Abstract
CypD (cyclophilin D) has been established as a critical regulator of the MPT (mitochondrial permeability transition) pore, and pharmacological or genetic inhibition of CypD attenuates MPT in numerous systems. However, it has recently been suggested that the inhibitory effects of CypD inhibition only manifest when Pi (inorganic phosphate) is present, and that inhibition is lost when Pi is replaced by Asi (inorganic arsenate) or Vi (inorganic vanadate). To test this, liver mitochondria were isolated from wild-type and CypD-deficient (Ppif−/−) mice and then incubated in buffer containing Pi, Asi or Vi. MPT was induced under both energized and de-energized conditions by the addition of Ca2+, and the resultant mitochondrial swelling was measured spectrophotometrically. For pharmacological inhibition of CypD, wild-type mitochondria were pre-incubated with CsA (cyclosporin A) before the addition of Ca2+. In energized and de-energized mitochondria, Ca2+ induced MPT regardless of the anion present, although the magnitude differed between Pi, Asi and Vi. However, in all cases, pre-treatment with CsA significantly inhibited MPT. Moreover, these effects were independent of mouse strain, organ type and rodent species. Similarly, attenuation of Ca2+ -induced MPT in the Ppif−/− mitochondria was still observed irrespective of whether Pi, Asi or Vi was present. We conclude that the pharmacological and genetic inhibition of CypD is still able to attenuate MPT even in the absence of Pi.
Keywords: arsenate, cyclophilin D, cyclosporin A, mitochondrial permeability transition (MPT), phosphate, vanadate
INTRODUCTION
The MPT (mitochondrial permeability transition) pore is a non-selective channel in the inner mitochondrial membrane, the opening of which is a critical event in the progression of cell death and therefore disease (see [1–4] for reviews). Although the specific molecular composition of the MPT pore is still highly debated, pharmacological, biochemical and genetic studies have demonstrated that the matrix peptidylprolyl isomerase CypD (cyclophilin D) is a key regulator of the MPT pore [5–12]. Consequently, pharmacological inhibitors of CypD such as CsA (cyclosporin A), sanglifehrin A and Debio 025 are effective in attenuating the MPT response [5,13,14], as is genetic disruption of the CypD-encoding Ppif (peptidylprolyl isomerase F) locus [10–12]. Such chemical/genetic tools have thus been used to establish a critical role for CypD-dependent MPT in the pathogenesis of several diseases, including cardiac [10,12], cerebral [11] and renal [15] ischaemia, muscular dystrophy [14] and neurodegenerative diseases such as Alzheimer’s disease [16] and multiple sclerosis [17].
Many factors, including Ca2+, reactive oxygen species, adenine nucleotides, pH, Mg2+, ubiquinones and voltage changes, regulate the MPT pore [1–4]. In particular, elevations in mitochondrial matrix Ca2+ are a primary trigger for MPT pore opening, and it has been well established that the presence of Pi (inorganic phosphate) can greatly increase the sensitivity of the MPT pore to the effects of this cation [5,18,19]. In contrast, inhibition of CypD appears to attenuate the MPT response by decreasing the sensitivity of the MPT pore to matrix Ca2+ [9–12].
A previous study, however, has suggested that Pi may have a dual effect on the MPT pore such that it can also act as a specific inhibitor of the MPT pore as well as a sensitizer [20]. In that study, it was found that inhibition of CypD, either by gene targeting or with CsA, only attenuated the MPT response when Pi was present, but not when Pi was replaced by other inorganic anions such as arsenate (Asi) or vanadate (Vi). It was consequently proposed that CypD normally masks a Pi-specific inhibitory site on the MPT pore such that blockade/removal of CypD unmasks this site and enables Pi to bind the pore and lower its sensitivity to Ca2+ [20]. However, a subsequent study has reported that substitution of Asi for Pi does not affect the ability of CsA to inhibit MPT in both energized and de-energized mitochondria [21].
Consequently, it has yet to be conclusively proven that the effects of CypD inactivation on MPT require an inhibitory action of Pi on the MPT pore. To this end, we wanted to test whether substitution of either Asi or Vi for Pi altered the ability of genetic and/or pharmacological CypD to inhibit MPT. We found that mouse liver mitochondria either treated with CsA or lacking CypD still exhibited an attenuated MPT response when Asi or Vi was used in lieu of Pi. Moreover, these findings were not dependent on the energetic state of the mitochondria, the organ or the strain of mouse from which they were isolated. Thus the ability of pharmacological and genetic inhibition of CypD to attenuate MPT does not appear to be dependent on Pi.
EXPERIMENTAL
Reagents
All chemicals/reagents were from either Fisher Scientific or Sigma–Aldrich.
Mice and rats
The pure strain FVB and C57BL/6 mice used for the CsA studies were purchased from Taconic. The Ppif+/+ and Ppif−/− mice on a mixed C57BL/6-sv129 background have been described previously [12]. Sprague–Dawley rats were purchased from Taconic. Both the mice and rats were used at ~8–9 weeks of age. All of the experiments involving mice and rats were approved by the University of Missouri Animal Care and Use Committee and conformed to NIH Guidelines for the Care and Use of Animals.
