Abstract
Isoflurane (ISO) is the most commonly used inhalational anesthetic for experimental interventions in mice and is preferred for imaging technologies that require the mouse to remain anesthetized for relatively long time periods. This study compares the stability of mean arterial pressure (MAP), heart rate (HR), and body temperature under ISO concentrations of 1%, 1.5%, and 2% (volume-to-volume, v/v) for up to 90 minutes postinduction. At all three levels of anesthesia, we examined evoked physiological responses to fractional inspiratory ratio variations of oxygen (FiO2) and nitrous oxide (N2O). In addition, we determined the hemodynamic effects of anesthesia on pH, glucose, insulin, glucocorticoids, and partial pressure of oxygen and of carbon dioxide in the blood (paO2, paCO2). The results indicate that the most appropriate ISO dose level was 1.5% v/v, yielding stable MAP and HR values comparable to those observed in the animal’s conscious state, with a minute-to-minute variability in MAP and HR of ≤11%. Based on such recordings, the optimal FiO2 appeared to be 50%. The additional use of N2O was associated with higher and more stable values of MAP and HR. Arterial pH values were within the physiological range and varied between 7.20 and 7.43. ISO anesthesia at 1.5% v/v was also associated with mild hyperglycemia (+47%), whereas insulin levels and corticosteroids remained unaltered. We conclude that the application of isoflurane as an inhalational anesthetic in the mouse can be optimized to attain stable hemodynamics by administering it at 1.5% v/v and by supplementing it with N2O.
Keywords: C57BL/6 mouse, cardiovascular physiology, inhalational anesthesia, isoflurane (ISO), fractional inspiratory ratio (FiO2), nitrous oxide (N2O)
Introduction
The mouse has become the preferred animal species for experimental cardiovascular research elucidating the effects of transgenic modifications (Gehrmann et al. 2000; Hoit 2001; Janssen and Smits 2002; MacGowan et al. 2001; Milano et al. 1994). Although phenotypic screening is increasingly conducted by telemetric techniques, many conventional invasive and noninvasive technologies that measure hemodynamic function are conducted under inhalational anesthesia (Erhardt et al. 1984; Hart et al. 2001; Kober et al. 2005; Ohnishi et al. 1974; Price and Ohnishi 1980). Yet anesthetics (injectable or inhalational) are known to depress cardiovascular function moderately to severely (Bosnjak et al. 1992; Connelly and Coronado 1994; Hart et al. 2001; Matsuda et al. 2007; Ohnishi et al. 1974; Pagel et al. 1991; Price and Ohnishi 1980; Szczesny et al. 2004). In addition, they have adverse physiological effects on circulating hormones as well as at the cellular level (Cornett et al. 2008; Lambert et al. 2005; Sato et al. 2006), affecting both calcium entry through L-type calcium ion (Ca2+) channels (Ohnishi et al. 1974; Orestes et al. 2009; Price and Ohnishi 1980) and the calcium sensitivity of the contractile proteins (Price and Ohnishi 1980). There are also prominent effects on the central and peripheral nervous system (Doursout and Chelly 1988), but most notably on metabolism, perfusion, and the uncoupling of oxidative phosphorylation by inhibition of adenosine triphosphate (ATP) synthesis (Chance 1963; Kohro et al. 2001; Rottenberg 1983).
For surgical intervention and short-term experimentation, isoflurane (ISO1) is the most frequently used anesthetic in studies with mice, in part because of its moderate cardiodepressive effects in comparison to those of the injectable agents pentobarbital and urethane or the ketamine/xylazine mixture (Janssen and Smits 2002; Janssen et al. 2004).
Advances in imaging modalities—magnetic resonance imaging (MRI) (Ruff et al. 1998), computed tomography (CT) (Badea et al. 2005), ultrasound (Chu et al. 2006; Roth et al. 2001), and micropositron emission tomography (microPET) (Toyama et al. 2004; Woo et al. 2008)—have also enabled the noninvasive study and phenotyping of the mouse cardiovascular system, with emphasis on the C57BL/6 strain, which is widely used for imaging studies. In such studies, it is imperative that the mouse be kept under prolonged anesthesia (up to 90 minutes) at the lowest possible level of ISO to minimize cardiodepressive effects.
Thermoregulation is vital for maintenance of homeostasis in mice, because of their small body size and large surface area. The effect of temperature on pH, blood flow, and peripheral or total arterial resistance and mean arterial pressure (MAP1) may also become significant (Barbee et al. 1992; Janssen and Smits 2002; Sarin et al. 1990). Previous studies have investigated the effects of thermoregulation and altered temperature patterns on the basal metabolic rate (rate of heat production) and intrinsic tissue sensitivity to β-adrenergic agonists linked to catechol-O-methyltransferase activity and its modulated response at lower body temperatures in the mouse (Ramirez et al. 1975). Other possible major heat loss mechanisms are attributed to metabolic reactions (anabolic or catabolic) (Dobson and Headrick 1995; Else and Hulbert 1981; Hulbert and Else 1989).
Despite the absence of evidence for direct effects between body temperature and glucose use, higher energetic and metabolic rates in small animals are attributed to their higher energetic O2 needs. Studies have shown that general inhalational anesthetics (isoflurane, halothane) decrease glucose metabolic rates as a result of inhibition of ATP synthesis in the mitochondria in both mice and rats (Kohro et al. 2001; Rottenberg 1983). Researchers have also reported that these effects impair glucose tolerance; raise baseline glucose, insulin, and glucagon levels; and lead to an enhanced plasma insulin response to glucose (attributed to increased hepatic glycogenolysis due to activated glycogen phosphorylase) (Bailey and Flatt 1980). Also prominent has been a rapid hyperglycemic effect (due to sympathetic liver innervation and immediate hormonal release) (Durand et al. 2009), including adrenomedullary catecholamine release. Similar dose-dependent anesthesia effects were noted for cerebral glucose metabolism in rats (Du et al. 2009; Kofke et al. 1987; Lenz et al. 1998; Ori et al. 1986; Stulken et al. 1977), with glucose metabolic rate and oxygen extraction decreases as low as 41–45% in cortical regions, corresponding increases in mean cerebral blood flow and plasma glucose, and reductions in heart rate. PET studies in mice have documented such findings, supporting dose-dependent, temporal uptake increases of ISO in heart and brain regions, indicative of coronary and cerebral blood flow increases and altered metabolism (Toyama et al. 2004).
Studies of the optimal settings of ISO administration for experimental use and for application in imaging studies are limited (Kober et al. 2005). Furthermore, little information is available on the effects of ISO on cardiovascular homeostasis in mice when the inhaled fractional inspiratory ratio of oxygen (FiO21) is altered or when anesthetic nitrous oxide (N2O1) is added as a balancing agent. Yet the use in humans of N2O as a balancing agent (“second anesthetic”) is widespread (Brunson 2008) and may have both cardiac and vascular effects. Price (1976) showed that N2O enhances sympathetic nervous activity, counteracting ISO-induced cardiodepressive effects. Other authors (e.g., Sanders et al. 2008) have reported that it elicits a vasoconstrictive effect, increasing peripheral vascular resistance and blood pressure. And, reporting a study in C57BL mice, Samarska and colleagues (2009) suggest that N2O stabilizes blood pressure by preventing downregulation of vascular cyclooxygenase expression.
