Abstract
Isolated immature maize (Zea mays L.) embryos have been shown to acquire tolerance to rapid drying between 22 and 25 d after pollination (DAP) and to slow drying from 18 DAP onward. To investigate adaptations in protein profile in association with the acquisition of desiccation tolerance in isolated, immature maize embryos, we applied in situ Fourier transform infrared microspectroscopy. In fresh, viable, 20- and 25-DAP embryo axes, the shapes of the different amide-I bands were identical, and this was maintained after flash drying. On rapid drying, the 20-DAP axes had a reduced relative proportion of α-helical protein structure and lost viability. Rapidly dried 25-DAP embryos germinated (74%) and had a protein profile similar to the fresh control axes. On slow drying, the α-helical contribution in both the 20- and 25-DAP embryo axes increased compared with that in the fresh control axes, and survival of desiccation was high. The protein profile in dry, mature axes resembled that after slow drying of the immature axes. Rapid drying resulted in an almost complete loss of membrane integrity in the 20-DAP embryo axes and much less so in the 25-DAP axes. After slow drying, low plasma membrane permeability ensued in both the 20- and 25-DAP axes. We conclude that slow drying of excised, immature embryos leads to an increased proportion of α-helical protein structures in their axes, which coincides with additional tolerance of desiccation stress.
Generally, seeds acquire desiccation tolerance during their development and before physiological maturity (Sun and Leopold, 1993; Sanhewe and Ellis, 1996). This desiccation tolerance is often inferred from the capacity of embryos to germinate after drying. In maize (Zea mays L.), excised, immature embryos acquire the ability to germinate at 14 DAP (Bochicchio et al., 1988). The rate of drying further determines how early in development isolated embryos acquire desiccation tolerance (Bochicchio et al., 1994b). Slow drying over a 6-d period renders them desiccation tolerant from 18 DAP onward, whereas rapid dehydration over a 2-d period is tolerated only from 22 DAP onward. The loss of viability in desiccation-sensitive embryos is often attributed to the loss of plasmalemma integrity after drying, as deduced from the excessive leakage of cytoplasmic solutes (Senaratna et al., 1988; Blackman et al., 1995). Disruption of membrane structures may lead to decompartmentalization of the cellular components, resulting in the release of enzymes that degrade cytoplasmic structures.
The induction and mechanism of desiccation tolerance in higher plant organs have been the subject of many biophysical and biochemical studies (for review, see Crowe et al., 1992; Vertucci and Farrant, 1995). Survival in the desiccated state requires protection of cytoplasmic proteins and retention of membrane structure upon dehydration and rehydration (Crowe et al., 1987; Hoekstra et al., 1997). Sugars may play a key role in this protection. The function of sugars in desiccation tolerance of anhydrous organisms, including seed embryos, is 2-fold. On the one hand, di- and oligosaccharides have been suggested to interact with dehydrating membranes and proteins, thus preventing conformational changes (Carpenter et al., 1987; Crowe et al., 1992, 1997). This has led to the formulation of the so-called “water-replacement hypothesis.” On the other hand, these carbohydrates could contribute to a glassy state in the dry cytoplasm at ambient temperatures (Burke, 1986; Williams and Leopold, 1989), which is considered important in preventing membrane fusion (Sun et al., 1996) and degradation of cytoplasmic components (Leopold et al., 1994; Hoekstra et al., 1997). In maize embryos raffinose increases upon slow drying of the embryos (Bochicchio et al., 1994a). However, no clear correlation has been found between the acquisition of desiccation tolerance in these excised embryos and either the Suc or raffinose content.
Slow drying of immature seeds also leads to the synthesis of Lea proteins, which are suggested to play a role in alleviating dehydration stress (Blackman et al., 1991, 1995; Ceccardi et al., 1994). It is striking that during normal development in the kernel, maize embryos initiate the transcription of a Lea protein RNA just at 22 DAP (Mao et al., 1995), which coincides with the moment that the embryos improve their survival of rapid drying (Bochicchio et al., 1994b). Specific secondary structures (α-helical) for some of these proteins have been predicted (Dure et al., 1989; Dure, 1993). The synthesis of Lea or other proteins during slow drying of excised immature embryos may thus alter the protein profile.
The secondary structure of proteins has been extensively studied using FTIR in the 1800 to 1500 cm−1 spectral region (Susi et al., 1967). Differences in the C=O stretching vibrations of the peptide groups (the amide-I region between 1600–1700 cm−1) provide information on the type of secondary structure, such as α-helix, β-strands, and different kinds of turn structures. In situ FTIR has been recently applied to study the overall protein secondary structure in dry pollen (Wolkers and Hoekstra, 1995) and seeds (Golovina et al., 1997b). The advantage of FTIR is that protein secondary structure is measured in the native environment of the proteins and that it is a noninvasive technique. The disadvantage is that FTIR only provides information on the average protein secondary structure.
In the present work maize embryos were excised at 20 and 25 DAP and exposed to slow drying, rapid drying, or flash drying, with embryos matured on the plant as the reference. The overall in situ secondary structure of proteins in dry embryo axes was studied using FTIR, in an attempt to link possible changes in structure to the acquisition of desiccation tolerance.
