Abstract
Objective
Development of an effective vaccine or topical compound to prevent HIV transmission remains a major goal for control of the AIDS pandemic. Using a nonhuman primate model of heterosexual HIV-1 transmission, we tested whether a topical microbicide that reduces viral infectivity can potentiate the efficacy of a T-cell-based HIV vaccine.
Design
A DNA prime and rAd5 virus boost vaccination strategy was employed, and a topical microbicide against the HIV nucleocapsid protein was used. To rigorously test the combination hypothesis, the vaccine constructs contained only two transgenes and the topical microbicide inhibitor was used at a sub-optimal dose. Vaccinees were exposed in the absence and presence of the topical microbicide to repeated vaginal R5 SHIVSF162P3 challenge at an escalating dose to more closely mimic high-risk exposure of women to HIV.
Methods
Infection status was determined by PCR. Antiviral immune responses were evaluated by gp120 ELISA and intracellular cytokine staining.
Results
A significant delay in SHIV acquisition (Log-rank test; p=0.0416) was seen only in vaccinated macaques that were repeatedly challenged in the presence of the topical microbicide. Peak acute viremia was lower (Mann-Whitney test; p=0.0387) and viral burden was also reduced (Mann-Whitney test; p=0.0252) in the combination-treated animals.
Conclusions
The combined use of a topical microbicide to lower the initial viral seeding/spread and a T-cell-based vaccine to immunologically contain the early virological events of mucosal transmission holds promise as a preventive approach to control the spread of the AIDS epidemic.
Keywords: HIV, vaccine, topical microbicide, nucleocapsid inhibitor, prevention
Introduction
As women account for close to 50% of newly acquired HIV-1 infections worldwide [1], the development of an effective and safe vaccine or topical compounds to prevent HIV transmission remains a major goal in the control of the AIDS pandemic. Although there has been some encouraging news on the development of a vaccine [2] and a topical microbicide [3] to prevent HIV-1 vaginal transmission, the effect seen is only partial. Thus, increasing emphasis is now placed on combining intervention strategies to enhance their efficacy [4–6]. While vaccines that elicit protective anti-HIV envelope antibodies are desirable, the challenges involved in eliciting broadly neutralizing antibodies remain formidable [7]. Thus, HIV vaccines have been developed primarily to elicit T-cell immunity and, as such, are unlikely to block establishment of the founder population. Rather, they are anticipated to restrict local expansion and/or dissemination of the transmitted virus, leading to lower set-point levels of HIV replication, slower disease progression, and reduction in secondary virus transmission [8]. Interventions that can lower the inoculum dose and/or slow amplification of the virus through virus inactivation, therefore, are likely to be good candidates for evaluation in combination with T-cell-based vaccines, as they would provide a wider window of opportunity within which a vaccine-induced immune response at the mucosal surface can be effective.
The HIV-1 nucleocapsid zinc fingers have long been considered prime targets for therapeutic and microbicide intervention because they are mutationally intolerant and are required for multiple steps of the viral replication cycle [9–15]. We have demonstrated that S-acyl-2-mercaptobenzamide thioester (SAMT) zinc finger inhibitors (ZFIs) penetrate cell-free HIV-1 virions and inactivate the zinc fingers via covalent modification and metal ejection [16–18]. SAMTs reduce virus infectivity and dissemination in cell-based assays and ex vivo cervical tissue explants [19–21]. In both an HIV-1 transgenic mouse model and an simian immunodeficiency virus (SIV) non-human primate (NHP) model, they reduced the level of infectious virus without a significant or consistent reduction in plasma virus RNA copy [22, 23] and a 1% gel formulation of SAMT-247 protected five of six rhesus macaques from vaginal challenge with a CCR5 (R5) -tropic SHIV [21]. Furthermore, this class of ZFI has recently been shown to have a unique mechanism of action in which the active drug is regenerated intracellularly [18] that may account for the prolonged reduction of the infectivity of the virus produced by infected cells [21].
Because a vaccine is considered the best strategy for controlling HIV infection, we initiated studies in a NHP model of vaginal HIV-1 transmission to investigate if the modest-protective effect of a prime-boost T-cell-based vaccine could be enhanced when used in combination with a ZFI topical microbicide. We hypothesized that by reducing the infectious dose, the length of the eclipse phase of infection prior to systemic viremia would be extended, allowing the vaccine a greater interval of efficacy. Indeed, Barouch and colleagues recently showed that by reducing the inoculum dose used in a mucosal challenge infection model, the time to the first positive peripheral plasma SIV RNA levels was lengthened [24]. To rigorously test our hypothesis, a vaccine containing only two transgenes (SIV Gag and Pol) was used to limit immunogenicity and effectiveness and a sub-optimal concentration (0.1%) of SAMT-247 was selected for the microbicide. This dose was shown not to have a significant effect on HIV infection in cervical explant assays but was effective at reducing the initial viral infectivity and infectivity of progeny virus [21]. A rhesus macaque model of vaginal infection consisting of weekly R5 SHIVSF162P3 exposures was employed. Our primary endpoint was prevention of SHIV acquisition, with post-infection viremia measurements serving as secondary endpoints. The results show that a poorly effective T cell-based HIV-1 vaccine may actually help to protect against the spread of the AIDS virus if combined with another prevention strategy that is able to reduce the challenge inoculum.