We present a detailed study of methods to ensure the physiological welfare of mice under ISO inhalational anesthesia and elucidate the temporal effects of FiO2 and N2O (up to 90 minutes postinduction) on cardiac function and integrative physiological response. Specifically, we assessed dose-dependent depressive effects of ISO anesthesia on cardiovascular function and physiological response, possible effects of FiO2, and effects of the evoked response on global cardiovascular function after administration of N2O. We also monitored corticosteroid levels, metabolic (insulin and glucose) hormones, and respiration and blood oxygenation parameters in order to assess metabolism and stress-induced responses.
Materials and Methods
Ethical Approval
All animal protocols were approved by the Veterinary Services of the Ministry of Agriculture of the Government of Cyprus in accordance with national rules set by the Ministry, European Animal Research directives,2 and international guidelines for animal research (NRC 1996). All experiments conformed to the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes.31
Animal Handling, Transportation, and Acclimation
Inbred mice were obtained from the Animal Housing Facility of the Cyprus Institute of Neurology and Genetics and housed in polypropylene cages (2–3 mice per cage) with food and water provided ad libitum. The mice were maintained on a 12:12 hour dark-light cycle with lights switched on at 6:00am. All procedures took place during the light phase.
Physiological Studies
The study involved 165 male C57BL/6 mice (weight, 22–32 g; age, 8–14 weeks). Anesthesia was initially induced by placing the mice for 2–3 minutes in a chamber filled with 3–5% volume-to-volume (v/v1) ISO (Piramal Healthcare Ltd., London, UK). The mice were then positioned on a specialized circuit board (Indus Instruments, Webster, Texas) that enabled controlled heating and ECG recording. Anesthesia was maintained by inhalation of different concentrations of ISO as described in detail in the Experimental Design section below. Mice breathed freely through a nose cone; gas mixtures were delivered via a constant volume ventilator (SAR-830, CWE Inc., Ardmore, Pennsylvania), with ventilation rate and airflow pressure settings that matched normal physiological respiratory conditions. Mouse respiration rates (counted based on visual inspection) led to measured tidal volumes of 0.12–0.15 ml and flows of 33.6–44 nl/min, in accordance with published reports of artificially ventilated (Gilson 2005) and freely breathing C57BL/6J mice (Schwartz 2000). The ventilator was connected to a calibrated continuous flow ISO vaporizer (Harvard Apparatus, Keighley, UK) and to O2, medical air, and N2O flow meters (PTFE, Cole-Parmer, Vernon Hills, Illinois). The depth of anesthesia was monitored from heart rate (HR1), respiration rates, and limb reflexes induced by paw and tail pinches applied periodically.
Heart rate was independently monitored using bipolar electrocardiograph (ECG) (Ambu Blue Sensor BRS, Ambu, Denmark) taped to the animal’s foot pads. Breathing flow rate was monitored using a respiration flow meter (TSD137A, Biopac, Goleta, California) attached to a commercially available Biopac recording system (Acknowledge, v. 3.8.2, Biopac) that allowed adjustment of inflow rates (≤12 ml/s) and gas mixture delivery pressures.
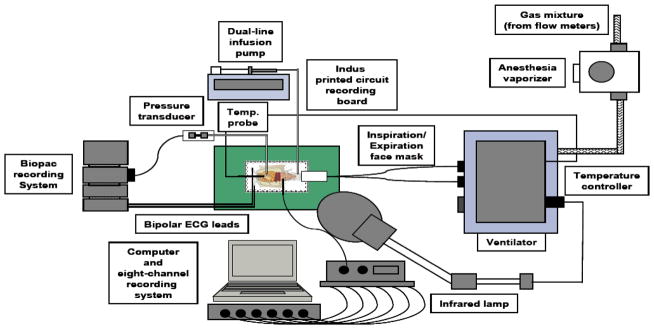
Temperature was monitored via a rectal probe (Physitemp, Clifton, New Jersey; and Biopac) and maintained at body temperature (approximately 37°C), using a feedback-controlled 175 W infrared lamp (Stoelting, Wood Dale, Illinois) via a temperature controller module (Digi-Sense, Cole-Parmer) (Figure 1). At the end of all procedures, mice were euthanized using the cervical dislocation technique.
Figure 1.
Diagram of experimental setup for laboratory mouse research. ADI, ADInstruments; ECG, electrocardiogram; exp, expiratory; insp, inspiratory; temp., temperature.
Microsurgical Procedures
For monitoring of mean arterial pressure, mice underwent an experimental microsurgical protocol under the guidance of a 100x dissecting magnification microscope (SZ61, Olympus, Japan) equipped with a digital camera (E-330, Olympus, Japan), with the help of a light source (KL1500LCD, Olympus, Mainz, Germany). A left carotid cutdown was performed with a 27 gauge needle to facilitate the insertion of an arterial catheter (PE10 polyethylene tubing, Intramedic, Clay Adams, Becton, Dickinson, Sparks, Maryland) attached to a pressure-transducer and connected to the Biopac recording system. Heparinized saline (154 mEq NaCl, 100 units/100 ml heparin) was administered to prevent clotting of the arterial line and to maintain blood volume.
Experimental Design
We performed six sets of studies as follows.
Study I: Effects of ISO Concentration Variation on MAP, HR, and Body Temperature
Twenty-one male C57BL/6 mice (weight, 25.6±7.3 g; age, 7.3±0.3 weeks) underwent the same experimental induction and surgical protocol for implantation of the carotid catheter. We then divided the mice into three groups of seven each and applied three different ISO concentrations—1%, 1.5%, and 2% v/v—for 40 to 90 minutes. Steady-state values of MAP and HR were measured and their variability estimated (as described in detail in the Statistical Analysis section below). In all these studies FiO2 was set to 100%.
Study II: Physiological Response to Fractional Inspiratory Ratio (FiO2)
After determining in study I that the optimal anesthetic level of ISO was 1.5% v/v, we conducted similar hemodynamic studies in 18 additional male C57BL/6 mice (weight, 26.7±1.4 g; age, 7.5±0.4; n = 25 including the 7 from study I) to determine the effects of O2 level variation (based on the variation of the FiO2 in the inhaled gas mixture). The three FiO2 levels that were applied were 50%, 75%, and 100%, in addition to separate recordings in pure medical air (FiO2, 20%). FiO2 levels were monitored throughout the studies with an oxygen analyzer and an externally mounted oxygen sensor (Hudson RCI, Temecula, California).
Study III: Titration of Nitrous Oxide in the Inspired Gas Mixture
We investigated the effects of anesthetic N2O as a balancing agent to the intermediate ISO anesthesia level (1.5% v/v) at two separate levels of O2 concentration—50% (weight, 28.7±1 g; age, 10.5±0.2 weeks) and 75% (weight, 29.5±0.7 g; age, 10.9±0.1 weeks)—in 10 male C57BL/6 mice, 5 in each group. The O2/N2O concentrations in the inhaled mixture were adjusted by altering the N2O flow rate with respect to the maximum O2 flow rate, using the oxygen analyzer-sensor assembly.