MATERIALS AND METHODS
Plant Material and Drying Treatments
Maize (Zea mays L.) plants from the inbred line Lo904 (1994, 1995, and 1996 harvests) were grown in Bergamo and Florence, Italy. Zygotic embryos were excised from the developing kernels at 20 and 25 DAP and from dry, mature kernels at approximately 65 DAP.
Isolated, immature embryos were dried to less than 5% water content (on a dry weight basis) by rapid or slow drying as described previously (Bochicchio et al., 1994b). Rapid drying occurred over 2 d, and slow drying over 6 d. Flash drying was performed by placing the excised embryos in a glove box that was continuously purged with dry air (RH < 3%) at ambient temperature for at least 24 h; embryos were dry within a few hours.
Desiccation-Tolerance Test
To assess desiccation tolerance of the 20- and 25-DAP excised embryos, we used the germination test according to the method of Bochicchio et al. (1988). Embryos were germinated aseptically in 9-cm Petri dishes (five embryos per dish) on a solid medium containing 0.8% (w/v) agar, 2% (w/v) Suc, and mineral salts at 25°C in a growth chamber for up to 15 d. Embryos were scored as germinated if their coleoptile and radicle or secondary roots had visibly grown (longer than 0.2 cm).
Membrane Integrity Measured by EPR Spin-Probe Technique
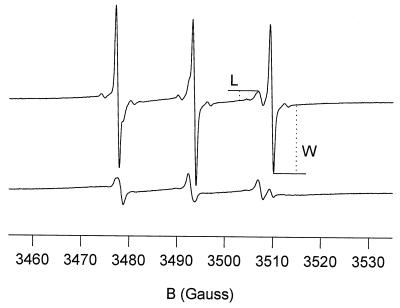
This membrane permeability assay is based on the differential penetration of amphipathic spin-probe molecules and the ions of the broadening agent ferricyanide (Miller, 1978; Golovina and Tikhonov, 1994; Golovina et al., 1997a). The ratio (L/W) between the line heights of the lipid (L) and water (W) components of the spectrum was used to quantitatively characterize membrane permeability. The intensity of the lipid signal serves as the reference for the amount of material under investigation, whereas the intensity of the water component is negatively correlated with the amount of ferricyanide molecules that penetrated the cells.
EPR measurements were performed on a spectrometer (300E EPR, Bruker, Billerica, MA). The EPR settings were: modulation amplitude of 0.5 G, field center at 3480 G with a scan range of 100 G, and a microwave frequency of 9.8 GHz. The microwave power was 2.02 mW. The Zeeman field modulation was 100 kHz, and the scan time was 80 s. Four scans were accumulated. The nitroxide radical Tempone (4-oxo-2,2,6,6-tetramethyl-1-piperidinyloxy [Sigma]) and potassium ferricyanide were used as the spin probe and the broadening agent, respectively (Miller, 1978).
A few embryos from each treatment were exposed to moisture-saturated air for 5 h and allowed to imbibe on filter paper in a small Petri dish for another 5 h. Fifteen minutes before the measurements, the embryos were dipped in a solution of 1 mm Tempone/100 mm potassium ferricyanide. Parts from the embryonic axis were prepared and directly loaded into 2-mm-diameter glass capillaries (with sample length of approximately 5 mm), and a small amount of solution was added to keep the material hydrated. The capillaries were accommodated within standard 4-mm-diameter quartz tubes.
Sugar Determinations by HPLC
Preweighed individual zygotic embryos (3–38 mg) were ground in small glass Potter-type homogenizers in 80% (v/v) methanol in the presence of approximately 0.5 to 1 mg of melezitose as the internal standard. The extracts were heated for 10 min at 75°C in a hot-water bath. The solvents were then removed by drying in a Speed-Vac (Savant Instruments Inc., Farmingdale, NY) for 2 h, and the final volume was adjusted to 1 mL with water. The debris was removed by centrifugation for 5 min in a centrifuge (Eppendorf), after which the extract was ready for analysis.
For the analysis of soluble sugars, we performed high-pH anion-exchange HPLC with pulsed electrochemical detection, using a gradient pump module (model GP40, Dionex, Sunnyvale, CA) and an ED40 pulsed electrochemical detector. Sugars were chromatographed on a CarboPac PA100 4- × 250-mm column (Dionex) preceded by a guard column (CarboPac PA100, 4 × 50 mm). The flow rate was 1 mL/min at 4°C.
FTIR
FTIR spectra were recorded on a spectrometer (model 1725, Perkin-Elmer) equipped with a liquid nitrogen-cooled mercury/cadmium/telluride detector and a Perkin-Elmer microscope as described previously (Wolkers and Hoekstra, 1995).
The embryos were cross-sectioned, and a slice of the embryo axis was pressed gently between two diamond windows and placed in a temperature-controlled brass cell for IR spectroscopy. To increase the transparency of dry slices, a small amount of fluorolube (Perkin-Elmer) was added. To avoid the interfering effects of water in the IR spectra of slices from fresh embryo axes, the intact embryos were also placed in D2O for 2 h before recording the FTIR spectra.
For protein studies, the spectral region 1800 to 1500 cm−1 was selected. This region contains the amide-I and amide-II absorption bands of the protein backbones. The FTIR spectra were recorded at room temperature. Deconvolved spectra were calculated using the interactive Perkin-Elmer routine for Fourier self-deconvolution. The parameters for the Fourier self-deconvolution were a smoothing factor of 15.0 and a width factor of 30.0 cm−1.
