INTRODUCTION
Delirium in the intensive care unit (ICU) represents an acute form of organ dysfunction, which manifests as a rapidly developing disturbance of both consciousness and cognition that tends to fluctuate throughout the course of a day.1 The American Psychiatric Association’s (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV defines four key features of delirium: 1) Disturbance of consciousness with reduced awareness of the environment and impaired ability to focus, sustain or shift attention; 2) Altered cognition (e.g., impaired memory, language disturbance or disorientation) or the development of a perceptual disturbance (e.g., hallucinations, delusions, or illusions) that is not better accounted for by preexisting or evolving dementia; 3) Disturbance develops over a short period of time (hours to days) and tends to fluctuate during the course of the day; and 4) Evidence of an etiologic cause (i.e., delirium due to general medical condition, substance induced delirium, delirium due to multiple etiologies, or delirium not otherwise specified).1
Delirium is common in the ICU, affecting 60–80% of mechanically ventilated patients and 20–50% of non-mechanically ventilated patients.2–8 Its presence heralds both short- and long-term adverse outcomes. In the hospital, delirious patients are at increased risk for prolonged mechanical ventilation, catheter removal, self-extubation and need for physical restraints.3,9,10 In addition, delirium predisposes patients to longer hospital stays, with greater healthcare costs, increased risk of death during the hospitalization and increased odds of institutionalization following discharge.10–15 Even after hospital discharge, the amount of time a patient was delirious in the ICU predicts long-term cognitive impairment, physical disability and death up to a year later.12,16–20
Given the common occurrence of delirium and the adverse outcomes associated with its presence, preventing delirium in the ICU is a key piece to enhancing the quality of care in ICUs worldwide. But, before considering preventive strategies, one must first ask whether delirium in the ICU can be prevented from developing in the first place? Though some ICU patients develop delirium due to a single, preventable risk factor—and thus recognition and avoidance or minimization of this risk factor may effectively prevent the patient from developing delirium—delirium more often occurs when a vulnerable patient (i.e., having multiple predisposing risk factors) encounters a large insult or insults (i.e., develops a precipitating risk factor).21 Frequently the insult causing delirium is the critical illness leading to ICU admission, such that a large number of patients are delirious prior to arrival in the ICU. Indeed, delirium commonly occurs in conjunction with other acute organ failures such as respiratory failure, shock, and/or renal failure. By the time of ICU admission, the ‘horse is out of the barn’ for many patients, because the syndrome has already developed and therefore cannot be prevented. Even in these patients, though, “preventive” strategies may be of benefit via their effect on duration of delirium. Multiple studies have found that the number of days an ICU patient is delirious is associated with numerous adverse outcomes, including cognitive impairment, physical disability and death in the year following a critical illness.
Thus, we review herein strategies to prevent not only the development of delirium in critically ill patients but also to prevent the persistence of delirium in the ICU, which may be a more attainable goal. Many of these strategies are part of the recently described “ABCDE” approach to ICU care, which clinicians can use to address modifiable risk factors associated with delirium and improve outcomes for their patients.
DELIRIUM RISK FACTORS
The average medical ICU patient has 11 or more risk factors for developing delirium.11 These risk factors can be divided into baseline (predisposing) and hospital-related (precipitating) factors.21 Baseline risk factors are those relating to a patient’s underlying characteristics and comorbidities, and hospital-related factors are those relating to the patient’s acute illness, its treatment and ICU management (Table 1). These risk factors combine to cause delirium in a given patient such that a highly vulnerable patient may develop delirium with only a minor insult (e.g., an elderly patient with underlying dementia may develop delirium from a simple urinary tract infection), whereas a less vulnerable patient often requires a greater insult or insults to develop delirium (e.g., a younger patient without predisposing risk factors who develops delirium in the setting of septic shock and ARDS requiring mechanical ventilation). In general, risk factors relating to the patient’s acute illness and its treatment are potentially more modifiable than baseline risk factors, and thus altering these risk factors may serve as one means to prevent delirium in the ICU.
Table 1.