Study IV: Stressor and Metabolic Hormone Assays
We used biochemical assays to compare associated effects of ISO at 1% and 2% v/v on steroid (cortisol, androstenedione) levels, metabolic (insulin) hormones, and glucose. Matched cohorts of male C57BL/6 mice (weight, 26.3±2.8 g; age, 10.2±1.3 weeks, n = 37 total) underwent anesthesia with 1% or 2% v/v ISO (mixed with 100% O2 postinduction) and the removal of aortic blood aliquots (1 ml), after which they were euthanized (with 3–5% v/v ISO) at t = 0, 20, 40, 60, and 80 minutes.
Study V: Temporal Variation of Blood Glucose
We studied the temporal effects of varying levels of ISO anesthesia (1%, 1.5%, 2% v/v) on blood glucose levels for up to 90 minutes postinduction. Male C57BL/6 mice (weight, 29±2 g; age, 9.8±0.6 weeks) were allowed to breathe freely ISO at 1% (n = 5), 1.5% (n = 7), or 2% (n = 8) mixed with 100% oxygen. The animals’ body temperature was maintained at 37.1±0.4°C and their ECG, HR, and respiratory flow rate values were constantly monitored. A 0.6 μl blood aliquot was extracted at 5-minute intervals (by a tail vein needle or by cutting the tail vein) for glucose measurements using a blood glucose meter (Ascensia Contour Meter, Bayer, Germany). These tail blood glucose levels (and their variation) were compared to those from 1 ml of carotid arterial blood sampled from two separate cohorts of male C57BL/6 mice (of similar age and weight ranges) anesthetized with 1% and 2% v/v ISO.
Study VI: Anesthesia-Dependent Respiration Effects
To assess possible respiratory distress as a result of anesthesia and optimize the ventilator and respiratory parameters, we used a separate mouse cohort (weight, 23–35 g; age, 9–12 weeks; n = 56), under the same induction protocol as in the other samples, to extract periodic 60 μl carotid artery blood samples in heparinized capillary tubes (Multicap, Siemens Healthcare Diagnostics Ltd, Surrey, UK) for analysis. Blood extracts were collected at t = 0 min (n = 7), t = 20–23 min (ISO = 2% v/v, n = 10; ISO = 1% v/v, n = 10), t = 60–64 min (ISO = 2% v/v, n = 9; ISO = 1% v/v, n = 5), t = 80–90 min (ISO = 2% v/v, n = 9; ISO = 1% v/v, n = 6) postinduction and underwent blood-gas (BG) analysis (pH, paCO2, paO2, Na+, K+, and hematocrit, or Hct1; Rapidlab 348, Siemens Healthcare Diagnostics, Deerfield, Illinois). In most of these experiments, mice were euthanized immediately after BG measurements.
Physiological Data Acquisition and Processing
Physiological data (MAP, ventilation, temperature, HR) were recorded in real time at 500–1000 samples/s using the Biopac system (Acknowledge v. 3.8.2). Concurrent recording of ECG activity was achieved with a separate, commercially available eight-channel recording module (Powerlab 8/30, ADInstruments, Oxford, UK) at 500–1000 samples/s, controlled via LabChart acquisition software (Lab Chart, v. 7, ADI). All files were saved and exported in text files. They were subsequently read by MATLAB (Natick, Massachusetts) for processing. Custom-written MATLAB routines with a front-end graphical user interface allowed temporal synchronization of recorded data, 1-minute interval analyses, and other signal analyses including computation of descriptive statistical and autocorrelation indices. ECG electrical parameters were quantified separately using the ECG analysis modules of LabChart with build-in algorithms that allowed R-wave detections, ECG signal averaging, and cluster classification.
Statistical Analysis
All results are reported as mean ± standard deviation (SD). Constancy of HR and MAP values is reported as the coefficient of variability1 (CVHR = 100. SDHR/MeanHR, CVMAP = 100. SDMAP/MeanMAP) of the 1-minute averaged samples. Two-way unbalanced analysis of variance (ANOVA) and repeated-measures, nonparametric two-tailed student t-tests, were also used (XLSTAT, Addinsoft, New York) to determine whether the various ISO doses were associated with significantly different temporal effects on the cardiovascular, metabolic, hormonal, and respiratory parameters (HR, MAP, temperature, glucose, insulin, androstenedione, cortisol, pH, paO2, paCO2, and electrolyte concentrations) at the 1%, 1.5%, and 2% v/v ISO levels.
Results
Study I
Figure 2 presents variations in HR, MAP, and body temperature at 1%, 1.5%, and 2% v/v ISO anesthesia during the 40- to 90-minute postinduction period. Body temperature was maintained at 36.4±2.1°C (for mice under 1% v/v ISO), 36.3±2.6°C (1.5%), and 36.6±1.5°C (2%). Mean heart rates—408±23 (1% v/v), 478±11.4 (1.5%), and 482±10 (2%)—were significantly lower at 1% v/v ISO than at 1.5% and 2% (p < 0.0001). Corresponding CVHR values were 14±2 (1%), 11±1 (1.5%), and 14±3 (2%) and MAP values were 85±3 (1% v/v), 92±2 (1.5%), and 72±9 millimeters of mercury (mmHg,1 a unit measuring force per unit area) (2%), differing significantly at 1.5% v/v from those at 1% and 2% (p < 0.0001). The corresponding CVMAP values were computed to be 13±3 (1% v/v), 8±2 (1.5%), and 33±2 (2%).
Figure 2.
Variation of heart rate (beats per minute, BPM), mean arterial pressure (MAP), and temperature at 1%, 1.5%, and 2% volume-to-volume (v/v) isoflurane (ISO) anesthesia 40–90 minutes postinduction at inspired O2 = 100%. Values are means ± standard deviation (SD), n = 7 male C57BL/6 mice per group. mmHg, millimeters of mercury
Study II
Fractional inspiratory ratio (FiO2) titration results at the intermediate anesthetic level of 1.5% v/v are depicted in Figure 3. Mean HR values in the 40- to 90-minute period were 478±11 (n = 7), 486±21 (n = 7), 476±22 (n = 6), and 477±27 (n = 5) for FiO2 at 100%, 75%, 50%, and 20%, respectively. Such values were not significantly different at the 5% significance. Corresponding CVHR values were 11±1, 10±2, 9±1, and 8±2, respectively; MAP values were 92±2 (n = 7), 83±4 (n = 7), 90±1 (n = 6), and 84±2 (n = 5); and CVMAP values were 8±2, 25±6, 6±2, and 10±4.
Figure 3.
Mean arterial pressure (MAP) and heart rate (beats per minute, BPM) fractional oxygen dependence at various levels of oxygen (O2)—100%, 75%, 50%, 20%—with the optimal isoflurane (ISO) anesthetic dose of 1.5% volume-to-volume (v/v), measured 40–90 minutes postinduction. Values are means ± standard deviation (SD); n = 5–7 male C57BL/6 mice per group. mmHg, millimeters of mercury
We observed that MAP values were significantly different for all FiO2 challenges and, in particular, for the 45–60 minute interval for FiO2 at both 75% and 20%. Figure 4 shows the effects of adding N2O (in the ISO/O2 gas mixture) on MAP and HR based on two experiments using 1.5% ISO v/v mixed with either N2O = 50% and O2 = 50% or N2O = 25% and O2 = 75%. Mean HR and MAP values (t = 40–90 min) were 489.5±14.8 (n = 5), 515.8±8.7 (n = 5) bpm (CVHR = 10±2; 7±2), and 94±3 mmHg for both groups of 5 male C57BL/6 mice (CVMAP = 6±3; 10±3) for the two mixtures (p = not significant). Mean HR and MAP values were significantly different from corresponding results in the FiO2 = 50–100% cases (p < 0.001). Mean body temperature was maintained at 37.6–37.8°C.
