Abstract
The switch of carcinoma cells from an epithelial to a mesenchymal-like phenotype, via a process designated “epithelial-to-mesenchymal transition (EMT),” has been recognized as a relevant step in the metastasis of solid tumors. Additionally, this phenotypic switch of carcinoma cells has been associated with the acquisition of tumor resistance mechanisms that reduce the anti-tumor effects of radiation, chemotherapy, and some small-molecule targeted therapies. As multiple signaling pathways and transcriptional regulators that play a role in this phenotypic switch are being identified, novel strategies can be designed to specifically target tumor cells with this metastatic and resistant phenotype. In particular, this review focuses on the potential use of cancer vaccine strategies to target tumor cells that exhibit a mesenchymal-like phenotype, with an emphasis on the characterization of a novel tumor antigen, Brachyury, which we have identified as a critical regulator of EMT in human cancer cells.
Keywords: metastasis, epithelial-to-mesenchymal transition, EMT, cancer vaccines, Brachyury
Introduction
A hallmark of malignant tumors is their ability to disseminate and to colonize sites that are distant from the location of the primary tumor mass.1 During this process, cancer cells undergo a sequence of events known as the metastatic cascade, which allows them to detach from the primary tumor mass and invade the surrounding tissue, enter into the circulation, home in on distant organs, and survive and proliferate at the site of metastases.1–3 Clinically, metastases can be detected either at the time of diagnosis of the primary tumor or they can emerge even a decade after the removal of the primary tumor mass.2 Tumor relapse is also frequently associated with the acquisition of resistance to currently available anti-tumor therapies, including chemotherapy4 and some novel targeted therapeutic agents.5, 6
As metastasis resistant to therapy is the main cause of death from cancer, it is essential to develop novel strategies focused at preventing and/or treating metastatic disease by targeting key regulators at the crossroad of metastasis and therapeutic resistance. Multiple studies have demonstrated the role of the acquisition of a mesenchymal-like phenotype by epithelial tumor cells in the metastasis of carcinomas, via a process designated as epithelial-to-mesenchymal transition (EMT). Comprehensive reviews on the mechanisms of EMT as well as its role in tumor progression can be found in the literature.7–9 This review focuses on the potential use of cancer vaccine strategies to target tumor cells with a mesenchymal-like, metastatic phenotype, with an emphasis on the characterization of a novel tumor antigen, Brachyury, as a critical regulator of EMT in human cancer cells.
Acquisition of a mesenchymal-like phenotype by epithelial tumor cells
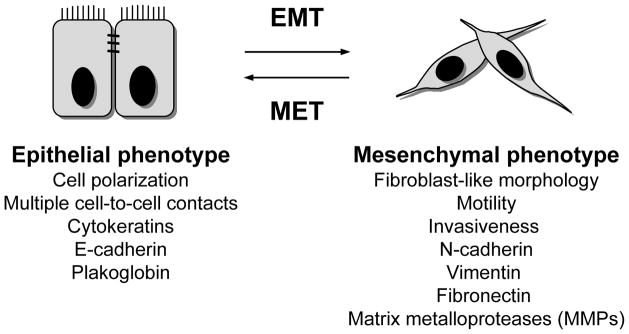
The phenotypic modulation of carcinoma cells, which involves downregulation of epithelial markers (cytokeratins, E-cadherin), loss of intercellular junctions and polarity, and upregulation of mesenchymal proteins (N-cadherin, Vimentin, Fibronectin), is designated as the epithelial-to-mesenchymal transition (EMT)10, 11 (Figure 1). As a consequence of the conversion from an epithelial to a mesenchymal-like phenotype, tumor cells undergoing EMT can acquire motility and the ability to invade the basal membrane and extracellular matrix (ECM), properties that are fundamental for the initial steps of the metastatic cascade.9, 12–14
Figure 1.
Relevant phenotypic changes defining the epithelial-to-mesenchymal transition (EMT) and its reverse process, the mesenchymal-to-epithelial transition (MET).