RESULTS
Desiccation Tolerance and Membrane Permeability of Immature Embryos
Embryos were excised at 20 and 25 DAP and subsequently tested for their germination capacity before and after slow, rapid, or flash drying. All fresh embryos were able to germinate. However, none of the rapidly or flash-dried 20-DAP embryos survived, whereas 90% of the slowly dried embryos germinated (Table I). Seventy-four percent of the rapidly dried 25-DAP embryos germinated, which could be further improved to 100% after slow drying. This indicates that another 5 d of development on the ear resulted in acquisition of tolerance to rapid drying and a higher percentage of embryos tolerating slow drying.
Table I.
Effect of slow, rapid, or flash drying of maize embryos excised on different DAP on germination, membrane permeability, and sugar content
| DAP | Treatment | Dry Wt | Germination | Permeability | Sugar
|
||
|---|---|---|---|---|---|---|---|
| Suc | Raffinose | Stachyose | |||||
| mg | % | L/W | % of dry wt | ||||
| 20 | SDa | 2.5 | 90.1 | 0.09 | 8.0 | 1.75 | 0.00 |
| 20 | RDb | 3.1 | 0 | 1.88 | 7.3 | 0.00 | 0.01 |
| 20 | FDc | 3.0 | 0 | 11.3 | 0.00 | 0.00 | |
| 25 | SD | 7.7 | 100 | 0.11 | 9.3 | 1.86 | 0.00 |
| 25 | RD | 8.2 | 73.9 | 0.54 | 8.0 | 0.03 | 0.03 |
| 25 | FD | 8.8 | 100d | 0.56 | 7.9 | 0.03 | 0.07 |
| 65 | Mature | 38 | 100 | 0.23 | 9.6 | 1.72 | 0.00 |
| lsd (P = 0.05) | 1.3 | 0.33 | 1.6 | 0.65 | 0.04 | ||
Germination percentages are means of at least two samples, each with more than 20 embryos. The sugar contents are averages of at least three extracts from individual embryos. The membrane permeability of embryonic axes of maize embryos was determined from EPR spectra and calculated as the ratio L/W in the high-field region of the EPR spectrum (see Fig. 1).
SD, Slow drying.
RD, Rapid drying.
FD, Flash drying.
Determined on five embryos only.
Membrane permeability was measured by an EPR spin-probe technique. Figure 1 shows EPR spectra of Tempone in 20-DAP embryo axes that were previously subjected to slow or rapid drying. The line height of the aqueous contribution (W) in the rapidly dried, 20-DAP embryos was considerably lower than that in the slowly dried, 20-DAP embryos because of the broadening effect of ferricyanide ions that had penetrated through the plasma membranes. From such EPR spectra the ratio of the line heights of the lipid peak to the water peak (L/W) was calculated, which is a measure of membrane permeability (Table I). The inability of the 20-DAP embryos to survive rapid drying coincided with high plasma membrane permeability (Table I). Low permeability was observed in the slowly dried, 20- and 25-DAP embryos, coinciding with a high degree of survival. Fast and rapidly dried, 25-DAP embryos had a slightly higher permeability than the slowly dried embryos. The mature embryos also had low plasma membrane permeability.
Figure 1.
EPR spectra of axes of maize embryos excised at 20 DAP and subjected to slow (upper spectrum) and rapid (lower spectrum) drying. The embryos were prehydrated from the vapor phase for 5 h and subsequently allowed to imbibe in H2O for 5 h. Before the measurements, the embryos were labeled in a mixture of Tempone (1 mm) and ferricyanide (100 mm) for 15 min. W, Line height of the H2O component; L, line height of the lipid component.
Sugar Contents in Immature Embryos
Sugar analyses of the differently dried, immature embryos showed that Suc was the major component (Table I). Slow drying was always accompanied by the synthesis of raffinose (close to 2% of the dry weight). After rapid drying, hardly any raffinose could be detected, even in the 25-DAP embryos. The raffinose content of mature, dry embryos was similar to that after slow drying of the immature embryos. Although traces of stachyose were detected, there was no clear correlation with the different drying treatments. The Suc content as a percentage of the dry weight was stable after the drying treatments, in spite of the fact that the dry matter more than doubled during development from 20 to 25 DAP.