Delirium Risk Factors
Critically ill patients are exposed to a multitude of risk factors for delirium relating to baseline comorbidities, acute illness and treatment in the hospital/ICU. These risk factors may be viewed as unmodifiable/unpreventable or potentially modifiable/preventable. Delirium prevention in the ICU should focus on reducing the number and duration of potentially modifiable/preventable risk factors.
| Unmodifiable/ Unpreventable Risk Factors | Potentially Modifiable/ Preventable Risk Factors | |
|---|---|---|
| Baseline Risk Factors | Age | |
| APOE-4 genotype | ||
| History of Hypertension | ||
| Pre-existing cognitive impairment | Sensory depravation (i.e., Hearing or Vision Impairment) | |
| History of Alcohol Use | ||
| History of Tobacco Use | ||
| History of Depression | ||
|
| ||
| Acute Illness-Related Risk Factors | High Severity of Illness | Anemia |
| Respiratory Disease | Acidosis | |
| Medical Illness (vs. Surgical) | Hypotension | |
| Need for mechanical ventilation | Infection/Sepsis | |
| Number of infusing medications | Metabolic disturbances (e.g. hypocalcemia, hyponatremia, azotemia, transaminitis, hyperamylasemia, hyperbilirubinemia) | |
| Elevated inflammatory biomarkers | ||
| High LNAA metabolite levels | Fever | |
|
| ||
| Hospital-Related Risk Factors | Lack of visitors | |
| Sedatives/Analgesics (e.g. benzodiazipines and opiates) | ||
| Lack of daylight | Immobility | |
| Isolation | Bladder catheters | |
| Vascular catheters | ||
| Gastric tubes | ||
| Sleep depravation | ||
APOE-4: apolipoprotien-E4 polymorphism; CRP: C-reactive protein; LNAA: Large neutral amino acids
DELIRIUM PREVENTION IN NON-ICU PATIENTS
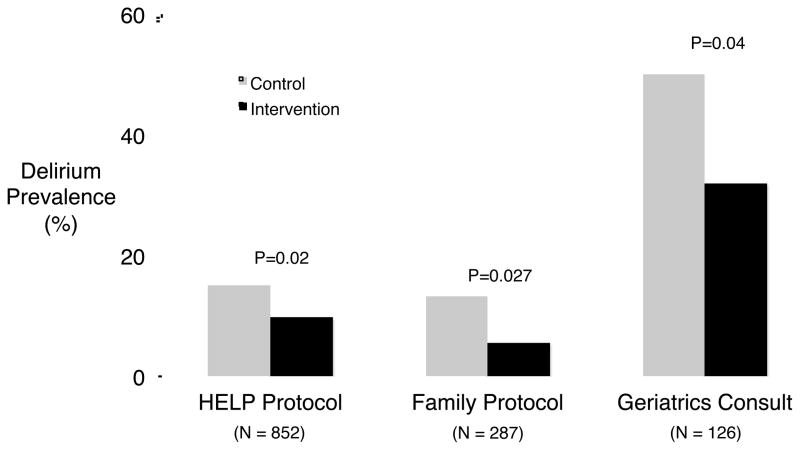
Multicomponent strategies to prevent the development of delirium have yet to be fully developed and studied in the ICU, but an overview of delirium prevention strategies that have been examined carefully in other populations where delirium is prevalent, namely hospitalized elderly patients and those undergoing hip fracture repair (Figure 1), is likely informative for ICU clinicians despite the current paucity of evidence in this setting. Inouye and colleagues, for example, studied a protocol aimed at reducing common risk factors for delirium among acutely ill elderly; the protocol targeted sleep depravation, disorientation, immobility, dehydration, and visual and hearing impairment. In a randomized controlled trial, this multi-faceted intervention was associated with a 40% relative reduction in the development of delirium in the intervention group.22 Interestingly, this intervention was more effective at preventing delirium than treating it once it had developed. In another randomized trial, the efficacy of a delirium prevention protocol delivered by patient family members was established. Family members were taught to recognize the signs and symptom of delirium, to reorient their loved ones by providing them with familiar objects such as family pictures, and to provide eyeglasses and hearing aids to avoid of sensory depravation. These interventions reduced the number of cases of incident delirium by half.23 A third randomized trial explored the effect of an early geriatrics consultation on the development of delirium among patients undergoing surgical hip fracture repair.24 The geriatricians took a protocolized approach to reducing the number of potentially deliriogenic medications, provided adequate analgesic medications, controlled blood pressure, prevented hypoxemia, and ensured the presence of eyeglasses and hearing aides. These interventions resulted in an 18% absolute reduction in incident delirium during the study period. In summary, all three of these well-designed, randomized trials demonstrated that non-pharmacologic interventions targeting multiple risk factors can reduce delirium prevalence in susceptible populations of non-critically ill patients.
Figure 1.