Numerous observations support the involvement of an epithelial-mesenchymal phenotypic switch in the progression of human carcinomas. For example, the loss of E-cadherin, a protein required for the formation of adherens junctions between epithelial cells, has been previously associated with tumor progression and metastasis.15–18 In breast tumors, reduction of the epithelial markers E-cadherin and cytokeratins, and upregulation of the mesenchymal markers Vimentin and N-cadherin, have been shown to positively correlate with a basal-like phenotype, high aggressiveness, and a high rate of metastasis.19 Similarly, progression of cervical carcinoma correlates with downregulation of E-cadherin and upregulation of Vimentin.20 Thus, the EMT program, which has long been studied for its role in embryogenesis and organogenesis,10, 21 as well as during wound repair and fibrosis of adult tissues,22, 23 is now emerging as a crucial process for the metastasis of carcinomas.8, 21, 24
The acquisition of mesenchymal-like characteristics by tumor cells is postulated to be only a temporary phenomenon; i.e., tumor cells undergoing EMT may or may not switch back to their epithelial phenotype once they have reached the site of metastasis, through a process designated as mesenchymal-to-epithelial transition (MET) (Figure 1).25 In experiments performed with mice inoculated at various sites with bladder carcinoma cell lines carrying either epithelial or mesenchymal-like characteristics, it has been demonstrated that the epithelial phenotype of tumor cells favors the last step of tumor metastasis (i.e, colonization), while a mesenchymal-like phenotype favors the spreading of tumor cells from the primary site to the metastasis site (i.e., the initial steps of the metastatic cascade).26 In human tissue samples, it has also been observed that epithelial E-cadherin is re-expressed in metastatic lesions of breast cancer patients, even when the primary tumor demonstrated low levels of E-cadherin.27 As of yet, the relevance of MET in the process of metastasis needs to be further demonstrated; for example, the expression of molecules that are able to drive the mesenchymal phenotype, such as Twist, has been seen in metastatic lesions of prostate cancer.28 Another point to consider regarding the involvement of EMT in the progression of solid tumors is that the switch from an epithelial to a mesenchymal-like phenotype may just involve a few cells at the invasive front of a tumor mass. This seems to be the case, for example, in human colorectal adenocarcinomas where nuclear accumulation of β-catenin, an indicator of EMT, is seen only at the invasive front of the tumor.29 The reversibility of EMT, together with the fact that only a few cells might be undergoing this phenotypic switch, are being mentioned as potential reasons for the small amount of data demonstrating EMT in human tumor tissue samples. Certainly, the identification of novel, specific markers of “tumor EMT” will help to unequivocally distinguish epithelial tumor cells undergoing a transition to a mesenchymal-like phenotype in human tumor samples.
Molecular control of EMT during tumor progression
Multiple growth factors, cytokines, and components of the extracellular matrix (ECM) have been shown to contribute to the induction of the EMT phenotype in epithelial cells. In many cases, tumor cells are responsible for the release of growth factors and cytokines that can act in an autocrine fashion via activation of receptor tyrosine kinase (RTK)-signaling pathways, inducing and maintaining the metastatic phenotype. Additionally, the tumor stroma or other cellular components of the tumor microenvironment, such as fibroblasts or immune cells, could provide the signals necessary to trigger EMT in the adjacent tumor cells, thus reinforcing the role of the tumor microenvironment in tumor progression. In particular, fibroblast growth factor (FGF), epidermal growth factor (EGF), insulin-like growth factors (IGFs), vascular growth factor (VEGF), platelet-derived growth factor (PDGF), tumor necrosis factor-alpha (TNF-α), Wnt ligands, as well as components of the ECM signaling through surface integrins, have all been implicated in the induction of EMT.8, 11, 30 One of the most studied inducers of EMT is the transforming growth factor-beta (TGF-β), which has been shown in multiple tumor types to cooperate with RTK-signaling pathways or other pathways to influence tumor cells towards a more mesenchymal, metastatic phenotype.31 For example, TGF-β signaling has been shown to be fundamental for the induction of EMT by oncogenic Ras32, 33 or in cooperation with platelet-derived growth factor (PDGF)34, thus reinforcing the idea of the contribution of several signaling pathways to the induction and maintenance of EMT. Also, TGF-β and EGF are known to synergistically drive the acquisition of a mesenchymal-like phenotype by ovarian cancer cells.35 Other signal transduction pathways that have been implicated in the induction of EMT include Notch,36 Wnt/β-catenin,37 and Hedgehog signaling.38 Recently, NF-Kβ has also been identified as a mediator of EMT in breast cancer progression, in conjunction with oncogenic Ras and TGF-β.39
EMT transcription factors
At the end of the multiple, redundant signaling pathways involved in EMT are a few transcriptional regulators designated as “EMT transcription factors.” Unlike oncogenes or tumor suppressor genes whose activation or inactivation, respectively, results in tumor initiation, the activation of EMT transcription factors is responsible for the acquisition of a metastatic phenotype by tumor cells. During the course of an EMT, the level of these transcription factors will be enhanced and, as a consequence, the expression of genes directly controlled by them will be either repressed (epithelial markers) or enhanced (mesenchymal markers). In general, the EMT transcription factors are highly expressed in the early embryo, where they participate in the control of developmental epithelial-mesenchymal transitions. The transcriptional repressors Snail and Slug, two zinc finger proteins directly involved in the repression of E-cadherin expression, have been shown to trigger EMT in epithelial tumor cells.40, 41 High levels of Snail expression have been shown to associate with breast or cervical cancer progression.20, 42 The expression of Twist, another regulator of EMT that participates in the repression of E-cadherin,14 is also increased in various types of cancer, including breast, prostate, and cervical cancer, with higher levels of Twist protein, for example, being detected in prostate cancer tissues of high Gleason score.28 Twist expression has also been shown to correlate with poor disease outcome in cervical cancer.43 Among the EMT transcription factors that also directly repress E-cadherin expression are the zinc finger proteins ZEB1 and ZEB2,44, 45 and E12/E47.46 In addition, some EMT transcription factors have been identified that are involved in the acquisition of mesenchymal traits by epithelial tumor cells and that do not participate in the direct control of epithelial E-cadherin. An example is FOXC2, which is upregulated in basal-like, aggressive breast cancer and has been shown to induce the expression of the mesenchymal markers N-cadherin, Vimentin, and Fibronectin.47 A list of EMT transcription factors that have been correlated with high-grade human tumors or with poor cancer prognosis is presented in Table 1.