Materials and Methods
Animals
46 female rhesus macaques (Macaca mulatta, RMs) of Indian origin housed individually at the Tulane National Primate Research Center (TNPRC) in compliance with the Guide for the Care and Use of Laboratory Animals were used. Studies were reviewed and approved by the Institutional Animal Care and Use Committee at TNPRC. Animals were confirmed negative for SIV, simian T-cell lymphotropic virus, and retrovirus type D. Additionally, animals were screened for the presence of the Mamu-A*01, Mamu-B*17 and Mamu-B*08 class I alleles expression using standard PCR with allele-specific primers [25]. The macaques were not treated with Depo-provera to thin the vaginal epithelium, and were sedated with ketamine-HCl (10mg/kg) for all procedures. At the end of the study period, the animals were euthanized by intravenous administration of ketamine-HCl followed by an overdose of sodium pentobarbital.
Vaccine construction and immunization
A DNA vaccine expressing SIVmac239 Gag and Pol as a fusion protein was constructed (see Figure, Supplemental Digital Content 1A). Using a published algorithm [26], the SIVmac239 gag and pol genes were optimized to reflect the codon characteristics of eukaryotic expression systems and synthesized by overlapping oligonucleotides. To reduce insertion fragment size for plasmid stablilization, the pol gene was truncated at both the N- (60aa) and C- (150aa) termini. The deleted regions were shown not to contain known T-cell epitopes (Los Alamos Data Base). The gag/pol fragment was cloned into the pVAX1 (Invitrogen, Carlsbad, CA) DNA vaccine vector. Recombinant E1/E3-deleted Ad5 construct was generated by cloning gag/pol fragments into a shuttle vector and subsequently transferring the entire expression cassette into the adenovirus genome vector (Ad-HQ system; Vector Laboratories, Eagleville, PA). Endotoxin-free DNA vaccine and rAd5 particles were prepared by Aldevron (Fargo, ND) and Vector Laboratories, respectively. RMs were primed intramuscularly with 5mg vaccine at weeks 0 and 4. All animals then received a single intramuscular immunization of 1011 viral particles of rAd5 vectors at week 12, with challenges beginning at week 24 (see Figure, Supplemental Digital Content 1B).
SHIV challenge
The challenge virus, SHIVSF162P3, was generated through successive rapid transfer in RMs of the CCR5 molecular clone SHIVSF162 [27]. The recovered virus was propagated and titered in RhPBMC. Weekly vaginal challenges were conducted for a total of 20 exposures or until the animals were confirmed to be infected. The initial 10 challenges were performed with 300 TCID50 (10.8×106 copies of SHIVSF162P3), followed by 5 inoculations with 1000 TCID50 virus and 5 with 3000 TCID50 (see Figure, Supplemental Digital Content 1C). Whole blood samples were collected weekly for 30 weeks following the first challenge and at biweekly intervals thereafter for an additional ten weeks. For ZFI-treated RMs, 2ml of 0.1% SAMT-247 formulated in 2% hydroxyl ethyl cellulose [28] was applied to the vaginal cavity 20min prior to each virus inoculation. Plasma virus was quantified by a branch DNA signal amplification assay [29] (Siemens Medical Solutions Diagnostic Clinical Lab, Emeryville, CA).