Mitochondrial isolation and swelling
Liver and kidney mitochondria were prepared from the different mice and rats by differential centrifugation in sucrose-based medium, as described previously [12]. The protein concentration was determined using the Bradford assay (Bio-Rad Laboratories), and the mitochondria were resuspended in swelling buffer at 0.25 mg/ml. For energized conditions, the buffer (pH 7.4) consisted of 120 mM KCl, 10 mM Tris/HCl, 5 mM glutamate, 2.5 mM malate and 5 mM KH2PO4, KH2 AsO4 or Na3VO4. The same buffer, but without glutamate and malate, was used for de-energized conditions. When necessary, the MPT inhibitor CsA (1 µM) was included in the swelling buffer. MPT was measured spectrophotometrically as a decrease in light scattering at 520 nm [12] and was induced by the addition of 25 µM (energized) or 250 µM (de-energized) CaCl2.
Statistical analyses
Statistical significance was calculated using Student’s t test. A P value <0.05 was considered statistically significant.
RESULTS
CsA can still inhibit MPT in the absence of Pi
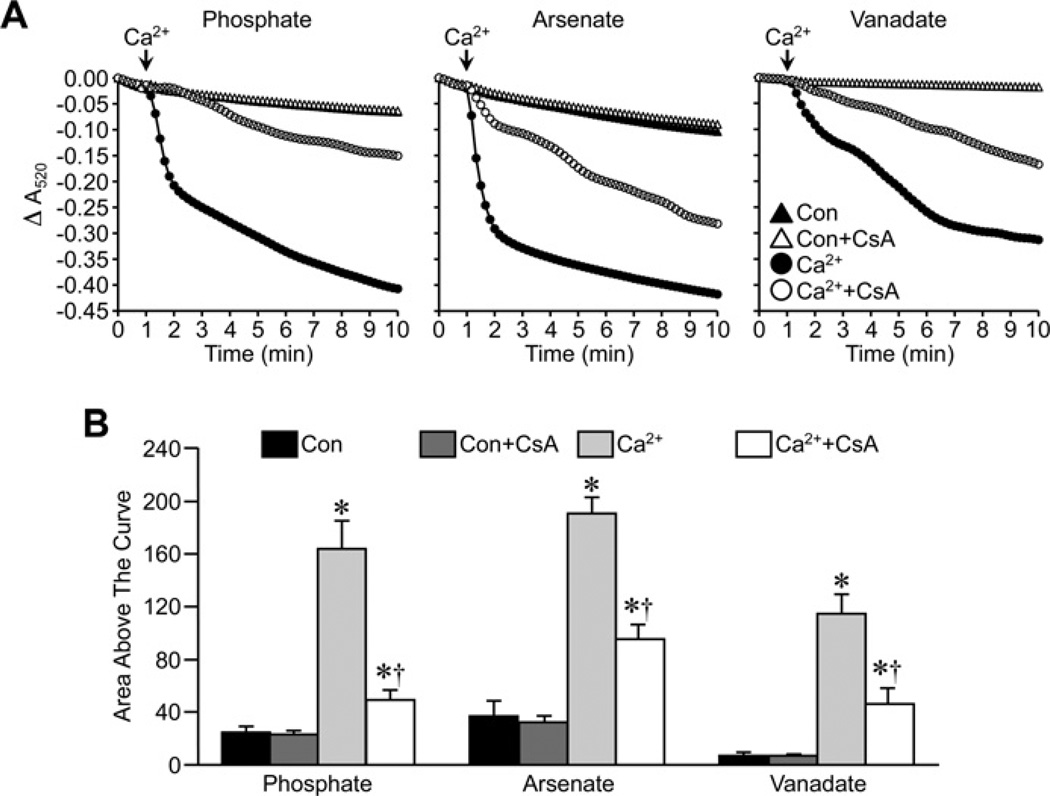
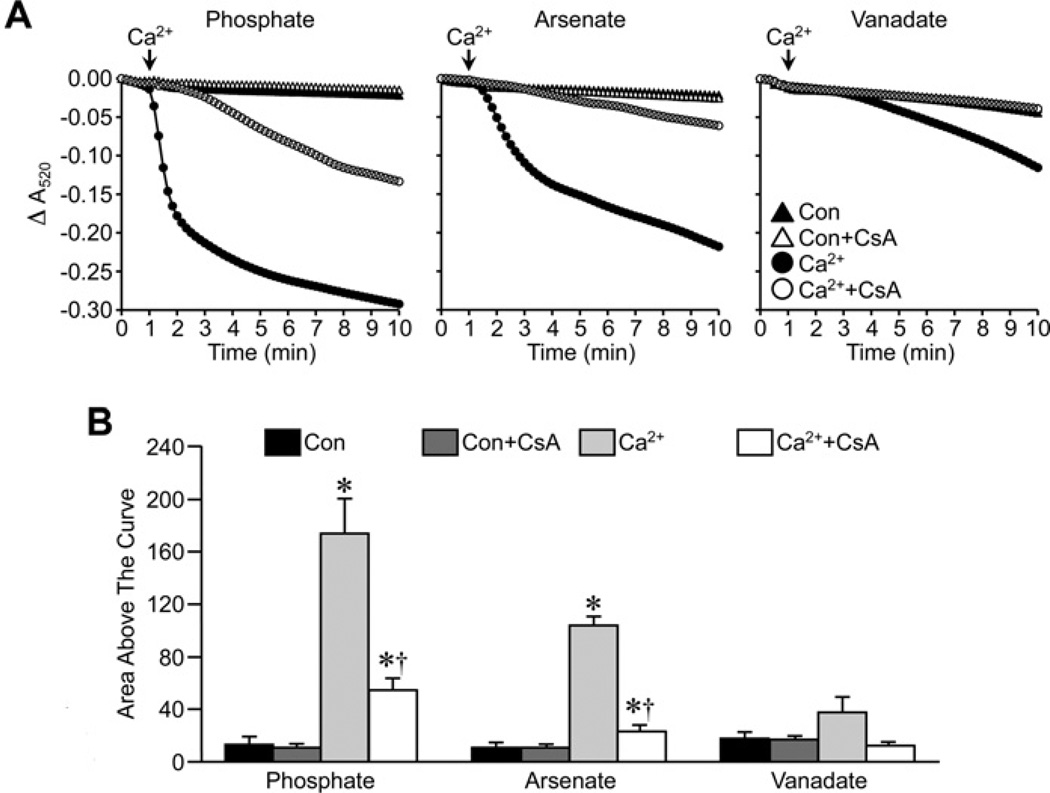
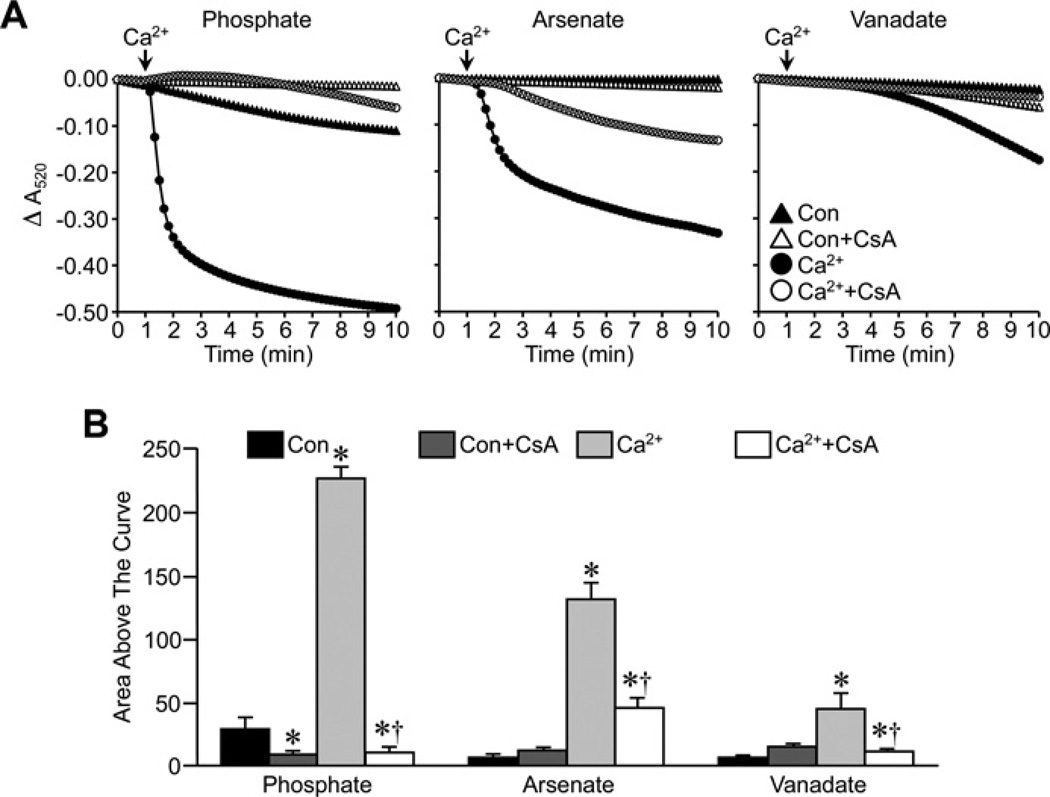
We first examined the effects of Pi replacement on the ability of CsA to attenuate MPT in energized liver mitochondria from FVB mice. Substitution of either Asi or Vi for Pi did not affect the ability of Ca2+ to induce large-amplitude swelling of