Prevalence of delirium in non-pharmacologic delirium prevention trials. Inouye and colleagues studied the Hospital Elder Life Program (HELP) protocol in hospitalized elderly and found a reduction in delirium prevalence from 15% among patients in the usual care group to 9.9% among patients in the intervention group.22 A similar protocol, studied by Martinez and colleagues, utilized family members to deliver the non-pharmacologic interventions to acutely ill elderly patients and found a reduction in delirium prevalence from 13.3% in the usual care group to 5.6% in the intervention group.23 Finally, Marcantonio and colleagues found that a geriatrics consultation in patients undergoing surgical fixation of hip fractures reduced delirium from 50% in patients not receiving a consultation to 32% in patients who received a consultation.24
Other trials examined whether pharmacologic interventions, namely prophylactic antipsychotics, can prevent delirium in post-operative populations; these trials yielded mixed results. Elderly hip-surgery patients, for example, who were randomized to haloperidol prophylaxis (rather than placebo) beginning prior to surgery and continuing for up to 3-days post-operatively did not experience any reduction in the incidence of post-operative delirium. The haloperidol group did, however, have significantly shorter duration of delirium and a shorter hospital stay, suggesting that interventions intended to prevent delirium may be beneficial despite the occurrence of delirium.25 A second trial in patients undergoing joint arthroplasty compared olanzapine with placebo and did find a significantly lower incidence of post-operative delirium in the olanzapine group.26 Finally, elective cardiac surgery patients randomized to risperidone immediately following surgery were less likely than those randomized to placebo to develop postoperative delirium compared with placebo.27 These trials suggest that prophylactic administration of antipsychotics may reduce the incidence of delirium in certain populations at high risk for developing delirium.
Importantly, the overall incidence of delirium in these non-ICU populations is much lower than those observed in the ICU, where patients are typically exposed to many more risk factors than those affecting non-ICU patients. Thus, the overall effectiveness of these preventative interventions may not be generalizable to the ICU, and further investigation of these strategies in the critically ill is needed. Nevertheless, non-pharmacologic interventions targeting specific modifiable risk factors such as removing catheters when no longer needed, providing reorientation to confused patients and ensuring the availability of eyeglasses and hearing aids are low risk, low cost approaches that may be easily implemented in the ICU and therefore warrant consideration as prevention strategies in the ICU. Prophylactic antipsychotic administration has been studied in small ICU studies, which we will discuss later in this article.
DELIRIUM PREVENTION STRATEGIES IN ICU PATIENTS
On the whole, the constellation of risk factors for delirium affecting individual ICU patients varies from patient to patient and thus an individualized delirium prevention strategy should be sought. Nonetheless, three risk factors in particular—sedatives, immobility and sleep disruption—are widespread in the ICU as a result of clinical practice habits in most ICUs and therefore serve as important targets for delirium prevention. Additionally, antipsychotics and cholinesterase inhibitors intended to prevent delirium in the ICU have been studied, though the results to date have not suggested their use is warranted.
Preventing delirium through management of sedatives
The use of sedatives is nearly ubiquitous among patients receiving mechanical ventilation in the ICU.28 A complete description of best practices for managing sedation in these patients is beyond the scope of this review and is the subject of forthcoming expert guidelines.29 We will focus therefore on two key components of sedation management that can improve brain function: performing coordinated daily spontaneous awakening and spontaneous breathing trials (Awake and Breathing Coordination) and avoiding administration of benzodiazepines.
The daily cessation of sedatives (whether given by infusion or bolus doses) combined with daily spontaneous breathing trials in the Awakening and Breathing Controlled (ABC) Trial resulted in a significant decrease in the number of days patients had acute brain dysfunction compared with the control group.5 Specifically, duration of coma was significantly reduced, whereas duration of delirium was not. When interpreting these findings, one must consider that delirium cannot be assessed in patients who are comatose. Thus, a reduction in coma among patients in the intervention group during the first few days following initiation of the ABC protocol meant that a higher number of patients could be assessed and diagnosed with delirium; i.e., delirium was no longer masked by sedative-induced coma, resulting in a bias comparison of delirium duration. Though the overall duration of delirium was similar between the two groups, patients in the intervention group were delirious earlier in their ICU stay and had resolution of acute brain dysfunction sooner than patients in the usual care group. Thus, compared with more traditional sedation strategies, daily interruption of sedatives as part of the Wake Up and Breathe protocol studied in the ABC Trial reduces the overall number of days ICU patients have acute brain dysfunction (coma plus delirium) and speeds recovery of normal brain function.
Not only can the general approach by which sedatives are administered and discontinued in the ICU affect brain dysfunction, but the type of sedatives administered can as well. Benzodiazepines have been associated with delirium in several studies across multiple ICU populations. Pandharipande and colleagues determined that receiving lorazepam was independently associated with risk of delirium the next day in a dose-dependent manner, such that patients who received 20 mg or more on a given day were nearly all delirious the following day (with the exception of those who were comatose and could not be assessed for delirium).30 Similarly, after adjusting for potential confounders in a population of surgical and trauma ICU patients, receipt of midazolam was associated with a 2.75-fold increase in the odds of developing delirium.7 Thus, one way to prevent delirium in the ICU may be to avoid administering benzodiazepines for routine sedation.