Immunology Assays
SHIV gp120-specific IgG antibodies was measured by ELISA using a 1:10 dilution of plasma, as previously described [30]. Briefly, 250ng of purified SF162 gp120 protein was coated onto 96-well Costar plates in PBS overnight at 4°C. After blocking in PBS containing 5% nonfat dry milk and 0.05% BSA, plasma samples were added and incubated at 37°C for 2h. Gp120-specific antibodies were detected by adding secondary HRP-conjugated anti-monkey IgG (Sigma-Aldrich) and the signal was developed by addition of TMB substrate (Invitrogen). The reaction was terminated by adding 1N H2SO4 and the absorbance at 450nm was measured. Between each incubation step, the plates were washed five times with PBS, 0.005% Tween-20. SHIV T cell-immunity was determined by intracellular cellular staining. Peripheral blood mononuclear cells (PBMCs) were isolated by ficoll gradient centrifugation from heparinized blood and resuspended in complete medium (RPMI 1640 supplemented with 10% FCS, 2mM L-glutamine, 100 U/ml penicillin and 100μg/ml streptomycin). 2 × 106 cells were incubated for 2 h at 37°C with medium as a negative control, overlapping 15-mer peptides spanning SIV Gag peptide (2 μg/ml), or staphylococcus enterotoxin B (SEB, 5μg/ml; Sigma-Aldrich, St. Louis, MO) as the positive control. Brefeldin A (10 μg/ml) was then added and incubation continued for an additional 5 h. Following activation, the cultured cells were stained with CD3, CD4 and CD8 specific antibodies. After being fixed and permeabilized using the GOLGI PLUG Kit (BD Biosciences; Franklin Lakes, NJ), cells were then stained with antibodies against gamma interferon (IFN-γ, clone 4S-B3) and interleukin-2 (IL-2, clone MQ1-17H12). Approximately 105 lymphocytes, gated through forward and side scatter, chosen to be large enough to include lymphocyte blasts were acquired on a LSR II instrument (BD Biosciences) and analyzed using FloJo software (Tree Star). Isotype controls were processed in parallel for each sample and used to set the gate for intracellular staining. Specific cytokine responses were determined by subtracting background staining seen in negative control (typically below 0.02% of gated CD4+ or CD8+ lymphocytes).
Statistical Analysis
Infection-free survival curves for the naïve untreated, vaccinated, ZFI- and combination-treated monkeys were estimated using the Kaplan-Meier method with the number of challenges treated as the time scale. Differences in time to infection between groups were assessed using Log-rank tests. Differences in peak and viral loads (log transformed) between naïve untreated, vaccinated, ZFI- and combination-treated animals were examined using Mann-Whitney U tests. A p-value less than 0.05 was considered statistically significant.
Results
Study Design
A prime-boost strategy with DNA prime and rAd5 virus boost was employed. This vaccine platform had previously been shown to suppress SHIV and SIV replication in the monkey model with vaccines that express multiple immunogens [31, 32]. To ensure that protection will be suboptimal, the constructs we used contained only SIV gag, pol without env, and the animals received two instead of three DNA immunizations. Vaccinees were exposed in the absence (n=8) and presence (n=15) of 0.1% SAMT-247, along with eight naïve controls and fifteen ZFI-treated monkeys, to repeated vaginal R5 SHIVSF162P3 challenges. The dose of the ZFI used was also designed to be largely ineffective [21], and 20 weekly exposures using escalating doses of the virus were performed to more closely mimic high-risk exposure of women to HIV as well as to increase the sensitivity of efficacy assessment [33]. Our criterion for infection was detectable plasma viremia (assay threshold of 165 RNA copies/ml) at two consecutive weeks and challenges were terminated upon systemic infection. Levels of SHIV replication in the infected macaques were monitored for over 20 weeks.
Combination treatment delays SHIV acquisition
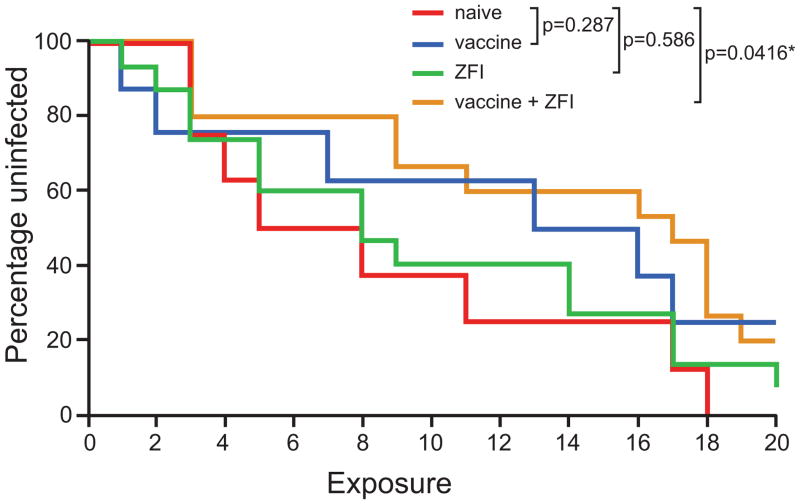
The eight naïve control macaques were infected after a median of 6.5 exposures (range 3–18) (Table 1). Among the vaccinated monkeys, six were infected after a median of 10 exposures (range 1–17), with two (25%) remaining free of systemic infection after 20 challenges. Fourteen of fifteen macaques challenged in the presence of the ZFI-microbicide alone were also infected, requiring a median of 8 exposures (range 1–20), with one (6%) showing no evidence of systemic infection. In comparison, twelve of fifteen vaccinees challenged in the presence of the ZFI-microbicide (combination) were infected after a median of 13.5 exposures (range 3–19), but three (20%) remained protected from systemic infection after receiving the maximum numbers of challenges in the study protocol. Kaplan-Meier infection-free survival curves for each group are given in Fig. 1. As anticipated from the study design, neither vaccine nor ZFI-microbicide alone reduced the rate of SHIVSF162P3 infection after repeated vaginal challenges. However, vaccine in combination with the ZFI-microbicide significantly delayed SHIV acquisition (Log-rank test, p=0.0416).