Protein Secondary Structure in Embryo Axes
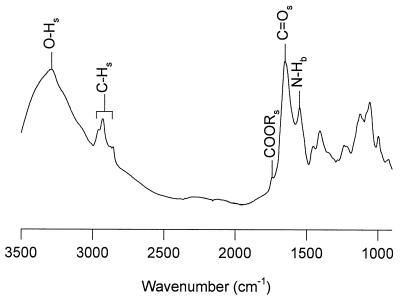
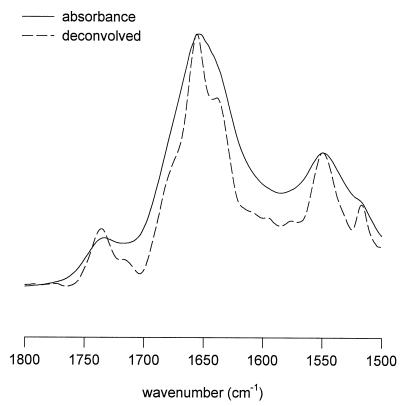
Figure 2 depicts a typical IR absorption spectrum of a dry embryo axis, which is composed mainly of proteins, lipids, and carbohydrates (sugar and cell wall material). The broad band around 3287 cm−1 corresponds to O–H stretching vibrations, mainly arising from proteins and carbohydrates. The bands at 2928 and 2856 cm−1 represent C–H stretching vibrations, arising mainly from neutral lipids, proteins, and carbohydrates. In the 1700 to 1500 cm−1 region, the amide-I band around 1650 cm−1 and the amide-II band around 1550 cm−1 can be observed, which are attributable to proteins (Wolkers and Hoekstra, 1995). The band around 1740 cm−1 in this region is attributable to ester bonds arising from lipids. We have focused on the amide-I band to study structural rearrangements in the protein secondary structure during drying of the embryos.
Figure 2.
FTIR absorption spectrum of the axis of a slowly dried maize embryo, 20 DAP. Characteristic group frequencies are indicated.
IR Spectra of Fresh 20- and 25-DAP Embryo Axes
Figure 3A depicts the protein region of IR spectra in axes excised from fresh (hydrated) embryos at 20 and 25 DAP. The spectral features resembled one another. To resolve possible differences in more detail, deconvolved spectra were calculated (Surewicz and Mantsch, 1988). Deconvolution revealed that the amide-I band around 1650 cm−1 is composed of several bands, but again, the band features in these deconvolved spectra were very similar (Fig. 3B). This indicates that no changes in type and relative proportion of the overall protein secondary structures were detectable during the 5 d of embryo development from 20 to 25 DAP in planta. However, the total amount of protein is expected to have increased because the dry matter increased from approximately 3 to 8 mg during this period (Table I). The differences in peak height around 1740 cm−1 were the result of uncontrolled losses of neutral lipid during the sandwiching of the sample between the diamond windows, and were not taken into consideration any further.
Figure 3.
Absorption (A) and deconvolved absorption (B) FTIR spectra of the axes of fresh maize embryos excised at 20 and 25 DAP.
The water in the fresh embryos is expected to absorb IR light around 1650 cm−1. To reduce possible interfering effects of this water, we also studied the protein structure of fresh embryo axes after the H2O was exchanged for D2O (Fig. 4, A and B). In contrast to H2O, D2O does not interfere with the amide-I band. Spectra in D2O of the embryo axes at 20 and 25 DAP also were very similar. However, the maximum band position occurred around 1646 cm−1, whereas in H2O this band position was around 1652 cm−1 (Table II). This lower band position can be attributed to the solvent effect (Haris et al., 1989). Furthermore, the bandwidth at 70% of the maximum band height was less in D2O than in H2O. This is an indication that in the fresh embryo axis the amide-I band was indeed broadened by the presence of some H2O. We did not attempt to subtract this water contribution to the amide-I band, because it is difficult to find an appropriate background for water in in situ spectra.
Figure 4.
Absorption (A) and deconvolved absorption (B) FTIR spectra of the axes of fresh maize embryos in D2O excised at 20 and 25 DAP.
Table II.
Characteristics of the amide-I band in original IR-absorption spectra from transverse slices of maize embryo axes
| DAP | Treatment | v | Δv |
|---|---|---|---|
| cm−1 | |||
| 20 | Fresh | 1651.9 | 55 |
| 25 | Fresh | 1652.1 | 54 |
| 20 | Fresh in D2O | 1646.3 | 51 |
| 25 | Fresh in D2O | 1646.7 | 47 |
| 20 | Slowly dried | 1653.5 | 52 |
| 25 | Slowly dried | 1653.5 | 46 |
| 20 | Rapidly dried | 1646.3 | 68 |
| 25 | Rapidly dried | 1652.2 | 57 |
| 20 | Flash dried | 1652.4 | 58 |
| 25 | Flash dried | 1652.3 | 58 |
| 65 | Mature | 1653.9 | 48 |
| lsd (P = 0.05) | 1.7 | 12 | |
The excised embryos were subjected to slow, rapid, or flash drying, or analyzed fresh in the presence of H2O or D2O. The absorption maximum, ν, and the linewidth, Δν, were determined at 70% of the total band height (means of at least two samples).