liver mitochondria (Figure 1). There were subtle differences in the magnitude of the swelling, with the greatest response observed with Asi, followed by Pi and then Vi. However, despite these differences, pre-treatment of the mitochondria with 1 µM CsA was able to significantly inhibit the swelling response irrespective of which anion was present in the buffer (Figure 1). We then repeated these studies in de-energized mitochondria. Interestingly, under these conditions, the largest swelling response was observed with Pi, rather than with Asi, with the least observed in the presence of Vi (Figure 2). Again, however, CsA was still able to significantly attenuate mitochondrial swelling whether the anion was Pi, Asi or Vi (Figure 2).
Figure 1. Inhibition of MPT by CsA in energized mitochondria does not require Pi.
(A) Energized liver mitochondria from FVB mice were incubated in swelling buffer containing 5 mM Pi, Asi or Vi with or without 1 µM CsA. Mitochondrial swelling was then induced by the addition of 25 µM CaCl2 and monitored as a decrease in absorbance (A520). (B) Group data, calculated as the area above the curve, for the swelling curves shown in (A). All results shown are representative of four independent experiments and are means ± S.E.M. *P < 0.05 compared with control (Con) and †P < 0.05 compared with Ca2+ alone.
Figure 2. Inhibition of MPT by CsA in de-energized mitochondria does not require Pi.