Table 1.
Susceptibility of macaques enrolled in this study. The number of exposures required for infection in each of the four study groups is indicated.
| Treatment | Animal | Number of challenges needed for infection | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||
| naive | EK80 | ○ | ○ | ● | |||||||||||||||||
| EE61 | ○ | ○ | ● | ||||||||||||||||||
| EE65 | ○ | ○ | ○ | ● | |||||||||||||||||
| CB31 | ○ | ○ | ○ | ○ | ● | ||||||||||||||||
| ER34 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||||||||||||
| BT71 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||||||||
| EL51 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||
| EM06 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||
| vaccine | EF56 | ● | |||||||||||||||||||
| EL52 | ○ | ● | |||||||||||||||||||
| V241 | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||||||||||||
| BR23 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||||||
| EJ99 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||||
| DF89 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||
| FG62 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| CL96 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| ZFI | CB34 | ● | |||||||||||||||||||
| T251 | ○ | ● | |||||||||||||||||||
| EA31 | ○ | ○ | ● | ||||||||||||||||||
| T749 | ○ | ○ | ● | ||||||||||||||||||
| CM18 | ○ | ○ | ○ | ○ | ● | ||||||||||||||||
| DV03 | ○ | ○ | ○ | ○ | ● | ||||||||||||||||
| AE41 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||||||||||||
| V234 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||||||||||||
| CM28 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||||||||||
| CK29 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||||||
| R186 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||||||
| CK94 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||
| CL70 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||
| BR68 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |
| EE77 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| vaccine + ZFI | FP24 | ○ | ○ | ● | |||||||||||||||||
| FN85 | ○ | ○ | ● | ||||||||||||||||||
| FK45 | ○ | ○ | ● | ||||||||||||||||||
| FH60 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||||||||||
| FT03 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||||||||||
| FG57 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||||||||
| EK82 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||||
| FC31 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||||
| EJ29 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||
| FC08 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||
| FN57 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | |||
| EE78 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ||
| FC28 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| FM42 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| FP96 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
Figure 1. Kaplan-Meier infection-free survival curves after repeated vaginal inoculations with SHIVSF162P3.
The percentage of animals remaining uninfected in the four study groups after each of the 20 inoculations with SHIVSF162P3 is shown. Statistical significance of the differences in the survival curves was determined by Log-rank test. Red: naïve (n=8); blue: vaccine (n=8) (p-value vs naïve=0.287); green: ZFI (n=15) (p- value vs naïve=0.586); orange: ZFI+vaccine (n=15) (p-value vs naïve=0.0416).