Table 1.
EMT transcription factors associated with human tumor progression*
| Transcription factor | Cancer type | Reference |
|---|---|---|
| Snail | Breast, cervical | 20, 42, 77, 78 |
| Slug | Lung, ovarian, colorectal | 78–80 |
| Twist | Breast, prostate, cervical | 14, 28, 43 |
| ZEB1/δEF1 | Uterine | 81 |
| ZEB2/SIP | Ovarian | 78 |
| FOXC2 | Breast (basal-like subtype) | 47 |
| Brachyury | Lung | 49 |
Only a partial list is included in this table.
EMT, epithelial-to-mesenchymal transition.
Brachyury, a novel EMT transcription factor
Recently, the T-box transcription factor Brachyury has been identified as a novel tumor antigen, highly expressed in various human tumors of epithelial origin, including lung, breast, colon, small intestine, stomach, kidney, bladder, uterus, ovary, and testis, and in tumor cell lines derived from lung, colon, and prostate carcinomas. The high levels of expression of Brachyury in tumors contrasted with its lack of expression in most normal adult tissues, with the exception of low levels observed in testis (Table 2).48
Table 2.
Expression of Brachyury mRNA in human tumors and normal tissues
| Positive expression | |
| Human carcinomas48, 49 | Lung, breast, colon, small intestine, stomach, kidney, bladder, uterus, ovary, testis, prostate |
| Other tumors48 | Chronic lymphocytic leukemia (CLL) |
| Normal tissues48 | Testis |
| Negative expression | |
| Normal tissues48 | Brain, heart, kidney, liver, lung, pancreas, placenta, skeletal muscle, colon, ovary, leukocytes, prostate, small intestine, spleen, thymus |
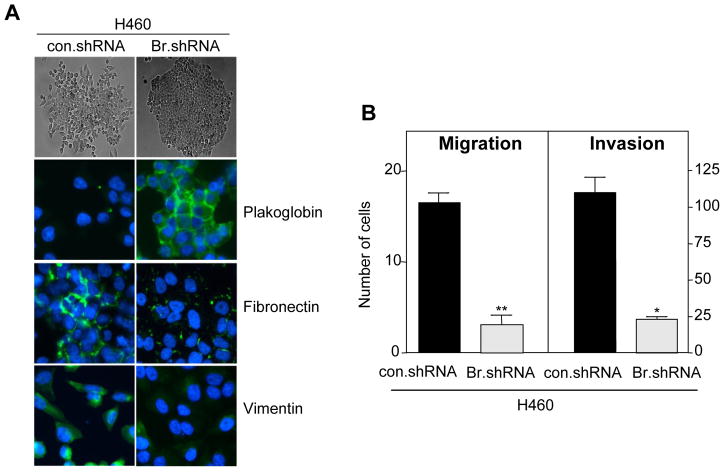
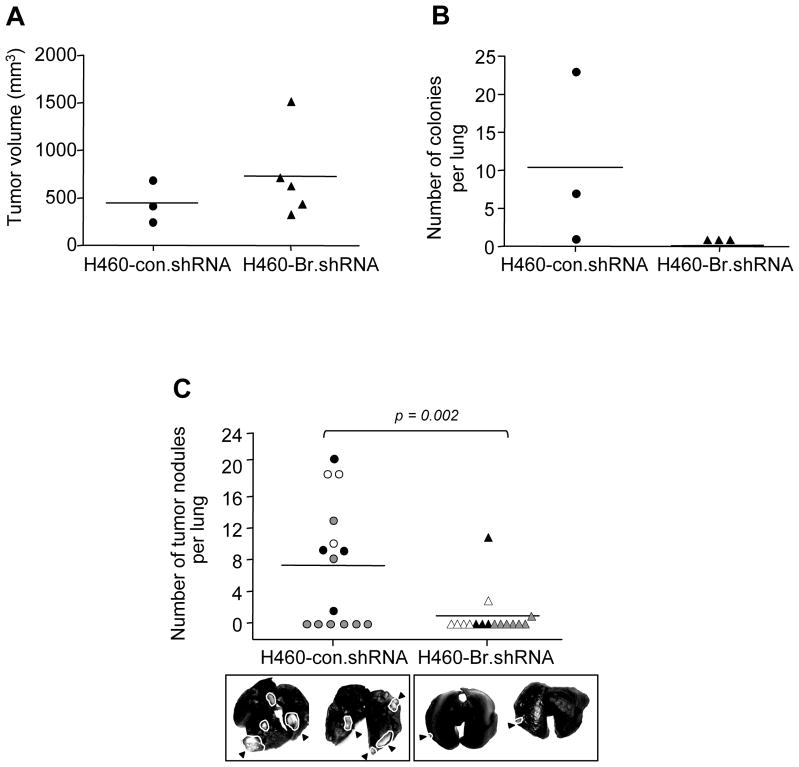
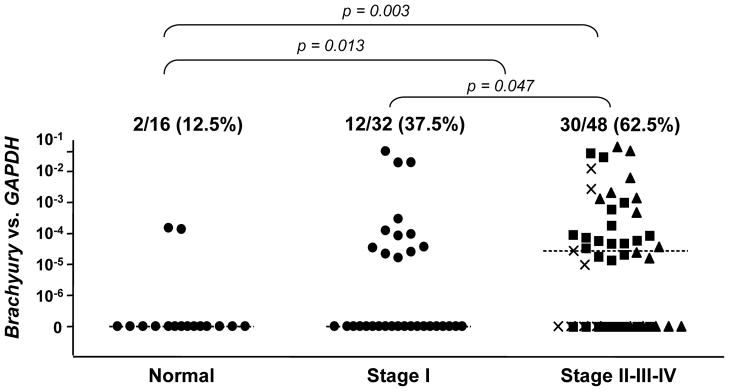
Brachyury, a gene highly active during early embryo development, has now been shown to induce the acquisition of a mesenchymal-like phenotype by epithelial tumor cells in vitro, promoting the expression of mesenchymal markers and downregulation of epithelial markers, with a concomitant increase in tumor cell migration and invasion (Figure 2).49 Brachyury over-expression in epithelial tumor cells was also shown to repress transcription of E-cadherin, an effect at least partly mediated by the cooperation of Brachyury with the transcriptional repressor Slug.49 In vivo, Brachyury-inhibited human tumor cells demonstrated a decreased ability to form experimental lung metastases after intravenous injection, as well as to disseminate from the primary, subcutaneous tumor to the site of metastases (Figure 3). The potential role of Brachyury in human lung cancer progression was suggested by a positive correlation between Brachyury expression and tumor stage; 62.5% of lung tumor tissues of stages II-IV were positive for Brachyury, compared to 37.5% of tissues from stage I lung cancer (Figure 4).49