(A) De-energized liver mitochondria from FVB mice were incubated in swelling buffer containing 5 mM Pi, Asi or Vi with or without 1 µM CsA. Mitochondrial swelling was then induced by the addition of 250 µM CaCl2 and monitored as a decrease in absorbance (A520). (B) Group data, calculated as the area above the curve, for the swelling curves shown in (A). All results shown are representative of five independent experiments and are means ± S.E.M. *P < 0.05 compared with control (Con) and †P < 0.05 compared with Ca2+ alone.
The Pi-independent effects of CsA are not strain-, tissue- or species-dependent
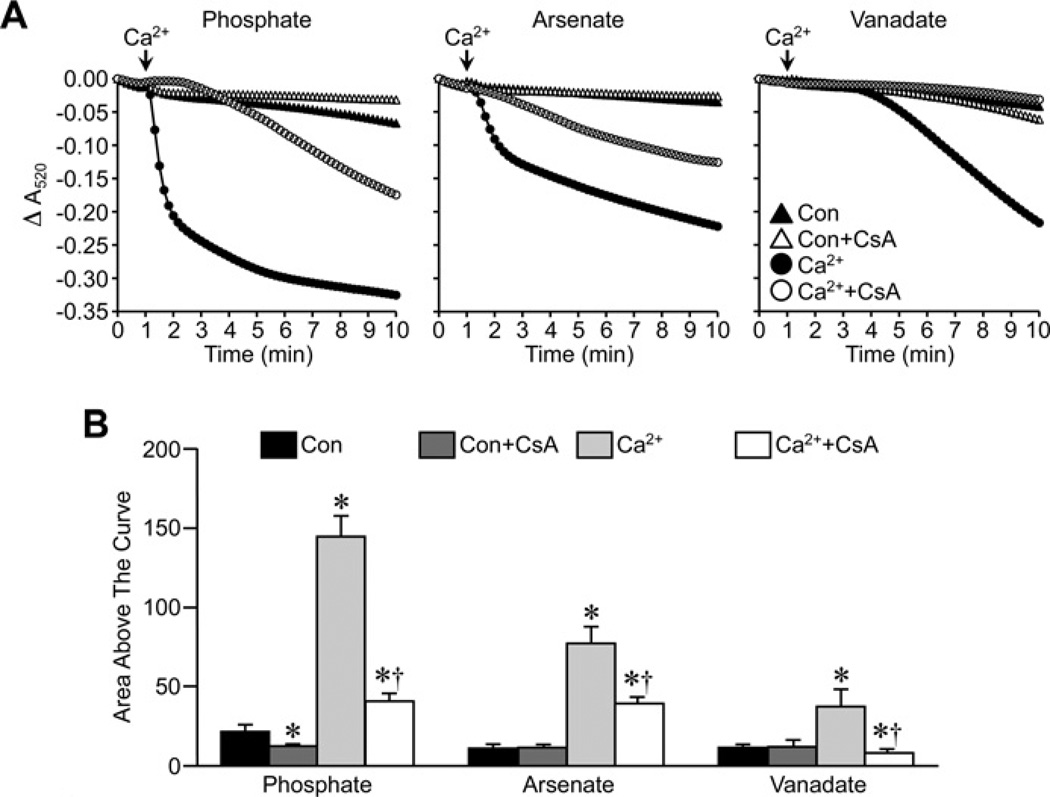
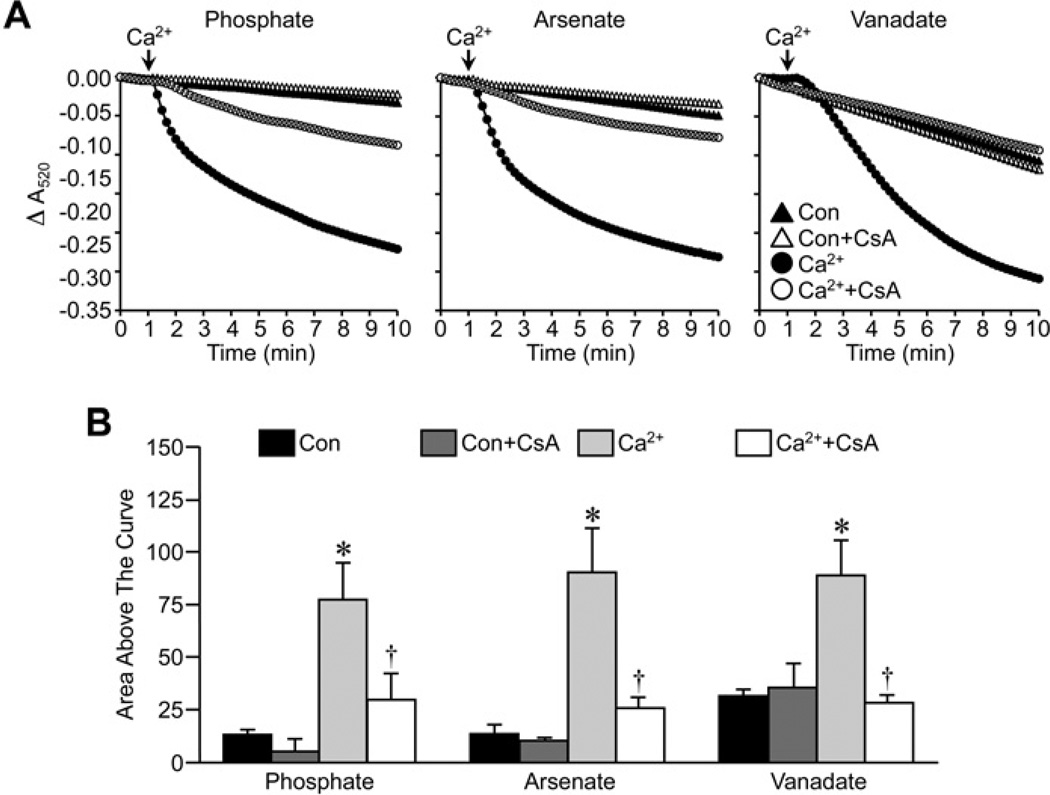
In the experiments described above, we utilized the FVB inbred mouse strain, as this is a strain commonly used for the generation of transgenic animals. However, the previous study suggesting an inhibitory role for Pi in MPT [20] used C57BL/6 mice. Therefore it was possible that the negative results observed in our FVB mitochondria were simply due to strain differences. To test for this we re-examined the effects of CsA in de-energized liver mitochondria isolated from C57BL/6 mice. The magnitude of the MPT response to Ca2+ observed in the presence of Pi, Asi or Vi followed the same pattern in the C57BL/6 liver mitochondria (Figure 3) as it did in the FVB liver mitochondria, i.e. Pi>Asi>Vi. Importantly, 1 µM CsA was still able to attenuate swelling in the C57BL/6 mitochondria independent of the species of anion (Figure 3). We also examined whether tissue differences existed by isolating kidney mitochondria. Interestingly, unlike liver mitochondria, the degree of swelling in response to Ca2+ was similar between the three anions in de-energized kidney mitochondria (Figure 4). However, in all three cases, CsA was still able to inhibit MPT (Figure 4). Finally, we assessed whether mouse liver mitochondria behaved differently from rat liver mitochondria. However, in the rat organelles we obtained results identical with those seen with the mice (Figure 5), both in terms of the Pi> Asi> Vi hierarchy of swelling amplitude and the ability of CsA to attenuate MPT independent of the anion.