SHIV replication is significantly reduced in combination-treated macaques
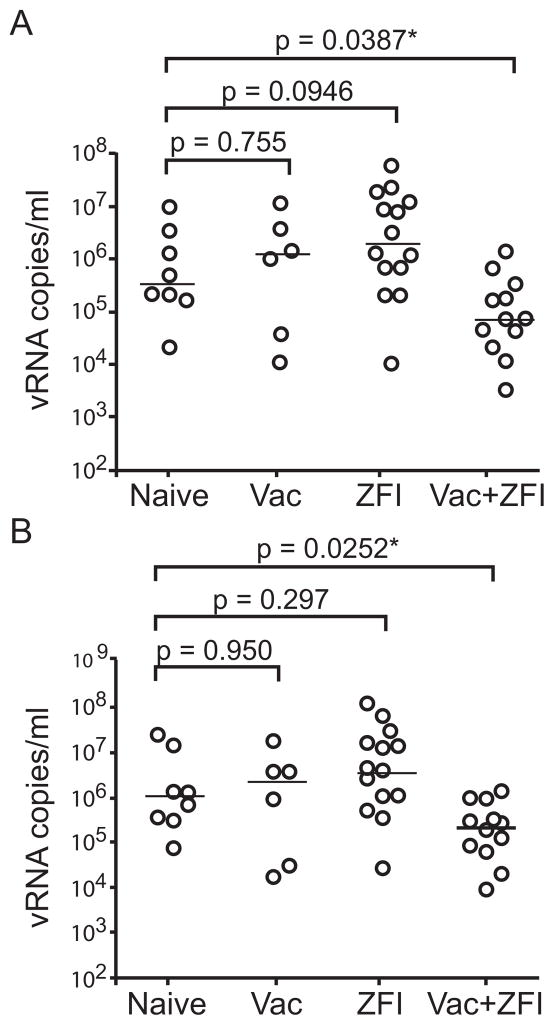
In comparison with infected naïve control animals, combination-treated animals had significantly lower peak plasma virus replication during the first three weeks of infection (Mann-Whitney U test, p= 0.0387) as well as reduced SHIV cumulative RNA levels during the first 20 weeks of infection (p=0.0252); this was not observed in monkeys that were vaccinated or treated with ZFI alone (Fig. 2). Peak plasma virus replication was lower in naïve control monkeys infected with low dose virus (300 TCID50) than in those infected with 10-fold higher doses, suggestive of a dose effect (Fig. 3). Moreover, the post-infection virus replication pattern was more variable and atypical in naïve macaques infected with low in comparison to high inoculum doses, with multiple spikes in viremia seen in the former. No obvious difference in replication pattern was seen among the ZFI-treated macaques infected with different inoculum doses, but the post-infection replication pattern in the infected vaccinees appeared to be the converse of that seen in the infected naïve controls. Plasma viremia was higher and the replication pattern was less variable in vaccinees infected with low dose in comparison to those infected with high inoculum sizes. In contrast, an effect of the vaccine in combination with the ZFI-microbicide in controlling SHIV viremia was readily apparent. Virus replication was attenuated in the combination-treated animals, regardless of inoculum size. None of the aviremic macaques seroconverted. Moreover, two infected monkeys in the combination-treated group which had transient and low viremia also failed to seroconvert (FN85, FP24; see Figure, Supplemental Digital Content 2 showing anti-SHIV gp120 antibody responses in infected macaques). Thus, if infection is determined by seroconversion, as is usually the case in HIV-1 infection in humans, then combination treatment reduced the risk of contracting SHIV by as much as a third, in comparison to none in the treatment-naive group, 25% in the vaccination alone group, and 6% in the ZFI alone group. Collectively, the data show that vaccination in combination with the ZFI-microbicide improved protection against infection and significantly reduced the levels of SHIV replication in macaques that were infected after repeated vaginal exposure.
Figure 2. Plasma virus levels in infected macaques.
(A) Peak acute viremia after infection with SHIVSF162P3. A scatter plot comparison of the peak plasma virus replication in vaccinated, ZFI-treated, combination and their naïve controls. (B) SHIV levels during the first 20 weeks of infection with SHIVSF162P3. A scatter plot comparison of the area under the curve (AUC) viremia in vaccinated, ZFI-treated, combination and their naïve controls during 20 weeks of infection. The line represents the median of each group. * highlights p values that are statistically significant.
Figure 3. SHIV infection pattern.
Plasma SHIV RNA levels in naïve control, vaccinated, ZFI- and combination-treated macaques infected with different inoculum doses are shown.
SHIV challenge elicits anamnestic cellular responses
Analysis of IFN-γ and IL-2 secretion from PBMCs freshly isolated from the group of combination-treated monkeys in response to SIV Gag antigen stimulation two weeks after rAd5 virus boost showed SHIV-specific T lymphocyte responses in a majority of the DNA-primed monkeys, demonstrating that the vaccine is immunogenic. Response was largely restricted to IFN-γ secretion, with comparable percentages of CD4+ and CD8+ cells responding (Fig. 4a). A SHIV-specific T-cell response could also be detected in peripheral blood 1–2 weeks after the first virus exposure (Fig. 4b), with a particularly robust response in four of fifteen animals: FP24, which was infected after 3 virus exposures, EE78 which required 19 exposures to be infected, and two of the three resistant monkeys (FM42, FP96) (Table 1). Notably, SIV gag-specific CD4+ T-cells were more readily demonstrable than CD8+ T-cells in the macaque that was infected early (FP24) than in those that were infected late (EE78) or resisted SHIV challenges (FM42, FP96). Because virus-specific CD4+ T-cells are the preferred targets for virus infection [34], the biased CD4 cell response in FP24 may have contributed to the ready susceptibility of this monkey to infection, but which was rapidly controlled. Conversely, the robust and balanced CD4 and CD8 anamnestic responses seen in EE78, FM42 and FP96 suggested that they may have played a role in delaying (EE78) or containing (FM42, FP96) systemic infection.