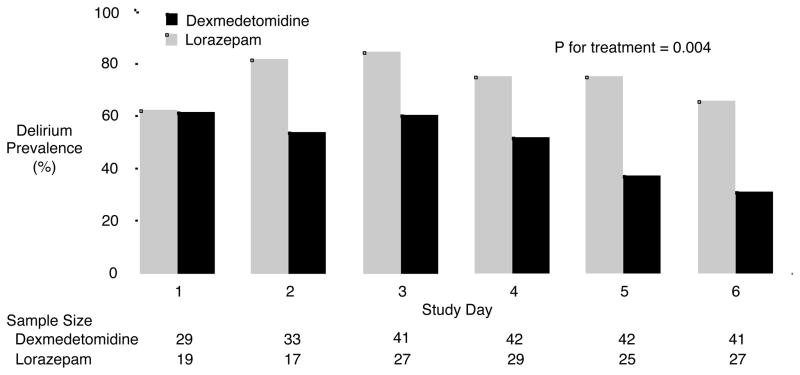
Although numerous randomized trials have demonstrated faster awakening times from sedation and shorter duration of mechanical ventilation among ICU patients sedated with alternative sedatives (e.g., propofol or dexmedetomidine) rather than benzodiazepines, only three trials have specifically measured delirium on a daily basis when comparing a benzodiazepine to an alternative sedative.6,31,32 The MENDS (Maximizing the Efficacy of Targeted Sedation and Reducing Neurologic Dysfunction) trial randomized mechanically ventilated medical/surgical ICU patients to sedation with lorazepam vs dexmedetomidine for up to 5 days and found that patients in the dexmedetomidine group had a median of 4 more days alive and free of delirium or coma than those sedated with lorazepam.6 Additionally, after the day of randomization, the daily prevalence of delirium was significantly lower for patients in the dexmedetomidine group compared with those in the lorazepam group (Figure 2). In a second randomized trial comparing dexmedetomidine with a benzodiazepine, the SEDCOM (Safety and Efficacy or Dexmedetomidine Compared with Midazolam) trial, patients sedated with midazolam had 23% higher delirium prevalence than those receiving dexmedetomidine.31 Finally, Maldonado and colleagues randomized patients undergoing elective cardiac valve operations to post-operative sedation with dexmedetomidine, midazolam or propofol and found the incidence of delirium in the dexmedetomidine group was significantly lower than either the midazolam or propofol groups.32 In contrast, the recently published MIDEX trial compared dexmedetomidine with midazolam and reported that a composite outcome of agitation, anxiety and delirium measured at a single point in time 48 hours after the cessation of sedative infusion was not different between the midazolam and dexmedetomidine groups.33 A simultaneously conducted trial comparing dexmedetomidine with propofol (the PRODEX trial) also found no difference between groups in agitation, anxiety and delirium 48 hours after sedative discontinuation, but neither MIDEX nor PRODEX is informative regarding delirium given that the outcome was assessed only once during these 45-day trials. The findings of the randomized trials which implemented granular delirium measurements indicate that the avoidance of benzodiazepines is an important strategy when seeking to both prevent delirium and reduce its duration.
Figure 2.
In the MENDS trial dexmedetomidine significantly reduced the prevalence of delirium over time. The sample size changes with study day as patients were extubated, died, discharged from the ICU or did not have delirium assessed. Reproduced with permission from Pandharipande et al., Critical Care 2010; 14: R38.
Preventing delirium through pain management
Pain is a modifiable risk factor for delirium and inadequate pain control is a frequent cause for agitation in the ICU. When pain is not assessed and treated, patients may be inappropriately given a sedative medication rather than an analgesic medication. Payen and colleagues found that ICU patients who were assessed for pain were less likely to receive sedatives, particularly deliriogenic benzodiazepines, and more likely to receive analgesic medications (non-opioids or opioids) than those who never had a pain assessment.34
The relationship between and opioid analgesics and delirium in the ICU may appear complex when examining the literature, given the seemingly conflicting results of observational studies. But, these results yield a consistent message when understood in light of the dual effects of opioid medications: analgesia and sedation. In ICU populations where opioids are used most often used to treat pain (e.g., in trauma and burn ICU populations), treatment with opioid analgesics has been associated with a reduced risk of delirium.7,35 Conversely, in ICU populations, such as in general medical and surgical ICU populations, where opioids are frequently used for sedation (either alone or in conjunction with other sedating medications, particularly benzodiazepines), treatment with opioid analgesics has been associated with an increased risk of delirium, especially when their use induces coma.3,7,13,36,37
In summary, these data suggest that opioids used to treat pain are protective against the development of delirium whereas those used at doses high enough to cause sedation may increase the risk of delirium. Therefore, patients should undergo regular pain assessments and when pain is detected, effective doses of an analgesic medication should be given taking care to avoid inducing heavy sedation.