Figure 3. Inhibition of MPT by CsA in mitochondria from C57BL/6 mice does not require Pi.
(A) De-energized liver mitochondria from C57BL/6 mice were incubated in swelling buffer containing 5 mM Pi, Asi or Vi with or without 1 µM CsA. Mitochondrial swelling was then induced by the addition of 250 µM CaCl2 and monitored as a decrease in absorbance (A520). (B) Group data, calculated as the area above the curve, for the swelling curves shown in (A). All results shown are representative of five independent experiments and are means ± S.E.M. *P < 0.05 compared with control (Con) and †P < 0.05 compared with Ca2+ alone.
Figure 4. Inhibition of MPT by CsA in mouse kidney mitochondria does not require Pi.
(A) De-energized kidney mitochondria from FVB mice were incubated in swelling buffer containing 5 mM Pi, Asi or Vi with or without 1 µM CsA. Mitochondrial swelling was then induced by the addition of 250 µM CaCl2 and monitored as a decrease in absorbance (A520). (B) Group data, calculated as the area above the curve, for the swelling curves shown in (A). All results shown are representative of four independent experiments and are means ± S.E.M. *P < 0.05 compared with control (Con) and †P < 0.05 compared with Ca2+ alone.
Figure 5. Inhibition of MPT by CsA in rat liver mitochondria does not require Pi.
(A) De-energized liver mitochondria from Sprague–Dawley rats were incubated in swelling buffer containing 5 mM Pi, Asi or Vi with or without 1 µM CsA. Mitochondrial swelling was then induced by the addition of 250 µM CaCl2 and monitored as a decrease in absorbance (A520). (B) Group data, calculated as the area above the curve, for the swelling curves shown in (A). All results shown are representative of four independent experiments and are means ± S.E.M. *P < 0.05 compared with control (Con) and †P < 0.05 compared with Ca2+ alone.