Figure 4.
SHIV-specific CD4+ and CD8+ T cell response in macaques with combined treatment prior to and following virus exposure. The percentages of CD4+ and CD8+ T cells producing IFN-γ and IL-2 in response to SIV Gag were determined by intracellular cytokine staining. Cytokine profiles of CD4+ and CD8+ T cells in the fifteen combination-treated macaques at 2 weeks after rAd5 boost (A), and at 1–2 weeks after first vaginal challenge with SHIVSF162P3 (B) are shown. The fifteen macaques are listed in the order of increasing time to infection, and grouped according to their infection status as well as the virus dose required to establish systemic infection.
Control of SHIV replication in combination-treated macaques cannot be explained by differences in the MHC class I loci
None of the animals used in this study expressed the restrictive MamuB*08 and B*17 genotypes, but three and five of the ZFI- and combination-treated SHIV-infected monkeys respectively expressed the MamuA*01 major histocompatibility complex. However, we did not find lower peak or chronic viremia in the SHIV-infected MamuA*01-positive animals in comparison to the MamuA*01-negative monkeys (see Figure, Supporting Digital Content 3 showing the effect of MamuA*01 allelic expression on SHIV replication), consistent with a previous report that this haplotype has a weak effect on viral control [35]. There was also no apparent correlation between virus replication levels and the allelic polymorphisms of TRIM5α (data not shown).
Discussion
This proof-of-concept study demonstrates that a partially-effective T-cell-based vaccine, in combination with a sub-optimal dose of a topically-applied microbicide, delays vaginal SHIV acquisition in macaques that were repeatedly challenged and weakens pathogenicity of the infection in those that became infected. Peak viremia and total RNA copies were significantly lower in the combination-treated infected macaques than in the naïve control monkeys. Since the levels of HIV viremia correlate with the likelihood of virus transmission and disease [36–39], our findings suggest that the combined use of a T-cell-based vaccination and a topical microbicide that reduces the viral challenge and infectivity could slow the spread of HIV-1.
We find that the SIVgag/pol DNA prime/rAd5 boost vaccine alone may afford some immune control. Although the differences did not reach statistical significance, only 6 of eight vaccinees were infected relative to all eight in the naïve control group, with increase number of exposures needed to establish systemic infection. As expected from the experimental design, 0.1% SAMT-247 alone was ineffective in preventing or controlling SHIV infection in comparison to the naive control macaques. Fourteen of the fifteen ZFI-treated macaques were infected, with no reduction in viremia as compared to the naïve control animals. In this regard, NCp7 inhibitor action has been associated with the inactivation of newly produced virus in the absence of decreases in viral RNA copy, p24 or supernatant reverse transcriptase activity in cellular models [20], transgenic mouse model of HIV-1 infection [40], and a therapeutic model in cynomolgous macaques [23]. Moreover, the dose of SAMT-247 used (0.1%) was chosen based on previous data showing that it did not significantly affect p24 production from cervical explants, but reduced the initial viral infectivity as well as infectivity of the progeny virus [21]. As recent studies show that the time to the first positive peripheral plasma SIV RNA levels was lengthened by reducing the infectious dose used in a mucosal challenge infection model [24], our finding of reduction of viremia in the combination group supports the hypothesis that the DNA prime-rAd virus boost vaccine established sufficient immunity for control of SHIV infection and pathogenesis if assisted by a microbicide that expands the potential window of vaccine effectiveness by reducing the initial virus seeding to slow virus spread and replication.
This proof-of-concept study does have several limitations. First, the SIV gag and pol in the immunogens and challenge virus were genetically matched, potentially overestimating efficacy. Thus, future studies will be required to evaluate the utility of vaccine/microbicide combination against heterologous virus challenge. Second, we made the assumption that the susceptibilities to infection were equal for all animals within each group. We monitored for MHC I and TRIM5α expression in the monkeys used in this study, and did not observe inherent genetic differences at these loci that could explain differences in SHIV susceptibility. Nevertheless, an examination of the extent of animal-to-animal variation in susceptibility will require a much larger number of animals than the estimation of prevention efficacy. Third, the number of animals used, in particular the vaccine arm alone group, was too few to allow a robust direct comparison of time to SHIV infection between the experimental groups. Lastly, SHIV T-cell immunity was analyzed using peripheral blood cells, which may not accurately reflect mucosal events. However, a repeat-challenge model prohibits sampling of mucosal and lymphoid tissue sites due to concerns that such procedures may alter SHIV susceptibility.