Figure 4.
Effect on heart rate (HR) and mean arterial pressure (MAP) of administered nitrous oxide (N2O) at various inspiration gas mixtures: N2O = 50%, oxygen (O2) = 50% (top) and N2O = 25%, O2 = 75% (bottom) at the optimal isoflurane (ISO) anesthesia level of 1.5% volume-to-volume (v/v). Values are means ± standard deviation (SD); n = 5 male C57BL/6 mice per group. BPM, beats per minute; mmHg, millimeters of mercury
Study III
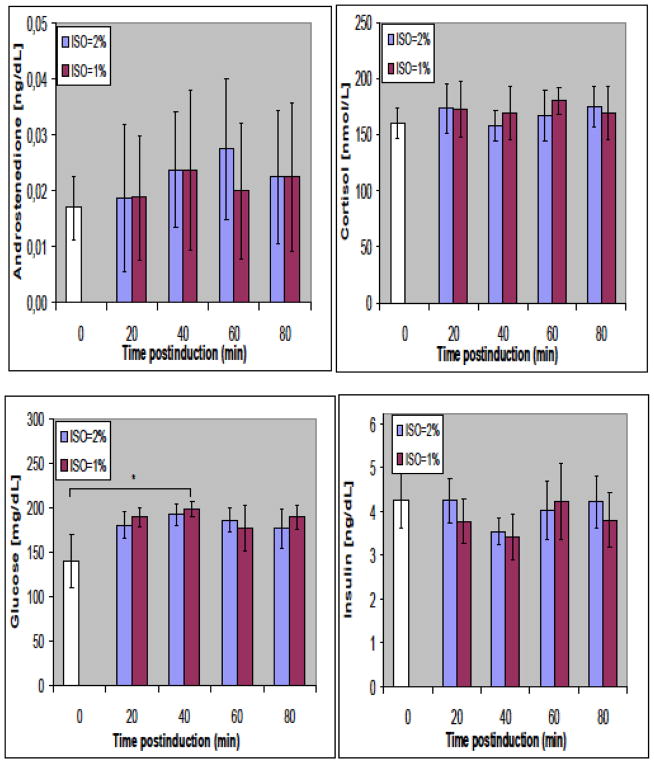
Figure 5 shows variations in arterial concentrations of glucose, insulin, cortisol, and androstenedione at 0, 20, 40, 60, and 80 minutes postinduction for 1% and 2% v/v ISO levels. No significant differences from baseline (t = 0) were observed among any of these parameters at either 1% or 2% v/v of ISO, suggesting the stability of physiological and metabolic status with the tested anesthesia protocol.
Figure 5.
Temporal variation of corticosteroid (androstenedione, cortisol), insulin, and glucose at 1% and 2% volume-to-volume (v/v) isoflurane (ISO) anesthesia up to 80 minutes postinduction in male C57BL/6 mice. Values are means ± standard deviation (SD); n = 4 per group (but for t = 0 [white bar] n = 5). Temporal variations are nonsignificant. mg/dl, milligrams per deciliter; ng/dl, nanograms per deciliter; nmol/l, nanomoles per liter
Study IV
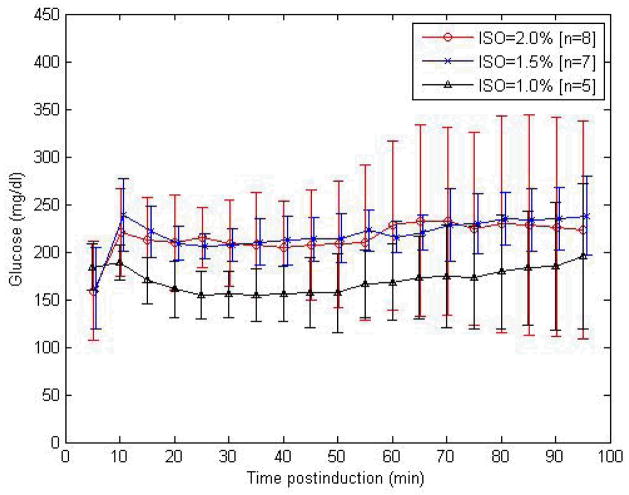
Figure 6 presents variations in tail vein glucose measurements conducted in C57BL/6J mice at 5-minute intervals at 1%, 1.5%, and 2% v/v ISO anesthesia postinduction. Glucose concentrations (mg/dl) ranged between 154.6±28.1 and 195.7±76.2 (1% v/v ISO, n = 7), between 161.7±42.9 and 239.0±38.3 (1.5%, n = 7), and between 158.7±51.9 and 204.6±48.8 (2%, n = 7). ANOVA analysis yielded significantly different tail vein glucose values at 1% v/v ISO compared to corresponding values at 1.5% or 2% v/v, at the 5% significance level (p < 0.0001). Mean glucose levels at ISO levels of 1.5% and 2% v/v were significantly higher compared to those at 1% (p < 0.0001).
Figure 6.
Temporal variation of tail vein glucose measurements at 5-minute intervals at 1%, 1.5%, and 2% volume-to-volume (v/v) isoflurane (ISO) anesthesia postinduction in male C57BL/6 mice (numbers per group as shown). mg/dl, milligrams per deciliter
Study V
Temporal variation of BG analyses (pH, paCO2, paO2, Na+, K+, Hct) at 1% and 2% v/v are shown in Figure 7, at 0, 20–43 (t1), 60–64 (t2), and 80–90 (t3) minutes postinduction. No significant changes were noted at the later time intervals for most of these indices either compared to normal baseline values or for different ISO concentrations, indicating adequate respiration and blood oxygenation. Significant change was observed, however, in paO2 values at the later time intervals (p < 0.0001), as expected after inspiration at 100% FiO2. The lack of significant changes in Hct values from baseline (49±1%) indicates unaltered homeostasis. Hct values for 2% v/v ISO were 46±4% (t1), 46±3% (t2), and 45±4% (t3), and for 1% v/v ISO were 45±5 % (t1), 46±2 % (t2), and 41±6% (t3).
Figure 7.
Temporal variation of respiratory (pH, paCO2, paO2) and electrolyte parameters (Na+, K+, Hct) at 1% and 2% volume-to-volume (v/v) isoflurane (ISO) anesthesia 0–90 minutes postinduction. Values are means ± standard deviation (SD); n = 5–11 male C57BL/6 mice per group. Temporal variations are nonsignificant (except for paO2). Hct, hematocrit; K+, potassium ion; mmHg, millimeters of mercury; mmol/l, millimoles per liter; Na+, sodium ion; paO2, partial pressure of oxygen in the blood; paCO2, partial pressure of carbon dioxide in the blood
Discussion
Taken together, the studies described above revealed the optimum level of isoflurane anesthesia to ensure stability of the murine cardiovascular function for up to 90 minutes. ISO effects were also considered in combination with administered oxygen (FiO2) and N2O. Based on 90-minute recordings, we observed that the most stable blood pressure and heart rate accompanied an ISO dose level of 1.5% v/v and that higher and less variable values of these measures resulted when FiO2 was 25–50% and N2O concentration was 50–75%. Table 1 shows these results alongside the major cardiovascular and biochemical indices measured in our studies.