Preventing delirium through early mobilization of ICU patients
Immobility has been identified as a risk factor for delirium in multiple non-ICU studies.21,22 But until recently, conventional wisdom held that critically ill patients, particularly those receiving mechanical ventilation, were too sick to participate in physical rehabilitation and mobility. In recent years, however, this belief was challenged and the safety and efficacy of providing ICU patients with physical rehabilitation at the earliest stages of their illness was demonstrated.38–44
Two studies of early mobilization assessed patients for delirium to examine the relationship between early mobility/physical rehabilitation and delirium.41,42 Schweickert et al., for example, performed a randomized trial of early physical and occupational therapy (PT/OT) beginning within the first 72 hours of endotracheal intubation and assessed patients for delirium on a daily basis.41 Patients in the early PT/OT group had half the number of days of delirium while in the ICU compared with those receiving usual care. A similar finding was noted in a quality improvement project at Johns Hopkins Hospital, where an emphasis was placed on reducing deep sedation and increasing the number of patients managed with early mobility. In the year following the implementation of this quality improvement project, patients spent more days without delirium and coma than those managed prior to project initiation.42 These data suggest a role for early mobility in the reduction of the duration of delirium among critically ill patients. Further study is needed to determine whether these interventions can prevent the development of delirium.
Preventing delirium through improving sleep in the ICU
Sleep depravation is nearly universal for ICU patients, with the average ICU patient sleeping between 2 and 8 hours in a 24-hour period.45 Sleep in the ICU is characterized by frequent interruptions and almost half occurs during the daytime hours; thus, very little sleep in the ICU is restorative, REM sleep.45,46 Reasons for poor sleep in the ICU include the continuous cycle of alarms, lights, beepers, care-related interruptions, pain, anxiety and ventilator dyssynchrony.47 Additionally, critically ill patients are frequently treated with medications that disrupt REM sleep including sedatives (particularly benzodiazepines), analgesics, vasopressors, beta-agonists, and corticosteroids.48 The association between sleep depravation and delirium is unclear, as both can present with symptoms of inattention, fluctuating mental status, memory impairment and cognitive dysfunction.49,50 Nevertheless, non-pharmacologic and pharmacologic sleep-promoting interventions for ICU patients have been studied, but only one trial assessed whether improving sleep reduces delirium in the ICU.51–53 Van Rompaey and colleagues randomized adult ICU patients to nighttime earplug use or no earplugs. Patients sleeping with earplugs reported better sleep during the first night in the ICU and fewer patients in this group demonstrated delirium or mild confusion during the five-night study period.51 Thus, while further study is needed into potential mechanisms linking sleep depravation and delirium, and future trials of interventions seeking to improve the quantity and quality of sleep in the ICU are needed and should include delirium measurement as outcomes, available evidence suggests that enhancing sleep through non-pharmacologic means can reduce the incidence of delirium. Noise-reduction strategies (such as earplugs), normalizing day-night illumination, minimizing care-related interventions during normal sleeping hours, and interventions promoting patient comfort and relaxation are low risk and often inexpensive and should be implemented to prevent delirium.
Preventing delirium through pharmacologic interventions
Pharmacologic interventions to prevent delirium are attractive given the ease with which a medication can be administered compared with the implementation of non-pharmacologic interventions such as early mobility and sleep enhancing protocols, but there are currently no medications Food and Drug Administration (FDA)-approved for the prevention or treatment of delirium. Those some studies of pharmacologic agents to prevent delirium have been performed, most included only cardiac surgery patients or post-operative hip fracture patients; since these populations rarely require long-term ICU treatment, findings from these studies may not be generalizable to medical and surgical ICU populations.54–57 Two studies, however, have specifically explored the role of antipsychotics for primary delirium prevention among critically ill, non-cardiac surgery patients. The Modifying the Incidence of Neurological Dysfunction (MIND) study randomized mechanically ventilated patients admitted to medical, surgical or trauma ICUs across six institutions to receive haloperidol, ziprazidone, or placebo, with treatment started immediately after enrollment (some patients were already delirious whereas others were not).58 Compared with placboe, neither of the antipsychotic drugs studied increased the number of days patients had normal brain function (i.e., were alive without coma or delirium). In contrast, Wang and colleagues randomized postoperative, non-cardiac surgery patients age 65 years and older to prophylactic haloperidol or placebo and found that patients receiving haloperidol prophylaxis were less likely to develop delirium during the seven-day study period.59 One major difference between these two studies, which may explain their disparate findings, is the severity of illness in study populations; the Wang study enrolled elective surgery patients who were not severely ill, whereas the MIND study included only critically ill patients on mechanical ventilation for acute respiratory failure. To date, these two studies represent the only two placebo-controlled trials of antipsychotics for prevention of ICU delirium. Though a large placebo-controlled trial of antipsychotics for the treatment delirium is ongoing,60 more data are needed before antipsychotics can be routinely recommended for the prevention of delirium in the ICU.
