LPS immunosorbent without loss of protection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Helenius A., McCaslin D. R., Fries E., Tanford C. Properties of detergents. Methods Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- Henning U., Jann K. Two-component nature of bacteriophage T4 receptor activity in Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):664–666. doi: 10.1128/jb.137.1.664-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Preparation and quantitative determination of antibodies against major outer mambranes proteins of Escherichia coli O26 K60. J Gen Microbiol. 1980 Apr;117(2):437–447. doi: 10.1099/00221287-117-2-437. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Van Tol J. D., Dankert J. Cross-reactivity of major outer membrane proteins of Enterobacteriaceae, studied by crossed immunoelectrophoresis. J Bacteriol. 1980 Jul;143(1):328–337. doi: 10.1128/jb.143.1.328-337.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Arrangement of proteins O-8 and O-9 in outer membrane of Escherichia coli K-12. Existence of homotrimers and heterotrimers. Eur J Biochem. 1979 Oct 15;100(2):321–328. doi: 10.1111/j.1432-1033.1979.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Sarvas M. Immunochemical characterization of major outer membrane components from Salmonella typhimurium. Infect Immun. 1981 Sep;33(3):750–757. doi: 10.1128/iai.33.3.750-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyman M. B., Stocker B. A., Roantree R. J. Evaluation of the immune response directed against the Salmonella antigenic factors O4,5 and O9. Infect Immun. 1979 Dec;26(3):956–965. doi: 10.1128/iai.26.3.956-965.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- Mutoh N., Furukawa H., Mizushima S. Role of lipopolysaccharide and outer membrane protein of Escherichia coli K-12 in the receptor activity for bacteriophage T4. J Bacteriol. 1978 Nov;136(2):693–699. doi: 10.1128/jb.136.2.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Ishii J., Tokunaga M. Subunit structure of functional porin oligomers that form permeability channels in the other membrane of Escherichia coli. J Biol Chem. 1979 Mar 10;254(5):1457–1461. [PubMed] [Google Scholar]
- Nurminen M., Lounatmaa K., Sarvas M., Mäkelä P. H., Nakae T. Bacteriophage-resistant mutants of Salmonella typhimurium deficient in two major outer membrane proteins. J Bacteriol. 1976 Aug;127(2):941–955. doi: 10.1128/jb.127.2.941-955.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornellas E. P., Roantree R. J., Steward J. P. The specificity and importance of humoral antibody in the protection of mice against intraperitoneal challenge with complement-sensitive and complement-resistant Salmonella. J Infect Dis. 1970 Feb;121(2):113–123. doi: 10.1093/infdis/121.2.113. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Randall L. L. Arrangement of protein I in Escherichia coli outer membrane: cross-linking study. J Bacteriol. 1978 Jan;133(1):279–286. doi: 10.1128/jb.133.1.279-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Yu F., Ichihara S., Mizushima S. A major outer membrane protein (O-8) of Escherichia coli K-12 exists as a trimer in sodium dodecyl sulfate solution. FEBS Lett. 1979 Apr 1;100(1):71–74. doi: 10.1016/0014-5793(79)81133-3. [DOI] [PubMed] [Google Scholar]