Table 1.
Cardiovascular and biochemical indices in inhalationally anesthetized male C57BL/6 mice
| HR (bpm) | CVHR | MAP (mmHg) | CVMAP | tRR (ms) | pH | Glucose (mg/dl) | Insulin (ng/ml) | ||
|---|---|---|---|---|---|---|---|---|---|
| ISO = 1.0% | O2 = 100% | 408±23 (n = 6/7) (65.5–90 min avg for 6 mice only) | 14±2 (n = 7) | 85±3 (n = 6/7) (63.5–90 min avg for 6 mice only) | 13±3 (n = 7) | 7.3±0.1 – 7.4±0.1 (n = 9/5) | 176.3±26.1 – 188.8±11.1 (n = 4/5) | 3.4±0.5 – 4.2±0.9 (n = 4/5) | |
| ISO = 1.5% | O2 = 100% | 478±11.4 (n = 7) | 11±1 (n = 7) | 92±2 (n = 7) | 8±2 (n = 7) | 114–134 (n = 4) | |||
| ISO = 2.0% | O2 = 100% | 482±10 (n = 5/7) (78–90 mean reflects avg for 5 mice only) | 14±3 (n = 7) | 72±9 (n = 5–7); (73.6–77.6 mean reflects avg for 6 mice only; 77.6–90 mean avg for 5 mice) | 33±2 (n = 7) | 7.3±0.1 – 7.3±0.2 (n = 9/6) | 139.4±30.1 – 192±11.9 (n = 4) | 3.54±0.3 – 4.24±0.6 (n = 4) | |
| ISO = 1.5% | O2 = 75% | 486±21 (n = 6/7) (89.4–90.4 mean reflects avg for 6 mice only) | 10±2 (n = 7) | 83±4 (n = 6/7) (89.4–90.4 mean reflects avg for 6 mice only) | 25±6 (n = 6/7) (89.4–90.4 mean reflects avg for 6 mice only) | ||||
| ISO = 1.5% | O2 = 50% | 475±21.6 (n = 6) | 9±1 (n = 6) | 90±1.3 (n = 6) | 6±2 (n = 6) | 108–150 (n = 3–6) | |||
| ISO = 1.5% | O2 = 20% | 477±27 (n = 3–5) (86.9–87.9 mean reflects avg for 4 mice only; 87.9 reflects avg for 3 mice only [last time point]) | 8±2 (n = 5) | 84±2 (n = 3–5) (86.9–87.9 mean reflects avg for 4 mice only; 87.9 reflects avg for 3 mice only [last time point]) | 10±4 (n = 5) | ||||
| ISO = 1.5% | O2 = 50% N2 O = 50% |
490±15.0 (n = 5) | 10±2 (n = 5) | 93±3 (n = 5) | 6±3 (n = 5) | 114–124 (n = 3–4) | |||
| ISO = 1.5% | O2= 75% N2 O = 25% |
516±8.7 (n = 5) | 7±2 (n = 5) | 94±3 (n = 5) | 10±3 (n = 5) |
avg, average; bpm, beats per minute; CV, coefficient of variability; Hct, hematocrit; HR, heart rate; ISO, isoflurane; MAP, mean arterial pressure; mmHg, millimeters of mercury; mg/ml, milligrams per milliliter; ms, milliseconds; ng/ml, nanograms per milliliter; N2O, nitrous oxide; O2, oxygen; tRR, time interval between successive QRS peaks
In theory, lower doses of ISO would closely match conscious physiological conditions, but our results indicated that lower levels of ISO may limit anesthetic depth and prohibit ease of animal handling, thus adversely affecting surgery, blood sampling, or restraint. Doses higher than 1.5% v/v seem to increase cardiodepression and thus are not advisable.
The ISO effects on MAP and HR are best explained through a reduction of sympathetic tone that reduces both cardiac output (CO) and total peripheral resistance (TPR). Direct dependence of MAP variations on beat-to-beat stroke volume (SV) variations is also possible under anesthetized conditions (Janssen and Smits 2002), notwithstanding reports of invasive, MRI, CT, and ultrasound studies that show relative constancy of temporospatial image-averaged SV values within physiological levels (Badea et al. 2005; Ruff et al. 1998). Thus ISO-induced changes in MAP are dependent on CO (the product of HR and SV) and TPR. Changes in the latter are dependent on ISO-related vasomotor effects and blood flow redistribution (Barbee et al. 1992; Rosenblum 1977; Sarin et al. 1990).
Toyama and colleagues (2004) used PET to assess the effects in mice of ISO on fluorodeoxyglucose (FDG) and its redistribution within 45 minutes postinduction. They observed a differential distribution of ISO with significantly increased uptake of FDG in heart and brain regions (91%) in comparison both to other regions and to awake controls. Their findings support the presence of ISO-induced coronary vasodilation and increased blood flow effects as well as altered metabolic patterns.
Researchers have proposed various mechanisms responsible for the ISO-mediated redistribution of blood flow: (1) direct effects on the vascular smooth muscle (induced release of endothelium-derived relaxing factor) (Loeb et al. 1998); (2) release of nitric oxide (NO) (Sarin et al. 1990; Loeb et al. 1998); (3) autoregulation and time-dependent adaptation (MAP-associated decreases as a result of vasodilation) (Crystal et al. 2000); and (4) metabolic changes. TPR changes have also been manifested through ISO-induced vasodilation leading to increases in coronary and cerebral blood flow (CBF). It appears that the CBF changes are the result of several interacting factors: the relaxing action of anesthetics on the vascular smooth muscle, the possible mediatory role of NO, time-dependent blood flow changes and redistribution, the influence of indirect metabolic mechanisms secondary to reduced cardiac work demand, pressure-flow autoregulation, and time-dependent vascular adaptation (Crystal et al. 2000).
Our results extend this ISO anesthesia titration study to FiO2 titration and administration of medical air in the inhaled mixture (Figure 3). The optimal FiO2 appears to be >50%, as reflected by the increased MAP values and their decreased variability. Additionally, the results shown in Figure 4 suggest that N2O acts as a balancing agent by antagonizing ISO-induced vascular vasodilatory responses and thus stabilizing the mouse’s physiological status. The optimal amount of administered N2O 25–75% results in higher and less variable MAP values than those observed in the absence of N2O or in the FiO2 titration study (Figure 3).
Adequate ventilation and oxygenation were also ensured through a careful match of the artificial ventilation parameters, inflow pressure, inspiratory and expiratory rates, and measured blood gas parameters. Slight deviations of paCO2 from normal ranges (>45 mmHg) at late time intervals postinduction are attributed to possible respiratory distress due to anesthesia; administration of 100% O2 led to a paO2 of 150 mmHg, indicating hyperoxic or nonphysiological responses. All reported values for electrolytes and respiration rates were within expected ranges. Measured pH values were between 7.28 and 7.42 at the ISO levels 1% and 2%) throughout the 90-minute postinduction period. Hematocrit values were between 40.5% and 48.7% and did not vary in the cases of multiple (2–3) serial blood extractions at different intervals.