Three studies of pharmacologic interventions intended to treat delirium during critical illness are worthy of mention. In an early trial, haloperidol was compared with the atypical antipsychotic olanzapine in delirious, critically ill adults and demonstrated a similar rate of improvement in delirium index scores, but no placebo comparator was used.61 A second small trial compared quetiapine to placebo among patients already receiving haloperidol and found faster resolution of delirium symptoms among patients treated with quetiapine.62 Finally, a randomized trial compared the efficacy of adding rivastigmine, a cholinesterase inhibitor, to haloperidol for the treatment of delirious ICU patients.63 This trial was stopped early for higher mortality among those patients receiving rivastigmine.
Thus, prophylactic administration of antipsychotics to prevent delirium may be indicated in elderly post-operative patients but there is no evidence currently supporting this approach in the broader population of critically ill patients. Routine treatment of delirium with antipsychotics is the subject of ongoing study. Current evidence from one small study suggests a potential benefit to the addition of quetiapine to haloperidol, but these findings need corroboration in a larger, multicenter trial. Finally, cholinesterase inhibitors should not be used to treat delirious patients in the ICU.
The ABCDE approach to combining best practices to prevent delirium
Delirium in the ICU is frequently multifactorial; thus, it is unlikely that a single intervention alone can prevent or reduce delirium with regularity. Therefore a bundled approach combining evidence-based practices in sedation management, ventilator weaning, delirium management and early mobility and exercise, which is referred to as the ABCDE approach, has been proposed to improve multiple outcomes, including preventing and reducing the duration of delirium in the ICU (Table 2).64–66 The ABCDEs combines Awakening and Breathing Coordination for liberation from sedation and mechanical ventilation, Choosing sedatives that are less likely to increase risk of delirium, Delirium management, and finally, Early mobility and Exercise. In addition to potential positive effects on delirium, components of the ABCDEs are individually associated with numerous improvements in outcomes, including shorter duration of mechanical ventilation, shorter ICU and hospital length of stay, improved functional outcomes and improved survival.
Table 2.
The ABCDEs of delirium prevention.
| Intervention | Improved Delirium Outcomes | Other Improved Outcomes |
|---|---|---|
|
Awakening and Breathing Coordination Combine daily spontaneous awakening trials with daily spontaneous breathing trials |
Shorter coma duration | Shorter duration of mechanical ventilation |
| Shorter ICU and hospital length of stay | ||
| Improved Survival at 1-year | ||
|
| ||
|
Choice of sedative agents Avoid benzodiazepines |
Lower prevalence of delirium | Shorter duration of mechanical ventilation |
| Shorter duration of acute brain dysfunction (duration or coma) | Greater Sedation Accuracy (more time at target level) | |
|
| ||
|
Delirium Monitoring & Management Frequently monitor patients for delirium and address modifiable/preventable risk factors and provide non-pharmacologic interventions (reorientation, cognitive stimulation, assess and treat pain, reduce sleep interruption and non-pharmacologic sleep enhancement) |
Increased detection of delirium | |
|
| ||
|
Early Mobility and Exercise Mobilize patients out of bed early in the course of their critical illness |
Shorter delirium duration | Increased return to functional independence at hospital discharge |
| Shorter ICU and hospital length of stay | ||
| Decreased odds of death/rehospitalization | ||
Awakening and Breathing Coordination
As discussed above, daily spontaneous awakening trials coordinated with daily spontaneous breathing trials were associated with a significant decrease in the overall duration of acute brain dysfunction in the ABC Trial. What is more, patients managed with this strategy were extubated an average of three days sooner and were discharged from the ICU and hospital an average of four days sooner than those managed with usual care. Remarkably, the coordination of daily spontaneous awakening trials and spontaneous breathing trials was associated with a 14% reduction in mortality at 1-year follow-up.5
Choice of Sedative Agent
The choice of sedating agent can similarly have important implications not only for delirium but also duration of mechanical ventilation and other outcomes. Both the MENDS and SEDCOM trials described above reported a decrease in delirium in the groups treated with dexmedetomidine compared with those treated with benzodiazepines. The SEDCOM trial also reported a 2-day reduction in time to extubation among patients treated with dexmedetomidine rather than midazolam. In the MIDEX study, treatment with dexmedetomidine also led to a shorter duration of mechanical ventilation than sedation with midazolam.33 Additionally, an a priori-specified subgroup analysis of patients with severe sepsis in the MENDS trial demonstrated that septic patients treated with dexmedetomidine had an increased number of ventilator-free days and a significantly lower 28-day mortality than septic patients treated with lorazepam.67 Thus, treatment with sedative agents other than benzodiazepines is associated with reductions in delirium development as well as reductions in duration of mechanical ventilation.