Figure 2. Brachyury-inhibition reduces expression of mesenchymal markers, migration, and invasion of human lung carcinoma cells.
(A) Bright field images of H460 lung cancer cells stably transfected with a control (con.shRNA) or a Brachyury-specific shRNA (Br.shRNA) grown on plastic surface (top, X10 magnification) and immunofluorescent analysis of EMT markers (bottom, X20 magnification); merged images with DAPI stained nuclei are shown. (B) In vitro cell migration and ECM invasion assays. Error bar = SEM of triplicate measurements. * p<0.05, **p<0.001, for con.shRNA vs. Br.shRNA. Reproduced with permission from the American Society for Clinical Investigation; see reference 49.
Figure 3. Brachyury inhibition reduces tumor metastasis.
(A) H460 cells stably transfected with con.shRNA (circle) or Br.shRNA (triangle) vectors were injected subcutaneously in nude mice. Graph shows tumor volumes at day 15 post-tumor implantation. (B) Lungs from animals bearing subcutaneous tumors were collected, homogenized, and cultured in puromycin containing medium. Graph shows visible colony counts. (C) Mice were inoculated with 7.5×105 H460 cells transfected as indicated via tail vein. Forty-five days after tumor implantation, animals were euthanized and lungs were evaluated for tumor nodules. Graph shows results from 3 independent experiments. Experiments 1–3 are denoted by black, grey, and white circles (con.shRNA) and triangles (Br.shRNA), respectively. Two representative lungs from each group are shown for comparison. White outlines and black arrowheads point to tumor masses. Reproduced with permission from the American Society for Clinical Investigation; see reference 49.
Figure 4. Brachyury expression in human lung tumor tissues.
Real-time PCR was performed for Brachyury on lung tumor tissue cDNA from 80 lung cancer patients of the indicated stages of disease. The Stage II, III, and IV cDNA samples are further represented by the symbols ν, σ, and ×, respectively. As controls, 16 samples of normal lung cDNA were also analyzed, each obtained from a pathologically normal section of lung from a cancer patient. All values and the medians for each group are expressed as a ratio to the endogenous control GAPDH. Reproduced with permission from the American Society for Clinical Investigation; see reference 49.