Reported physiological pH levels range between 7.2 and 7.43 in a number of mouse strains. Specifically, studies (Dalkara et al. 1995; Kapinya et al. 2002; Zuurbier et al. 2002) have indicated normal physiological murine arterial oxygen (paO2) tensions greater than 65 mmHg (for 50% hemoglobin oxygen saturation) and 30–45 mmHg of paCO2, given pressures of 5–20 cmH2O at the inspiratory site, ventilator breathing rates of 80–280 breaths per minute, and minute volume values of 0.1–0.6 ml in multiple strains under isoflurane and urethane-α-chloralose anesthesia. Earlier publications on pH variation in the mouse (Bernstein 1966; Dalkara et al. 1995; Kapinya et al. 2002; Lahiri 1975; Siemann 1983; Weir 1949; Wolfe 1959) indicate that such variations are attributed to H2O intake, food consumption, environment, temperature, or metabolic adaptations. Some studies have reported Hct values of 50–53% (John et al. 1996).
To ensure proper physiological conditions, it is important that blood oxygenation, myocardial oxygen consumption, and stress levels (as indicated by measures of hormones such as cortisol and androstenedione) not deviate from expected physiological ranges (Janssen and Smits 2002). Figure 5 shows cortisol and androstenedione levels immediately after induction of anesthesia; the levels suggest that the mice were handled with care and that their stress was not high (Tuli et al. 1995).
Blood glucose and insulin measurements from our studies show anesthesia-induced effects on mouse glucose metabolism (Figures 5 and 6): ISO levels of 1.5% produce a disruption of glucose metabolism upon induction, a mild hyperglycemic anesthesia-induced effect possibly associated with vasodilatory and increased blood flow effects. ISO-induced (1.5%, 2%) glucose increases of 38–47% were observed within the first 5 minutes after anesthesia administration, although the values stabilized for the remainder of the study, as shown in Figure 6. Such glucose changes and percentage increases are consistent with those reported in a recent study of age-, sex-, and strain-matched male mice upon 2% ISO administration (Durand et al. 2009).
From our study it is not possible to determine whether the mechanism of action involves transient sympathetic activation, steroid release, a direct effect of ISO, or a combination of such effects. The overall effects of ISO may be the result of opposing vasodilatory and vasoconstrictive effects, either directly (vasodilation) or secondary to an anesthesia-induced decrease of the heart’s metabolic demand, inferences supported by numerous published studies (Crystal et al. 1995; Kober et al. 2005; Ogushi et al. 1993; Park et al. 1994).
Conclusion
The protocol we have described for physiological studies of mice under anesthesia has the potential for high reproducibility in diagnostic modalities (including MRI, microCT, ultrasound, and microPET). Our results show that the optimal ISO anesthetic regimen for mice is a dose of 1.5% v/v mixed with 25–50% O2 and 75–50% N2O. This regimen can be useful in phenotypic screening and pharmacological studies of cardiac function in mice and can facilitate the migration of such work to noninvasive imaging platforms, with tremendous potential for both basic science and translational research.
Acknowledgments
We thank Professors Laurence Hedlund and George Allan Johnson at the Center for In Vivo Microscopy (CIVM) at Duke University for their instrumental role and critical advice and consultation for setting up the infrastructure, purchasing, calibrating equipment, and conducting the experimental work and for Prof. Hedlund’s critical review of the manuscript. Special thanks are owed to Mrs. Sally Zimney at CIVM for editorial assistance. Particular thanks are also due to Drs. Robert Balaban and Alan Koretsky at NIH and to Mr. Felix Onojafe, Mr. Daryl DesPres, and Drs. Martin Lizak and Brenda Klaunberg at the NIH Mouse Imaging Facility for their initial support and guidance in mouse physiological studies. Mr. Anastasios Nicolaou and Dr. Charis Petevinos are thanked for their integral role in the setting, citing, and initial physiological work at the Laboratory of Physiology and Biomedical Imaging [LBI]. We also appreciate the support of the Veterinary Services of the Ministry of Agriculture in Cyprus and its director Drs. Giorgos Neophytou and Charalambos Kakoyiannis and veterinarian Dr. Theophanis Pieridis for their invaluable support. We are indebted to the Director of the Meteorological Service (Dr. Kyriacos Theophilou) and to the Agricultural Research Institute (Drs. Marios Kyriacou and Polycarpos Polycarpou) for their invaluable help in the early stages of the laboratory commissioning. Dr. Stavros Malas, of the Animal Facility at the Cyprus Institute of Neurology and Genetics, has contributed immensely to our effort with consultation and further support throughout our studies.
Grants
This work was supported by a grant on International Cooperation (STOXOS/0308/02) from the Research Promotion Foundation, Cyprus. Additional support was received from the Hellenic Bank through a “HEART” grant.
References
- Badea CT, Fubara B, Hedlund LW, Johnson GA. 4-D micro-CT of the mouse heart. Mol Imag. 2005;4:110–116. doi: 10.1162/15353500200504187. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Flatt PR. Insulin and glucagon during pentobarbitone anaesthesia. Diabete Metabol (Paris) 1980;6:91–95. [PubMed] [Google Scholar]
- Barbee RW, Perry BD, Re RN, Murgo TP. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am J Physiol Regulatory Integr Comp Physiol. 1992;263:R728–R733. doi: 10.1152/ajpregu.1992.263.3.R728. [DOI] [PubMed] [Google Scholar]
- Bernstein SE. Physiological characteristics. In: Green EL, editor. Biology of the Laboratory Mouse. New York: McGraw-Hill; 1966. pp. 337–348. [Google Scholar]
- Bosnjak ZJ, Aggarwal A, Turner LA, Kampire JM, Kampire JP. Differential effects of halothane, enflurane, and isoflurane on Ca2+ transients and papillary muscle tension in guinea pigs. Anesthesiology. 1992;76:123–131. doi: 10.1097/00000542-199201000-00018. [DOI] [PubMed] [Google Scholar]
- Brunson DB. Pharmacology of inhalation anesthetics. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and Analgesia in Laboratory Animals. 2. Chapter 3. London: Elsevier; 2008. pp. 83–93. [Google Scholar]
- Chance B, Williams GR, Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progresterone, and methylene glycol. J Biol Chem. 1963;278:418–431. [PubMed] [Google Scholar]
- Chu D, Jordan M, Kim J, Couto M, Roos K. Comparing isoflurane with Tribomoethanol anesthesia for echocardiographic phenotyping of transgenic mice. JAALAS. 2006;45:8–13. [PubMed] [Google Scholar]