Delirium Monitoring and Management
Delirium is commonly overlooked by ICU practitioners unless a validated screening tool is used to detect its presence.68,69 Thus, clinicians should monitor patients for the presence of delirium using a delirium screening tool designed for use in the ICU. Recent expert guidelines advocate the use of the Confusion Assessment Method for the ICU (CAM-ICU) or the Intensive Care Unit Delirium Screening Checklist (ICDSC).29 Whereas delirium monitoring is not associated with preventing the syndrome or shortening its duration, the presence of a positive screening test alerts the clinician to look for a reversible or treatable risk factor (Table 1). Only after identifying and treating reversible causes and attempting non-pharmacologic management of patients at risk for or with delirium (e.g., addressing sleep depravation, immobility, dehydration, visual and hearing impairment) should the clinician consider pharmacologic interventions.
Early Mobility and Exercise
The beneficial effects of early physical rehabilitation/early mobility in reducing delirium were presented above. Early mobility is associated with numerous other beneficial outcomes in addition to effects on cognition. Morris and colleagues found that patients treated with an early mobility protocol were out of bed nearly 1 week earlier, were discharged from the ICU 2 days earlier, and were discharged from the hospital 3.5 days earlier than those receiving usual care.39 The effects of this intervention were long lasting, as patients who were treated by the early mobility team while in the ICU were less likely to be readmitted to the hospital or die in the year following their index hospitalization.40 Schewickert and collaborators conducted the first randomized trial of early physical and occupational therapy in ICU patients, during which patients in the intervention group first received therapy within 72-hours of intubation, nearly 6 days sooner than those in the usual care group.41 In addition to a reduction in delirium duration, receipt of early PT/OT was associated with an increased probability of return to baseline functional status at the time of hospital discharge. Finally, Needham and colleagues reported a 2-day shorter average length of ICU stay and a 3-day shorter hospital length of stay compared with the previous year following the implementation of a quality improvement initiative that emphasized freeing patients from deep sedation and routine consultation of physical therapy, occupational therapy and physiatry for critically ill patients.42
CONCLUSION
Delirium in the ICU is exceedingly common and risk factors for delirium among the critically ill are nearly ubiquitous. Nevertheless, addressing modifiable risk factors including sedation management, deliriogenic medications, immobility, sleep disruption can help to prevent and reduce the duration of this deadly syndrome. The ABCDE approach to critical care is a bundled approach that clinicians can implement for many patients treated in their ICUs to prevent the adverse outcomes associated with delirium and critical illness.
Delirium in the ICU is exceedingly common and risk factors for delirium among the critically ill are nearly ubiquitous.
Addressing modifiable risk factors including sedation management, deliriogenic medications, immobility, sleep disruption can help to prevent and reduce the duration of this deadly syndrome.
The ABCDE approach to critical care is a bundled approach that clinicians can implement for many patients treated in their ICUs to prevent the adverse outcomes associated with delirium and critical illness.
Acknowledgments
Funding Source: None
Footnotes
The authors have no financial conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric A. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 2.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001 Dec 5;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 3.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001 May;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 5.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 6.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007 Dec 12;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 7.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guenther U, Popp J, Koecher L, et al. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care. 2010 Mar;25(1):144–151. doi: 10.1016/j.jcrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Micek ST, Anand NJ, Laible BR, Shannon WD, Kollef MH. Delirium as detected by the CAM-ICU predicts restraint use among mechanically ventilated medical patients. Crit Care Med. 2005;33(6):1260–1265. doi: 10.1097/01.ccm.0000164540.58515.bf. [DOI] [PubMed] [Google Scholar]
- 10.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care unit patients. Crit Care Med. 2010 Sep 9; doi: 10.1097/CCM.0b013e3181f85759. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004 Apr 14;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 13.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 14.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 61 non-ventilated patients. Crit Care. 2005 Aug;9(4):R375–381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 16.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 17.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Elseviers M, Bossaert L. Long term outcome after delirium in the intensive care unit. J Clin Nurs. 2009 Dec;18(23):3349–3357. doi: 10.1111/j.1365-2702.2009.02933.x. [DOI] [PubMed] [Google Scholar]
- 19.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brummel NE, Jackson JC, Torres R, Shintani A, Ely EW, Girard TD. Does Duration of ICU Delirium Predict Long-Term Functional Impairment? Am J Respir Crit Care Med. 2011;183:A2653. [Google Scholar]
- 21.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996 Mar 20;275(11):852–857. [PubMed] [Google Scholar]