EMT, therapeutic resistance, and tumor “stemness”
Numerous reports indicate a direct correlation between the acquisition of a mesenchymal-like phenotype by carcinoma cells (i.e., EMT) and enhanced resistance to a variety of cell death-inducing signals. For example, induction of oxaliplatin-resistance in colorectal cancer cell lines by chronic exposure to oxaliplatin has been reported to induce a phenotypic change indicative of EMT.50 Similarly, paclitaxel-resistance51 and radio-resistance52 in ovarian cancer cells, and gemcitabine-resistance in pancreatic carcinoma cells53 have also been associated with a switch from an epithelial to a mesenchymal-like phenotype. In these studies, cell lines have been exposed to the various chemotherapeutic agents or radiation to select for a population of cells “resistant” to each agent. The opposite experiments were also conducted in which, for example, breast cancer cells were induced into EMT by downregulation of epithelial E-cadherin or over-expression of Twist;54 the resulting mesenchymal-like tumor cells were evaluated for their sensitivity to a variety of chemotherapeutic agents, demonstrating that EMT induces resistance to a broad range of drugs. Interestingly, some of the transcriptional regulators of EMT, including Snail55 and Brachyury49 have been shown not only to trigger the acquisition of mesenchymal traits by epithelial tumor cells but also to negatively regulate cell cycle progression. Therefore, a lower proliferation rate of mesenchymal-like tumor cells may protect them from various stressful conditions, such as nutrient deprivation and genotoxic injuries like the ones induced by radiation or certain chemotherapies.
Recently, the induction of EMT in neoplastic epithelial cells has also been shown to result in the enrichment of a population of cells with stem cell-like properties.56, 57 It has been reported that the induction of EMT in human mammary epithelial cells (HMLEs) resulted in the acquisition of a mesenchymal-like phenotype by those cells and concomitantly increased the proportion of stem-like cells, characterized by a CD44high/CD24low phenotype.56 The clinical significance of this association was recently proposed by the demonstration that residual breast tumor cell populations surviving post-conventional treatments were enriched in CD44+/CD24−/low cells that also exhibited mesenchymal features.58
Targeting of EMT as a means to reduce metastasis
Because of the relevant role of EMT in the development of carcinoma metastasis, novel anti-cancer strategies can be designed for specifically targeting tumor cells that exhibit a mesenchymal-like phenotype. It can be hypothesized that, if employed at early stages of disease, strategies targeting EMT might be effective at preventing tumor dissemination; once the tumor cells have disseminated, however, targeting of EMT might be beneficial to alleviate tumor resistance and to improve the efficacy of conventional anti-tumor therapies, such as radiation and chemotherapy.
Three general strategies can be proposed for eliminating tumor cells undergoing EMT: a) the reversal of EMT by blocking of relevant signaling pathways that trigger and sustain the mesenchymal-like phenotype of carcinoma cells; b) the elimination of tumor cells undergoing EMT by cancer vaccine approaches that target essential regulators of the EMT process, such as the EMT transcription factors, and c) the use of selective inhibitors of tumor cells that have undergone an EMT transition.
Reversal of EMT
The blockade of the multiple cytokine, growth factors, and integrin-initiated signaling pathways that are responsible for the initiation and maintenance of EMT constitutes one approach to revert the mesenchymal phenotype of tumor cells and to alleviate resistance to cell death. For example, inhibition of the EGFR signaling pathway by using the EGFR tyrosine kinase inhibitor, erlotinib, has been shown to revert the mesenchymal phenotype and to inhibit motility and invasiveness of breast metastatic cancer cells in vitro, as well as to inhibit spontaneous lung metastasis in a xenograft model of breast cancer.59 Another strategy to revert the mesenchymal-like phenotype of carcinoma cells is that of blocking TGF-β signaling via anti-TGF-β monoclonal antibodies, TGF-β antisense oligonucleotides, or by using TGF-β receptor I (TGF-βRI) kinase inhibitors. For example, it has been demonstrated that various TGF-βRI inhibitors are able to revert the TGF-β-induced EMT in murine mammary epithelial cells60 or human cancer cell lines, in vitro.61 As EMT initiation and maintenance in various tumor types may depend on the cooperation of multiple pathways, reversal to a fully epithelial phenotype might not be achieved by blockade of individual pathways. Instead, it might be necessary to develop novel combinatorial strategies aimed at simultaneously targeting multiple signaling pathways that contribute to the metastatic phenotype.