Effect of Drying Rate and Developmental Age
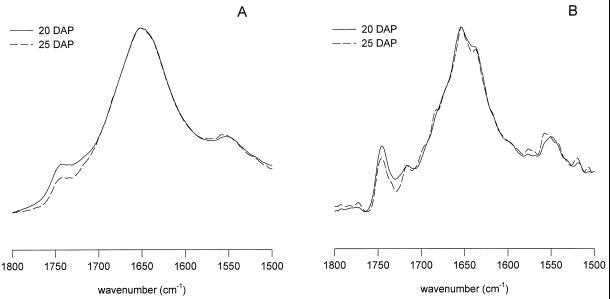
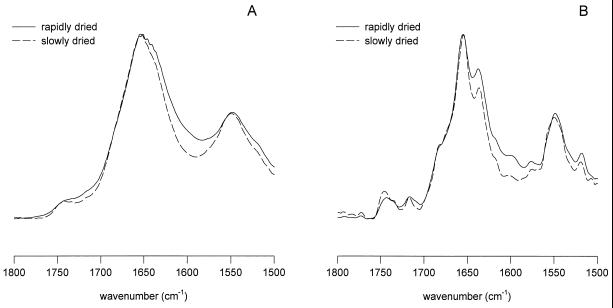
In Figure 5A, the IR spectra of 25-DAP embryo axes after slow and rapid drying are shown. The amide-I band maxima were located at 1653.5 and 1652.2 cm−1, respectively. Also with respect to the fresh control, these maxima were almost identical. Deconvolution (Fig. 5B) shows that the major component of the amide-I region is a band around 1655 cm−1, which can be assigned to α-helical structures. The other bands around 1637 and 1680 cm−1 reflect β-sheet and turn structures (Surewicz et al., 1993). The slight difference in band position and bandwidth between the 25-DAP rapidly and slowly dried embryos as shown in Figure 5A and Table II stem from the difference in relative proportion of the assigned secondary structures (Fig. 5B). These differences are illustrated in Figure 8.
Figure 5.
Absorption (A) and deconvolved absorption (B) FTIR spectra of the axes of maize embryos excised at 25 DAP and subjected to rapid and slow drying.
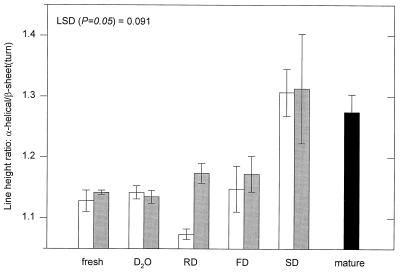
Figure 8.
Line-height ratios of the bands denoting α-helical structure and β-sheet/turn structure. The line heights were determined from deconvolved IR absorption spectra of fresh, deuterated (D2O), rapidly dried (RD), slowly dried (SD), and flash-dried (FD) axes of maize embryos excised at 20 DAP (white bars) and 25 DAP (shaded bars). Data points are means of at least two samples ± se.
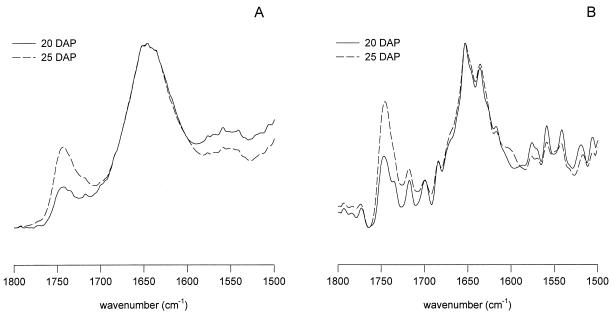
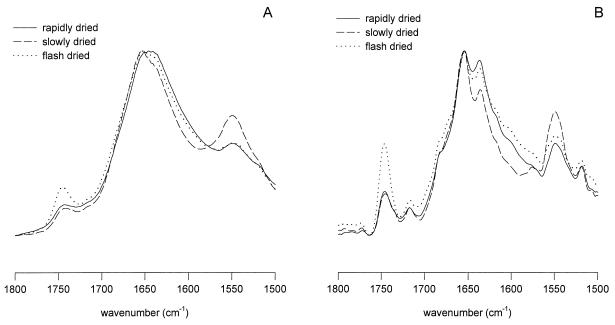
However, when embryos excised at 20 DAP were subjected to slow, rapid, or flash drying, major differences in the overall protein secondary structure were observed (Fig. 6A). Compared with slow drying, rapid drying resulted in a shift of the amide-I band to lower wave number position and an increased line width (see also Table II). Flash drying of these 20-DAP embryos gave an intermediate amide-I band pattern. The deconvolved spectra in Figure 6B show that this shift to lower wave number in the amide-I band was caused by a decrease of the α-helical band around 1656 cm−1 relative to the β-sheet (and turn structures) band around 1639 cm−1. The decrease of the α-helical contribution to the amide-I region of the spectrum was also reflected in a decrease of the amide-II line height. On slow drying, the protein profiles of the 20- and 25-DAP embryo axes were fairly similar (compare Figs. 5 and 6). For comparison, the spectrum (original and deconvolved) of an axis from a mature, dry, 65-DAP embryo is presented in Figure 7. This spectrum resembles the spectra of the slowly dried, immature embryo axes (both 20 and 25 DAP).
Figure 6.
Absorption (A) and deconvolved absorption (B) FTIR spectra of the axes of maize embryos excised at 20 DAP and subjected to slow, rapid, and flash drying.
Figure 7.
Absorption and deconvolved absorption FTIR spectra of axes of dry, mature maize embryos (65 DAP).
Statistical verification of the differences in position and width of the amide-I band for the differently treated embryos is given in Table II. The low-wave-number position of the amide-I band in D2O-treated fresh embryos stems from solvent effects (Haris et al., 1989). Considerable differences were observed only between slowly and rapidly dried 20-DAP embryos. This indicates a major difference in the overall protein secondary structure between them. Deconvolved spectra were used to further resolve the changes in specific protein secondary structures.