Inhibition of MPT by Ppif gene targeting is still present in the absence of Pi
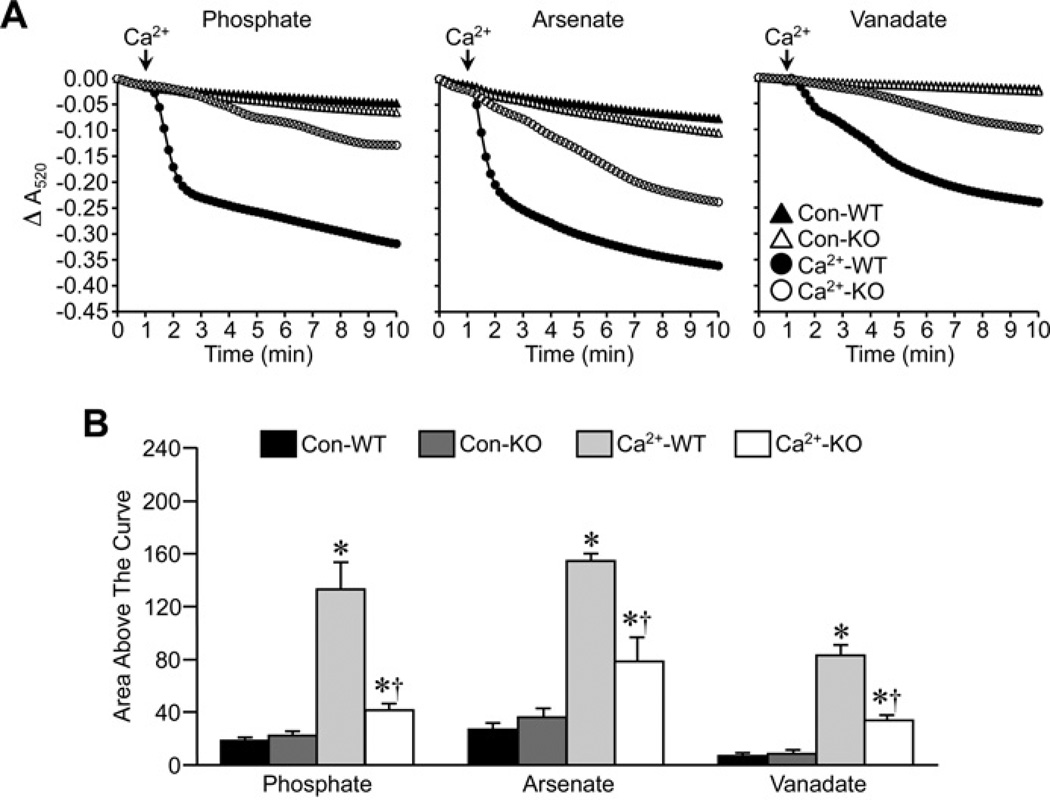
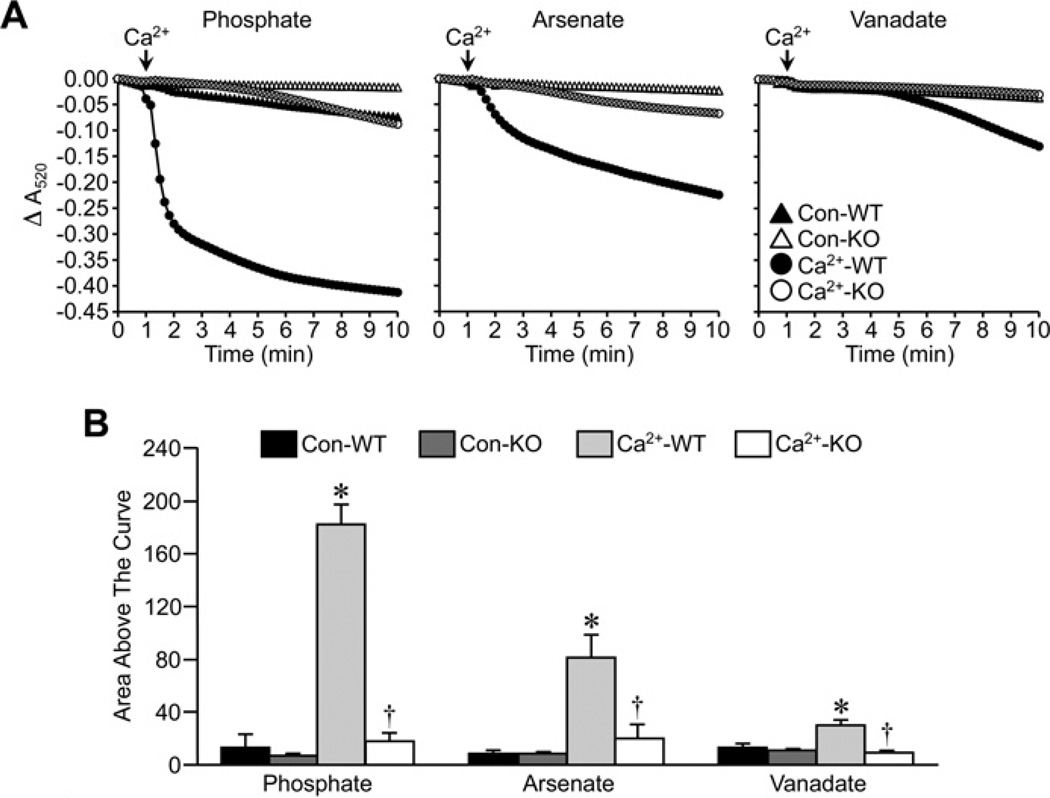
In the final set of experiments, we tested the effects of Pi substitution on the inhibitory effect of Ppif (the locus encoding CypD) gene ablation on MPT. Ca2+ was able to induce robust swelling of energized Ppif+/+ liver mitochondria in buffer that contained Pi, Asi or Vi (Figure 6), following the same pattern as that seen in the CsA experiments. In all three cases, the degree of swelling was markedly blunted in the CypD-lacking Ppif−/− mitochondria (Figure 6). This inhibitory effect was even more pronounced in de-energized Ppif−/− mitochondria, and was again independent of the anion species (Figure 7).
Figure 6. Inhibition of MPT by Ppif gene targeting in energized mitochondria does not require Pi.
(A) Energized liver mitochondria from Ppif+/+ (WT) and Ppif−/− (KO) mice were incubated in swelling buffer containing 5 mM Pi, Asi or Vi. Mitochondrial swelling was then induced by the addition of 25 µM CaCl2 and monitored as a decrease in absorbance (A520). (B) Group data, calculated as the area above the curve, for the swelling curves shown in (A). All results shown are representative of four independent experiments and are means ± S.E.M. *P < 0.05 compared with control (Con-WT) and †P < 0.05 compared with Ca2+ -WT.