In summary, the combined use of a topical microbicide to lower the initial viral seeding/spread and a modestly-effective T-cell-based vaccine to immunologically contain the early virological events of mucosal transmission holds promise as a preventive approach to control the spread of the AIDS epidemic. Conceivably, the use of a more potent topical gel (20–50% efficacy) in combination with a more effective vaccine that includes an Env component could increase protection against infection. Our findings represent an important first step towards improving HIV-1 prevention measures, and have implications for the design of combination packages against HIV-1. A 31% protection against HIV-1 acquisition was reported in the recently completed RV144 study in Thailand using a recombinant poxvirus vector combined with a recombinant envelope protein, with subgroup analyses suggesting that the vaccine may be more effective at blocking low risk than high risk HIV-1 transmission [2]. Thus, it is tempting to speculate that the modest protective effect of the RV144 vaccine might be enhanced if the individuals were using a microbicide at the same time. Furthermore, a 1% vaginal gel formulation of Tenofovir reduced HIV acquisition by an estimated 39% overall and by 54% in women with high gel adherence [3], suggesting that a major obstacle to microbicide effectiveness will be adherence. A vaccine could compensate for compliance issues in the use of topical microbicides. Further studies will be required to determine if the additive or possibly synergistic protection provided by the combination of a modestly-effective vaccine and a topical microbicide depends on the mechanism of action of the microbicide or the vaccine components.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH Intramural AIDS Targeted Antiretroviral Program (IATAP) (EA), the Tulane National Primate Research Center Base grant RR00164 and NIH grant AI069991 (CCM). The authors thank L. Stamatatos for sgp120, and T.M. Rasmussem and R. Hayashi for technical assistance. SIV gag peptides were obtained through the NIH AIDS Research and Reagent Program, Division of AIDS, NIAID, NIH. CCM, YH, MLS, and EA conceived experiments. AG, LT, WR, MS, STS, NT, and JB performed experiments. YL performed statistical analysis. CCM, YH, LMMJ, MLS, and EA prepared the manuscript.
Footnotes
CONFLICTS OF INTEREST: This work was funded by the NIH Intramural AIDS Targeted Antiretroviral Program (IATAP) (EA) and Tulane National Primate Research Center Base grant RR00164 and NIH grant AI069991 (CCM). The authors declare no competing financial interests or conflicts of interest.
References
- 1.UNAIDS. Joint United Nations Programme on HIV/AIDS (UNAIDS) 2008. 2008 Report on the Global AIDS Epidemic. [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merson M, Padian N, Coates TJ, Gupta GR, Bertozzi SM, Piot P, et al. Combination HIV prevention. Lancet. 2008;372:1805–1806. doi: 10.1016/S0140-6736(08)61752-3. [DOI] [PubMed] [Google Scholar]
- 5.Piot P, Bartos M, Larson H, Zewdie D, Mane P. Coming to terms with complexity: a call to action for HIV prevention. Lancet. 2008;372:845–859. doi: 10.1016/S0140-6736(08)60888-0. [DOI] [PubMed] [Google Scholar]
- 6.Excler JL, Rida W, Priddy F, Gilmour J, McDermott AB, Kamali A, et al. AIDS vaccines and preexposure prophylaxis: is synergy possible? AIDS Res Hum Retroviruses. 2011;27:669–680. doi: 10.1089/aid.2010.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. 2010;464:224–231. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- 8.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 9.de Rocquigny H, Shvadchak V, Avilov S, Dong CZ, Dietrich U, Darlix JL, et al. Targeting the viral nucleocapsid protein in anti-HIV-1 therapy. Mini Rev Med Chem. 2008;8:24–35. doi: 10.2174/138955708783331603. [DOI] [PubMed] [Google Scholar]
- 10.Goldschmidt V, Miller Jenkins LM, De Rocquigny H, Darlix JL, Mely Y. The nucleocapsid protein of HIV-1 as a promising therapeutic target for antiviral drugs. HIV Therapy. 2010;4:179. [Google Scholar]
- 11.Gorelick RJ, Nigida SM, Jr, Bess JW, Jr, Arthur LO, Henderson LE, Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990;64:3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rein A, Henderson LE, Levin JG. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem Sci. 1998;23:297–301. doi: 10.1016/s0968-0004(98)01256-0. [DOI] [PubMed] [Google Scholar]