- 22.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FT, Tobar C, Beddings CI, Vallejo G, Fuentes P. Preventing delirium in an acute hospital using a non-pharmacological intervention. Age Ageing. 2012 Sep;41(5):629–634. doi: 10.1093/ageing/afs060. [DOI] [PubMed] [Google Scholar]
- 24.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005 Oct;53(10):1658–1666. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 26.Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010 Sep-Oct;51(5):409–418. doi: 10.1176/appi.psy.51.5.409. [DOI] [PubMed] [Google Scholar]
- 27.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007 Oct;35(5):714–719. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 28.Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009 Mar;37(3):825–832. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barr J, Fraser GL, Puntillo K, et al. Clincial Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Critical Care Medicine. 2012 doi: 10.1097/CCM.0b013e3182783b72. in press. [DOI] [PubMed] [Google Scholar]
- 30.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009 Feb 4;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 32.Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009 May-Jun;50(3):206–217. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 33.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012 Mar 21;307(11):1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 34.Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post Hoc analysis of the DOLOREA study. Anesthesiology. 2009 Dec;111(6):1308–1316. doi: 10.1097/ALN.0b013e3181c0d4f0. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal V, O’Neill PJ, Cotton BA, et al. Prevalence and risk factors for development of delirium in burn intensive care unit patients. J Burn Care Res. 2010 Sep-Oct;31(5):706–715. doi: 10.1097/BCR.0b013e3181eebee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13(3):R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37(1):177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 39.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 40.Morris PE, Griffin L, Berry M, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–377. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010 Apr;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010 Jul-Aug;17(4):271–281. doi: 10.1310/tsr1704-271. [DOI] [PubMed] [Google Scholar]
- 44.Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–1690. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 45.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117(3):809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 46.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167(5):708–715. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 47.Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest. 2009;136(1):284–294. doi: 10.1378/chest.08-1546. [DOI] [PubMed] [Google Scholar]
- 48.Bourne RS, Mills GH. Sleep disruption in critically ill patients--pharmacological considerations. Anaesthesia. 2004;59(4):374–384. doi: 10.1111/j.1365-2044.2004.03664.x. [DOI] [PubMed] [Google Scholar]
- 49.Mistraletti G, Carloni E, Cigada M, et al. Sleep and delirium in the intensive care unit. Minerva Anestesiol. 2008 Jun;74(6):329–333. [PubMed] [Google Scholar]
- 50.Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: Delirium in ICU patients - importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012 May 4;16(3):R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu RF, Jiang XY, Zeng YM, Chen XY, Zhang YH. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care. 2010;14(2):R66. doi: 10.1186/cc8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12(2):R52. doi: 10.1186/cc6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714–719. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 55.Gamberini M, Bolliger D, Lurati Buse GA, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery--a randomized controlled trial. Crit Care Med. 2009;37(5):1762–1768. doi: 10.1097/CCM.0b013e31819da780. [DOI] [PubMed] [Google Scholar]
- 56.Hudetz JA, Patterson KM, Iqbal Z, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009 Oct;23(5):651–657. doi: 10.1053/j.jvca.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of Delirium with Dexmedetomidine Compared with Morphine Based Therapy after Cardiac Surgery: A Randomized Controlled Trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009 doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 58.Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38(2):428–437. doi: 10.1097/ccm.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med. 2012 Mar;40(3):731–739. doi: 10.1097/CCM.0b013e3182376e4f. [DOI] [PubMed] [Google Scholar]
- 60.Ely EW. MIND-ICU Study. [Accessed: August 31, 2012.];Delirium and Dementia in Veterans Surviving ICU Care. [Google Scholar]
- 61.Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004 Mar;30(3):444–449. doi: 10.1007/s00134-003-2117-0. [DOI] [PubMed] [Google Scholar]
- 62.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419–427. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 63.van Eijk MM, Roes KC, Honing ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010 Nov 27;376(9755):1829–1837. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- 64.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Curr Opin Crit Care. 2011 Feb;17(1):43–49. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 65.Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness--crossing the quality chasm. Chest. 2010 Nov;138(5):1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vasilevskis EE, Pandharipande PP, Girard TD, Ely EW. A screening, prevention, and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med. 2010 Oct;38(10 Suppl):S683–691. doi: 10.1097/CCM.0b013e3181f245d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spronk PE, Riekerk B, Hofhuis J, Rommes JH. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276–1280. doi: 10.1007/s00134-009-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009 Jun;37(6):1881–1885. doi: 10.1097/CCM.0b013e3181a00118. [DOI] [PubMed] [Google Scholar]