Cancer vaccines
The goal of vaccine-based cancer immunotherapy approaches is to induce a long-lasting, tumor-specific T-cell mediated immune response in the patient that ultimately will reduce tumor burden. Among the various types of vaccines, a common modality consists of the active immunization of the patient against a specific molecule (designated as a “tumor antigen”) that is either exclusively expressed in the tumor cells or that is over-expressed in cancerous versus normal tissues. As a result, an immune response could be elicited against the antigen that will lead to tumor elimination and cure or that will limit tumor growth, therefore prolonging patient survival.
Tumor antigens fall into one of two major categories. The first category corresponds to “tumor-specific antigens,” which are molecules expressed de novo by the tumor cells, such as virally-derived products in tumors driven by infectious agents, or mutated proteins like the proto-oncogene ras62 and the tumor suppressor p53.63 The second category corresponds to “tumor-associated antigens,” constituted by proteins that are over-expressed in tumor cells but show low levels of expression among normal tissues. Most tumor antigens so far identified belong to the latter category; the immune system is normally tolerant to these “self proteins” and different strategies must be employed to enhance antigen recognition and establish an effective immune response against them. A comprehensive review of the various cancer vaccine modalities and their current status in the clinic can be found in the literature.64–67
Cancer cells acquire several mechanisms that allow them to evade immune recognition or to negatively affect the functionality of effector T cells,68 a fact that would limit the efficiency of cancer vaccine approaches. For example, a strategy used by tumors to escape immune recognition and destruction is the complete or partial loss of an antigen(s).69 Both in experimental animal models70 and in human cancer69 it has been shown that “antigen-negative tumor variants,” characterized by the loss of the targeted antigen, can appear subsequent to an immune intervention. Cancer vaccines may be able to overcome this antigenic loss by initiating an antigen cascade. This phenomenon, which has been documented both in preclinical71 and clinical studies,72 involves the establishment of an immune response against antigens in the tumor that are not included in the vaccine formulation, as a result of tumor cell lysis and cross-priming of T cells. Another way to overcome this problem is the targeting of “functionally relevant antigens,” defined as proteins with an essential role during tumor initiation, growth, survival, or metastasis.73 It can be hypothesized that an immune intervention against a “functioning” tumor antigen would greatly reduce the emergence of antigen-negative variants, since cells that have lost the antigen will fail to grow, survive, or metastasize.
Cancer vaccines against Brachyury
With a critical role in the establishment of a metastatic phenotype, EMT transcription factors could be explored as novel “functionally relevant tumor antigens” for the targeting of metastatic tumor cells. Because of their intracellular localization, transcription factors cannot be targeted by modalities that depend on the recognition of surface-expressed proteins, such as therapeutic antibodies. Additionally, in the drug discovery field, transcription factors are currently regarded as “undruggable” targets for classical small molecule drug modalities. In contrast, T cells are able to recognize short peptide fragments of an antigen that, following intracellular processing, are presented on the cell surface in the form of complexes with the major histocompatibility complex (MHC) class I and II molecules. Thus, T cells induced by cancer vaccines may be a suitable means to specifically target transcriptional regulators of the EMT process.
Tumor specificity is a first condition for a molecule to be used as a cancer vaccine target, i.e., the molecule should be over-expressed in tumor cells but its levels must be relatively low among all normal adult tissues. A second requisite is that pre-existing immune tolerance against the molecule could be overcome and that an effective T-cell immune response could be generated against it. This latter point may be of great importance with EMT transcription factors, as they are molecules normally expressed during embryo development (“self antigens”) and it is expected that the immune system would be tolerant against them.
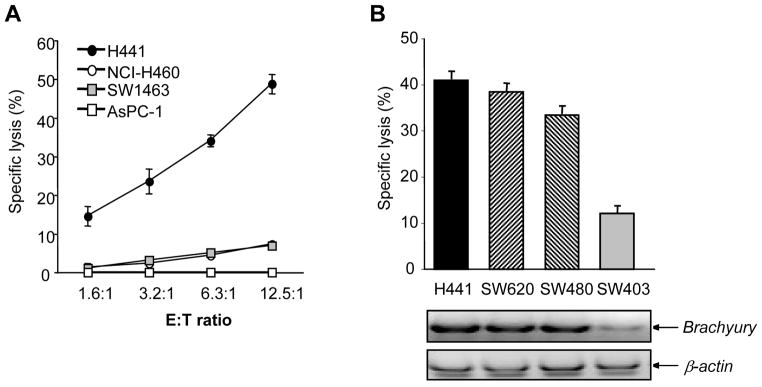
An example of a functionally relevant tumor antigen critically involved in the acquisition of a mesenchymal-like phenotype by human cancer cells is Brachyury. This EMT transcription factor has been demonstrated to be tumor specific, highly expressed in various human carcinomas but absent in most human normal adult tissues tested.48, 49 Moreover, Brachyury has been shown to be immunogenic. A CD8 T-cell epitope of Brachyury was recently identified that was successfully used to expand Brachyury-specific T cells from the peripheral blood of cancer patients.48 The Brachyury-specific T cells have been used to efficiently lyse, in vitro, human tumor cells that naturally express Brachyury (Figure 5).