Figure 8 shows histograms of the line-height ratios between the α-helical structure and β-sheet/turn structure as derived from the deconvolved absorption spectra. The ratios were significantly higher in the axes of the slowly dried embryos (20 and 25 DAP) and in the mature embryos compared with those in axes of all the other embryos. This indicates an elevated proportion of α-helical structures in the axes of slowly dried and mature embryos. In the rapidly dried embryos excised at 20 DAP, the line-height ratio was significantly lower than those for all of the other embryos, indicating a declined contribution of α-helical structures. The rapidly dried embryos excised at 25 DAP and the 20- and 25-DAP flash-dried embryos had line- height ratios that were not significantly different from those for the fresh controls (in H2O and D2O).
To gain insight into the extent that the relative amounts of α-helical structures increase after slow drying of the immature embryos, a curve-fitting procedure was applied on the amide-I band (for details, see Wolkers and Hoekstra, 1995). It was calculated on the basis of the relative absorbance that the α-helical structures increased from an average of 35% of the total protein secondary structures in the flash-dried axes to 51% in the slowly dried axes (both 20 and 25 DAP).
DISCUSSION
Slow drying of excised, immature maize embryos confers tolerance to desiccation at a stage of development in which rapid drying leads to debilitation (Bochicchio et al., 1994b). Thus, it was shown in Table I that viable, 20-DAP, excised embryos survive slow drying but do not tolerate rapid drying. This acquired tolerance to desiccation is accompanied by a considerably reduced leakage of endogenous solutes during rehydration and an increased level of raffinose, apart from the Suc that is already present in the fresh material. Apparently, accumulation of raffinose is typically the result of slow drying, because it was also observed in the 25-DAP, slowly dried embryos and in the mature embryos after maturation drying on the ear. Acquisition of desiccation tolerance often is associated with the synthesis of oligosaccharides such as raffinose and stachyose (Horbowicz and Obendorf, 1994). Nevertheless, a considerable percentage (74%) of the 25-DAP embryos survived rapid drying, in spite of the fact that raffinose was almost absent. Therefore, raffinose is not a prerequisite for desiccation tolerance, as was stipulated earlier for immature maize embryos (Bochicchio et al., 1994b) and primed cauliflower seeds (Hoekstra et al., 1994). As expected, membrane permeability of the rehydrated, 25-DAP, rapidly dried embryos was much lower than that of the rapidly dried, 20-DAP embryos. However, it was higher than after slow drying of the 25 DAP embryos, which also is reflected in the lower germination percentage (74% compared with 100%).
Coinciding with the acquisition of desiccation tolerance in seeds, new proteins such as Lea proteins are generally synthesized. In higher plants Lea proteins are ubiquitous and also may be induced to higher levels of expression in other tissues by ABA and/or desiccation stress (Close et al., 1989). They are considered to play a role in the protection against desiccation stress (Blackman et al., 1995), but the exact protective mechanism is unknown. In fresh, immature maize embryos, transcripts of a Lea protein appear from 22 DAP onward, and ABA can stimulate the level of transcription (Mao et al., 1995). In our experiments the detachment of the embryos from the plant and/or the slow drying might have induced the synthesis of such proteins. Although some of the Lea proteins were predicted to exist as amphipathic helical structures (Dure et al., 1989; Dure, 1993), the direct surroundings, such as ionic strength of the solution, have a considerable influence on their secondary structure (Russouw et al., 1995).
Using FTIR we characterized changes in the overall protein secondary structure in developing maize embryo axes that were exposed to different drying regimes. In fresh, immature, 20- and 25-DAP maize embryo axes, the protein profiles were very similar (see Figs. 3 and 4). Because moisture contents are still high in these fresh axes, the amide-I band might be distorted because of the interfering effect of water in the amide-I region. This problem was circumvented by using D2O instead of H2O, because D2O absorbs at a much lower wave number (Haris et al., 1989). Also, in D2O the results indicate that the proportion of the different protein secondary structures did not change between 20 and 25 DAP, irrespective of the considerable dry matter gain during these 5 d of development on the plant.
Changes in the protein profile of the embryos can be observed, both in line width and proportion of α-helical structures, depending on the drying treatment. The relative proportion of ordered α-helical structures increased upon slow drying, which was also observed after normal maturation on the plant. We infer this from the reduced line width of the original amide-I band (Table II) and the increased line height of the band around 1655 cm−1 in the deconvolved spectra (Fig. 8).
Rapid drying of the immature embryos resulted in a proportionally lower α-helical content and higher contribution of β-sheet/turn structures. This was particularly prominent in the 20-DAP embryo axes. Such high contribution of β-sheet structures was not observed after flash drying (see Figs. 6 and 8). Apparently, flash drying fixes the protein profile that is present in fresh embryos. If breakdown of the α-helix is responsible for the decreased ratio of α-helix to β-sheet/turn structures in the rapidly dried 20-DAP embryos, then such a fixation after the flash drying would seem logical, simply because there would be less time available for protein breakdown. This idea of breakdown is supported by the high membrane permeability of the 20-DAP rapidly dried axes. Proteases may have been released already during the rapid drying process, to cleave the α-helical structure, increasing the proportion of β-sheet/turn structures. Although our permeability measurements were performed after rehydration, high permeability might have occurred before the embryos were dry. In desiccation-sensitive somatic embryos, phospholipid breakdown already occurred before drying was completed (Tetteroo et al., 1996), which may be linked with high membrane permeability.