- 13.Rice WG, Schaeffer CA, Harten B, Villinger F, South TL, Summers MF, et al. Inhibition of HIV-1 infectivity by zinc-ejecting aromatic C-nitroso compounds. Nature. 1993;361:473–475. doi: 10.1038/361473a0. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JA, Gorelick RJ. Nucleocapsid protein function in early infection processes. Virus Res. 2008;134:39–63. doi: 10.1016/j.virusres.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turpin JA, Schito ML, Jenkins LM, Inman JK, Appella E. Topical microbicides: a promising approach for controlling the AIDS pandemic via retroviral zinc finger inhibitors. Adv Pharmacol. 2008;56:229–256. doi: 10.1016/S1054-3589(07)56008-4. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins LMM, Byrd JC, Hara T, Srivastava P, Mazur S, Stahl SJ, et al. Studies on the inactivation of the HIV-1 nucleocapsid protein NCp7 with 2-mercaptobenzamide thioesters. J Med Chem. 2005;48:2487–2458. doi: 10.1021/jm0492195. [DOI] [PubMed] [Google Scholar]
- 17.Turpin JA, Song Y, Inman JK, Huang M, Wallqvist A, Maynard A, et al. Synthesis and biological properties of novel pyridinioalkanoyl thiolesters (PATE) as anti-HIV-1 agents that target the viral nucleocapsid protein zinc fingers. J Med Chem. 1999;42:67–86. doi: 10.1021/jm9802517. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins LM, Ott DE, Hayashi R, Coren LV, Wang D, Xu Q, et al. Small-molecule inactivation of HIV-1 NCp7 by repetitive intracellular acyl transfer. Nat Chem Biol. 2010;6:887–889. doi: 10.1038/nchembio.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava P, Schito M, Fattah RJ, Hara T, Hartman T, Buckheit RW, Jr, et al. Optimization of unique, uncharged thioesters as inhibitors of HIV replication. Bioorg Med Chem. 2004;12:6437–6450. doi: 10.1016/j.bmc.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Turpin JA, Terpening SJ, Schaeffer CA, Yu G, Glover CJ, Felsted RL, et al. Inhibitors of human immunodeficiency virus type 1 zinc fingers prevent normal processing of gag precursors and result in the release of noninfectious virus particles. J Virol. 1996;70:6180–6189. doi: 10.1128/jvi.70.9.6180-6189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace GS, Cheng-Mayer C, Schito ML, Fletcher P, Miller Jenkins LM, Hayashi R, et al. Human immunodeficiency virus type 1 nucleocapsid inhibitors impede trans infection in cellular and explant models and protect nonhuman primates from infection. J Virol. 2009;83:9175–9182. doi: 10.1128/JVI.00820-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schito ML, Goel A, Song Y, Inman JK, Fattah RJ, Rice WG, et al. In vivo antiviral activity of novel human immunodeficiency virus type 1 nucleocapsid p7 zinc finger inhibitors in a transgenic murine model. AIDS Res Hum Retroviruses. 2003;19:91–101. doi: 10.1089/088922203762688595. [DOI] [PubMed] [Google Scholar]
- 23.Schito ML, Soloff AC, Slovitz D, Trichel A, Inman JK, Appella E, et al. Preclinical evaluation of a zinc finger inhibitor targeting lentivirus nucleocapsid protein in SIV-infected monkeys. Curr HIV Res. 2006;4:379–386. doi: 10.2174/157016206777709492. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knapp LA, Lehmann E, Piekarczyk MS, Urvater JA, Watkins DI. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Krasnitz M, Rabadan R, Witten DM, Song Y, Levine AJ, et al. A recoding method to improve the humoral immune response to an HIV DNA vaccine. PLoS One. 2008;3:e3214. doi: 10.1371/journal.pone.0003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harouse JM, Gettie A, Eshetu T, Tan RC, Bohm R, Blanchard J, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21:845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 29.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 30.Stamatatos L, Werner A, Cheng-Mayer C. Differential regulation of cellular tropism and sensitivity to soluble CD4 neutralization by the envelope gp120 of human immunodeficiency virus type 1. J Virol. 1994;68:4973–4979. doi: 10.1128/jvi.68.8.4973-4979.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letvin NL, Huang Y, Chakrabarti BK, Xu L, Seaman MS, Beaudry K, et al. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J Virol. 2004;78:7490–7497. doi: 10.1128/JVI.78.14.7490-7497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regoes RR, Longini IM, Feinberg MB, Staprans SI. Preclinical assessment of HIV vaccines and microbicides by repeated low-dose virus challenges. PLoS Med. 2005;2:e249. doi: 10.1371/journal.pmed.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 35.Zhang ZQ, Fu TM, Casimiro DR, Davies ME, Liang X, Schleif WA, et al. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J Virol. 2002;76:12845–12854. doi: 10.1128/JVI.76.24.12845-12854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 37.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 38.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 39.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 40.Schito ML, Kennedy PE, Kowal RP, Berger EA, Sher A. A human immunodeficiency virus-transgenic mouse model for assessing interventions that block microbial-induced proviral expression. J Infect Dis. 2001;183:1592–1600. doi: 10.1086/320716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.