Figure 5. Cytotoxic activity of Brachyury-specific CTLs against tumor targets.
(A) Brachyury-specific, cytotoxic T lymphocytes from a normal donor were used as effectors against various tumor targets in an 111In 16-hour release assay, as indicated. The following tumor cell lines were used: H441 (HLA-A2+/Brachyury+), NCI-H460 (HLA-A2−/Brachyury+), SW1463 (HLA-A2+/Brachyury−), ASPC-1 (HLA-A2−/Brachyury−). (B) T cells derived from PBMCs of a cancer patient were used as effectors against various HLA-A2+ tumor targets, as indicated. Also shown is the expression of Brachyury and β-actin mRNA by RT-PCR in each tumor cell line. Reproduced with permission from the American Association for Cancer Research; see reference 48.
Potentially, other transcription factors critically involved in the control of EMT during human tumor progression could be explored as novel tumor antigens for the targeting of metastatic disease, provided that they also meet the criteria of tumor specificity and immunogenicity described above. Because T cells recognize a target in the form of short peptides presented in the context of the major histocompatibility complex (MHC), T-cell mediated immunotherapy can be used to target molecules irrespective of their cellular localization, and thus poses an advantage over other forms of targeted modalities, such as therapeutic antibodies that depend on the recognition of surface-expressed molecules.
Future perspectives
Progress in understanding the molecular mechanisms that govern the process of metastasis, including a full understanding of the role of EMT (and its reverse process, MET) in the progression of human carcinomas, will help in designing therapies aimed at preventing and/or treating metastatic disease. Cancer vaccines able to specifically target metastatic tumor cells constitute a very attractive methodology. Recently, cancer immunotherapy has demonstrated encouraging results in preclinical as well as in clinical studies, 74, 75 and the field has entered a new era with the approval of the first therapeutic cancer vaccine for the treatment of metastatic prostate cancer (Provenge, sipuleucel-T, Dendreon Corporation).76 Unlike other modalities, cancer vaccines may be able to generate a long-lasting anti-tumor response, and so far they have demonstrated no associated toxicities. As multiple preclinical and clinical studies are demonstrating the feasibility and advantage of employing combinatorial therapies for the treatment of cancer, it can be envisioned that the elimination of metastatic tumor cells via targeting of EMT may rely on the combination of the various approaches described above.
Acknowledgments
The authors thank Debra Weingarten for editorial assistance in the preparation of this manuscript.
Grant support: Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH
Footnotes
Author contributions: All authors participated in the writing and review of the manuscript.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341–52. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 4.Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, Sommer H, Pantel K. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18(1):80–6. doi: 10.1200/JCO.2000.18.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, Iwata KK, Gibson N, Haley JD. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65(20):9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 6.Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25(8):843–54. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]
- 7.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23(10):912–23. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 8.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi: 10.172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120(11):1351–83. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 12.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3(3):155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–9. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39(3):305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 16.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13(23):7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 17.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 18.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68(7):2329–39. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68(4):989–97. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 20.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14(15):4743–50. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 21.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19(3):294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101(4):830–9. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition--does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5(5):280–90. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101(2):293–9. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66(23):11271–8. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 27.Bukholm IK, Nesland JM, Borresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients [see comments] J Pathol. 2000;190(1):15–9. doi: 10.1002/(SICI)1096-9896(200001)190:1<15::AID-PATH489>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, Wong YC, Wang X. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65(12):5153–62. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 29.Jung A, Schrauder M, Oswald U, Knoll C, Sellberg P, Palmqvist R, Niedobitek G, Brabletz T, Kirchner T. The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear beta-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am J Pathol. 2001;159(5):1613–7. doi: 10.1016/s0002-9440(10)63007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4(8):657–65. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 32.Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beug H, Mikulits W. Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J Cell Sci. 2002;115(Pt 6):1189–202. doi: 10.1242/jcs.115.6.1189. [DOI] [PubMed] [Google Scholar]