Figure 7. Inhibition of MPT by Ppif gene targeting in de-energized mitochondria does not require Pi.
(A) De-energized liver mitochondria from Ppif+/+ (WT) and Ppif−/− (KO) mice were incubated in swelling buffer containing 5 mM Pi, Asi or Vi. Mitochondrial swelling was then induced by the addition of 250 µM CaCl2 and monitored as a decrease in absorbance (A520). (B) Group data, calculated as the area above the curve, for the swelling curves shown in (A). All results shown are representative of four independent experiments and are means ± S.E.M. *P < 0.05 compared with control (Con-WT) and †P < 0.05 compared with Ca2+ -WT.
DISCUSSION
It has long been known that Pi can sensitize the MPT pore to the effects of matrix Ca2+. Consequently, the majority of studies examining the MPT response in isolated mitochondria, especially with regard to CypD inhibition, include Pi in the assay medium. However, Bernardi’s group reported that the ability of CypD inhibition (genetic or pharmacological) to negatively regulate the MPT pore is surprisingly lost when Pi is omitted from the buffer and replaced with other inorganic anions (in order to maintain Ca2+ uptake into the mitochondria) [20]. This was consistent with earlier studies suggesting that Pi could strengthen the inhibitory effect of CsA on MPT in isolated mitochondria [22], and that Asi-dependent MPT was less sensitive to CsA [23].
To confirm these findings, we repeated the experiments in mouse liver mitochondria that were either treated with CsA or were completely lacking CypD (i.e. from Ppif−/− mice). However, in direct contrast with the previous study [20], we found that these mitochondria still exhibited a diminished MPT response compared with untreated/wild-type mitochondria regardless of whether it was Pi, Asi or Vi, in the assay buffer. Consequently, our findings are, in fact, more in line with another recent study in rat liver mitochondria where the anti-MPT effects of CsA were apparent in the presence of either Pi or Asi [21]. Brand’s group has also reported a maintained ability of CsA to block MPT in the absence of Pi in brown adipose mitochondria [24].
The reason for these discrepancies is not immediately apparent. We used the same species (mouse), organ (liver) and substitute anions (Asi and Vi) as Bernardi’s group. One concern was that our initial studies (Figures 1 and 2) were implemented in inbred FVB mice, which we traditionally use for generating transgenic mice, whereas the study by Basso et al. [20] utilized C57BL/6 mice. Our Ppif+/+ and Ppif−/− mice are also in a mixed C57BL/6-sv129 background as opposed to pure C57BL/6. Thus the differences in results could purely be due to mouse strain differences. Indeed, profound differences have been reported between FVB, C57BL/6 and/or sv129 strains with regard to their sensitivity to cardiac [25] and cerebral [26] ischaemia/reperfusion injury as well as neuronal excitotoxicity [27], all of which involve opening of the MPT pore.
However, we found that CsA was still able to inhibit MPT in the presence of Asi or Vi in liver mitochondria isolated from C57BL/6 mice. Therefore strain differences do not appear to account for the disparate datasets. Furthermore, this Pi-independent effect of CsA was still observable in mitochondria from another tissue (kidney) and even another species (rat); the latter recapitulating the findings of the earlier rat study [18].
Another difference is that we utilized mitochondrial swelling as our index of MPT, whereas Bernardi’s group employed the CRC (calcium-retention capacity) assay [20]. Although unlikely, it is possible that these different assays could yield different results. However, Varanyuwatana and Halestrap [21] simultaneously measured swelling and CRC in energized mitochondria, and found that, in both cases, replacement of Pi did not affect the inhibitory effect of CsA. Also consistent with that study, we found that the anion-independent inhibition of MPT by CsA was observed in both energized and de-energized mitochondria. We observed a similar pattern in the Ppif−/− mitochondria too. We did use a higher concentration of anion than Basso et al. [20] and Varanyuwatana and Halestrap [21] (5 mM compared with 1 mM). It is therefore possible that we have bypassed the ‘inhibition’ aspect of Pi with this higher amount, such that we are only in the ‘activation’ range. However, this would still fail to account for the fact that, in our study, both CsA and CypD deletion were effective even in the absence of Pi.
Interestingly, in the course of these studies, we observed that, whereas the magnitude of the swelling response to Ca2+ followed an Asi> Pi> Vi pattern in energized liver mitochondria, it instead followed a Pi> Asi> Vi pattern under de-energized conditions. Indeed, both of the previous studies reported an Asi> Pi response in energized mitochondria [20,21] and Varanyuwatana and Halestrap [21] also found that this was reversed in de-energized mitochondria. Why the sensitizing effects of Pi and Asi differ depending on energetic state is not clear. Changes in the ability of each anion to bind matrix Ca2+ or Mg2+ under the different conditions would influence their ability to affect MPT, as has been speculated [21]. Alternatively, under energized conditions, the mitochondrial ATP synthase would use the Asi to create ADP–As adducts instead of ATP [28]. The non-enzymatic hydrolysis of these adducts would create a futile cycle between ADP and ADP–As [29]. This, coupled with a reduction in ATP, would reduce the inhibitory effects of adenine nucleotide levels on the MPT pore, thereby further sensitizing it to Ca2+. It should be noted, however, that the swelling amplitude in the kidney mitochondria was independent of the anion present, i.e. the response was much the same whether it was Pi, Asi or Vi present. The reasons for this are not clear, but may suggest different regulatory factors/proteins in the kidney compared with the liver.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health [grant number HL094404 (to C.P.B.)].