- Connelly TJ, Coronado R. Activation of the Ca2+ release channel of cardiac sarcoplasmic reticulum by volatile anesthetics. Anesthesiology. 1994;81:459–469. doi: 10.1097/00000542-199408000-00025. [DOI] [PubMed] [Google Scholar]
- Cornett PM, Matta JA, Ahern GP. General anesthetics sensitize the capsaicin receptor transient receptor potential V1. Mol Pharmacol. 2008;74:1261–1268. doi: 10.1124/mol.108.049684. [DOI] [PubMed] [Google Scholar]
- Crystal GJ, Khoury E, Gurevicious J, Salem MR. Direct effects of halothane on coronary blood flow, myocardial oxygen consumption, and myocardial segmental shortening in in situ canine hearts. Anesth Analg. 1995;80:256–262. doi: 10.1097/00000539-199502000-00010. [DOI] [PubMed] [Google Scholar]
- Crystal GJ, Zhou X, Gurevicius J, Czinn E, Salem MR, Alam S, Piotrowski A, Hu G. Direct coronary vasomotor effects of sevoflurane and desflurane in in situ canine hearts. Anesthesiology. 2000;92:1103–1113. doi: 10.1097/00000542-200004000-00029. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Irikura K, Huang Z, Panachian N, Moskowitz MA. Cerebrovascular responses under controlled and monitored physiological conditions in the anesthetized mouse. J Cereb Blood Flow Metabol. 1995;15:631–638. doi: 10.1038/jcbfm.1995.78. [DOI] [PubMed] [Google Scholar]
- Dobson GP, Headrick JP. Bioenergetic scaling: Metabolic design and body-size constraints in mammals. Proc Natl Acad Sci U S A. 1995;92:7317–7321. doi: 10.1073/pnas.92.16.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doursout MF, Chelly JE. Effects of basal anaesthesia on cardiac function. Br J Anaesth. 1988;60:119S–122S. doi: 10.1093/bja/60.suppl_1.119s. [DOI] [PubMed] [Google Scholar]
- Du F, Zhang Y, Iltis I, Morjanska M, Zhu XH, Henry PG, Chen W. In vivo proton MRS to quantify anesthesia effects of pentobarbital on cerebral metabolism and brain activity in rat. Magn Res Med. 2009;62:1385–1393. doi: 10.1002/mrm.22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand JL, Hosinking W, Jelicks LA. Time course of inhalational anesthesia on blood glucose level in male and female C57BL/6 mice. Horm Metab Res. 2009;41:339–341. doi: 10.1055/s-0028-1112114. [DOI] [PubMed] [Google Scholar]
- Else PL, Hulbert AJ. Comparison of the “mammal machine” and the “reptile machine”: Energy production. Am J Physiol. 1981;240:R3–R9. doi: 10.1152/ajpregu.1981.240.1.R3. [DOI] [PubMed] [Google Scholar]
- Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blumel G. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylaxine, carfentanyl-etomidate) Res Exp Med. 1984;84:159–169. doi: 10.1007/BF01852390. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol. 2000;279:H733–H740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- Gilson WD, Yang Z, French BA, Epstein FH. Measurement of myocardial mechanics in mice before and after infarction using multislice displacement-encoded MRI with 3D motion encoding. Am J Physiol Heart Circ Physiol. 2005;288:H1491–H1497. doi: 10.1152/ajpheart.00632.2004. [DOI] [PubMed] [Google Scholar]
- Hart CYT, Burnett JC, Redfield MM. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol. 2001;281:H1938–H1945. doi: 10.1152/ajpheart.2001.281.5.H1938. [DOI] [PubMed] [Google Scholar]
- Hoit BD. New approaches to phenotypic analysis in adult mice. J Mol Cell Cardiol. 2001;33:27–35. doi: 10.1006/jmcc.2000.1294. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Else PL. Evolution of mammalian endothermic metabolism: Mitochondrial activity and cell composition. Am J Physiol. 1989;256:R63–R69. doi: 10.1152/ajpregu.1989.256.1.R63. [DOI] [PubMed] [Google Scholar]
- Janssen BJA, Smits JFM. Autonomic control of blood pressure in mice: Basic physiology and effects of genetic modification. Am J Physiol Reg Integrat Comp Physiol. 2002;282:R1545–R1564. doi: 10.1152/ajpregu.00714.2001. [DOI] [PubMed] [Google Scholar]
- Janssen BJA, Celle TD, Debets J, Brouns A, Callahan M, Smith T. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol. 2004;287:H1618–H1624. doi: 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- John SWM, Veress AT, Honrath U, Chong CK, Peng L, Smithies O, Sonnenberg H. Blood pressure and fluid-electrolyte balance in mice with reduced or absent ANP. Am J Physiol Reg Integr Comp Physiol. 1996;271:R109–R114. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- Kapinya KJ, Prass K, Dirnagl U. Isoflurane induced prolonged protection against cerebral ischemia in mice: A redox sensitive mechanism? Neuropharmacol Neurotox. 2002;13:1431–1435. doi: 10.1097/00001756-200208070-00017. [DOI] [PubMed] [Google Scholar]
- Kober F, Iltis I, Cozzone PJ, Bernard M. Myocardial blood flow mapping in mice using high resolution spin labeling magnetic resonance imaging: Influence of ketamine/xylazine and isoflurane anesthesia. Magn Reson Med. 2005;53:601–606. doi: 10.1002/mrm.20373. [DOI] [PubMed] [Google Scholar]
- Kofke WA, Hawkins RA, Davis DW, Biebuyck JF. Comparison of the effects of volatile anesthetics on brain glucose metabolism in rats. Anesthesiology. 1987;66:810–813. doi: 10.1097/00000542-198706000-00016. [DOI] [PubMed] [Google Scholar]
- Kohro S, Hogan QH, Nakae Y, Yamakage M, Bosnjak ZJ. Anesthetic effects on mitochondrial ATP-sensitive K channel. Anesthesiology. 2001;95:1435–1440. doi: 10.1097/00000542-200112000-00024. [DOI] [PubMed] [Google Scholar]
- Lahiri S. Blood oxygen affinity and alveolar ventilation in relation to body weight in mammals. Am J Physiol. 1975;229:529–536. doi: 10.1152/ajplegacy.1975.229.2.529. [DOI] [PubMed] [Google Scholar]
- Lambert S, Arras M, Vogt KE, Rudolph U. Isoflurane-induced surgical tolerance mediated only in part by β3-containing GABAA receptors. Eur J Pharmacol. 2005;516:23–27. doi: 10.1016/j.ejphar.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Lenz C, Rebel A, van Ackern K, Kuschinsky W, Waschke KF. Local cerebral blood flow, local cerebral glucose utilization and flow-metabolism coupling during sevoflurane versus isoflurane in rats. Anesthesiology. 1998;89:1480–1488. doi: 10.1097/00000542-199812000-00026. [DOI] [PubMed] [Google Scholar]
- Loeb AL, Raj NR, Longnecker DE. Cerebellar nitric oxide is increased during isoflurane anesthesia compared to halothane anesthesia. Anesthesiology. 1998;89:723–730. doi: 10.1097/00000542-199809000-00024. [DOI] [PubMed] [Google Scholar]