- 33.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156(2):299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotzmann J, Fischer AN, Zojer M, Mikula M, Proell V, Huber H, Jechlinger M, Waerner T, Weith A, Beug H, Mikulits W. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene. 2006;25(22):3170–85. doi: 10.1038/sj.onc.1209083. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Jiang Y, Steed H, Davidge S, Fu Y. TGFbeta and EGF synergistically induce a more invasive phenotype of epithelial ovarian cancer cells. Biochem Biophys Res Commun. 2010;401(3):376–81. doi: 10.1016/j.bbrc.2010.09.059. [DOI] [PubMed] [Google Scholar]
- 36.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yee DS, Tang Y, Li X, Liu Z, Guo Y, Ghaffar S, McQueen P, Atreya D, Xie J, Simoneau AR, Hoang BH, Zi X. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol Cancer. 2010;9:162. doi: 10.1186/1476-4598-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1(6–7):338–51. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114(4):569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 41.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 42.Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21(20):3241–6. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 43.Shibata K, Kajiyama H, Ino K, Terauchi M, Yamamoto E, Nawa A, Nomura S, Kikkawa F. Twist expression in patients with cervical cancer is associated with poor disease outcome. Ann Oncol. 2008;19(1):81–5. doi: 10.1093/annonc/mdm344. [DOI] [PubMed] [Google Scholar]
- 44.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7(6):1267–78. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 45.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24(14):2375–85. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem. 2001;276(29):27424–31. doi: 10.1074/jbc.M100827200. [DOI] [PubMed] [Google Scholar]
- 47.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007;104(24):10069–74. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP, Schlom J. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13(8):2471–8. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 49.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120(2):533–44. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, Gray MJ, Cheng H, Hoff PM, Ellis LM. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12(14 Pt 1):4147–53. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 51.Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31(2):277–83. [PubMed] [Google Scholar]
- 52.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27(9):2059–68. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 53.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14(12):3629–37. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 54.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18(10):1131–43. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 58.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang D, LaFortune TA, Krishnamurthy S, Esteva FJ, Cristofanilli M, Liu P, Lucci A, Singh B, Hung MC, Hortobagyi GN, Ueno NT. Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res. 2009;15(21):6639–48. doi: 10.1158/1078-0432.CCR-09-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng SB, Yan L, Xia X, Watkins SA, Brooks HB, Beight D, Herron DK, Jones ML, Lampe JW, McMillen WT, Mort N, Sawyer JS, Yingling JM. Kinetic characterization of novel pyrazole TGF-beta receptor I kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochemistry. 2005;44(7):2293–304. doi: 10.1021/bi048851x. [DOI] [PubMed] [Google Scholar]
- 61.Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7(5):509–21. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abrams SI, Stanziale SF, Lunin SD, Zaremba S, Schlom J. Identification of overlapping epitopes in mutant ras oncogene peptides that activate CD4+ and CD8+ T cell responses. Eur J Immunol. 1996;26(2):435–43. doi: 10.1002/eji.1830260225. [DOI] [PubMed] [Google Scholar]
- 63.Offringa R, Vierboom MP, van der Burg SH, Erdile L, Melief CJ. p53: a potential target antigen for immunotherapy of cancer. Ann N Y Acad Sci. 2000;910:223–33. doi: 10.1111/j.1749-6632.2000.tb06711.x. discussion 33–6. [DOI] [PubMed] [Google Scholar]
- 64.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13(13):3776–82. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373(9664):673–83. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palena C, Schlom J. Vaccines against human carcinomas: strategies to improve antitumor immune responses. J Biomed Biotechnol. 2010 doi: 10.1155/2010/380697. Epub 2010 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. Strategies for cancer vaccine development. J Biomed Biotechnol. 2010 doi: 10.1155/2010/596432. Epub 2010 Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 70.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol. 2007;37(3):675–85. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11(6):2416–26. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 72.Lechleider RJ, Arlen PM, Tsang KY, Steinberg SM, Yokokawa J, Cereda V, Camphausen K, Schlom J, Dahut WL, Gulley JL. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14(16):5284–91. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirohashi Y, Torigoe T, Inoda S, Kobayasi J, Nakatsugawa M, Mori T, Hara I, Sato N. The functioning antigens: beyond just as the immunological targets. Cancer Sci. 2009;100(5):798–806. doi: 10.1111/j.1349-7006.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, Cereda V, Vergati M, Steinberg SM, Halabi S, Jones E, Chen C, Parnes H, Wright JJ, Dahut WL, Schlom J. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 77.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8(3):197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 78.Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103(8):1631–43. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 79.Shih JY, Tsai MF, Chang TH, Chang YL, Yuan A, Yu CJ, Lin SB, Liou GY, Lee ML, Chen JJ, Hong TM, Yang SC, Su JL, Lee YC, Yang PC. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res. 2005;11(22):8070–8. doi: 10.1158/1078-0432.CCR-05-0687. [DOI] [PubMed] [Google Scholar]
- 80.Shioiri M, Shida T, Koda K, Oda K, Seike K, Nishimura M, Takano S, Miyazaki M. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94(12):1816–22. doi: 10.1038/sj.bjc.6603193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spoelstra NS, Manning NG, Higashi Y, Darling D, Singh M, Shroyer KR, Broaddus RR, Horwitz KB, Richer JK. The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res. 2006;66(7):3893–902. doi: 10.1158/0008-5472.CAN-05-2881. [DOI] [PubMed] [Google Scholar]