Our in situ FTIR data show an increased proportion in α-helical structures after slow and maturation drying. This may be attributable to the effect of drying per se on the overall protein secondary structure. However, because flash drying cannot evoke the elevated α-helical content, there must be other reasons for this phenomenon. De novo synthesis of proteins associated with the defense against desiccation stress may have caused this increase. Synthesis of storage proteins is unlikely here because it was reported earlier that it ceases upon slow drying (Jiang et al., 1996). Proteins other than Leas also may be involved in the increase in α-helical structures. It should be stressed that our FTIR analysis was performed in situ and that no other technique can provide information about structure of proteins in their native state. However, the averaging character of FTIR makes it difficult to directly link changes in the overall protein secondary structure to the synthesis of specific proteins.
We conclude that slow drying of immature maize embryos (20 and 25 DAP) causes adaptations in the cytoplasmic protein profile, membranes, and sugar composition, which also can be found in embryos of dry, mature seeds. These adaptations cannot be completed during rapid drying. The fact that the 25-DAP embryos germinate to a certain extent after rapid drying without an increased proportion of α-helical structures in their protein profile suggests that the increased proportion of α-helices as observed in slowly dried embryos may not be a prerequisite for desiccation tolerance. However, the total amount of protein is expected to have increased considerably during the additional 5 d of development from 20 to 25 DAP, including α-helical structures. Furthermore, in comparison with the slowly dried, 25-DAP embryos, the rapidly dried, 25-DAP embryos are more sensitive to desiccation stress, as shown by the higher membrane permeability. These considerations contribute to the paradigm that the acquisition of full desiccation tolerance is a gradual process during maturation (Hong and Ellis, 1992; Sun and Leopold, 1993), and involves the adaptations mentioned above. Germination is nevertheless possible, even if not all of these adaptations have been completed. The full benefit of all these adaptations may become apparent in an increased storage longevity.
ACKNOWLEDGMENTS
We thank Mark Alberda for excellent technical assistance and acknowledge the Istituto Sperimentale di Cerealicoltura, Sez. Maiscoltura at Bergamo, Italy, where part of the plants were grown and hand pollination was carried out.
Abbreviations:
- DAP
days after pollination
- D2O
2H2O
- EPR
electron paramagnetic resonance
- FTIR
Fourier transform IR spectroscopy
- Lea
late embryogenesis abundant
Footnotes
This research was supported by the Life Sciences Foundation, which is subsidized by the Netherlands Organization for Scientific Research (W.F.W.), and by European Community (EC) grant PL 920248 from the EC AIR program to A.B. and G.S.
LITERATURE CITED
- Blackman SA, Obendorf RL, Leopold AC. Desiccation tolerance in developing soybean seeds: the role of stress proteins. Physiol Plant. 1995;93:630–638. doi: 10.1104/pp.100.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman SA, Wettlaufer SH, Obendorf RL, Leopold AC. Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol. 1991;96:868–874. doi: 10.1104/pp.96.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochicchio A, Rizzi E, Balconi C, Vernieri P, Vazzana C. Sucrose and raffinose contents and acquisition of desiccation tolerance in immature maize embryos. Seed Sci Res. 1994a;4:123–126. [Google Scholar]
- Bochicchio A, Vazzana C, Raschi A, Bartels D, Salamini F. Effect of desiccation on isolated embryos of maize: onset of desiccation tolerance during development. Agronomie. 1988;8:29–36. [Google Scholar]
- Bochicchio A, Vernieri P, Puliga S, Balducci F, Vazzana C. Acquisition of desiccation tolerance by isolated maize embryos exposed to different conditions: the questionable role of endogenous abscisic acid. Physiol Plant. 1994b;91:615–622. [Google Scholar]
- Burke MJ (1986) The glassy state and survival of anhydrous biological systems. In AC Leopold, ed, Membranes, Metabolism and Dry Organisms. Cornell University Press, Ithaca, NY, pp 358–363
- Carpenter JF, Martin B, Crowe LM, Crowe JH. Stabilization of phosphofructokinase during air drying with sugars and sugar/transition metal mixtures. Cryobiology. 1987;24:455–464. doi: 10.1016/0011-2240(87)90049-6. [DOI] [PubMed] [Google Scholar]
- Ceccardi TL, Meyer NC, Close TJ. Purification of a maize dehydrin. Protein Expression and Purification. 1994;5:266–269. doi: 10.1006/prep.1994.1040. [DOI] [PubMed] [Google Scholar]
- Close TJ, Kortt AA, Chandler PM. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989;13:95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Carpenter JF, Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987;242:1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Carpenter JF, Prestrelski SJ, Hoekstra FA. Anhydrobiosis: cellular adaptations to extreme dehydration. In: Dantzler WH, editor. Handbook of Physiology, Section 13: Comparative Physiology, Vol II. Oxford, UK: Oxford University Press; 1997. pp. 1445–1477. [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:570–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Dure L., III A repeating 11-mer amino acid motif and plant desiccation. Plant J. 1993;3:363–369. doi: 10.1046/j.1365-313x.1993.t01-19-00999.x. [DOI] [PubMed] [Google Scholar]