Abbreviations used
- Asi
inorganic arsenate
- CRC
calcium-retention capacity
- CsA
cyclosporin A
- CypD
cyclophilin D
- MPT
mitochondrial permeability transition
- Pi
inorganic phosphate
- Ppif
peptidylprolyl isomerase F
- Vi
inorganic vanadate
Footnotes
AUTHOR CONTRIBUTION
Christopher Baines conceived and designed the studies. Allison McGee and Christopher Baines performed the experiments and analysed the data. Christopher Baines wrote the paper.
REFERENCES
- 1.Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc. Res. 2009;83:213–225. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim. Biophys. Acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 4.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787:1395–13401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halestrap AP, Davidson AM. Inhibition of Ca2+ -induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis–trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connern CP, Halestrap AP. Purification and N-terminal sequencing of peptidyl-prolyl cis–trans-isomerase from rat liver mitochondrial matrix reveals the existence of a distinct mitochondrial cyclophilin. Biochem. J. 1992;284:381–385. doi: 10.1042/bj2840381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J. Biol. Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 8.Andreeva L, Tanveer A, Crompton M. Evidence for the involvement of a membrane-associated cyclosporin-A-binding protein in the Ca2+ -activated inner membrane pore of heart mitochondria. Eur. J. Biochem. 1995;230:1125–1132. doi: 10.1111/j.1432-1033.1995.tb20664.x. [DOI] [PubMed] [Google Scholar]
- 9.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 11.Schinzel A, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 13.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin D at a different site from cyclosporin A. J. Biol. Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 14.Tiepolo T, Angelin A, Palma E, Sabatelli P, Merlini L, Nicolosi L, Finetti F, Braghetta P, Vuagniaux G, Dumont JM, et al. The cyclophilin inhibitor Debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in Col6a1−/− myopathic mice. Br. J. Pharmacol. 2009;157:1045–1052. doi: 10.1111/j.1476-5381.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am. J. Physiol. Renal Physiol. 2009;297:F749–F759. doi: 10.1152/ajprenal.00436.2003. [DOI] [PubMed] [Google Scholar]
- 16.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, Yu X, Fowlkes J, Rahder M, Stem K, et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 2003;278:19062–10970. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 19.Kowaltowski AJ, Castilho RF, Grijalba MT, Bechara EJ, Vercesi AE. Effect of inorganic phosphate concentration on the nature of inner mitochondrial membrane alterations mediated by Ca2+ ions: a proposed model for phosphate-stimulated lipid peroxidation. J. Biol. Chem. 1996;271:2929–2934. doi: 10.1074/jbc.271.6.2929. [DOI] [PubMed] [Google Scholar]
- 20.Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 2008;283:26307–26311. doi: 10.1074/jbc.C800132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varanyuwatana P, Halestrap AP. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion. 2011 doi: 10.1016/j.mito.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chávez E, Moreno-Sánchez R, Zazueta C, Rodríguez JS, Bravo C, Reyes-Vivas H. On the protection by inorganic phosphate of calcium-induced membrane permeability transition. J. Bioenerg. Biomembr. 1997;29:571–577. doi: 10.1023/a:1022483018482. [DOI] [PubMed] [Google Scholar]
- 23.Bravo C, Chávez E, Rodríguez JS, Moreno-Sánchez R. The mitochondrial membrane permeability transition induced by inorganic phosphate or inorganic arsenate: a comparative study. Comp. Biochem. Physiol. Biochem. Mol. Biol. 1997;117:93–99. doi: 10.1016/s0305-0491(96)00257-x. [DOI] [PubMed] [Google Scholar]
- 24.Crichton PG, Parker N, Vidal-Puig AJ, Brand MD. Not all mitochondrial carrier proteins support permeability transition pore formation: no involvement of uncoupling protein 1. Biosci. Rep. 2009;30:187–192. doi: 10.1042/BSR20090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnabei MS, Palpant NJ, Metzger JM. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol. Genomics. 2010;42A:103–113. doi: 10.1152/physiolgenomics.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keum S, Marchuk DA. A locus mapping to mouse chromosome 7 determines infarct volume in a mouse model of ischemic stroke. Circ. Cardiovasc. Genet. 2009;2:591–598. doi: 10.1161/CIRCGENETICS.109.883231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finn R, Kovács AD, Pearce DA. Altered sensitivity to excitotoxic cell death and glutamate receptor expression between two commonly studied mouse strains. J. Neurosci. Res. 2010;88:2648–2660. doi: 10.1002/jnr.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gresser MJ. ADP-arsenate:formation by submitochondrial particles under phosphorylating conditions. J. Biol. Chem. 1981;256:5981–5983. [PubMed] [Google Scholar]
- 29.Chinopoulos C, Konrád C, Kiss G, Metelkin E, Töröcsik B, Zhang SF, Starkov AA. Modulation of F0F1-ATP synthase activity by cyclophilin D regulates matrix adenine nucleotide levels. FEBS J. 2011;278:1112–1125. doi: 10.1111/j.1742-4658.2011.08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]