- MacGowan GA, Du C, Cowan DB, Stamm C, McGowan FX, Solaro RJ, Koretsky AP, DelNido PJ. Ischemic dysfunction in transgenic mice expressing troponin I lacking protein kinase C phosphorylation sites. Am J Physiol Heart Circ Physiol. 2001;280:H835–H843. doi: 10.1152/ajpheart.2001.280.2.H835. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Ohsaka K, Yamamoto H, Jiyouraku K, Natsume K, Hirabayashi S, Kounoike M, Inoue M. NARCOBIT: A newly developed inhalational anesthesia system for mice. Exp Anim. 2007;56:131–137. doi: 10.1538/expanim.56.131. [DOI] [PubMed] [Google Scholar]
- Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Enhanced myocardial function in transgenic mice overexpressing the beta-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- NRC [National Research Council] Guide for the Care and Use of Laboratory Animals. Washington: National Academy Press; 1996. [Google Scholar]
- Ogushi T, Kashimoto S, Yamaguchi T, Nakamura T, Kumazawa T. Is Pentobarbital appropriate for basal anesthesia in the working rat heart model? J Pharmacol Toxicol Meth. 1993;29:37–43. doi: 10.1016/1056-8719(93)90049-k. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Pressman GS, Price HL. A possible mechanism of anesthetic-induced myocardial depression. Biochem Biophys Res Comm. 1974;57:316–322. doi: 10.1016/s0006-291x(74)80392-x. [DOI] [PubMed] [Google Scholar]
- Orestes P, Bojadzic D, Chow RM, Todorovic SM. Mechanisms and functional significance of inhibition of neuronal T-type calcium channels by isoflurane. Mol Pharmacol. 2009;75:542–554. doi: 10.1124/mol.108.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori C, Dam M, Sizzoloto G, Battistin L, Giron G. Effects of isoflurane anesthesia on local cerebral glucose utilization in the rat. Anesthesiology. 1986;65:152–156. doi: 10.1097/00000542-198608000-00004. [DOI] [PubMed] [Google Scholar]
- Pagel P, Kampire JP, Schmeling WT, Warltier DC. Influence of volatile anesthetics on myocardial contractility in vivo: Desflurane versus isoflurane. Anesthesiology. 1991;74:900–907. doi: 10.1097/00000542-199105000-00016. [DOI] [PubMed] [Google Scholar]
- Park KW, Dai HB, Lowenstein E, Darvish A, Selke FW. Heterogeneous vasomotor responses of rabbit coronary microvessels to isoflurane. Anesthesiology. 1994;81:1190–1197. doi: 10.1097/00000542-199411000-00012. [DOI] [PubMed] [Google Scholar]
- Price HL. Myocardial depression by nitrous oxide and its reversal by Ca++ Anesthesiology. 1976;44:211–215. doi: 10.1097/00000542-197603000-00009. [DOI] [PubMed] [Google Scholar]
- Price HL, Ohnishi TS. Effects of anesthetics on the heart. Fed Proc. 1980;39:1575–1579. [PubMed] [Google Scholar]
- Ramirez HM, Ryan CF, Buckner CK. Studies on the temperature-dependent sensitivity of mouse atria to adrenergic drugs. Eur J Pharmacol. 1975;30:73–78. doi: 10.1016/0014-2999(75)90205-8. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. Regional cerebral blood flow in the anesthetized mouse as measured by local hydrogen clearance. Stroke. 1977;8:103–106. doi: 10.1161/01.str.8.1.103. [DOI] [PubMed] [Google Scholar]
- Roth D, Swaney J, Dalton N, Gilpin E, Ross J. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002;282:H2134–H2140. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. Uncoupling of oxidative phosphorylation in rat liver mitochondria by general anesthesia. Proc Nat Acad Sci U S A. 1983;80:3313–3317. doi: 10.1073/pnas.80.11.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff J, Wiessmann F, Hiller KH, Voll S, von Kienlin M, Bauer WR, Rommel E, Neubauer S, Haase A. Magnetic resonance microimaging for noninvasive quantification of myocardial function and mass in the mouse. Magn Res Med. 1998;40:43–48. doi: 10.1002/mrm.1910400106. [DOI] [PubMed] [Google Scholar]
- Samarska IV, van Meurs M, Buikerma H, Houwertjes MC, Wulfert FM, Molema G, Epema AH, Henning RH. Adjunct nitrous oxide normalizes vascular reactivity changes after hemorrhagic shock in mice under isoflurane anesthesia. Anesthesiology. 2009;111:600–608. doi: 10.1097/ALN.0b013e3181b31c8e. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide. Anesthesiology. 2008;109:707–22. doi: 10.1097/ALN.0b013e3181870a17. [DOI] [PubMed] [Google Scholar]
- Sarin SK, Sabba C, Groszmann RJ. Splanchnic and systemic hemodynamics in mice using a radioactive microsphere technique. Am J Physiol Gastrointest Liver Physiol. 1990;258:G365–G369. doi: 10.1152/ajpgi.1990.258.3.G365. [DOI] [PubMed] [Google Scholar]
- Sato Y, Seo N, Kobayashi E. Genetic background differences between FVB and C57BL/6 mice affect hypnotic susceptibility to pentobarbital, ketamine and nitrous oxide, but not isoflurane. Acta Anaesthesiol Scand. 2006;50:553–556. doi: 10.1111/j.1399-6576.2006.001002.x. [DOI] [PubMed] [Google Scholar]
- Schwartz LA, Zuurbier CJ, Ince C. Mechanical ventilation of mice. Basic Res Cardiol. 2000;95:510–520. doi: 10.1007/s003950070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann DW. Quantitative changes in the arterial blood gases of mice following localized irradiation of the lungs. Radiat Res. 1983;93:560–566. [PubMed] [Google Scholar]
- Stulken EH, Jr, Milde JH, Michenfelder JD, Tinker JH. The nonlinear responses of cerebral metabolism to low concentrations of halothane, enflurane, isoflurane, and thiopental. Anesthesiology. 1977;46:28–34. doi: 10.1097/00000542-197701000-00007. [DOI] [PubMed] [Google Scholar]
- Szczesny G, Veihelmann, Massberg S, Nolte D, Messmer K. Long-term anaesthesia using inhalatory isoflurane in different strains of mice: The haemodynamic effects. Lab Anim. 2004;38:64–69. doi: 10.1258/00236770460734416. [DOI] [PubMed] [Google Scholar]
- Toyama H, Ichise M, Liow JS, Modell K, Vines D, Seneca N, Modell K, Seidel J, Green MS, Innis RB. Evaluation of anesthesia effects on (18F)FDG uptake in mouse brain and heart using small animal PET. Nucl Med Biol. 2004;31:251–256. doi: 10.1016/S0969-8051(03)00124-0. [DOI] [PubMed] [Google Scholar]
- Tuli JS, Smith A, Morton DB. Stress measurements in mice after transportation. Lab Anim. 1995;29:132–138. doi: 10.1258/002367795780740249. [DOI] [PubMed] [Google Scholar]
- Weir JA. Blood pH as a factor in genetic resistance to mouse typhoid. J Inf Dis. 1949;84:252–274. doi: 10.1093/infdis/84.3.252. [DOI] [PubMed] [Google Scholar]
- Wolfe HG. Blood-pH differences in two inbred strains of mice. J Hered. 1959;50:155–158. [Google Scholar]
- Woo SK, Lee T, Kim K, Kim JY, Jung JH, Knag J, Cheon G, Choi C, Lim S. Anesthesia condition for 18F-FDG imaging of lung metastasis tumors using small animal PET. Nucl Med Biol. 2008;35:143–150. doi: 10.1016/j.nucmedbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Zuurbier CJ, Emons VM, Ince C. Hemodynamics of anesthetized ventilated mouse models: Aspects of anesthetics, fluid support, and strain. Am J Physiol Heart Circ Physiol. 2002;282:H2099–H2105. doi: 10.1152/ajpheart.01002.2001. [DOI] [PubMed] [Google Scholar]