- Dure L, III, Crouch M, Harada J, Ho THD, Mundy J, Quatrano R, Thomas T, Sung ZR. Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol. 1989;12:475–486. doi: 10.1007/BF00036962. [DOI] [PubMed] [Google Scholar]
- Golovina EA, Tikhonov AN. The structural differences between the embryos of viable and nonviable wheat seeds as studied with the EPR spectroscopy of lipid-soluble spin labels. Biochim Biophys Acta. 1994;1190:385–392. doi: 10.1016/0005-2736(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Golovina EA, Tikhonov AN, Hoekstra FA. An electron paramagnetic resonance spin probe study of membrane permeability with seed aging. Plant Physiol. 1997a;114:383–389. doi: 10.1104/pp.114.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina EA, Wolkers WF, Hoekstra FA. Long term stability of protein secondary structure in dry seeds. Comp Biochem Physiol. 1997b;117A:343–348. [Google Scholar]
- Haris PI, Coke M, Chapman D. Fourier transform infrared spectroscopic investigation of rhodopsin structure and its comparison with bacteriorhodopsin. Biochim Biophys Acta. 1989;995:160–167. doi: 10.1016/0167-4838(89)90075-7. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Haigh AM, Tetteroo FAA, Van Roekel T. Changes in soluble sugars in relation to desiccation tolerance in cauliflower seeds. Seed Sci Res. 1994;4:143–147. [Google Scholar]
- Hoekstra FA, Wolkers WF, Buitink J, Golovina EA, Crowe JH, Crowe LM. Membrane stabilization in the dry state. Comp Biochem Physiol. 1997;117A:335–341. [Google Scholar]
- Hong TD, Ellis RH. Development of desiccation tolerance in Norway maple (Acer platanoides L.) seeds during maturation drying. Seed Sci Res. 1992;2:169–172. [Google Scholar]
- Horbowicz M, Obendorf RL. Seed desiccation tolerance and storability. Dependence on flatulence-producing oligosaccharides and cyclitols: review and survey. Seed Sci Res. 1994;4:385–406. [Google Scholar]
- Jiang L, Abrams SR, Kermode AR. Vicilin and napin storage-protein gene promoters are responsive to abscisic acid in developing transgenic tobacco seed but lose sensitivity following premature desiccation. Plant Physiol. 1996;110:1135–1144. doi: 10.1104/pp.110.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold AC, Sun WQ, Bernal-Lugo I. The glassy state in seeds: analysis and function. Seed Sci Res. 1994;4:267–274. [Google Scholar]
- Mao Z, Paiva R, Kriz AL, Juvik JA. Dehydrin gene expression in normal and viviparous embryos of Zea mays during seed development and germination. Plant Physiol Biochem. 1995;33:649–653. [Google Scholar]
- Miller RW. Osmotically induced removal of water from fungal cells as determined by a spin probe technique. Plant Physiol. 1978;62:741–745. doi: 10.1104/pp.62.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russouw PS, Farrant J, Brandt W, Maeder D, Lindsey GG. Isolation and characterization of a heat-soluble protein from pea (Pisum sativum) embryos. Seed Sci Res. 1995;5:137–144. [Google Scholar]
- Sanhewe AJ, Ellis RH. Seed development and maturation in Phaseolus vulgaris. I. Ability to germinate and to tolerate desiccation. J Exp Bot. 1996;47:949–958. [Google Scholar]
- Senaratna T, Gusse JF, McKersie BD. Age-induced changes in cellular membranes of imbibed soybean seed axes. Physiol Plant. 1988;73:85–91. [Google Scholar]
- Sun WQ, Leopold AC. Acquisition of desiccation tolerance in soybeans. Physiol Plant. 1993;87:403–409. [Google Scholar]
- Sun WQ, Leopold AC, Crowe LM, Crowe JH. Stability of dry liposomes in sugar glasses. Biophys J. 1996;70:1769–1776. doi: 10.1016/S0006-3495(96)79740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewicz WK, Mantsch HH. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim Biophys Acta. 1988;952:115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- Surewicz WK, Mantsch HH, Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993;32:389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- Susi H, Timasheff SN, Stevens L. Infrared spectra and protein conformations in aqueous solutions. I. The amide I band in H2O and D2O solutions. J Biol Chem. 1967;242:5460–5466. [PubMed] [Google Scholar]
- Tetteroo FAA, de Bruijn AY, Henselmans RNM, Wolkers WF, van Aelst AC, Hoekstra FA. Characterization of membrane properties in desiccation-tolerant and -intolerant carrot somatic embryos. Plant Physiol. 1996;111:403–412. doi: 10.1104/pp.111.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW, Farrant JM (1995) Acquisition and loss of desiccation tolerance. In J Kigel, G Galili, eds, Seed Development and Germination, Marcel Dekker, New York, pp 237–271
- Williams RJ, Leopold AC. The glassy state in corn embryos. Plant Physiol. 1989;89:977–981. doi: 10.1104/pp.89.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkers WF, Hoekstra FA. Aging of dry desiccation-tolerant pollen does not affect protein secondary structure. Plant Physiol. 1995;109:907–915. doi: 10.1104/pp.109.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]